Abstract
Novel therapies for the treatment of acute myeloid leukemia are required to overcome disease resistance and to provide potentially less toxic therapies for older adults. Prior clinical trials involving patients with non-small cell lung cancer have demonstrated the safety and biologic activity of the administration of EGFR inhibitors in carefully selected patients. The potential efficacy of this approach in patients with acute myeloid leukemia is unknown. The effects of gefitinib on differentiation induction and cell viability in AML cell lines and primary patient AML cells were previously reported and cell viability was inhibited in a clinically achievable range. To determine if EGFR inhibitors would be therapeutically efficacious in advanced AML, we performed a phase II trial in which 18 patients with a median age of 72 (range, 57 to 84 years) were treated with gefitinib (750 mg orally daily). While there were no unexpected toxicities, no patients experienced an objective response, though one had stable disease lasting 16 months. We conclude that in spite of pre-clinical activity and anecdotal cases of response to EGFR inhibitors, routine use of the EGFR inhibitor gefitinib as a single agent for advanced AML is not appropriate.
Keywords: EGFR, leukemia, chemotherapy, gefitinib
INTRODUCTION
Acute myeloid leukemia (AML) is relatively rare in adults under 40 years of age, with an incidence of about 1 per 100,000, but increases dramatically to an incidence of about 15 per 100,000 at age 75 or older. There are approximately 13,780 cases of AML in the United States annually, which results in about 10,200 deaths per year.1 Without treatment AML is invariably fatal with a median survival of less than two months. While response rates to induction chemotherapy in newly diagnosed younger patients range from 50 to 70 percent the majority will relapse with the overall survival at 5 years ranging from 20 to 30 percent.2,3 Intrinsic AML resistance and concomitant organ system diseases result in markedly worse outcome in older patients.4 The development of novel and less toxic drugs with anti-leukemic activity is critical to the successful treatment of AML, particularly for these higher risk groups of patients.
AML is characterized by defects both in proliferation and differentiation with current therapy primarily focused on the proliferation defect.5 The remarkable success of all-trans retinoic acid (ATRA) therapy in the treatment of patients with acute promyelocytic leukemia (APL) supports the concept of differentiation therapy for the treatment of other subtypes of AML.6 However, clinical study of ATRA use in patients with non-APL subtypes of AML has shown little treatment efficacy. Unfortunately, at the present time, few other pharmacologic triggers of differentiation have been identified. Thus, there is a need to find novel inducers of myeloid blast differentiation.
Efforts to identify new AML differentiation agents have been hampered by lack of knowledge of an appropriate ‘druggable’ target and the inherent challenges of cell-based phenotypic screening. New genomic approaches have allowed unbiased high-throughput screening strategies which can be applied to identify and validate small-molecule inducers of AML differentiation.7,8 In a small-molecule library screen to identify AML differentiating agents by assessing for a differentiation-associated gene expression signature, an epidermal growth factor receptor (EGFR) inhibitor, 4,5-dianilinophthalimide (DAPH1), was found to induce myeloid maturation.9 The EGFR kinase inhibitor, gefitinib (Iressa), was shown to induce myeloid blast maturation and inhibit viability in AML cell lines, as well as in primary patient AML blasts, at low micromolar concentrations9. Subsequent studies confirmed the in vitro activity of gefitinib, and the structurally related EGFR inhibitor erlotinib, in AML cell lines and primary patient AML and myelodysplastic syndrome (MDS) blasts.10 Moreover, erlotinib prolonged survival in an AML xenograft study.11
Thus far, there have been no clinical trials published evaluating the efficacy of EGFR inhibitors in patients with AML. In light of the above pre-clinical data, and the excellent safety profile of these molecules, we hypothesized that this compound class is promising for AML therapy.
METHODS
Study Design and Patient population
Patients were eligible for protocol entry if they had AML and were either over 60 years and not considered a candidate for myelosuppressive chemotherapy due to age or comorbid disease, or had relapsed or refractory AML and not likely to require cytoreductive therapy within 30 days. Eligible patients had a Zubrod performance status of 2 or less, and exclusion criteria included pregnancy, active psychiatric illness, uncontrolled active infection, and prior history of pancreatitis, cerebrovascular accident or hemorrhage, or evidence of infection with HIV. Patients were required to have preserved hepatic and renal function. A total of 18 patients were enrolled between September 2005 and October 2007. The study was conducted according to the Declaration of Helsinki and its amendments. Before study entry, all patients signed an informed consent document approved by the Institutional Review Board at each institution.
Therapy
Patients who met the eligibility requirements were treated with gefitinib at a dose of 750 mg orally (three 250 mg tabs) daily until either disease progression or the development of intolerable toxicity. Gefitinib was taken with a full 8 oz glass of water. Patients were instructed to avoid the use of grapefruit juice.
Statistical methods
A true response rate of 25% would be considered active in this particular patient population. A two-stage design was used for the study to ensure that the number of patients exposed to this new treatment was minimized. If there were at least three responses among the first twenty patients, an additional twenty patients would have been entered. If at least six patients among the forty patients responded to the treatment, then the gefitinib administration would be considered a promising treatment. The probability of stopping early if the true response rate was 10% was 0.68, and 0.09, if the true response rate was 25%. If the true response rate was 25%, the probability of concluding that the drug had sufficient activity was 0.89 and 0.15 if the true rate was 10%.
The 90% confidence interval for the true response rate of the gefitinib administration, assuming that this trial continued to forty patients with observed response rate of 10%, was between 7% and 34%. If the observed response rate was 25% (10/40), the 90% confidence interval for the true response rate was between 15% and 40%.
Response and toxicity assessment
Hematologic response was defined by standard IWG criteria.12 The Common Terminology Criteria for Adverse Events (CTCAE) version 2.0 was used for coding toxicities. Toxicity data reflect all submitted data, regardless of patient exclusion from other analyses.
Immunoblotting
EGFR expression-negative MV4-11 cells and EGFR expression-positive HCC827 cells were kindly provided by Dr. Scott Armstrong and Dr. Matthew Meyerson, respectively. Primary patient AML blasts were collected from peripheral blood or bone marrow aspirate after obtaining patient informed consent. Mononuclear cells were isolated using Ficoll-Paque Plus (Amersham Biosciences) followed by red blood cell lysis. Cells were lysed in cold 1x Cell Signaling Lysis Buffer (Cell Signaling) containing Complete, EDTA-free Protease Inhibitor Cocktail Tablet (Roche Diagnostics), resolved by electrophoresis on 7% NuPAGE Novex Tris-Acetate Gels (Invitrogen) and transferred to nitrocellulose membranes (BioRad Laboratories). All proteins were detected using chemiluminescence and antibodies to EGFR (Santa Cruz, SC-03) and Actin (Neomarker, MS1295-P).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from MV4-11, HCC827 and primary patient AML cells using TRIZOL Reagent (Invitrogen). cDNA was synthesized from 1 μg of total RNA from each sample using SuperScript III Reverse Transcriptase (Invitrogen) and oligo d(T)16 primers in a 20 μL reaction system. One microliter of cDNA was amplified using HotStarTaq DNA Polymerase (Qiagen, Valencia, CA) in the MBS Satellite 0.2G Thermal Cycler (Thermo Electron Corporation, Milford, MA) in a 20 μL reaction system. PCR was performed at 94°C for 9 minutes followed by 40 cycles of denaturation at 94°C for 30 seconds, annealing at 50°C for 30 seconds, and extension at 72°C for 30 seconds. The primer pairs were as follows: glyceraldehyde-3- phosphate dehydrogenase (GAPDH): 5’-TGCGTGCCCAGTTGAACCA-3’, 5’-CAGGCGGTGACTCGGACC-3’; EGFR (epidermal growth factor receptor): 5’-GAGGGCAAATACAGCTTTGG-3’, 5’-TGGAGGTGCAGTTTTTGAAG-3’.
RESULTS
Patient Characteristics
A total of 18 patients were enrolled. Median age was 72 (range 51 to 84). Eight patients were female (44%). All patients were Caucasian and none were Hispanic or Latino. Fifteen of the patients were PS 1 (83%); two patients were PS 0 and one was PS 2. FAB classification was available for 16 of the 18 patients; among those reporting FAB there were 5 pts with M0; 4 with M1; 2 with M2; 3 with M4; and 2 with M6 AML. Ten patients had prior MDS, of whom two had been treated. Eleven patients had previously been treated (median 3 prior AML regimens, range 1 to 5). None of the patients had favorable cytognetics and 9 (50%) had poor risk cytogenetics.13 Due to competing trials, all of the patients were FLT3-ITD and D835 negative. The median time between registration and treatment initiation was 2.5 days (range 0 to 6 days). At study entry, the median percent cellularity of the biopsy sample was 45%, range 10% to 90%, n=17; median percent blasts in the marrow biopsy was 35% (range 5% to 90%), n= 12; and the median percent blasts in the marrow aspirate was 45% (range 11% to 78%), n=17. The patient with only 5% blasts in the bone marrow biopsy had a diagnosis of AML with 35% blasts in the aspirate as the biopsy was small and hypocellular.
Overall Outcomes
Five patients received a single month of gefitinib; 7 received two months; 3 received 3 months; and a single patient who experienced a prolonged stable disease, received a total of 16 months of treatment. This patient had progressive AML following two cycles of anthracycline based chemotherapy followed by gemtuzumab. His initial white blood cell count was 2,400/μL with 2% myeloblasts, hematocrit of 34% and a platelet count of 96,000/μL. His bone marrow revealed 30–40% myelobalsts throughout until he developed progressive disease. He went on to receive additional investigational therapy without benefit. There were no patients, however, who experienced an objective response on this study (0/18), so the point estimate for response is 0% and the upper 90% exact binomial confidence bound is 12%. This means that with 90% confidence we conclude that the objective response rate with single agent gefitinib in this setting is less than 12%. For this reason, the study closed to accrual when 18 patients had enrolled and had been assessed.
Toxicity
Table 2 summarizes adverse events (AEs) that were identified as definitely, probably, or possibly attributed to study drug. The most common grade 3 or higher non-hematologic AE was diarrhea, which occurred in 4 patients (22%). No serious adverse events (SAE) were noted at any time during this study.
Table 2.
Gefitinib Related Adverse Events.
| TOXICITY TYPE | GRADE(#) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| Hemoglobin | 0 | 3 | 0 | 0 | 3 |
| Neutrophils | 0 | 0 | 1 | 3 | 4 |
| Platelets | 2 | 0 | 0 | 1 | 3 |
| Fatigue | 4 | 0 | 0 | 0 | 4 |
| Weight loss | 1 | 0 | 0 | 0 | 1 |
| Dry skin | 1 | 0 | 0 | 0 | 1 |
| Rash/desquamation | 0 | 4 | 0 | 0 | 4 |
| Anorexia | 1 | 1 | 0 | 0 | 2 |
| Constipation | 1 | 0 | 0 | 0 | 1 |
| Diarrhea w/o prior colostomy | 6 | 2 | 4 | 0 | 12 |
| Muco/stomatitis (symptom) oral cavity | 1 | 0 | 0 | 0 | 1 |
| Nausea | 3 | 0 | 1 | 0 | 4 |
| Vomiting | 2 | 0 | 1 | 0 | 3 |
| GI-other | 1 | 0 | 0 | 0 | 1 |
| Infection Gr0-2 neut, skin | 0 | 1 | 0 | 0 | 1 |
| Hypoalbuminemia | 0 | 1 | 0 | 0 | 1 |
| Alkaline phosphatase | 2 | 0 | 0 | 0 | 2 |
| ALT, SGPT | 1 | 1 | 1 | 0 | 3 |
| AST, SGOT | 5 | 0 | 0 | 0 | 5 |
| Bicarbonate | 0 | 1 | 0 | 0 | 1 |
| Bilirubin | 1 | 1 | 0 | 0 | 2 |
| Hypocalcemia | 2 | 0 | 0 | 0 | 2 |
| Creatinine | 1 | 0 | 0 | 0 | 1 |
| Hyperglycemia | 3 | 0 | 0 | 0 | 3 |
| Hypomagnesemia | 1 | 0 | 0 | 0 | 1 |
| Hypokalemia | 1 | 0 | 1 | 0 | 2 |
| Hyperuricemia | 2 | 0 | 0 | 0 | 2 |
| Dizziness | 2 | 0 | 0 | 0 | 2 |
| Tearing | 0 | 1 | 0 | 0 | 1 |
| Oral cavity, pain | 0 | 1 | 0 | 0 | 1 |
| Cough | 1 | 0 | 0 | 0 | 1 |
EGFR Assessment
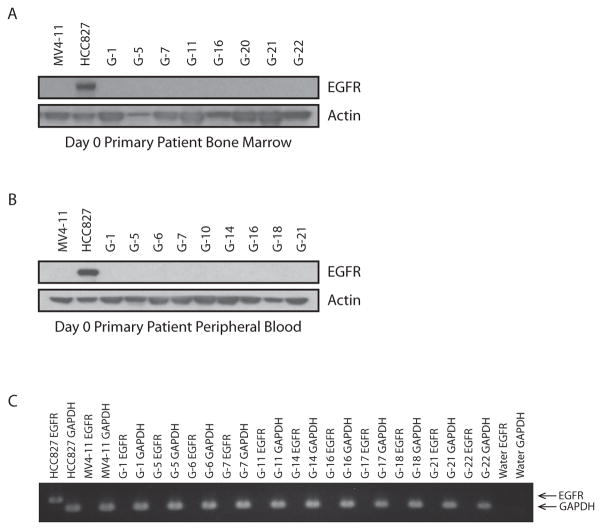
Peripheral blood and bone marrow aspirates were analyzed for the expression of the epidermal growth factor receptor (EGFR) in a subset of patients. Only patients with circulating peripheral blood myeloblasts and cellular bone marrow aspirates were analyzed. The presence of either EGFR transcript or protein could not be identified by either RT-PCR or western blotting, respectively (Figure 1).
Figure 1. Primary patient AML blasts do not express EGFR transcript or protein based on western immunoblotting and RT-PCR.
(A) Primary patient AML bone marrow samples were assessed by western immunoblotting for the expression of EGFR protein. An EGFR-expressing positive control HCC827, and an EGFR-non-expressing negative control, MV4-11, were included. Actin was evaluated as a loading control.
(B) Primary patient AML peripheral blood mononuclear cells were assessed in the same manner as in (A) for expression of EGFR protein.
(C) RT-PCR was performed to evaluate for EGFR transcript expression in primary patient AML cells. GAPDH was amplified as a control gene and an EGFR-expressing positive control, HCC827, and an EGFR-non-expressing negative control, MV4-11, were included.
DISCUSSION
The inability to cure most patients with relapsed or refractory AML dictates that novel treatment strategies for these patients must be investigated. In particular, tyrosine kinase inhibition poses an attractive approach for targeted therapy. Imatinib, in the treatment of chronic myeloid leukemia (CML), has proven this approach effective.14,15 Other tyrosine kinases have been implicated in the pathogenesis of myeloid leukemias.16 The most common tyrosine kinase abnormalities reported in AML result in the activation of FLT-3, a member of the PDGFR family of tyrosine kinases.17–19 Trials are ongoing to evaluate the effects of FLT-3 inhibitors.20,21 Undoubtedly, there are yet undiscovered tyrosine kinase dependencies involved in the pathogenesis of AML.
In a small-molecule library screen for compounds inducing an AML differentiation program, EGFR inhibitors were found to have anti-AML activity.9 Subsequently, gefitinib, and the structurally related EGFR inhibitor erlotinib, have been shown by multiple investigators to have significant anti-leukemic activity in AML cell lines and in primary patient AML and MDS blasts in vitro.10,11,22,23 Since the EGFR inhibitor gefitinib was previously approved for the treatment of patients with metastatic non-small cell lung cancer24–26 and this molecule emerged as a leading candidate for AML therapy, we proposed to test its use in patients with a poor prognosis and few alternative treatment strategies.
There have been three anectodal reports of responses to erlotinib in patients with MDS/AML.11,27,28 The in vivo effects of erlotinib were evaluated in a patient diagnosed concurrently with metastatic EGFR-positive NSCLC and MDS (RAEB-2) who received erlotinib as a single agent and only supportive care for the MDS. 11 The patient had a transient, objective hematopoietic improvement in platelet and neutrophil count (IWG-2006 criteria), though the blast percentage remained stable at approximately 15%, during the 34 days of erlotinib therapy. Platelet and blast neutrophil counts declined after erlotinib therapy was discontinued. A second patient was a 68-year old male smoker who presented with a concurrent diagnosis of NSCLC and AML (FAB classification M1).27 Because of the patient’s poor performance status, he received erlotinib as a single agent at 150 mg orally daily. Three months into treatment, he had normal complete blood counts, no circulating blasts, and a bone marrow biopsy revealing less than 3% myeloblasts, maturing trilineage hematopoiesis and mild hypocellularity for age. He ultimately died of NSCLC although his AML remained in a complete remission. A third publication reported a 68-year old male smoker also with a concurrent diagnosis of NSCLC and M1-AML.28 Because of the patient’s poor performance status, he similarly received erlotinib as a single agent at 150 mg orally daily. After three months of erlotinib, he too had normal complete blood counts and no circulating blasts, and at 7 months remained clinically well with a mildly hypocellular remission bone marrow with normal cytogenetics. Interestingly, EGFR was undetectable in myeloblasts from both of these patients, suggesting that erlotinib was acting on a non-EGFR kinase in these patients.
Despite rare reports of EGFR mutations in primary AML/MDS samples,29,30 multiple prior studies of AML cell lines and primary patient AML blasts suggest that the activity of gefitinib and erlotinib is frequently via an off-target effect. First, higher concentrations of the EGFR inhibitors are needed to achieve anti-AML activity in AML cell lines compared to the concentrations needed to achieve activity in NSCLC where EGFR is amplified or mutated. Second, both by PCR and by western immunoblotting in several responsive AML cell lines, EGFR was not identified nor was it identified by immunohistochemistry in a collection of primary patient AML blasts.9,11,23,27 Similarly, in our clinical trial, we did not identify EGFR expression in the primary patient AML cells using conventional PCR and western immunoblotting although we cannot completely exclude low levels of expression below the detection limits of these assays. Indeed, a recent publication reports the expression of EGFR in a minority of patients with AML.31 Furthermore, several alternative direct and indirect targets of these EGFR inhibitors have been proposed including JAK2, SYK, BTK, and SRC.11,32–34 Of these potential targets, there is a literature supporting a role for SRC, SYK and JAK2 activation in AML. While activating mutations in SRC and SYK have not been documented in AML, activation of these kinases and AML dependency are reported. For example, SRC family kinases (SFKs) have been reported to regulate STAT activation in AML35 with LYN demonstrated to be a major, activated SFK family member in AML cells,36 and the SFK HCK reported to be highly differentially expressed in primary human leukemia stem cells compared to human normal hematopoietic stem cells.37 SRC has also been reported to be a signaling mediator of FLT3-ITD,38 and high levels of SRC pathway activation have been reported in elderly patients with AML.39 SYK activation and dependency have been reported in AML, and integrin β2 induces SYK-dependent activation of STAT3 and STAT5, which in turn induces AML cell survival and proliferation.32,40 By contrast, while rare, mutations in JAK2 have been reported in AML.41
There are several possible explanations for the disappointing results with gefitinib in advanced AML despite the promising pre-clinical studies. First, there may be a fundamental difference in the nature of the AML in the primary samples tested in vitro and the anecdotal responders and the patients in this clinical trial. All three of the anecdotal responders had de novo disease, whereas most of the patients on this trial had relapsed disease and had received prior AML-directed chemotherapy. However, a study comparing previously untreated and relapsed patient AML samples did not observe a difference in response to gefitinib treatment in vitro though the sample size was small. 23 A second explanation is that the necessary target(s) were not expressed or activated in the patients enrolled in the trial. Unfortunately, we did not obtain adequate blasts from the one patient with sustained disease stability for 16 months to evaluate for expression of EGFR or other potential off-target proteins. A third possibility is that while the necessary target may have been expressed, we were unable to achieve adequate, sustained inhibition in vivo with gefitinib at this dose and schedule despite treating at the MTD.
In conclusion, the results of this study do not support the use of gefitinib as a single agent for patients with AML. However, EGFR inhibitors may be useful in combination with other active drugs; synergy has been reported with the combination of etoposide and gefitinib and erlotinib and azacitidine in AML23,42 and gefitinib has been reported to enhance the effects of ATRA- and arsenic trioxide-induced AML differentiation.43,44 These combination studies have been conducted in vitro. In light of the negative findings in this clinical trial, it will be important to clarify which of these combinations is also highly active in AML in vivo.
Table 1.
Patient Demographics and Disease Characteristics
| N | % | |
|---|---|---|
| Age (median, range) | 72 | (51, 84) |
| Gender | ||
| Male | 10 | 56 |
| Female | 8 | 44 |
| Race | ||
| White | 18 | 100 |
| Ethnicity | ||
| Hispanic | 0 | 0 |
| Non-Hispanic | 18 | 100 |
| Prior Diagnosis of MDS | 10 | 56 |
| Refractory AML | 11 | 61 |
| Performance Status | ||
| 0 | 2 | 11 |
| 1 | 15 | 83 |
| 2 | 1 | 6 |
| Cytogenetics | ||
| Favorable-risk | 0 | 0 |
| Intermediate-risk | 9 | 50 |
| Poor-risk | 9 | 50 |
| BM cellularity (median, range) | 45% | (10–90%) |
| BM aspirate blasts (median, range) | 45% | (11–78%) |
Footnotes
ClinicalTrials.gov Identifier: NCT00130702
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–62. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 3.Schiller G. Treatment of resistant acute myeloid leukemia. Blood Rev. 1991;5:220–6. doi: 10.1016/0268-960x(91)90012-2. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilliland DG. Hematologic malignancies. Curr Opin Hematol. 2001;8:189–91. doi: 10.1097/00062752-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Ades L, Guerci A, Raffoux E, et al. Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–6. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- 7.Stegmaier K. Genomic approaches in acute leukemia. Best Pract Res Clin Haematol. 2006;19:263–8. doi: 10.1016/j.beha.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Stegmaier K, Ross KN, Colavito SA, et al. Gene expression-based high-throughput screening(GE-HTS) and application to leukemia differentiation. Nat Genet. 2004;36:257–63. doi: 10.1038/ng1305. [DOI] [PubMed] [Google Scholar]
- 9.Stegmaier K, Corsello SM, Ross KN, et al. Gefitinib induces myeloid differentiation of acute myeloid leukemia. Blood. 2005;106:2841–8. doi: 10.1182/blood-2005-02-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehrer S, Ades L, Galluzzi L, et al. Erlotinib and gefitinib for the treatment of myelodysplastic syndrome and acute myeloid leukemia: a preclinical comparison. Biochem Pharmacol. 2008;76:1417–25. doi: 10.1016/j.bcp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Boehrer S, Ades L, Braun T, et al. Erlotinib exhibits antineoplastic off-target effects in AML and MDS: a preclinical study. Blood. 2008;111:2170–80. doi: 10.1182/blood-2007-07-100362. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 14.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 15.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 16.Lainey E, Thepot S, Bouteloup C, et al. Tyrosine kinase inhibitors for the treatment of acute myeloid leukemia: delineation of anti-leukemic mechanisms of action. Biochem Pharmacol. 2011;82:1457–66. doi: 10.1016/j.bcp.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Gilliland DG, Griffin JD. Role of FLT3 in leukemia. Curr Opin Hematol. 2002;9:274–81. doi: 10.1097/00062752-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–42. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 19.Grosjean-Raillard J, Ades L, Boehrer S, et al. Flt3 receptor inhibition reduces constitutive NFkappaB activation in high-risk myelodysplastic syndrome and acute myeloid leukemia. Apoptosis. 2008;13:1148–61. doi: 10.1007/s10495-008-0243-4. [DOI] [PubMed] [Google Scholar]
- 20.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–76. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 21.Stone RM, De Angelo J, Galinsky I, et al. PKC 412 FLT3 inhibitor therapy in AML: results of a phase II trial. Ann Hematol. 2004;83 (Suppl 1):S89–90. doi: 10.1007/s00277-004-0850-2. [DOI] [PubMed] [Google Scholar]
- 22.Lindhagen E, Nygren P, Larsson R. The fluorometric microculture cytotoxicity assay. Nat Protoc. 2008;3:1364–9. doi: 10.1038/nprot.2008.114. [DOI] [PubMed] [Google Scholar]
- 23.Lindhagen E, Eriksson A, Wickstrom M, et al. Significant cytotoxic activity in vitro of the EGFR tyrosine kinase inhibitor gefitinib in acute myeloblastic leukaemia. Eur J Haematol. 2008;81:344–53. doi: 10.1111/j.1600-0609.2008.01120.x. [DOI] [PubMed] [Google Scholar]
- 24.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. Jama. 2003;290:2149–58. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 25.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 26.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 27.Chan G, Pilichowska M. Complete remission in a patient with acute myelogenous leukemia treated with erlotinib for non small-cell lung cancer. Blood. 2007;110:1079–80. doi: 10.1182/blood-2007-01-069856. [DOI] [PubMed] [Google Scholar]
- 28.Pitini V, Arrigo C, Altavilla G. Erlotinib in a patient with acute myelogenous leukemia and concomitant non-small-cell lung cancer. J Clin Oncol. 2008;26:3645–6. doi: 10.1200/JCO.2008.17.0357. [DOI] [PubMed] [Google Scholar]
- 29.Loriaux MM, Levine, Tyner JW, et al. High-throughput sequence analysis of the tyrosine kinome in acute myeloid leukemia. Blood. 2008;111:4788–96. doi: 10.1182/blood-2007-07-101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejar R, Stevenson K, Abdel-Wahab O, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364:2496–506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JZ, Lu Y, Xu Y, et al. Epidermal growth factor receptor expression in acute myelogenous leukaemia is associated with clinical prognosis. Hematol Oncol. 2012;30:89–97. doi: 10.1002/hon.1002. [DOI] [PubMed] [Google Scholar]
- 32.Hahn CK, Berchuck JE, Ross KN, et al. Proteomic and genetic approaches identify Syk as an AML target. Cancer Cell. 2009;16:281–94. doi: 10.1016/j.ccr.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boehrer S, Galluzzi L, Lainey E, et al. Erlotinib antagonizes constitutive activation of SRC family kinases and mTOR in acute myeloid leukemia. Cell Cycle. 2011;10:3168–75. doi: 10.4161/cc.10.18.16599. [DOI] [PubMed] [Google Scholar]
- 34.Weber C, Schreiber TB, Daub H. Dual phosphoproteomics and chemical proteomics analysis of erlotinib and gefitinib interference in acute myeloid leukemia cells. J Proteomics. 2012;75:1343–56. doi: 10.1016/j.jprot.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Ozawa Y, Williams AH, Estes ML, et al. Src family kinases promote AML cell survival through activation of signal transducers and activators of transcription (STAT) Leuk Res. 2008;32:893–903. doi: 10.1016/j.leukres.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 36.Dos Santos C, Demur C, Bardet V, et al. A critical role for Lyn in acute myeloid leukemia. Blood. 2008;111:2269–79. doi: 10.1182/blood-2007-04-082099. [DOI] [PubMed] [Google Scholar]
- 37.Saito Y, Kitamura H, Hijikata A, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17ra9. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leischner H, Albers C, Grundler R, et al. SRC is a signaling mediator in FLT3-ITD- but not in FLT3-TKD-positive AML. Blood. 2012;119:4026–33. doi: 10.1182/blood-2011-07-365726. [DOI] [PubMed] [Google Scholar]
- 39.Rao AV, Valk PJ, Metzeler KH, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:5580–6. doi: 10.1200/JCO.2009.22.2547. [DOI] [PubMed] [Google Scholar]
- 40.Oellerich T, Oellerich MF, Engelke M, et al. beta2 integrin-derived signals induce cell survival and proliferation of AML blasts by activating a Syk/STAT signaling axis. Blood. 2013;121:3889–99. S1–66. doi: 10.1182/blood-2012-09-457887. [DOI] [PubMed] [Google Scholar]
- 41.Steensma DP, McClure RF, Karp JE, et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 2006;20:971–8. doi: 10.1038/sj.leu.2404206. [DOI] [PubMed] [Google Scholar]
- 42.Lainey E, Wolfromm A, Marie N, et al. Azacytidine and erlotinib exert synergistic effects against acute myeloid leukemia. Oncogene. 2012 doi: 10.1038/onc.2012.469. [DOI] [PubMed] [Google Scholar]
- 43.Miranda MB, Duan R, Thomas SM, et al. Gefitinib potentiates myeloid cell differentiation by ATRA. Leukemia. 2008;22:1624–7. doi: 10.1038/leu.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noh EK, Kim H, Park MJ, et al. Gefitinib enhances arsenic trioxide (AS2O3)-induced differentiation of acute promyelocytic leukemia cell line. Leuk Res. 2010;34:1501–5. doi: 10.1016/j.leukres.2010.02.016. [DOI] [PubMed] [Google Scholar]