Abstract
Objective
To improve the likelihood for survival with favorable neurologic function after cardiac arrest, we assessed a new advanced life support approach using active compression-decompression cardiopulmonary resuscitation plus an intrathoracic pressure regulator.
Design
Prospective animal investigation.
Setting
Animal laboratory.
Subjects
Female farm pigs (n = 25) (39 ± 3 kg).
Interventions
Protocol A: After 12 minutes of untreated ventricular fibrillation, 18 pigs were randomized to group A—3 minutes of basic life support with standard cardiopulmonary resuscitation, defibrillation, and if needed 2 minutes of advanced life support with standard cardiopulmonary resuscitation; group B—3 minutes of basic life support with standard cardiopulmonary resuscitation, defibrillation, and if needed 2 minutes of advanced life support with active compression-decompression plus intrathoracic pressure regulator; and group C—3 minutes of basic life support with active compression-decompression cardiopulmonary resuscitation plus an impedance threshold device, defibrillation, and if needed 2 minutes of advanced life support with active compression-decompression plus intrathoracic pressure regulator. Advanced life support always included IV epinephrine (0.05 μg/kg). The primary endpoint was the 24-hour Cerebral Performance Category score. Protocol B: Myocardial and cerebral blood flow were measured in seven pigs before ventricular fibrillation and then following 6 minutes of untreated ventricular fibrillation during sequential 5 minutes treatments with active compression-decompression plus impedance threshold device, active compression-decompression plus intrathoracic pressure regulator, and active compression-decompression plus intrathoracic pressure regulator plus epinephrine.
Measurements and Main Results
Protocol A: One of six pigs survived for 24 hours in group A versus six of six in groups B and C (p = 0.002) and Cerebral Performance Category scores were 4.7 ± 0.8, 1.7 ± 0.8, and 1.0 ± 0, respectively (p = 0.001). Protocol B: Brain blood flow was significantly higher with active compression-decompression plus intrathoracic pressure regulator compared with active compression-decompression plus impedance threshold device (0.39 ± 0.23 vs 0.27 ± 0.14 mL/min/g; p = 0.03), whereas differences in myocardial perfusion were not statistically significant (0.65 ± 0.81 vs 0.42 ± 0.36 mL/min/g; p = 0.23). Brain and myocardial blood flow with active compression-decompression plus intrathoracic pressure regulator plus epinephrine were significantly increased versus active compression-decompression plus impedance threshold device (0.40 ± 0.22 and 0.84 ± 0.60 mL/min/g; p = 0.02 for both).
Conclusion
Advanced life support with active compression-decompression plus intrathoracic pressure regulator significantly improved cerebral perfusion and 24-hour survival with favorable neurologic function. These findings support further evaluation of this new advanced life support methodology in humans.
Keywords: active compression-decompression, cardiac arrest, cardiopulmonary resuscitation, impedance threshold device, intrathoracic pressure regulation, left ventricular function, neurologic function
When optimally performed, blood flow to the heart and brain generated by manual closed chest compressions or standard cardiopulmonary resuscitation (CPR) is less than 30% of normal (1–3). Consequently, pharmacological therapy during advanced life support (ALS), including use of vasopressor agents like epinephrine, is often used to increase blood pressure, but despite such efforts, outcomes remain poor (4, 5). Fresh approaches to ALS are needed.
Several new physiological methods have been developed to augment circulation during CPR including the combination of active compression-decompression (ACD) CPR device plus an impedance threshold device (ITD) (6, 7) and an intrathoracic pressure regulator (ITPR) (8, 9); both provide intrathoracic pressure regulation therapy. These devices lower intrathoracic pressures during the decompression phase (ACD-ITD) or continuously (ITPR) during CPR after each positive pressure ventilation thereby augmenting the refilling of the heart after each compression. They also help to reduce intracranial pressure (ICP) and thus reduce resistance to forward cerebral blood flow (9–11). ACD-ITD has been shown to significantly increase survival with favorable neurologic function when compared with standard CPR in animals and in patients with out-of-hospital cardiac arrest (OHCA) (12–15). Standard CPR + ITPR has also been reported to increase circulation and short-term survival in pigs in cardiac arrest and circulation, end-tidal CO2 (ETCO2), and resuscitation rates in humans compared with standard CPR (8, 9).
Based on previously identified mechanisms of action, the primary focus of this investigation was to compare ALS with standard CPR alone versus ALS with ACD-CPR plus the ITPR (ACD-ITPR). We hypothesized that the use of combination of ACD-ITPR in a porcine model of prolonged untreated cardiac arrest would provide superior perfusion during ALS and thereby increase survival with favorable neurologic function compared with standard CPR alone. This is the first time that the combination of ACD-ITPR has been assessed during cardiac arrest. In addition, we evaluated the potential benefit of ACD-ITPR as an ALS rescue therapy when resuscitation was not possible with ALS using standard CPR.
METHODS
This study was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center. All animal care was compliant with the National Research Council’s 1996 Guidelines for the Care and Use of Laboratory Animals. All studies were performed by a qualified, experienced research team in York-shire female farm bred pigs weighing 39 ± 3 kg. A certified and licensed veterinarian assured the protocols were performed in accordance with the National Research Council’s Guidelines.
Preparatory Phase
The surgical preparation, anesthesia, data monitoring, and recording procedures used in this study have been previously described (10, 16). Under aseptic surgical conditions, initial sedation was achieved with intramuscular ketamine (10 mL of 100 mg/mL) followed by inhaled isoflurane (0.8–1.2%). Pigs were intubated with a 7.0F endotracheal tube and then ventilated with room air, using an anesthesia machine (Narkomed, Telford, PA), with a tidal volume of 10 mL/kg and a respiratory rate adjusted to continually maintain a PaCO2 of 40 mm Hg and PaO2 of 80 mm Hg (blood oxygen saturation > 95%). Normothermia was maintained with a warming blanket (Bair Hugger; Augustine Medical, Eden Prairie, MN). Central aortic blood pressure was recorded continuously with a catheter (Mikro-Tip Transducer; Millar Instruments, Houston, TX) in the descending thoracic aorta. Another catheter was inserted into the right atrium. An ultrasound flow probe (Transonic 420 series multichannel; Transonic Systems, Ithaca, NY) was used to quantify carotid blood flow (mL/min). Animals were fasted overnight. They received up to 1,000 mL of normal saline solution prior to the induction of ventricular fibrillation (VF) in order to maintain the mean right atrial pressure between 3 and 5 mm Hg. Animals received an IV heparin bolus (100 U/kg). Arterial blood gases (Gem 3000; Instrumentation Laboratory, Bedford, MA) were obtained at baseline, 15, 30, 60, and 4 hours after return of spontaneous circulation (ROSC). Electrocardiograms were continuously recorded. Hemodynamic data were continuously monitored and recorded (BIOPAC MP 150; BIOPAC Systems, Goleta, CA). Coronary perfusion pressure (CPP) was calculated as the difference between aortic and right atrial pressures during the decompression phase. ETCO2, tidal volume, minute ventilation, and blood oxygen saturation were continuously measured (COSMO Plus; Novametrix Medical Systems, Wallingford, CT). ROSC was defined using the Utstein guidelines for uniform reporting in animal research as maintenance of systolic pressure of more than or equal to 60 mm Hg for more than or equal to 10 consecutive minutes (17).
Experimental Protocol
Following the surgical preparation, VF was induced by delivering direct intracardiac current via a temporary pacing wire. Standard CPR and ACD-CPR were performed with a pneumatically driven automatic piston device (Pneumatic Compression Controller; Ambu International, Glostrup, Denmark) as previously described (6). During standard CPR, uninterrupted chest compressions were performed at a rate of 100 compressions/min, with a 50% duty cycle and a compression depth of 25% of the anteroposterior chest diameter. After each compression, the chest wall was allowed to fully recoil passively. With ACD-CPR, after each compression, the chest was actively pulled upward with suction cup attached to the skin with a decompression force of ~20 lbs (6, 18).
If basic life support (BLS) was unsuccessful, as defined as refractory VF or pulseless electrical activity after three sequential shocks, then ACD-ITPR therapy was started immediately. An ITPR device called the CirQLATOR (Advanced Circulatory Systems, Roseville, MN) was used and has been described previously (9, 19, 20). This ITPR device was connected to an endotracheal tube, a positive pressure ventilation source, and a regulated vacuum source. It actively generates a subatmospheric pressure within the thorax after each positive pressure ventilation. After each positive pressure breath (tidal volume of ~10 mL/kg and rate of 10 breaths/min) with a manual resuscitator bag, an internal mechanical switch immediately closes within the CirQLATOR, providing a means to actively withdraw respiratory gases at a pressure of −9 mm Hg relative to atmospheric pressure during the entire expiratory phase. This device generates a continuous negative pressure during the entire cycle between two positive pressure ventilations. By contrast, the ITD provides a negative pressure only during the chest recoil or decompression phase of CPR. The development of negative intrathoracic pressure during CPR with the ITD is completely dependent on the recoil of the chest; this is not the case with the CirQLATOR. As invasive ICP and cerebral perfusion measurements can adversely affect survival and neurologic outcome, we used two protocols as described below.
Protocol A
Protocol A was used to assess differences in 24-hour neurologic function based on the ALS. Following 12 minutes of untreated VF, 18 animals were randomized to three groups: group A received BLS and ALS with standard CPR, group B received BLS with standard CPR and ALS with ACD-ITPR, and group C received BLS with ACD-ITD and ALS with ACD-ITPR (Fig. 1). We examined the new ALS method of ACD-ITPR with group C, as some emergency medical services systems use ACD-ITD during BLS.
Figure 1.
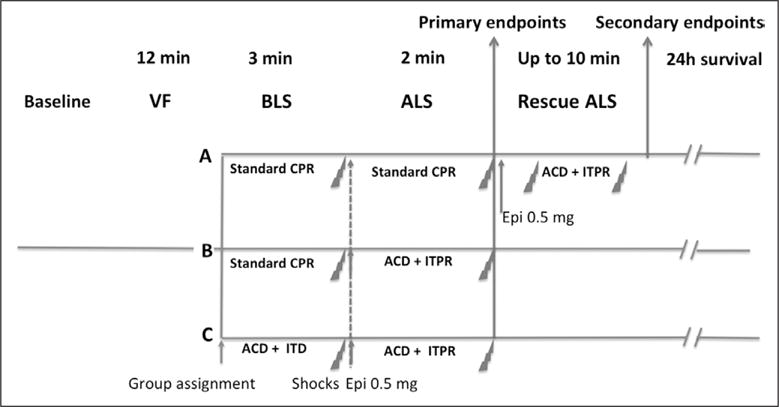
Study protocol. After 12 min of ventricular fibrillation (VF) and 3 min of basic life support (BLS) up to three 275 joules, biphasic defibrillatory shocks were delivered (Lifepak 15; Physio-Control, Redmond, WA). If return of spontaneous circulation (ROSC) was not achieved after 1–3 defibrillations, advanced life support (ALS) was started based on a randomization plan that was unblinded after induction of VF. In all groups, epinephrine (Epi) was administered as a 0.5 mg (~15 μg/kg) bolus 15 s after starting ALS through the jugular vein. A bolus of 25 mg of amiodarone was also administered in all groups after epinephrine injection. After 2 min of ALS, 1–3 shocks were delivered, as needed. As a secondary endpoint, when ROSC was not achieved in animals in group A using standard cardiopulmonary resuscitation (CPR) during ALS, then active compression-decompression (ACD) plus intrathoracic pressure regulator (ITPR) was initiated as a rescue therapy along with a second dose of 0.5 mg of epinephrine. A defibrillatory shock was delivered every 2 min thereafter until ROSC was achieved or for up to the point in time when a total of 15 min of CPR had been performed. ITD = impedance threshold device.
Post-ROSC care
After ROSC, animals were mechanically ventilated. The carotid flow probe and jugular venous catheter were removed. Supplemental oxygen was added only if arterial saturation was lower than 90% with a SaO2 target between 90% and 94%. Animals were maintained under general anesthesia with isoflurane for 4 hours. Therapeutic hypothermia was induced and sustained between 32°C and 34°C using IV cold saline (1,000 mL) and external cooling during a 4-hour period. Animals that had a stable post-ROSC rhythm but were hypotensive (mean arterial pressure, < 50 mm Hg) received increments of 0.1–0.2 mg IV epinephrine every 5 minutes until the mean arterial pressure rose above 50 mm Hg. If the arterial blood pH was lower than 7.2, then 50–100 mEq of NaHCO3 were given IV.
Animals were weaned off the ventilator 4 hours post ROSC and extubated. They were returned to their cages and observed for the first 6 hours for signs of clinical deterioration. Controlled rewarming (0.5°C/hr) to normothermia was performed at that point using a blanket and a heating lamp. The total duration of hypothermia was about 12 hours. Survivors received nonsteroidal anti-inflammatory medication, as previously described, and had free access to water and food (21).
Neurologic assessment
Twenty-four hours after ROSC, a certified veterinarian, blinded to the intervention, assessed the pigs’ neurologic function based on a Cerebral Performance Category (CPC) scoring system modified for pigs. The following scoring system was used: 1 = normal; 2 = slightly disabled; 3 = severely disabled but conscious; 4 = vegetative state; a 5 was given to animals that died in the laboratory due to unachievable ROSC or died in the cage following ROSC (21). If the veterinarian determined the pigs were in severe distress prior to the 24-hour assessment, they were euthanized per the Animal Care Committee protocol. Dichotomous assessment of good (CPC ≤ 2) versus poor (CPC > 2) outcomes was also evaluated. The Swine Neurologic Deficit Score (NDS) was also used to evaluate level of consciousness, respiratory pattern, cranial nerve function, motor and sensory function, and behavior evaluation, in 24-hour survival animals (Supplemental Table 1, http://links.lww.com/CCM/B224) (22).
Echocardiographic evaluation of left ventricular function
A transthoracic echocardiogram was obtained on all survivors 1, 4, and 24 hours post ROSC (23). Ejection fraction was assessed using Simpson’s method of volumetric analysis by an independent clinical echocardiographer blinded to the treatments (24).
Protocol B
Protocol B assessed blood flow to the heart and brain. Protocol B and A surgical procedures were similar except that in B a second femoral artery catheter was placed through which a 7F pigtail catheter was positioned in the left ventricle.
Protocol B was as follows: after 6 minutes of untreated VF, and 2 minutes of ACD-ITD CPR as a stabilization period, seven pigs received the following sequence of interventions: 5 minutes of ACD-ITD CPR, 5 minutes of ACD-ITPR CPR, and then 0.5 mg of epinephrine followed by 5 minutes of ACD-ITPR. ACD-ITD CPR was used to provide BLS in protocol B based on the results from protocol A comparing standard CPR versus ACD-ITD CPR. Cerebral and myocardial perfusion was assessed using microspheres as previously described (6, 25, 26). In the current study, 15 μm diameter neutron-activated Lanthanum (140La), Gold (198Au), Ytterbium (175Yb), and Lutetium (177Lu) microspheres (STERIspheres; BioPAL; BioPhysics Assay Laboratory, Worcester, MA) were injected into the left ventricle under stable baseline conditions just prior to the induction of VF and then 1 minute after the start of each of the study interventions (ACD-ITD, ACD-ITPR, ACD-ITPR + epinephrine). The number of microspheres injected for each intervention was computed as follow:
where μ is the required number of microspheres and ω is the pig weight.
Concurrently with the microsphere injections, reference blood samples were withdrawn from the descending aorta at a collection rate of 10 mL/min. At the end of the procedure, animals were euthanatized and brain and heart samples were obtained. Samples were desiccated and sent to the reference BioPhysics Assay Laboratory for analysis (26).
Statistical Analysis
Data were expressed as mean ± SD. Kruskal-Wallis nonparametric test and post hoc analysis were used to determine statistical significance of differences of continuous variables between groups and pairwise comparison of subgroups. The chi-square or Fisher exact test was used for comparison of proportions. All statistical tests were two-sided, and a p value of less than 0.05 was required to reject the null hypothesis. Survival was assessed at the end of the ALS phase, prior to the rescue protocol for group A. A paired t test was used to compare hemodynamic variables before and after rescue ALS.
In protocol B, significant differences between interventions were compared by paired t test. Statistical analysis was performed using SPSS 21 (IBM Corporation, Armonk, NY).
RESULTS
Protocol A
All animals were included in this analysis. No adverse device events were observed. There were no significant baseline differences between treatment groups (Table 1). During BLS and ALS, compression rates were controlled by the automated CPR device at 100 per minute. The compression depths were similar for groups A, B, and C during ALS: 5.9 ± 1.1 cm, 5.6 ± 0.8 cm, and 5.3 ± 0.2 cm, respectively (p = 0.39). The compression and decompression phase airway pressure, a surrogate for intrathoracic pressure in groups A–C, are shown in Table 1. Airway pressure during decompression was negative with ACD-ITD (−10.5 ± 0.8 mm Hg for group C during BLS) and ACD-ITPR (−11.3 ± 1.8 and −12.3 ± 2.1 for groups B and C during ALS).
TABLE 1.
Summary of Hemodynamic Variables, Survival Rates, Defibrillation Attempts, and Epinephrine Use During Basic Life Support and Advanced Life Support in Groups A, B, and C
| Group of CPR | Measure | Baseline | BLS 2 Min 30 CPR | ALS 5 Min CPR | ROSC 1 Hr | No. of Shocks | ROSC at BLS | ROSC BLS + ALS | 24-Hr Survival |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n = 18 | n = 18 | n = 16 | n = 13 | ||||||
| Group A | SBP | 102 ± 20 | 54 ± 9 | 74 ± 23 | 47 | 6 | 0/6 | 1/6 | 1/6 |
| BLS: standard | DBP | 76 ± 17 | 31 ± 11 | 44 ± 12 | 32 | ||||
| ALS: standard | CPP | 77 ± 18 | 26 ± 12 | 36 ± 12 | 25 | ||||
| CBF | 240 ± 69 | 27 ± 12 | 25 ± 9 | 99 | |||||
| ETCO2 | 39 ± 2 | 21 ± 5 | 22 ± 8 | 43 | |||||
| Airway pressure maximum | 2.6 ± 0.4 | 2.2 ± 2.4 | 2.2 ± 2.1 | 3.1 | |||||
| Airway pressure minimum | 1.5 ± 0.4 | −0.3 ± 1.4 | −0.3 ± 1.2 | 1.8 | |||||
|
| |||||||||
| Group B | SBP | 103 ± 16 | 85 ± 29 | 119 ± 28a | 80 ± 9 | 6 ± 6 | 0/6 | 6/6a | 6/6a |
| BLS: standard | DBP | 71 ± 14 | 33 ± 18 | 56 ± 19 | 44 ± 8 | ||||
| ALS: ACD-ITPR | CPP | 68 ± 20 | 29 ± 17 | 50 ± 16 | 44 ± 8 | ||||
| CBF | 243 ± 53 | 25 ± 22 | 37 ± 28 | 163 ± 48 | |||||
| ETCO2 | 40 ± 1 | 28 ± 8 | 41 ± 9a | 45 ± 4 | |||||
| Airway pressure maximum | 2.9 ± 0.8 | 1.3 ± 0.9 | −4.2 ± 3.2a | 3.2 ± 0.6 | |||||
| Airway pressure minimum | 1.7 ± 0.4 | −0.9 ± 0.4 | −11.3 ± 1.8a | 1.5 ± 0.8 | |||||
|
| |||||||||
| Group C | SBP | 107 ± 18 | 97 ± 23a | 117 ± 15a | 106 ± 25a | 5 ± 4 | 2/6 | 6/6a | 6/6a |
| BLS: ACD plus impedance threshold device | DBP | 77 ± 18 | 43 ± 19 | 57 ± 6 | 63 ± 15 | ||||
| ALS: ACD-ITPR | CPP | 76 ± 17 | 37 ± 19 | 46 ± 11 | 59 ± 16a | ||||
| CBF | 273 ± 80 | 42 ± 22 | 37 ± 18 | 208 ± 70 | |||||
| ETCO2 | 40 ± 1 | 43 ± 10a | 42 ± 4a | 45 ± 5 | |||||
| Airway pressure maximum | 3.1 ± 0.5 | 4.3 ± 2.5 | −1.9 ± 6 | 3.3 ± 0.7 | |||||
| Airway pressure minimum | 2 ± 0.4 | −10.5 ± 0.8a,b | −12.3 ± 2.1a | 1.7 ± 0.8 | |||||
CPR = cardiopulmonary resuscitation, BLS = basic life support, ALS = advanced life support, ROSC = return of spontaneous circulation, SBP = systolic blood pressure, DBP = diastolic blood pressure, CPP = coronary perfusion pressure, CBF = carotid blood flow, ETCO2 = end-tidal CO2, ACD = active compression-decompression, ITPR = intrathoracic pressure regulator.
p < 0.05 compared with group A.
p < 0.05 compared with group B.
Airway pressure was measured between positive pressure ventilation. Values are shown as mean ± SD. All pressures are in mm Hg and all flows in mL/min.
CPR Hemodynamics
Systolic blood pressure and ETCO2 were higher with group C compared with group A during BLS (p = 0.009 and p = 0.002, respectively) (Table 1). During ALS, systolic blood pressure and ETCO2 were higher in the group B compared with group A (p = 0.021 and p = 0.007, respectively) and systolic blood pressure and ETCO2 were higher with group C during ALS compared with group A (p = 0.034 and p = 0.031, respectively). Arterial blood gas values for PO2, PCO2, pH, and base deficit were not statistically different between groups A, B, and C—15, 30, 60 and 240 minutes after ROSC.
24-Hour Survival
ROSC was not achieved in any pigs in groups A and B during BLS. By contrast, ROSC was achieved in one of six pigs in group A and six of six pigs in group B with ALS (p = 0.015). Two pigs had a ROSC with BLS alone in group C and the four remaining had a ROSC after ALS. Thus, all pigs in groups B and C were resuscitated by the end of the ALS period, and all lived for 24 hours whereas one of six pigs in group A survived for 24 hours (p = 0.002) prior to the rescue therapy protocol. Neurologic function was better in pigs treated with the new ALS treatment: CPC scores were 4.7 ± 0.8 for group A (prior to the rescue therapy protocol), 1.7 ± 0.8 for the group B, and 1.0 for group C, respectively (p = 0.001). In the group B, five of six pigs had a good neurologic outcome (CPC 1 or 2) versus 0 in group A (p = 0.015).
Left Ventricular Function
Among survivors, there was no difference between groups in the left ventricular ejection fraction, respectively, for groups A (n = 1), B (n = 6), and C (n = 6) at 1 hour (55%, 50% ± 14%, and 53% ± 10%; p = 0.99) at 4 hours (40%, 57% ± 10%, and 47% ± 14%; p = 0.14), and at 24 hours (55%, 65% ± 0%, and 46% ± 10%; p = 0.12).
Rescue ALS With ACD-ITPR Protocol
If standard CPR during ALS was not effective in group A pigs, ALS with ACD-ITPR was applied as a rescue therapy. With the new ALS technique, three of five pigs achieved a ROSC when ACD-ITPR was used after ALS using standard CPR failed. As anticipated, time to ROSC trended longer in the group A with rescue ALS compared with groups B and C, 446 ± 109 versus 326 ± 51 and 265 ± 76 seconds, respectively (p = 0.07), and more epinephrine was necessary to achieve a ROSC when ACD-ITPR was used as a rescue therapy ALS in group A compared with groups B and C (1.2 ± 0.5 mg, 0.6 ± 0.2 and 0.3 ± 0.3 mg, respectively; p = 0.006).
The three pigs that were successfully resuscitated with the rescue protocol had a good neurologic outcome at 24 hours (CPC 1 and 2) (Fig. 2). The NDS evaluated only on 24-hour survivor animals showed no differences between groups (10 ± 14, 6 ± 9 and 1 ± 2 in the group A after rescue ALS, groups B and C, respectively; p = 0.138).
Figure 2.
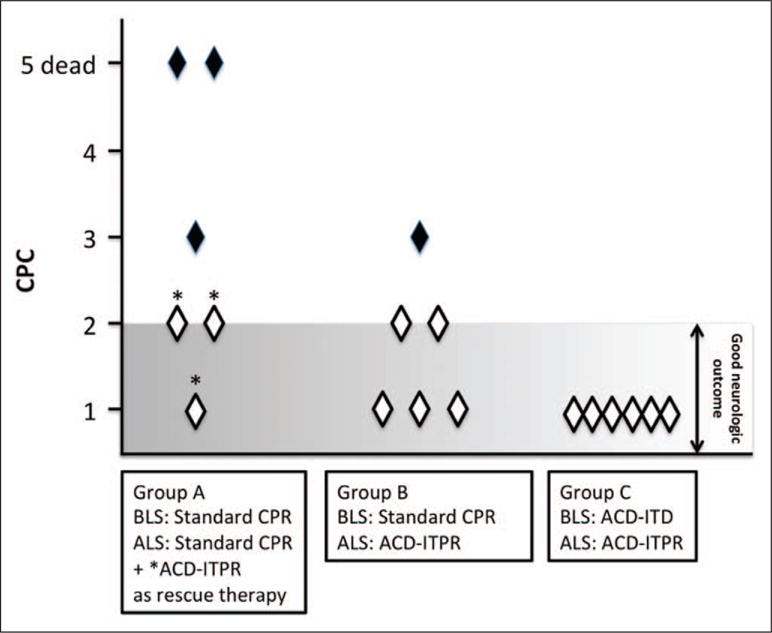
Twenty-four-hour Cerebral Performance Category (CPC) score (1= normal, 2 = mild deficit, 3 = severe deficit, 4 = coma, and 5 = dead). CPC is significantly different between groups (p = 0.001). ACD = active compression-decompression, ALS = advanced life support, BLS = basic life support, CPR = cardiopulmonary resuscitation, ITD = impedance threshold device, ITPR = intrathoracic pressure regulator.
Protocol B
ACD-ITD provided around 40% of baseline flow to heart and brain. As shown in Table 2, ACD-ITPR provided 44% more brain flow compared with ACD-ITD (0.39 ± 0.23 vs 0.27 ± 0.14 mL/min/g; p = 0.03). There were no significant differences in myocardial perfusion with comparing ACD-ITPR versus ACD-ITD. The administration of epinephrine did not significantly increase blood flow (mL/min/g) to the heart or brain compared with ACD-ITPR alone. By contrast, brain and myocardial blood flow were increased with ACD-ITPR with epinephrine versus ACD-ITD alone (0.40 ± 0.22 vs 0.27 ± 0.14 for the brain and 0.84 ± 0.60 vs 0.42 ± 0.36 for the heart [p = 0.02 for both], respectively).
TABLE 2.
Blood Flow to the Heart and Brain Measured by Neutron-Activated Microsphere
| Organ | Baseline | ACD Plus Impedance Threshold Device | ACD-ITPR | ACD-ITPR + Epinephrine |
|---|---|---|---|---|
| Brain | 0.65 ± 0.36 | 0.27 ± 0.14a | 0.39 ± 0.23b | 0.41 ± 0.22b |
| Heart | 0.89 ± 0.28 | 0.42 ± 0.36c,d | 0.65 ± 0.81 | 0.83 ± 0.60b |
ACD = active compression-decompression, ITPR = intrathoracic pressure regulator.
p < 0.05 comparing the different interventions with ACD-ITPR.
p < 0.05 comparing the different interventions with ACD plus impedance threshold device.
p < 0.05 comparing the different interventions with baseline.
p < 0.05 comparing the different interventions with ACD-ITPR + epinephrine.
DISCUSSION
Recent studies have called into question the clinical utility of current ALS approaches to patients in cardiac arrest (4, 5). No current clinical ALS algorithm has been shown to increase favorable neurologic outcomes after cardiac arrest (4, 27–30). The current study evaluated a fundamentally new approach to ALS. This new ALS approach focused on combining a new mechanical means to enhance circulation with a low dose of epinephrine. The results demonstrated that after failure of two different BLS CPR techniques (standard CPR and ACD-CPR), the use of ACD-ITPR during ALS improved hemodynamics, ROSC rates, neurologic function, and 24-hour survival rates compared with the use of standard CPR during ALS. The new ACD-ITPR ALS approach also resulted in the lowest amount of epinephrine needed to achieve ROSC when comparing the study groups. Use of ACD-ITD for BLS and ACD-ITPR for ALS provided the shortest time to ROSC and the highest overall survival with favorable 24-hour neurologic outcome.
In the present study, the untreated VF time was 12 minutes, and no pigs were successfully resuscitated after BLS or ALS with standard CPR alone or standard CPR with epinephrine, the conventional ALS protocol recommended by current American Heart Association guidelines (29). When ACD-ITPR plus epinephrine was used as a rescue therapy following failure of BLS and ALS in group A pigs treated with standard CPR for BLS and ALS, three of five pigs were successfully resuscitated and survived for 24 hours with good neurologic function.
The ITPR device has been previously shown to improve hemodynamics, blood flow, vital organ perfusion pressures, and short-term survival rates during VF cardiac arrest and standard CPR in pigs when used as the first intervention in an experimental model with 8 minutes of untreated VF without the need for exogenous epinephrine administration (9). In the earlier study, ICPs during the decompression phase of CPR were reduced compared with non-ITPR device and cerebral perfusion pressures were 60% higher. In addition, standard CPR and the ITPR device has been recently shown to significantly increase ETCO2 and ROSC rates in patients in prolonged cardiac arrest, thus demonstrating the first proof of clinical concept for the use of ITPR in humans during ALS CPR (8).
The current study is the first to report on the use of the combination of the ITPR and ACD-CPR and to measure heart and brain blood flow as well as hemodynamics and 24-hour survival rates. For the microsphere blood flow portion of the study, we compared ACD-ITD versus ACD-ITPR, as ACD-ITD provided the best outcomes in the 24-hour survival protocol. ACD-ITD has been previously shown to provide greater vital organ blood flow in animal models of cardiac arrest and higher survival rates with favorable neurologic function in patients with OHCA than standard CPR (6, 25). In the current study, we observed a 44% increase in cerebral perfusion using a microsphere technique with ACD-ITPR versus ACD-ITD. These observations provide important new mechanistic insight into the likely physiological basis for the improved 24-hour neurologic outcomes observed with the new ALS approach in the current study.
The biophysical effect of the ITPR device is conceptually similar to ACD-ITD, but the continuous negative intrathoracic pressure generated with the ITPR following each positive pressure ventilation is generated by actively withdrawing respiratory gases from the lungs rather than by active chest decompression (10). The pressure waveforms generated with standard CPR, ACD-ITD CPR, and ACD-ITPR are shown in Figure 3. During ACD-ITD, intrathoracic pressure is reduced during each CPR decompression phase, whereas with ACD-ITPR, intrathoracic pressure is continuously negative except when a positive pressure breath is delivered. We speculate that the combination of ACD-ITPR further harnesses the intrathoracic pump compared with ACD-ITD and should therefore provide additional clinical benefit during CPR, by both augmenting forward flow and by reducing ICP during the decompression phase.
Figure 3.
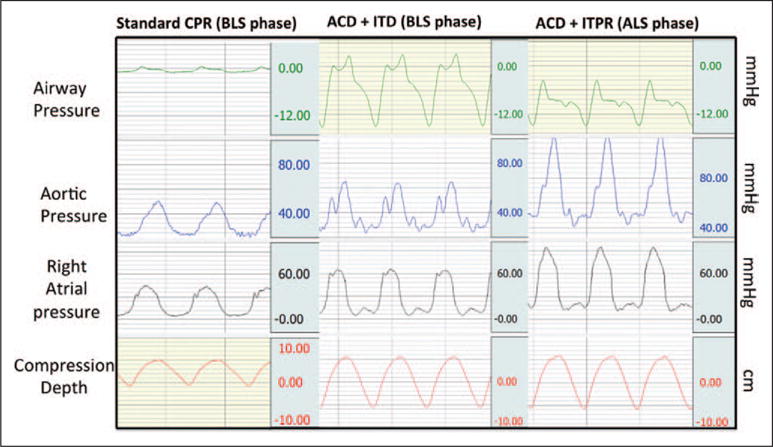
Representative example of pressure curve during the study. ACD = active compression-decompression, ALS = advanced life support, BLS = basic life support, CPR = cardiopulmonary resuscitation, ITD = impedance threshold device, ITPR = intrathoracic pressure regulator.
A potential advantage of the new ALS approach is that less epinephrine is needed to achieve 24-hour survival with favorable brain function. While epinephrine use has been associated with improved ROSC rate, higher doses may worsen outcomes (4, 27, 28). After ROSC, microcirculatory blood flow has been shown to be markedly reduced after epinephrine, resulting in greater brain ischemia (31). ITPR application in noncardiac models of hypotension increased microcirculation blood flow without exogenous epinephrine (32). These observations may help to further explain the improved neurologic outcomes observed in this current study.
LIMITATIONS
In the current study, the method of CPR could not be blinded, but the compression rate and depth were automated, well controlled, and the same between groups. Anticipating this potential limitation, the primary endpoint, 24 neurological survival, was evaluated by a veterinarian, blinded to the intervention. There was a trend toward higher CPPs and carotid flow with the ITPR: the lack of statistical significance of these findings could be related to a lack of power to demonstrate improvement for these endpoints. Another limitation relates to the design of study protocol B as it only focused on a comparison of ACD + ITD versus ACD + ITPR, as ACD + ITD has been widely studied and shown to increase survival with favorable neurologic function (12, 13). Future studies are needed to determine if the increased perfusion observed with ACD + ITPR versus ACD + ITD during ALS will result in superior neurologically sound survival outcomes.
CONCLUSION
Use of ACD-ITPR during ALS improved hemodynamics, ROSC rates, and neurological favorable survival, the primary study endpoint, compared with conventional ALS using standard CPR. ACD-ITPR also significantly improved brain blood flow compared with ACD-ITD. These positive findings provide strong support for further ALS research with ACD-ITPR.
Acknowledgments
Supported, in part, by the National Institutes of Health (grant, 1R43HL110517).
Dr. Debaty received a grant from the Region Rhône-Alpes (France) and from the Société Française de Medecine d’Urgence for a postdoctoral fellowship. He is employed by the University Hospital of Grenoble and received support for article research from the National Institutes of Health (NIH). His institution received grant support from the NIH. Dr. Metzger is an employee of Advanced Circulatory Systems (ACSI) (employed by manufacturer of devices studied—active compression-decompression, impedance threshold device [ITD], intrathoracic pressure regulator [ITPR]) and received support for article research from the NIH. Her institution received grant support and support for travel from the NIH. Dr. Rees is employed by the University of Minnesota and received support for article research from the NIH. Her institution received grant support from the NIH and Department of Defense. Mr. McKnite received support for article research from the NIH. His institution received grant support from the NIH. Dr. Puertas is employed by Advanced Circulatory. Dr. Yannopoulos is employed by the University of Minnesota and received support for article research from the NIH. He and his institution received grant support from the NIH grants for research and Medtronic Foundation grant for resuscitation. Dr. Lurie served as a board member for ACSI (he founded ACSI which makes the ITD and ITPR device used in this research), received grant support from the NIH, has a patent (coinvented the inspiratory ITD and the ITPR used in this research), has stock options with ACSI, and received support for article research from the NIH. His institution received grant support from the NIH.
References
- 1.Cohen TJ, Tucker KJ, Lurie KG, et al. Active compression-decompression. A new method of cardiopulmonary resuscitation Cardiopulmonary Resuscitation Working Group. JAMA. 1992;267:2916–2923. doi: 10.1001/jama.267.21.2916. [DOI] [PubMed] [Google Scholar]
- 2.Lurie KG, Lindner KH. Recent advances in cardiopulmonary resuscitation. J Cardiovasc Electrophysiol. 1997;8:584–600. doi: 10.1111/j.1540-8167.1997.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 3.Duggal C, Weil MH, Gazmuri RJ, et al. Regional blood flow during closed-chest cardiac resuscitation in rats. J Appl Physiol (1985) 1993;74:147–152. doi: 10.1152/jappl.1993.74.1.147. [DOI] [PubMed] [Google Scholar]
- 4.Olasveengen TM, Sunde K, Brunborg C, et al. Intravenous drug administration during out-of-hospital cardiac arrest: A randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 5.Stiell IG, Wells GA, Field B, et al. Ontario Prehospital Advanced Life Support Study Group Advanced cardiac life support in out-of-hospital cardiac arrest. N Engl J Med. 2004;351:647–656. doi: 10.1056/NEJMoa040325. [DOI] [PubMed] [Google Scholar]
- 6.Lurie KG, Coffeen P, Shultz J, et al. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91:1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 7.Lurie KG, Zielinski T, McKnite S, et al. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation. 2002;105:124–129. doi: 10.1161/hc0102.101391. [DOI] [PubMed] [Google Scholar]
- 8.Segal N, Parquette B, Ziehr J, et al. Intrathoracic pressure regulation during cardiopulmonary resuscitation: A feasibility case-series. Resuscitation. 2013;84:450–453. doi: 10.1016/j.resuscitation.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Yannopoulos D, Nadkarni VM, McKnite SH, et al. Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation. 2005;112:803–811. doi: 10.1161/CIRCULATIONAHA.105.541508. [DOI] [PubMed] [Google Scholar]
- 10.Metzger AK, Herman M, McKnite S, et al. Improved cerebral perfusion pressures and 24-hr neurological survival in a porcine model of cardiac arrest with active compression-decompression cardiopulmonary resuscitation and augmentation of negative intrathoracic pressure. Crit Care Med. 2012;40:1851–1856. doi: 10.1097/CCM.0b013e318246b9ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aufderheide TP, Lurie KG. Vital organ blood flow with the impedance threshold device. Crit Care Med. 2006;34:S466–S473. doi: 10.1097/01.CCM.0000246013.47237.86. [DOI] [PubMed] [Google Scholar]
- 12.Plaisance P, Lurie KG, Vicaut E, et al. A comparison of standard cardiopulmonary resuscitation and active compression-decompression resuscitation for out-of-hospital cardiac arrest. French Active Compression-Decompression Cardiopulmonary Resuscitation Study Group. N Engl J Med. 1999;341:569–575. doi: 10.1056/NEJM199908193410804. [DOI] [PubMed] [Google Scholar]
- 13.Aufderheide TP, Frascone RJ, Wayne MA, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: A randomised trial. Lancet. 2011;377:301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolcke BB, Mauer DK, Schoefmann MF, et al. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108:2201–2205. doi: 10.1161/01.CIR.0000095787.99180.B5. [DOI] [PubMed] [Google Scholar]
- 15.Plaisance P, Lurie KG, Vicaut E, et al. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61:265–271. doi: 10.1016/j.resuscitation.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Lurie KG, Yannopoulos D, McKnite SH, et al. Comparison of a 10-breaths-per-minute versus a 2-breaths-per-minute strategy during cardiopulmonary resuscitation in a porcine model of cardiac arrest. Respir Care. 2008;53:862–870. [PubMed] [Google Scholar]
- 17.Idris AH, Becker LB, Ornato JP, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a Task Force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Resuscitation. 1996;33:69–84. doi: 10.1016/s0300-9572(96)01055-6. [DOI] [PubMed] [Google Scholar]
- 18.Lindner KH, Pfenninger EG, Lurie KG, et al. Effects of active compression-decompression resuscitation on myocardial and cerebral blood flow in pigs. Circulation. 1993;88:1254–1263. doi: 10.1161/01.cir.88.3.1254. [DOI] [PubMed] [Google Scholar]
- 19.Convertino VA, Ryan KL, Rickards CA, et al. Optimizing the respiratory pump: Harnessing inspiratory resistance to treat systemic hypotension. Respir Care. 2011;56:846–857. doi: 10.4187/respcare.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yannopoulos D, Aufderheide TP, McKnite S, et al. Hemodynamic and respiratory effects of negative tracheal pressure during CPR in pigs. Resuscitation. 2006;69:487–494. doi: 10.1016/j.resuscitation.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Yannopoulos D, Matsuura T, Schultz J, et al. Sodium nitroprusside enhanced cardiopulmonary resuscitation improves survival with good neurological function in a porcine model of prolonged cardiac arrest. Crit Care Med. 2011;39:1269–1274. doi: 10.1097/CCM.0b013e31820ed8a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bircher N, Safar P. Cerebral preservation during cardiopulmonary resuscitation. Crit Care Med. 1985;13:185–190. doi: 10.1097/00003246-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Marino BS, Yannopoulos D, Sigurdsson G, et al. Spontaneous breathing through an inspiratory impedance threshold device augments cardiac index and stroke volume index in a pediatric porcine model of hemorrhagic hypovolemia. Crit Care Med. 2004;32:S398–S405. doi: 10.1097/01.ccm.0000139950.39972.68. [DOI] [PubMed] [Google Scholar]
- 24.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 25.Voelckel WG, Lurie KG, Sweeney M, et al. Effects of active compression-decompression cardiopulmonary resuscitation with the inspiratory threshold valve in a young porcine model of cardiac arrest. Pediatr Res. 2002;51:523–527. doi: 10.1203/00006450-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 26.Reinhardt CP, Dalhberg S, Tries MA, et al. Stable labeled microspheres to measure perfusion: Validation of a neutron activation assay technique. Am J Physiol Heart Circ Physiol. 2001;280:H108–H116. doi: 10.1152/ajpheart.2001.280.1.H108. [DOI] [PubMed] [Google Scholar]
- 27.Hagihara A, Hasegawa M, Abe T, et al. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA. 2012;307:1161–1168. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs IG, Finn JC, Jelinek GA, et al. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation. 2011;82:1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 30.Gueugniaud PY, David JS, Chanzy E, et al. Vasopressin and epinephrine vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J Med. 2008;359:21–30. doi: 10.1056/NEJMoa0706873. [DOI] [PubMed] [Google Scholar]
- 31.Ristagno G, Sun S, Tang W, et al. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35:2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 32.Segal N, Rees J, Convertino VA, et al. Improving microcirculation with therapeutic intrathoracic pressure regulation in a porcine model of hemorrhage. Resuscitation. 2011;82(Suppl 2):S16–S22. doi: 10.1016/S0300-9572(11)70146-0. [DOI] [PubMed] [Google Scholar]


