Abstract
Postural instability represents a main source of disability in Parkinsonian syndromes and its pathophysiology is poorly understood. Indirect probes (i.e., mental imagery) of brain involvement support the role of prefrontal cortex as a key cortical region for postural control in older adults with and without Parkinsonian syndromes. Using functional near infrared spectroscopy (fNIRs) as a direct online cortical probe, this study aimed to compare neural activation patterns in prefrontal cortex, postural stability, and their respective interactions, in (1) patients with Parkinsonian syndromes; (2) those with mild parkinsonian signs; (3) and healthy older adults. Among 269 non-demented older adults (76.41±6.70 years, 56% women), 26 individuals presented with Parkinsonian syndromes (Unified Parkinson’s disease rating scale (UPDRS): 11.08±3.60), 117 had mild parkinsonian signs (UPDRS: 3.21±2.49), and 126 individuals were included as a healthy control group. Participants were asked to stand upright and count silently for ten seconds while changes in oxygenated hemoglobin levels over prefrontal cortex were measured using fNIRs. We simultaneously evaluated postural stability with center of pressure velocity data recorded on an instrumented walkway. Compared to healthy controls and patients with mild parkinsonian signs, patients with Parkinsonian syndromes demonstrated significantly higher prefrontal oxygenation levels to maintain postural stability. The pattern of brain activation and postural control of participants with mild parkinsonian signs were similar to that of normal controls. These findings highlight the online role of the prefrontal cortex in postural control in patients with Parkinsonian syndromes and afford the opportunity to improve therapeutic options for postural instability.
Keywords: Parkinsonian syndromes, Prefrontal cortex, Aging, Postural control, Functional near infrared, spectroscopy
1. Introduction
Postural instability represents a main limitation of older adults with Parkinson’s disease (PD; Post et al., 2007; Muslimovic et al., 2008) as it contributes to falls (Kerr et al., 2010; Johnson et al., 2013), gait disorders (Chastan et al., 2009), disability (Muslimovic et al., 2008) and death (Auyeung et al., 2012; Cilia et al., 2014). Postural control mechanisms depend on sensory information received from the visual, proprioceptive, and vestibular systems as well as appropriate motor outputs. As postural conditions become more challenging (e.g., standing on a narrow support, unipedal stance, or even dual-tasking), regions including the prefrontal cortex (PFC; Mihara et al., 2008) and parietal lobes (Mihara et al., 2008; Huang and Hwang, 2013) become progressively more involved in its monitoring. An increase in cortical involvement has been demonstrated in normal aging (Zwergal et al., 2012; Sullivan et al., 2009), especially during challenging conditions (Goble et al., 2011). However, the conclusions of these studies demonstrating the cortical involvement on postural control have been limited by small sample sizes and do not include patients with Parkinsonian syndromes (PS). Studying the online neural correlates of postural control represents a technical challenge. Most previous studies examining postural control have employed indirect methods such as mental imagery of standing (Zwergal et al., 2012; Malouin et al., 2003; Jahn et al., 2004), virtual reality (Basso Moro et al., 2014; Ferrari et al., 2014) or even simulated active balance during supine position (Karim et al., 2014) instead of measuring activity online during actual standing. Findings from these studies employing indirect methods to assess cortical postural control suggest that the prefrontal cortex plays a critical role in healthy younger adults (Basso Moro et al., 2014; Ferrari et al., 2014) and in patients with neurological conditions like stroke (Fujimoto et al., 2014). However, in order to better understand the mechanism of postural instability in healthy older adults and in patients with PS, direct online cortical measurement in the prefrontal regions during upright standing is needed. Functional near-infrared spectroscopy (fNIRs) is a non-invasive neuroimaging technique that enables the direct measurement of cerebral activity in the prefrontal regions during standing, and helps circumvent the limitations of other neuroimaging methods to measure or assess prefrontal activity directly during task performance (Basso Moro et al., 2014; Fujimoto et al., 2014; Karim et al., 2013a).
The current study addressed the knowledge gap regarding online prefrontal neural correlates of postural control in PS. Studying PS patients not only has clinical relevance as it a common neurodegenerative condition in aging but also contrasting this disease group with individuals with normal aging and mild parkinsonian signs provides insights to aging effects on prefrontal postural control mechanisms. Oxygenated hemoglobin activation in the prefrontal areas was measured directly using fNIRs during upright standing in non-demented older adults. Specifically, we compared brain activation patterns in the PFC during postural performance, in patients with PS, to participants with mild parkinsonian signs (MPS) – transitional state between normal aging and Parkinsonian syndromes – and to healthy older participants without any MPS. Based on the role of the PFC in postural control in neurological conditions (Fujimoto et al., 2014; Mihara et al., 2012) and the neural inefficiency hypothesis which posits that greater brain activation is required to perform equal or worse behavioral performance (Holtzer et al., 2009), we hypothesize that patients with PS would demonstrate greater prefrontal activation and worse postural stability throughout the postural control task, compared to both healthy older adults and individuals with MPS.
2. Results
2.1. Demographics
A total of 269 non-demented adults age 65 and older were included in the current study (mean age: 76.41±6.70 years, 56% women). All participants were considered to be non-demented as determined by their AD8 scores (Galvin et al., 2005) and consensus diagnostic case conference (Holtzer et al., 2008). Additionally, participants were relatively healthy and cognitively intact as determined by their overall global health status score (GHS; 1.15±1.11) and overall cognitive functioning standard score on the Repeatable Battery for Assessment of Neuropsychological Status (RBANS; 92±12). All participants were categorized into one of three groups: MPS, PS, or healthy control (i.e., normal). As in our previous studies (Allali et al., 2014a; Mahoney et al., 2014), MPS were systematically ascertained in participants by the study clinician using the motor evaluation portion (Part III) of the original version of the Unified Parkinson’s Disease Rating Scale (UPDRS; Fahn and Elton, 1987). MPS diagnosis was based on the presence of any one of the four cardinal features (bradykinesia, rigidity, rest tremor, or postural instability and gait disturbance, regardless of its severity (see Section 4 for specific details). For a diagnosis of PS, we applied the United Kingdom Parkinson’s disease society brain bank clinical diagnostic criteria (Hughes et al., 1992) where presence of moderate to severe bradykinesia was required in addition to the presence of one additional cardinal feature. All other participants without presence of any cardinal features constituted the normal group. Of the 269 participants, 126 were considered normal, 117 presented with MPS, and 26 were diagnosed with PS.
Baseline characteristics of the sample are provided in Table 1 for each of the three diagnostic groups. Bradykinesia and rigidity were present in all PS patients, whereas PIGD was present in 50% and tremor in 15%. Within the MPS group, rigidity was the most common MPS domain (37%), followed by bradykinesia (23%), PIGD (19%), and tremor (6%). Participants in the control group were significantly younger than those in both the MPS and PS groups; as well, individuals in the MPS group were significantly younger than those in the PS group. Compared to healthy normal adults, those with MPS had significantly lower performance on the RBANS and those with PS endorsed significantly more symptoms of depression.
Table 1.
Sample characteristics.
| Normal (n=126) | MPS (n=117) | PS (n=26) | ||
|---|---|---|---|---|
| Demographics# | Age (years)* | 74.41 (6.12) | 77.50 (6.72) | 81.23 (5.93) |
| Education (years) | 14.44 (3.00) | 14.36 (3.08) | 13.81 (2.40) | |
| % Female | 55.00 | 57.00 | 58.00 | |
| % Caucasian | 89.00 | 83.00 | 96.00 | |
| GHS score (0–10) | 0.99 (0.97) | 1.26 (1.26) | 1.39 (0.94) | |
| GDS score (0–30)\widehat | 4.12 (3.14) | 4.90 (4.04) | 6.15 (3.88) | |
| RBANS total standard score (55–145)¥ | 93.75 (11.10) | 89.82 (12.85) | 91.04 (11.12) | |
| MPS Domains# | Bradykinesia (% present) | 0.00 | 31.00 | 100.00 |
| Rigidity (% present) | 0.00 | 63.00 | 100.00 | |
| Tremor (% present) | 0.00 | 9.00 | 15.00 | |
| PIGD (% present) | 0.00 | 32.00 | 50.00 | |
| MPS severity score (0–36) | 0.00 (0.00) | 3.21 (2.49) | 11.08 (3.60) | |
| fNIRS# | Mean HbO2 value | 0.03 (0.21) | 0.07 (0.21) | 0.05 (0.27) |
| Balance# | COP velocity (cm/s) | 1.51 (3.14) | 1.56 (1.92) | 2.82 (7.45) |
Abbreviations: MPS=mild parkinsonian signs; PS=parkinsonian syndrome; PIGD=postural instability and gait disturbances; GHS=global health score; GDS=geriatric depression scale; RBANS=Repeatable Battery for the Assessment of Neuropsychological Status; HbO2 =oxygenated hemoglobin averaged across 16 channels and 8 second recording time; COP=Center of pressure average across 8 second recording time.
Mean (SD) unless otherwise noted.
Group differences in demographics: significant difference at the p<0.01 for all group comparisons (normal vs. MPS, normal vs. PS, & MPS vs. PS).
\widehat: Group differences in demographics: significant difference at the p<0.01 for normal vs. PS.
Group differences in demographics: significant differences at the p<0.01 for normal vs. MPS.
Oxygenated hemoglobin (HbO2) data recorded from 16 fNIRs channels were used to characterize changes in activation over prefrontal cortex during the postural control task. Here, relative changes in the concentrations of HbO2 were obtained by comparing the measurements made during the first two seconds to those made during the remaining eight seconds of our ten-second postural control task. Sampling interval of fNIRs activity was set at 500 ms, which afforded 16 time points during the eight-second task. In terms of overall HbO2 values averaged across all time points and channels, healthy controls demonstrated significantly less oxygenated hemoglobin levels compared to both individuals with MPS (p=0.04) and patients with PS (p<0.001).
Postural control assessments were conducted on an instrumented walkway that utilizes ProtoKinetics Movement Analysis Software (PKMAS; Zenometrics, LLC; Peekskill, NY) and the center of pressure (COP) velocity (cm/s) was used a proxy for postural control and sway while participants were ask to stand and count silently in numerical order. In terms of postural stability (i.e., overall COP velocity (cm/s; averaged across all time points), healthy controls and individuals with MPS demonstrated similar levels of sway (p=0.81) that were significantly less than patients with PS (p<0.001).
2.2. Linear mixed effects model results
Three separate linear mixed effects models (LMEMs), each adjusted for age, gender, and ethnicity were used to examine the main effects of group, time and HbO2, as well as their second- and third-order interaction effects. All LMEMs employed a first-order autoregressive covariance type and a random intercept that was included to allow for variability across individuals (i.e., subject was treated as a random effect). The advantage of the linear mixed effects model is that the heterogeneity and correlation of repeated measures under different conditions are taken into account (Laird and Ware, 1982). A preliminary LMEM model to test a main effect of lateralization reports no main effect of HbO2 by hemi-sphere or interaction with the group status.
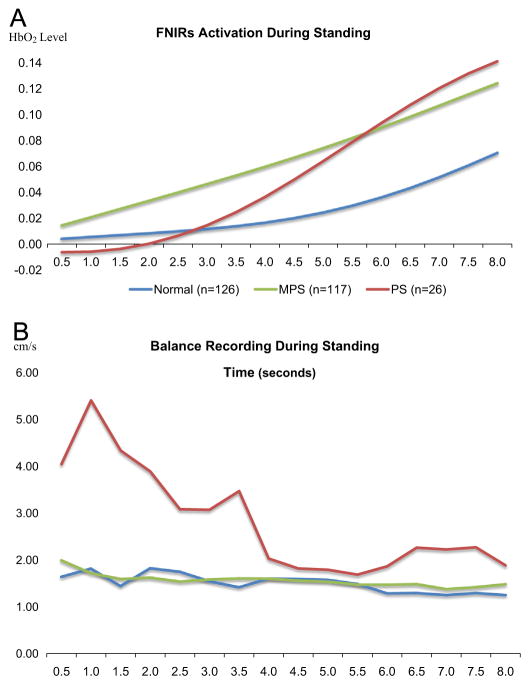
Our first LMEM aimed to test the hypothesis that patients with PS would demonstrate greater prefrontal activation throughout the postural control task compared to healthy older adults. This model examined the effect of group status (MPS, PS, and normal), time (8 s), and their interaction on averaged HbO2 activation levels during the postural control task. Results indicated no main effect of group status (p=0.17) on overall level of prefrontal activation; however there was a significant overall effect of time (p<0.01). Further, there were significant differences in prefrontal activation levels between controls and individuals with MPS during the second half of the task (i.e., seconds 5.5–8) relative to the prefrontal activation at 0.5 s (Table 2). Similarly, there were significant differences in prefrontal activation levels between controls and patients with PS from seconds 6 to 8 relative to the prefrontal activation at 0.5 s. In fact, the differences in prefrontal activation between controls and patients with PS (β=0.08) was nearly twice as large as the differences in prefrontal activation between controls and participants with MPS (β=0.04). These specific effects reveal that compared to controls, individuals with MPS, and more specifically PS demonstrate a need for significantly greater HbO2 activation (relative HbO2 activation at the first time point) in prefrontal regions in order to successfully complete this postural control task (see Fig. 1 – Panel A).
Table 2.
Linear mixed effect model of mean HbO2 with the following predictors: (1) group; (2) time; and (3) group × timea.
| Parameter | Estimate | Std. error | df | t | Sig. | 95% confidence interval
|
||
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| (1) | Control vs. MPS | 0.01 | 0.02 | 795.69 | 0.24 | 0.81 | −0.04 | 0.05 |
| Control vs. PS | −0.02 | 0.04 | 784.09 | −0.55 | 0.58 | −0.10 | 0.06 | |
| (2) | Time: 0.5 vs. 1.0 | 0.00 | 0.02 | 2260.27 | 0.08 | 0.94 | −0.04 | 0.04 |
| Time: 0.5 vs. 1.5 | 0.00 | 0.02 | 2409.06 | 0.15 | 0.88 | −0.03 | 0.04 | |
| Time: 0.5 vs. 2.0 | 0.00 | 0.02 | 2573.27 | 0.23 | 0.82 | −0.03 | 0.04 | |
| Time: 0.5 vs. 2.5 | 0.01 | 0.02 | 2753.28 | 0.32 | 0.75 | −0.03 | 0.04 | |
| Time: 0.5 vs. 3.0 | 0.01 | 0.02 | 2948.54 | 0.43 | 0.67 | −0.03 | 0.04 | |
| Time: 0.5 vs. 3.5 | 0.01 | 0.02 | 3157.09 | 0.56 | 0.57 | −0.02 | 0.04 | |
| Time: 0.5 vs. 4.0 | 0.01 | 0.02 | 3374.89 | 0.75 | 0.46 | −0.02 | 0.05 | |
| Time: 0.5 vs. 4.5 | 0.02 | 0.02 | 3594.97 | 0.99 | 0.32 | −0.02 | 0.05 | |
| Time: 0.5 vs. 5.0 | 0.02 | 0.02 | 3806.79 | 1.32 | 0.19 | −0.01 | 0.05 | |
| Time: 0.5 vs. 5.5 | 0.03 | 0.01 | 3995.93 | 1.77 | 0.08 | 0.00 | 0.05 | |
| Time: 0.5 vs. 6.0 | 0.03 | 0.01 | 4144.73 | 2.36 | 0.02 | 0.01 | 0.06 | |
| Time: 0.5 vs. 6.5 | 0.04 | 0.01 | 4234.39 | 3.18 | <0.01 | 0.02 | 0.06 | |
| Time: 0.5 vs. 7.0 | 0.05 | 0.01 | 4248.54 | 4.35 | <0.01 | 0.03 | 0.07 | |
| Time: 0.5 vs. 7.5 | 0.06 | 0.01 | 4177.48 | 6.21 | <0.01 | 0.04 | 0.07 | |
| Time: 0.5 vs. 8.0 | 0.07 | 0.01 | 4021.58 | 10.07 | <0.01 | 0.05 | 0.08 | |
| (3) | [Time: 0.5 vs. 1.0]*[Control vs. MPS] | 0.00 | 0.03 | 2260.27 | 0.17 | 0.86 | −0.05 | 0.06 |
| [Time: 0.5 vs. 1.5]*[Control vs. MPS] | 0.01 | 0.03 | 2409.06 | 0.36 | 0.72 | −0.04 | 0.06 | |
| [Time: 0.5 vs. 2.0]*[Control vs. MPS] | 0.01 | 0.03 | 2573.27 | 0.55 | 0.58 | −0.04 | 0.07 | |
| [Time: 0.5 vs. 2.5]*[Control vs. MPS] | 0.02 | 0.03 | 2753.28 | 0.75 | 0.45 | −0.03 | 0.07 | |
| [Time: 0.5 vs. 3.0]*[Control vs. MPS] | 0.02 | 0.03 | 2948.54 | 0.95 | 0.34 | −0.03 | 0.07 | |
| [Time: 0.5 vs. 3.5]*[Control vs. MPS] | 0.03 | 0.02 | 3157.09 | 1.16 | 0.25 | −0.02 | 0.08 | |
| [Time: 0.5 vs. 4.0]*[Control vs. MPS] | 0.03 | 0.02 | 3374.89 | 1.36 | 0.17 | −0.01 | 0.08 | |
| [Time: 0.5 vs. 4.5]*[Control vs. MPS] | 0.04 | 0.02 | 3594.97 | 1.57 | 0.12 | −0.01 | 0.08 | |
| [Time: 0.5 vs. 5.0]*[Control vs. MPS] | 0.04 | 0.02 | 3806.79 | 1.78 | 0.07 | 0.00 | 0.08 | |
| [Time: 0.5 vs. 5.5]*[Control vs. MPS] | 0.04 | 0.02 | 3995.93 | 2.00 | 0.04 | 0.00 | 0.08 | |
| [Time: 0.5 vs. 6.0]*[Control vs. MPS] | 0.04 | 0.02 | 4144.73 | 2.24 | 0.03 | 0.01 | 0.08 | |
| [Time: 0.5 vs. 6.5]*[Control vs. MPS] | 0.04 | 0.02 | 4234.39 | 2.51 | 0.01 | 0.01 | 0.08 | |
| [Time: 0.5 vs. 7.0]*[Control vs. MPS] | 0.04 | 0.02 | 4248.54 | 2.86 | <0.01 | 0.01 | 0.08 | |
| [Time: 0.5 vs. 7.5]*[Control vs. MPS] | 0.04 | 0.01 | 4177.48 | 3.39 | <0.01 | 0.02 | 0.07 | |
| [Time: 0.5 vs. 8.0]*[Control vs. MPS] | 0.04 | 0.01 | 4021.58 | 4.57 | <0.01 | 0.02 | 0.06 | |
| [Time: 0.5 vs. 1.0]*[Control vs. PS] | 0.00 | 0.05 | 2260.27 | −0.02 | 0.98 | −0.09 | 0.09 | |
| [Time: 0.5 vs. 1.5]*[Control vs. PS] | 0.00 | 0.05 | 2409.06 | −0.01 | 0.99 | −0.09 | 0.09 | |
| [Time: 0.5 vs. 2.0]*[Control vs. PS] | 0.00 | 0.04 | 2573.27 | 0.05 | 0.96 | −0.09 | 0.09 | |
| [Time: 0.5 vs. 2.5]*[Control vs. PS] | 0.01 | 0.04 | 2753.28 | 0.16 | 0.88 | −0.08 | 0.09 | |
| [Time: 0.5 vs. 3.0]*[Control vs. PS] | 0.01 | 0.04 | 2948.54 | 0.31 | 0.76 | −0.07 | 0.10 | |
| [Time: 0.5 vs. 3.5]*[Control vs. PS] | 0.02 | 0.04 | 3157.09 | 0.51 | 0.61 | −0.06 | 0.10 | |
| [Time: 0.5 vs. 4.0]*[Control vs. PS] | 0.03 | 0.04 | 3374.89 | 0.75 | 0.45 | −0.05 | 0.11 | |
| [Time: 0.5 vs. 4.5]*[Control vs. PS] | 0.04 | 0.04 | 3594.97 | 1.03 | 0.30 | −0.04 | 0.12 | |
| [Time: 0.5 vs. 5.0]*[Control vs. PS] | 0.05 | 0.04 | 3806.79 | 1.35 | 0.18 | −0.02 | 0.12 | |
| [Time: 0.5 vs. 5.5]*[Control vs. PS] | 0.06 | 0.04 | 3995.93 | 1.70 | 0.09 | −0.01 | 0.13 | |
| [Time: 0.5 vs. 6.0]*[Control vs. PS] | 0.07 | 0.03 | 4144.73 | 2.07 | 0.04 | 0.00 | 0.13 | |
| [Time: 0.5 vs. 6.5]*[Control vs. PS] | 0.07 | 0.03 | 4234.39 | 2.50 | 0.01 | 0.02 | 0.13 | |
| [Time: 0.5 vs. 7.0]*[Control vs. PS] | 0.08 | 0.03 | 4248.54 | 2.99 | <0.01 | 0.03 | 0.13 | |
| [Time: 0.5 vs. 7.5]*[Control vs. PS] | 0.08 | 0.02 | 4177.48 | 3.69 | <0.01 | 0.04 | 0.12 | |
| [Time: 0.5 vs. 8.0]*[Control vs. PS] | 0.08 | 0.02 | 4021.58 | 5.08 | <0.01 | 0.05 | 0.11 | |
Adjusted for age, gender, and ethnicity.
Fig. 1.
(A) Averaged oxygenated hemoglobin (HbO2) levels across 16 consecutive seconds for normal controls (blue trace), individuals with MPS (green trace), and individuals with PS (red trace). (B) Averaged COP velocity across 16 consecutive seconds for normal controls (blue trace), individuals with MPS (green trace), and individuals with PS (red trace). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Our second LMEM model tested the hypothesis that patients with PS would demonstrate greater postural instability throughout the task compared to healthy older adults. This model examined the effect of group status (MPS, PS, and normal), time (8 s), and their interaction on COP velocity. Results indicated a main effect of group status on overall COP velocity (p<0.01), but no significant effect of time (p=0.44). The main effect of group status was primarily driven by the difference in overall COP velocity between controls and patients with PS (p<0.01; Table 3). The overall group status × time interaction was not significant (p=0.68), but there were significant differences between controls and patients with PS at 5.5 s. This effect reveals that patients with PS demonstrated significantly more postural instability (increased COP velocity specifically at the beginning of the task) during the postural control task compared to controls (see Fig. 1 – Panel B), relative to the first 0.5 s. There were no significant main effects or interactions between healthy controls and individuals with MPS for this analysis.
Table 3.
Linear mixed effect model of mean COP velocitya with the following predictors: (1) group; (2) time; (3) Group × timeb.
| Parameter | Estimate | Std. error | df | t | Sig. | 95% confidence interval
|
||
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| 1) | Control vs. MPS | 0.04 | 0.08 | 1875.69 | 0.53 | 0.60 | −0.12 | 0.20 |
| Control vs. PS | 0.46 | 0.14 | 1854.75 | 3.41 | <0.01 | 0.20 | 0.73 | |
| 2) | Time: 0.5 vs. 1.0 | 0.04 | 0.08 | 1938.67 | 0.56 | 0.58 | −0.11 | 0.20 |
| Time: 0.5 vs. 1.5 | 0.02 | 0.08 | 1951.40 | 0.26 | 0.80 | −0.13 | 0.17 | |
| Time: 0.5 vs. 2.0 | −0.02 | 0.08 | 1970.08 | −0.23 | 0.82 | −0.17 | 0.14 | |
| Time: 0.5 vs. 2.5 | 0.04 | 0.08 | 1997.40 | 0.50 | 0.61 | −0.11 | 0.19 | |
| Time: 0.5 vs. 3.0 | 0.02 | 0.08 | 2037.25 | 0.32 | 0.75 | −0.13 | 0.18 | |
| Time: 0.5 vs. 3.5 | −0.03 | 0.08 | 2095.29 | −0.35 | 0.73 | −0.18 | 0.13 | |
| Time: 0.5 vs. 4.0 | −0.05 | 0.08 | 2179.71 | −0.63 | 0.53 | −0.20 | 0.10 | |
| Time: 0.5 vs. 4.5 | −0.08 | 0.08 | 2302.49 | −1.10 | 0.27 | −0.24 | 0.07 | |
| Time: 0.5 vs. 5.0 | 0.01 | 0.08 | 2480.94 | 0.12 | 0.90 | −0.14 | 0.16 | |
| Time: 0.5 vs. 5.5 | 0.04 | 0.08 | 2738.86 | 0.50 | 0.62 | −0.11 | 0.19 | |
| Time: 0.5 vs. 6.0 | −0.10 | 0.07 | 3103.44 | −1.37 | 0.17 | −0.25 | 0.04 | |
| Time: 0.5 vs. 6.5 | −0.08 | 0.07 | 3581.95 | −1.08 | 0.28 | −0.22 | 0.06 | |
| Time: 0.5 vs. 7.0 | −0.10 | 0.07 | 4075.94 | −1.49 | 0.14 | −0.23 | 0.03 | |
| Time: 0.5 vs. 7.5 | −0.05 | 0.06 | 4231.16 | −0.76 | 0.45 | −0.16 | 0.07 | |
| Time: 0.5 vs. 8.0 | −0.08 | 0.05 | 3630.88 | −1.61 | 0.11 | −0.17 | 0.02 | |
| 3) | [Time: 0.5 vs. 1.0]*[Control vs. MPS] | −0.03 | 0.11 | 1938.67 | −0.27 | 0.78 | −0.25 | 0.19 |
| [Time: 0.5 vs. 1.5]*[Control vs. MPS] | 0.02 | 0.11 | 1951.40 | 0.15 | 0.88 | −0.20 | 0.24 | |
| [Time: 0.5 vs. 2.0]*[Control vs. MPS] | 0.09 | 0.11 | 1970.08 | 0.79 | 0.43 | −0.13 | 0.31 | |
| [Time: 0.5 vs. 2.5]*[Control vs. MPS] | 0.01 | 0.11 | 1997.40 | 0.11 | 0.91 | −0.21 | 0.23 | |
| [Time: 0.5 vs. 3.0]*[Control vs. MPS] | 0.08 | 0.11 | 2037.25 | 0.70 | 0.48 | −0.14 | 0.30 | |
| [Time: 0.5 vs. 3.5]*[Control vs. MPS] | 0.12 | 0.11 | 2095.29 | 1.03 | 0.30 | −0.10 | 0.34 | |
| [Time: 0.5 vs. 4.0]*[Control vs. MPS] | 0.13 | 0.11 | 2179.71 | 1.20 | 0.23 | −0.08 | 0.35 | |
| [Time: 0.5 vs. 4.5]*[Control vs. MPS] | 0.10 | 0.11 | 2302.49 | 0.94 | 0.35 | −0.11 | 0.32 | |
| [Time: 0.5 vs. 5.0]*[Control vs. MPS] | 0.05 | 0.11 | 2480.94 | 0.41 | 0.68 | −0.17 | 0.26 | |
| [Time: 0.5 vs. 5.5]*[Control vs. MPS] | −0.06 | 0.11 | 2738.86 | −0.56 | 0.57 | −0.28 | 0.15 | |
| [Time: 0.5 vs. 6.0]*[Control vs. MPS] | 0.11 | 0.11 | 3103.44 | 1.04 | 0.30 | −0.10 | 0.32 | |
| [Time: 0.5 vs. 6.5]*[Control vs. MPS] | 0.09 | 0.10 | 3581.95 | 0.90 | 0.37 | −0.11 | 0.30 | |
| [Time: 0.5 vs. 7.0]*[Control vs. MPS] | 0.06 | 0.10 | 4075.94 | 0.61 | 0.54 | −0.13 | 0.25 | |
| [Time: 0.5 vs. 7.5]*[Control vs. MPS] | 0.02 | 0.09 | 4231.16 | 0.27 | 0.79 | −0.15 | 0.19 | |
| [Time: 0.5 vs. 8.0]*[Control vs. MPS] | 0.07 | 0.07 | 3630.88 | 0.98 | 0.33 | −0.07 | 0.20 | |
| [Time: 0.5 vs. 1.0]*[Control vs. PS] | −0.19 | 0.19 | 1938.67 | −0.99 | 0.32 | −0.56 | 0.18 | |
| [Time: 0.5 vs. 1.5]*[Control vs. PS] | −0.20 | 0.19 | 1951.40 | −1.06 | 0.29 | −0.57 | 0.17 | |
| [Time: 0.5 vs. 2.0]*[Control vs. PS] | −0.17 | 0.19 | 1970.08 | −0.90 | 0.37 | −0.54 | 0.20 | |
| [Time: 0.5 vs. 2.5]*[Control vs. PS] | −0.15 | 0.19 | 1997.40 | −0.82 | 0.41 | −0.52 | 0.22 | |
| [Time: 0.5 vs. 3.0]*[Control vs. PS] | −0.09 | 0.19 | 2037.25 | −0.49 | 0.63 | −0.46 | 0.28 | |
| [Time: 0.5 vs. 3.5]*[Control vs. PS] | −0.10 | 0.19 | 2095.29 | −0.53 | 0.60 | −0.47 | 0.27 | |
| [Time: 0.5 vs. 4.0]*[Control vs. PS] | −0.23 | 0.19 | 2179.71 | −1.21 | 0.23 | −0.59 | 0.14 | |
| [Time: 0.5 vs. 4.5]*[Control vs. PS] | −0.19 | 0.19 | 2302.49 | −1.03 | 0.30 | −0.56 | 0.17 | |
| [Time: 0.5 vs. 5.0]*[Control vs. PS] | −0.25 | 0.19 | 2480.94 | −1.35 | 0.18 | −0.61 | 0.11 | |
| [Time: 0.5 vs. 5.5]*[Control vs. PS] | −0.40 | 0.18 | 2738.86 | −2.20 | 0.03 | −0.76 | −0.04 | |
| [Time: 0.5 vs. 6.0]*[Control vs. PS] | −0.11 | 0.18 | 3103.44 | −0.60 | 0.55 | −0.46 | 0.24 | |
| [Time: 0.5 vs. 6.5]*[Control vs. PS] | −0.13 | 0.17 | 3581.95 | −0.74 | 0.46 | −0.47 | 0.21 | |
| [Time: 0.5 vs. 7.0]*[Control vs. PS] | −0.03 | 0.16 | 4075.94 | −0.17 | 0.86 | −0.35 | 0.29 | |
| [Time: 0.5 vs. 7.5]*[Control vs. PS] | −0.16 | 0.15 | 4231.16 | −1.09 | 0.28 | −0.45 | 0.13 | |
| [Time: 0.5 vs. 8.0]*[Control vs. PS] | −0.15 | 0.11 | 3630.88 | −1.28 | 0.20 | −0.37 | 0.08 | |
Natural log (COP velocity).
Adjusted for age, gender, and ethnicity.
Finally, the last LMEM was designed to test the neural inefficiency hypothesis (Holtzer et al., 2009) and determine whether group status moderated the relationship between level of HbO2 activation and postural control ( Table 4). Here, in order to examine the effect of time on postural control, we compared the mean HbO2 activation levels acquired during the first and second halves of the recording period. The actual model consisted of a three-level group (MPS, PS, and normal) and a two level time period (first 4 s vs. second 4 s). Overall, there was a main effect of group (p=0.03), time (p<0.01), and level of HbO2 activation (p<0.01). The group × time × HbO2 activation interaction was significant (p<0.01) and suggested that compared to patients with PS, healthy controls and individuals with MPS require less HbO2 activation to perform similar levels of postural stability in the second half of the postural control task, compared to the first half. In terms of the two-level interactions, the time period × HbO2 activation interaction, group × HbO2 activation interaction, and group × time period interaction were all significant at the p<0.01 level; note that these interactions are explained by the above three level interaction. Taken together, these results suggest that group status does indeed moderate the relationship between PFC activation levels and postural stability performance in older adults with PS compared to healthy controls.
Table 4.
Linear mixed effect model of mean COP velocitya with the following predictors: (1) group; (2) time period; (3) HbO2; (4) time × group; (5) time × HbO2; (6) HbO2 × group; and (7) HbO2 × time × groupb.
| Parameter | Estimate | Std. error | df | t | Sig. | 95% confidence interval
|
||
|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||
| (1) | PS vs. MPS | −0.29 | 0.08 | 317.29 | −3.87 | <0.01 | −0.44 | −0.14 |
| PS vs. control | −0.39 | 0.08 | 297.47 | −5.18 | <0.01 | −0.54 | −0.24 | |
| (2) | [Time period 1 vs. 2]c | −0.46 | 0.08 | 161.88 | −5.79 | <0.01 | −0.62 | −0.31 |
| (3) | HbO2 | −0.77 | 0.17 | 288.18 | −4.48 | <0.01 | −1.11 | −0.43 |
| (4) | [Time period 1 vs. 2]*[PS vs. MPS] | 0.35 | 0.09 | 159.56 | 4.02 | <0.01 | 0.18 | 0.53 |
| [Time period 1 vs. 2]*[PS vs. control] | 0.44 | 0.09 | 156.86 | 5.08 | <0.01 | 0.27 | 0.61 | |
| (5) | [Time period 1 vs. 2]*[HbO2] | 1.62 | 0.19 | 309.81 | 8.48 | <0.01 | 1.24 | 1.99 |
| (6) | [HbO2]*[PS vs. MPS] | 0.88 | 0.20 | 288.55 | 4.32 | <0.01 | 0.48 | 1.28 |
| [HbO2]*[PS vs. control] | 0.86 | 0.20 | 288.27 | 4.25 | <0.01 | 0.46 | 1.25 | |
| (7) | [HbO2]*[time period 1 vs. 2] * [PS vs. MPS] | −1.10 | 0.23 | 298.26 | −4.86 | <0.01 | −1.55 | −0.66 |
| [HbO2]*[time period 1 vs. 2]*[PS vs. control] | −1.07 | 0.22 | 306.31 | −4.80 | <0.01 | −1.51 | −0.63 | |
Natural log (COP velocity).
Adjusted for age, gender, and ethnicity.
Time period 1=average of COP velocity over first 8 time points (first half); Time period 2=average of COP velocity over the second 8 time points (second half).
3. Discussion
In this study, we tested, in a large group of non-demented older adults, the hypothesis that patients with Parkinsonian syndromes require increased prefrontal activation to maintain an upright standing position in comparison to healthy older adults. The main finding demonstrated that indeed patients with PS required increased prefrontal oxygenation in comparison to both healthy older adults and individuals with MPS in order to maintain postural control. In fact, prefrontal activation between controls and patients with PS was nearly twice as large as the difference in prefrontal activation between controls and participants with MPS. By increasing their prefrontal oxygenation levels, patients with PS were able to improve their postural control (by decreasing their COP velocity) towards the latter part of the task.
3.1. The role of prefrontal cortex in postural control
The increased activation in the prefrontal cortex required by patients with Parkinsonian syndromes highlights the role of this region in postural control. In young and middle-aged healthy adults, the cerebellum, especially the vermis, but also the visual associative cortex are mainly activated during an upright standing task (Ouchi et al., 2001, 1999). However, mental imagery studies of locomotion (i.e. gait) in aging have demonstrated the role of the prefrontal cortex using different neuroimaging methods (Holtzer et al., 2014a) like functional MRI (Allali et al., 2014b; Blumen et al., 2014) or PET-Scan (la Fougere et al., 2010). However, few studies address this issue in older adults with PS using direct online assessments of prefrontal brain regions (Maillet et al., 2014; Thevathasan et al., 2012). In the PS population, extracortical regions, like the pedunculopontine nucleus (Thevathasan et al., 2012) or cerebellar regions (Maillet et al., 2014) appear to play a key role in the cerebral networks involved in the control of gait. In terms of postural control, previous studies (Goble et al., 2011; Karim et al., 2013b) showed that older adults recruit cerebral networks involving temporal and prefrontal regions, as well as the subcortical areas. Interestingly, in PD, decreased cholinergic innervations in the pedunculopontine nucleus and in the thalamus, but not in the cortical regions, affect postural control (Muller et al., 2013). The close connection between the pedunculopontine nucleus and the thalamus with the prefrontal cortex (Maillet et al., 2012) could contribute to the explanation of the increased oxygenation of the prefrontal cortex to maintain postural control in patients with PS. Neuropathological studies have reported that older adults with parkinsonian signs not due to PD have specific neuronal loss in the substantia nigra (Ross et al., 2004; Buchman et al., 2012), that projects via the striatum and the thalamus to the prefrontal cortex (Obeso et al., 2008; Krack et al., 2010). The nigral neuronal loss that consecutively affects the cortico-basal ganglia-thalamo-cortical circuits, including the motor circuit, could explain the compensatory need for increased prefrontal activation in patients with PS in order to optimize their postural control. However, in contrast to participants with MPS who were able to maintain a similar postural performance relative to healthy older adults, as suggested by the neural inefficiency model (Holtzer et al., 2009), patients with PS fail to maintain the same postural performance as the healthy controls, although they increased prefrontal activation during the postural task. Interestingly, in regard to the similar postural performance between MPS and healthy older participants, a previous report showed that MPS can be reversible in 38% at a follow-up of 1 year (Mahoney et al., 2014).
Another indirect illustration of the suspected role of the prefrontal cortex in postural control follows the effect of deep brain stimulation on postural control in PD: as subthalamic nucleus and internal globus pallidus stimulations did not improve posture (St. George et al., 2014). Therefore, one could argue that these targets are too deep to affect postural control controlled by cortical regions, especially the prefrontal cortex, as suggested in the present study. Taken together, findings from neuropathological studies in older adults as well as clinical studies in PD could explain the observed role of increased cortical activation in the PFC during a postural control task for individuals with PS.
3.2. Postural instability in Parkinsonian syndromes
Although participants with MPS did not show any postural instability during the course of the task, patients with PS were unable to maintain similar postural control to the healthy older participants at the beginning of the task. Poor postural control was previously associated with MPS in older adults (Louis et al., 2006). Unlike the objective quantitative measure of postural control in the present study, this previous report assessed balance by subjective complaints and the use of an assisted device, which could explain the contradictory findings (Louis et al., 2006). Different factors from disease processes to compensatory strategies contribute to postural instability in older adults with PS. In addition to the cholinergic system (Muller et al., 2013), defective adrenergic innervations (Grimbergen et al., 2009) were also suspected to contribute to postural control in neurodegenerative diseases like PD. Other external factors, like cerebrovascular risk factors, especially diabetes (Kotagal et al., 2013), or comorbidities (Williams-Gray et al., 2013) contribute to postural instability in patients with Parkinsonian syndromes. Independent of these factors, the involvement of the frontal lobe on postural control in PD patients has been suggested in many reports (Wang et al., 2012; Jacobs, 2014); however, the current study highlights for the first time, to our knowledge, the online role of prefrontal regions during actual standing in patients with Parkinsonian syndromes.
3.3. Strengths and limitations
Measuring online prefrontal activation using fNIRs during actual standing in a large cohort of non-demented older adults constitutes the main strength of this study. Furthermore, including a transitional state-participants with MPS –adds to the understanding of the involvement of the prefrontal lobe in postural control from the spectrum from normal aging to PS. Co-registration of fNIRs with a standard morphological neuroimaging method would permit the identification of exact brain regions involved in postural control. Future studies should include a longer period of postural control task in order to confirm the present findings. To prevent participants from daydreaming during the postural task, we used a very simple counting task during the recording of the postural control task. However, we cannot exclude the hypothetical contribution (even minimal) of the prefrontal activation by the counting task. Since a validated scale to quantify mild parkinsonian signs in aging does not exist, we used the UPDRS specifically designed for patients with Parkinson’s disease and not for older adults in general, as performed in previous studies (Allali et al., 2014a; Louis and Bennett, 2007). Finally, a future longitudinal study, including yearly clinical follow-up, would enable us to make causal inferences and assess if healthy participants with greater prefrontal activation during the postural task will develop Parkinsonian syndromes. Such a longitudinal study could also include a specific assessment of the dopamine responsiveness of participants with Parkinsonian syndrome in regard to the prefrontal activation.
3.4. Conclusions
In conclusion, this study revealed that non-demented older adults with Parkinsonian syndromes require increased prefrontal activation in comparison to healthy older adults and individuals with MPS in order to maintain postural control. These findings afford the opportunity to refine therapeutic options for postural instability in patients with PS.
4. Experimental procedure
4.1. Participants
A total of 405 non-demented adults age 65 and older, recruited in an ongoing cohort study entitled Central Control of Mobility in Aging (CCMA) from June 2011 to January 2014 were included in the current study. The CCMA study aims to determine the cognitive and brain predictors of mobility decline and disability in aging. The study procedures have been previously described (Holtzer et al., 2014b, 2014c, 2015). Briefifly, participants enrolled in the CCMA study are non-demented older adults residing in lower Westchester County who have successfully passed a structured telephone-screening interview where verbal assent, medical history, mobility function (Baker et al., 2003) are assessed and dementia is ruled out (Galvin et al., 2005). Exclusion criteria include significant loss of vision and/or hearing, inability to ambulate independently, current or history of neurological or psychiatric disorders, participants on dopaminergic drugs (i.e. levodopa or dopamine agonists) and recent or anticipated medical procedures that may affect mobility. Individuals who passed the telephone interview and agreed to participate in the study were invited to two in-person study visits at our research center, each lasting approximately three hours. During the visits, participants received comprehensive neuropsychological, cognitive, psychological, and mobility assessments as well as a structured neurological examination. Consensus diagnostic case conferences were conducted to assure that participants did not meet criteria for dementia (Holtzer et al., 2008).
Of the 405 participants who completed the in-house evaluations, participants without valid fNIRs recordings (n=46), without valid postural control recordings (n=53), with idiopathic Parkinson’s disease (PD) at baseline (n=2), with dementia at baseline (n=4), with history of stroke or TIA (n=27), prescribed dopamine-blocking agents/neuroleptics (n=1), and/or unable to stand without an assistive device during the postural control task (n=3) were excluded from this analysis. Following exclusions, 269 non-demented older adults were included in the current analysis (mean age: 76.41±6.70 years, 56% women). The institutional review board of the Albert Einstein College of Medicine approved the experimental procedures and all participants provided written informed consent in accordance with the tenets of the Declaration of Helsinki.
4.2. Clinical assessment
Comprehensive neurological examination included assessment for clinical gait abnormalities (Verghese et al., 2002), mild parkinsonian signs (MPS), and medical illnesses. As stated earlier, all participants were categorized into one of three groups: MPS, PS, or healthy control (i.e., normal). As in our previous studies (Allali et al., 2014a; Mahoney et al., 2014), MPS were systematically ascertained in participants by the study clinician using the motor evaluation portion (Part III) of the original version of the Unified Parkinson’s Disease Rating Scale (UPDRS; (Fahn and Elton, 1987)). Accordingly, clinician ratings (0–4) within 4 core domains were recorded: (1) bradykinesia in extremities and body (UPDRS items 23–26 and 31); (2) rigidity in extremities and neck (UPDRS item 22); (3) rest tremor in extremities (UPDRS item 20); and (4) postural instability and gait disturbance (PIGD) (UPDRS items 29–30); where scores greater than zero in any one of these domains suggest the presence of MPS. Consistent with our previous work on MPS (Allali et al., 2014a; Mahoney et al., 2014), we diagnosed MPS based on the presence of any one of the four cardinal features of MPS regardless of its severity (1–4 points). This approach has shown good internal consistency (Allali et al., 2014a; Mahoney et al., 2014). Although more conservative methods have been employed by other investigators (Louis et al., 2004, 2005), we employed a sensitive definition in an effort to identify early markers of MPS. Participants with MPS can present one or more abnormal scores in the 4 core domains of the UPDRS, as long as they did not meet the clinical criteria of parkinsonism defined by the United Kingdom Parkinson’s disease society brain bank clinical diagnostic criteria (Hughes et al., 1992). We applied the United Kingdom Parkinson’s disease society brain bank clinical diagnostic criteria (Hughes et al., 1992) to define PS, where presence of bradykinesia (≥2 points) was required in addition to the presence of one additional core feature (rigidity, tremor, or PIGD). All other participants without presence of bradykinesia, rigidity, tremor, or PIGD constituted the healthy control group.
4.3. fNIRs acquisition during the postural control task
fNIRs Imager 1000 (fNIRs Devices, LLC, Potomac, MD) was used to monitor changes in hemodynamic activity in the prefrontal cortex (specifically oxygenated hemoglobin levels) of participants during the ten-second postural control task, where participants were asked to stand upright, fixate on the wall directly in front of them, and count silently in their head, as previously described (Holtzer et al., 2015). The counting task, a very simple task, was strategically included to ensure that all participants were engaged in the same task while standing and not just daydreaming. Participants were not asked to complete a complex task, like reciting alternate letters, during standing.
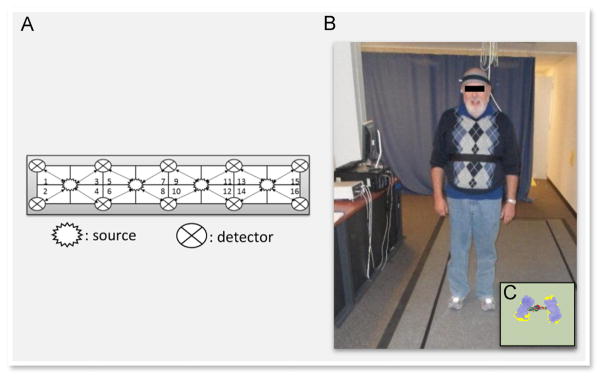
The fNIRs system consists of a flexible circuit board (102 gr) that is placed on each participant’s forehead using standard procedures, a control box for data acquisition and a computer for data collection and storage. The system collects data at a sampling rate of 2 Hz. The fNIRs sensor consists of four LED light sources and ten photodetectors with a source-detector separation of 2.5 cm (see Fig. 2A). This configuration forms 16 channels of recording. The light sources on the sensor (Epitex Inc. type L4 × 730/4 × 805/4 × 850-40Q96-I) contain three built-in LEDs with peak wavelengths at 730, 805, and 850 nm, with an overall outer diameter of 9.2±0.2 mm. The photodetectors (Bur Brown, type OPT101) are monolithic photodiodes with a single-supply transimpedance amplifier. Light sources and detectors are built on a flexible printed circuit board that is covered in silicone for sealing, durability, comfort and hygiene (see Fig. 2B). Light levels are individually calibrated based on skin color to ensure valid fNIRs recordings between 700 and 3500 nm. The flexibility of the sensor permits components to move and adapt to the various contours of the participants’ foreheads, such that the sensor elements maintain an orthogonal orientation to the skin surface, which ultimately improves light coupling efficiency and signal strength. Minimal migration of sensors was assured by placement of a firm band around each participant’s head (see Fig. 2). There is a standard sensor placement procedure followed in all of our studies. The fNIRs is placed on the forehead so that the horizontal axis is centered on the midline of the head, and the vertical axis is centered right above the eyebrows, such that according to the international 10–20 system FP1 and FP2 locations are approximately positioned in-line with the lower row of channels (Ayaz et al., 2006). Given the sensitivity of the fNIRs recording device, the lighting in the test room was reduced such that the mean illumination of the forehead was approximately 150 lux, which is about one-third of typical office lighting.
Fig. 2.
(A) The fNIRs sensor with 4 light sources and 16 light detectors (i.e., channels; see also Holtzer et al. (2015, 2011)) and their approximate placement over prefrontal cortex. (B) This panel depicts a participant wearing the fNIRs sensor while standing on the PKMAS instrumented walkway. (C) This panel shows the participants’ footprints with varying levels of pressure (violet). The red and green dots in between the feet represent the center of mass and center of pressure values respectively at a given time point. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4.3.1. Preprocessing and hemodynamic signal extraction
First, data from each of the 16 fNIRs channels were carefully inspected and recordings were removed from analysis if saturation or dark-current conditions were identified. The raw intensity measurements at 730 and 850 nm that were not saturated or at dark-current levels were then low-pass filtered with a finite impulse response filter that had a cut-off frequency of 0.14 Hz to eliminate possible contamination from respiration and heart rate signals as well as any unwanted high-frequency noise (Izzetoglu et al., 2010). Oxygenated hemoglobin (HbO2), deoxygenated hemoglobin (Hb), oxygenation or oxygen index (HbO2−Hb) and total hemoglobin (HbO2+Hb) signals can be calculated from the artifact-removed raw intensity measurements at 730 and 850 nm using the modified Beer–Lambert law for each channel (see Boas et al., 2002). In the current experiment, only HbO2 values were used to characterize changes in the prefrontal cortex during the postural control task, given that they have been found to be more reliable and less sensitive to movement-related changes in cerebral blood flow (Harada et al., 2009). The use of a single index for task-related hemodynamic changes also reduces the number of comparisons, and thus the probability of increased Type I error.
Baseline corrections over a wide range of 1–15 s have been used in previous fNIRs studies (Csibra et al., 2004; Izzetoglu et al., 2007). In the current study, relative changes in the concentrations of HbO2 were obtained by comparing the measurements made during the first 2 s to those made during the remaining 8 s of our 10 s postural task. Given that the time for a peak hemodynamic response during a motor task is typically ~6 s post stimulus, with an onset delay of about 2 s (Jasdzewski et al., 2003), and the fact that we are specifically investigating the time window of 2–10 s post stimulation, we are confident that we captured the peak hemodynamic response in the current dataset. The sampling interval of fNIRs activity was set at 500 ms, which afforded 16 time points during the eight-second postural stability task.
4.3.2. fNIRs data collection during the postural control task
Individual mean HbO2 data were extracted separately for each of the 16 channels during a synchronized eight-second recording of concurrent fNIRs and postural activation data. A central “hub” computer with E-Prime 2.0 software was used to send synchronized triggers to both the fNIRs system (via serial port) and the Zenometrics quantitative gait system (via parallel port). The fNIRs acquisition software (COBI Studio) accepted numerical triggers from E-prime. The gait acquisition software (PKMAS) accepted TTL (transistor–transistor-logic; 5 V) pulses (square waves) that indicated the beginning and end of each recording.
4.4. Postural recordings
Postural control assessments were conducted by a research assistant (blinded to the group status) via an instrumented walkway that utilizes ProtoKinetics Movement Analysis Software (PKMAS) (Zenometrics, LLC; Peekskill, NY). The postural measure was collected simultaneously with fNIRs recordings. Quantitative measures collected on this instrumented walkway are based on location and mathematical parameters between footfalls (i.e., geometric arrangement, spatial and temporal relationship, relative pressures). As in previous studies in both aging and Parkinsonian syndromes (Muller et al., 2013; Mancini et al., 2012; Eikema et al., 2013), the center of pressure (COP) velocity (cm/s), a proxy for postural control and sway, was the criterion measure (see Fig. 2C for pressure sensor data). COP velocity, as a measure of the mean speed of the COP, represents a highly reliable parameter of postural control in healthy older adults (Lin et al., 2008; Moghadam et al., 2011), as well as in patients with neurological conditions (Gray et al., 2014; Tamburella et al., 2014). In line with the fNIRs data, the sampling interval of the COP velocity during the eight-second recording was set at 500 ms. Testing was conducted in a quiet room and participants wore comfortable footwear with the fNIRs sensor attached to their forehead.
4.5. Additional testing procedures
As in our previous studies, global health status (GHS; range 0–10) was obtained from dichotomous rating (presence or absence) of medical illnesses including: diabetes, chronic heart failure, arthritis, hypertension, depression, stroke, PD, chronic obstructive pulmonary disease, angina, and myocardial infarction (Verghese et al., 2007). Global cognitive status was assessed using the RBANS total score. The RBANS, a brief cognitive test with alternate forms, measures immediate and delayed memory, attention, language, and visuospatial abilities, which also provides a total index score (Duff et al., 2008). Additionally, depression was measured using the Geriatric Depression Scale (GDS) in which a cutoff score>9 was used to define the presence of any depression symptomology from mild to severe (Yesavage et al., 1982).
4.6. Statistics
Descriptive statistics (M and SD) were calculated for each of the three (control, MPS, and PS) groups. Data were inspected graphically, as well as with descriptive statistics, and model assumptions (e.g., normality) were formally tested. Log transformation of COP velocity was performed to achieve normality and variance stabilization of differences across participants. All statistical analyses were run using IBM’s Statistical Package for the Social Sciences (SPSS), Version 20.0 (Corp., 2011).
As stated earlier, three separate linear mixed effects models (LMEMs), each adjusted for age, gender, and ethnicity were used to examine the main effects of group, time and HbO2, as well as their second- and third-order interaction effects. As previously noted, fNIRs light levels are individually calibrated to ensure valid recordings; however, ethnicity was included as a covariate in each of our statistical models as a means of adjusting for skin color, where individuals with darker skin required increased levels.
The first LMEM was designed to test the hypothesis that patients with PS would demonstrate greater prefrontal activation throughout the postural control task compared to healthy older adults; it examined the effect of group status, time, and their interaction on averaged HbO2 activation levels (criterion variable) during the postural control task. To identify the contributors to postural control and determine whether participants with PS would demonstrate greater postural instability throughout the task compared to healthy older adults, a second LMEM examined the effect of group status, time, and their interaction on COP velocity (criterion variable). Both models consisted of a three-level group (control, MPS, and PS) and a 16-level time variable.
To test the neural inefficiency hypothesis (Holtzer et al., 2009) we employed a final LMEM to examine the effects of group status, time, averaged HbO2 activation level over first and second time periods, and their interactions on averaged COP velocity. This final LMEM was designed to determine whether the relation between group status and postural control throughout the task is moderated by HbO2 activation level. Here, in order to examine the effect of time on postural control, we compared the mean HbO2 activation levels acquired during the first and second halves of the recording period. The actual model consisted of a three-level group (control, MPS, and PS) and a two level time period (first vs. second half). All three LMEMs employed a first-order autoregressive covariance type and a random intercept that was included to allow for variability across individuals (i.e., subject was treated as a random effect). The advantage of the linear mixed effects model is that the heterogeneity and correlation of repeated measures under different conditions are taken into account (Laird and Ware, 1982).
Finally, to assess the effect of lateralization, we conducted a separate LMEM to determine whether participants demonstrated lateralized (right vs. left hemisphere) prefrontal activation (averaged across time and channels) during our postural control task.
Acknowledgments
Funding
This study was supported by funds from the National Institutes of Health, National Institute on Aging (R01AG036921-01A1 & R01AG044007-01A1). Gilles Allali was supported by a Grant from the Resnick Gerontology Center, Albert Einstein College of Medicine, Yeshiva University and the Geneva University Hospitals.
Special thanks to the CCMA research assistants and clinicians for their assistance with data collection and to Constantin Trantzas (Zenometrics, LLC) for his assistance.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Auyeung M, Tsoi TH, Mok V, Cheung CM, Lee CN, Li R, et al. Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson’s disease patients. J Neurol, Neurosurg, Psychiatry. 2012;83(6):607–611. doi: 10.1136/jnnp-2011-301590. [DOI] [PubMed] [Google Scholar]
- Allali G, Verghese J, Mahoney JR. Contributions of mild parkinsonian signs to gait performance in the elderly. Age. 2014a;36(4):9678. doi: 10.1007/s11357-014-9678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol Ser A, Biol Sci Med Sci. 2014b;69(11):1389–1398. doi: 10.1093/gerona/glt207. [DOI] [PubMed] [Google Scholar]
- Ayaz H, Izzetoglu M, Platek SM, Bunce S, Izzetoglu K, Pourrezaei K, et al. Registering fNIR data to brain surface image using MRI templates. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference; 2006. pp. 2671–2674. [DOI] [PubMed] [Google Scholar]
- Basso Moro S, Bisconti S, Muthalib M, Spezialetti M, Cutini S, Ferrari M, et al. A semi-immersive virtual reality incremental swing balance task activates prefrontal cortex: a functional near-infrared spectroscopy study. Neuro Image. 2014;85(Pt. 1):451–460. doi: 10.1016/j.neuroimage.2013.05.031. [DOI] [PubMed] [Google Scholar]
- Blumen HM, Holtzer R, Brown LL, Gazes Y, Verghese J. Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum Brain Mapp. 2014;35(8):4090–4104. doi: 10.1002/hbm.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, et al. Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol. 2012;71(2):258–266. doi: 10.1002/ana.22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PS, Bodner EV, Allman RM. Measuring life-space mobility in community-dwelling older adults. J Am Geriatr Soc. 2003;51(11):1610–1614. doi: 10.1046/j.1532-5415.2003.51512.x. [DOI] [PubMed] [Google Scholar]
- Boas D, Franceschini M, Dunn A, Strangman G. Non-invasive imaging of cerebral activation with diffuse optical tomography. In: Frostig RD, editor. In-Vivo Optical Imaging of Brain Function. CRC Press; New York: 2002. pp. 193–221. [Google Scholar]
- Chastan N, Westby GW, Yelnik J, Bardinet E, Do MC, Agid Y, et al. Effects of nigral stimulation on locomotion and postural stability in patients with Parkinson’s disease. Brain: J Neurol. 2009;132(Pt. 2):172–184. doi: 10.1093/brain/awn294. [DOI] [PubMed] [Google Scholar]
- Cilia R, Cereda E, Klersy C, Canesi M, Zecchinelli AL, Mariani CB, et al. Parkinson’s disease beyond 20 years. J Neurol, Neurosurg, Psychiatry. 2014;86(8):849–855. doi: 10.1136/jnnp-2014-308786. [DOI] [PubMed] [Google Scholar]
- Csibra G, Henty J, Volein Á, Elwell C, Tucker L, Meek J, et al. Near infrared spectroscopy reveals neural activation during face perception in infants and adults. J Pediatr Neurol. 2004;2(2):85–59. [Google Scholar]
- Corp., I. SPSS Statistics for Windows. IBM Corp; Armonk, NY: 2011. Released. [Google Scholar]
- Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol: Off J Natl Acad Neuropsychol. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikema DJ, Hatzitaki V, Konstantakos V, Papaxanthis C. Elderly adults delay proprioceptive reweighting during the anticipation of collision avoidance when standing. Neuroscience. 2013;234:22–30. doi: 10.1016/j.neuroscience.2012.12.053. [DOI] [PubMed] [Google Scholar]
- Ferrari M, Bisconti S, Spezialetti M, Basso Moro S, Di Palo C, Placidi G, et al. Prefrontal cortex activated bilaterally by a tilt board balance task: a functional near-infrared spectroscopy study in a semi-immersive virtual reality environment. Brain Topogr. 2014;27(3):353–365. doi: 10.1007/s10548-013-0320-z. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Mihara M, Hattori N, Hatakenaka M, Kawano T, Yagura H, et al. Cortical changes underlying balance recovery in patients with hemiplegic stroke. Neuro Image. 2014;85(Pt. 1):547–554. doi: 10.1016/j.neuroimage.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale. MacMillan Healthcare Information; Florham Park, NJ: 1987. [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, et al. Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci: Off J Soc Neurosci. 2011;31(45):16344–16352. doi: 10.1523/JNEUROSCI.4159-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson’s disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9(2):279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- Gray VL, Ivanova TD, Garland SJ. Reliability of center of pressure measures within and between sessions in individuals post-stroke and healthy controls. Gait Posture. 2014;40(1):198–203. doi: 10.1016/j.gaitpost.2014.03.191. [DOI] [PubMed] [Google Scholar]
- Huang CY, Hwang IS. Behavioral data and neural correlates for postural prioritization and flexible resource allocation in concurrent postural and motor tasks. Hum Brain Mapp. 2013;34(3):635–650. doi: 10.1002/hbm.21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Rakitin BC, Steffener J, Flynn J, Kumar A, Stern Y. Age effects on load-dependent brain activations in working memory for novel material. Brain Res. 2009;1249:148–161. doi: 10.1016/j.brainres.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol, Neurosurg, Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol Ser A, Biol Sci Med Sci. 2014a;69(11):1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney J, Verghese J. Intraindividual variability in executive functions but not speed of processing or conflict resolution predicts performance differences in gait speed in older adults. J Gerontol Ser A, Biol Sci Med Sci. 2014b;69(8):980–986. doi: 10.1093/gerona/glt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications. Age. 2014c;36(1):373–381. doi: 10.1007/s11357-013-9570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuro Image. 2015;112:152–159. doi: 10.1016/j.neuroimage.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Goldin Y, Zimmerman M, Katz M, Buschke H, Lipton RB. Robust norms for selected neuropsychological tests in older adults. Arch Clin Neuropsychol: Off J Natl Acad Neuropsychol. 2008;23(5):531–541. doi: 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193(3):445–454. doi: 10.1007/s00221-008-1643-y. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol Ser A, Biol Sci Med Sci. 2011;66(8):879–887. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzetoglu M, Chitrapu P, Bunce S, Onaral B. Motion artifact cancellation in NIR spectroscopy using discrete Kalman filtering. Biomed Eng Online. 2010;9:16. doi: 10.1186/1475-925X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzetoglu M, Bunce SC, Izzetoglu K, Onaral B, Pourrezaei K. Functional brain imaging using near-infrared technology. IEEE Eng Med Biol Mag. 2007;26(4):38–46. doi: 10.1109/memb.2007.384094. [DOI] [PubMed] [Google Scholar]
- Johnson L, James I, Rodrigues J, Stell R, Thickbroom G, Mastaglia F. Clinical and posturographic correlates of falling in Parkinson’s disease. Mov Disord: Off J Mov Disord Soc. 2013;28(9):1250–1256. doi: 10.1002/mds.25449. [DOI] [PubMed] [Google Scholar]
- Jahn K, Deutschlander A, Stephan T, Strupp M, Wiesmann M, Brandt T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. Neuro Image. 2004;22(4):1722–1731. doi: 10.1016/j.neuroimage.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Jacobs JV. Why we need to better understand the cortical neurophysiology of impaired postural responses with age, disease, or injury. Front Integr Neurosci. 2014;8:69. doi: 10.3389/fnint.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasdzewski G, Strangman G, Wagner J, Kwong KK, Poldrack RA, Boas DA. Differences in the hemodynamic response to event-related motor and visual paradigms as measured by near-infrared spectroscopy. Neuro Image. 2003;20(1):479–488. doi: 10.1016/s1053-8119(03)00311-2. [DOI] [PubMed] [Google Scholar]
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75(2):116–124. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- Karim HT, Sparto PJ, Aizenstein HJ, Furman JM, Huppert TJ, Erickson KI, et al. Functional MR imaging of a simulated balance task. Brain Res. 2014;1555:20–27. doi: 10.1016/j.brainres.2014.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim H, Fuhrman SI, Sparto P, Furman J, Huppert T. Functional brain imaging of multi-sensory vestibular processing during computerized dynamic posturography using near-infrared spectroscopy. Neuro Image. 2013a;74:318–325. doi: 10.1016/j.neuroimage.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, Fuhrman SI, Furman JM, Huppert TJ. Neuroimaging to detect cortical projection of vestibular response to caloric stimulation in young and older adults using functional near-infrared spectroscopy (fNIRS) Neuro Image. 2013b;76:1–10. doi: 10.1016/j.neuroimage.2013.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33(10):474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kotagal V, Albin RL, Muller ML, Koeppe RA, Frey KA, Bohnen NI. Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Park Relat Disord. 2013;19(5):522–526. doi: 10.1016/j.parkreldis.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- Louis ED, Schupf N, Marder K, Tang MX. Functional correlates of mild parkinsonian signs in the community-dwelling elderly: poor balance and inability to ambulate independently. Mov Disord: Off J Mov Disord Soc. 2006;21(3):411–416. doi: 10.1002/mds.20735. [DOI] [PubMed] [Google Scholar]
- Louis ED, Bennett DA. Mild Parkinsonian signs: an overview of an emerging concept. Mov Disord: Off J Mov Disord Soc. 2007;22(12):1681–1688. doi: 10.1002/mds.21433. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Mayeux R. Parkinsonian signs in older people in a community-based study: risk of incident dementia. Arch Neurol. 2004;61(8):1273–1276. doi: 10.1001/archneur.61.8.1273. [DOI] [PubMed] [Google Scholar]
- Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch Neurol. 2005;62(2):297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- Lin D, Seol H, Nussbaum MA, Madigan ML. Reliability of COP-based postural sway measures and age-related differences. Gait Posture. 2008;28(2):337–342. doi: 10.1016/j.gaitpost.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B, de Haan RJ. Determinants of disability and quality of life in mild to moderate Parkinson disease. Neurology. 2008;70(23):2241–2247. doi: 10.1212/01.wnl.0000313835.33830.80. [DOI] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Role of the prefrontal cortex in human balance control. Neuro Image. 2008 doi: 10.1016/j.neuroimage.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19(1):47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Miyai I, Hattori N, Hatakenaka M, Yagura H, Kawano T, et al. Cortical control of postural balance in patients with hemiplegic stroke. Neuroreport. 2012;23(5):314–319. doi: 10.1097/WNR.0b013e328351757b. [DOI] [PubMed] [Google Scholar]
- Mahoney JR, Verghese J, Holtzer R, Allali G. The evolution of mild parkinsonian signs in aging. J Neurol. 2014;261(10):1922–1928. doi: 10.1007/s00415-014-7442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet A, Thobois S, Fraix V, Redoute J, Le Bars D, Lavenne F, et al. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson’s disease: a kinesthetic imagery approach. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller ML, Albin RL, Kotagal V, Koeppe RA, Scott PJ, Frey KA, et al. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain: J Neurol. 2013;136(Pt. 11):3282–3289. doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet A, Pollak P, Debu B. Imaging gait disorders in parkinsonism: a review. J Neurol, Neurosurg, Psychiatry. 2012;83(10):986–993. doi: 10.1136/jnnp-2012-302461. [DOI] [PubMed] [Google Scholar]
- Mancini M, Carlson-Kuhta P, Zampieri C, Nutt JG, Chiari L, Horak FB. Postural sway as a marker of progression in Parkinson’s disease: a pilot longitudinal study. Gait Posture. 2012;36(3):471–476. doi: 10.1016/j.gaitpost.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam M, Ashayeri H, Salavati M, Sarafzadeh J, Taghipoor KD, Saeedi A, et al. Reliability of center of pressure measures of postural stability in healthy older adults: effects of postural task difficulty and cognitive load. Gait Posture. 2011;33(4):651–655. doi: 10.1016/j.gaitpost.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Okada H, Yoshikawa E, Futatsubashi M, Nobezawa S. Absolute changes in regional cerebral blood flow in association with upright posture in humans: an orthostatic PET study. J Nucl Med: Off Publ Soc Nucl Med. 2001;42(5):707–712. [PubMed] [Google Scholar]
- Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain: J Neurol. 1999;122(Pt. 2):329–338. doi: 10.1093/brain/122.2.329. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson’s disease. Mov Disord: Off J Mov Disord Soc Suppl. 2008;3:S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- Post B, Merkus MP, de Haan RJ, Speelman JD. Prognostic factors for the progression of Parkinson’s disease: a systematic review. Mov Disord: Off J Mov Disord Soc. 2007;22(13):1839–1851. doi: 10.1002/mds.21537. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, et al. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol. 2004;56(4):532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A. Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging. 2009;30(5):793–807. doi: 10.1016/j.neurobiolaging.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George RJ, Carlson-Kuhta P, Nutt JG, Hogarth P, Burchiel KJ, Horak FB. The effect of deep brain stimulation randomized by site on balance in Parkinson’s disease. Mov Disord: Off J Mov Disord Soc. 2014;29(7):949–953. doi: 10.1002/mds.25831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain: J Neurol. 2012;135(Pt. 1):148–160. doi: 10.1093/brain/awr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburella F, Scivoletto G, Iosa M, Molinari M. Reliability, validity, and effectiveness of center of pressure parameters in assessing stabilometric platform in subjects with incomplete spinal cord injury: a serial cross-sectional study. J Neuroeng Rehabil. 2014;11:86. doi: 10.1186/1743-0003-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol, Neurosurg, Psychiatry. 2007;78(9):929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Gray CH, Mason SL, Evans JR, Foltynie T, Brayne C, Robbins TW, et al. The CamPaIGN study of Parkinson’s disease: 10-year outlook in an incident population-based cohort. J Neurol, Neurosurg, Psychiatry. 2013;84(11):1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- Wang HC, Hsu JL, Leemans A. Diffusion tensor imaging of vascular parkinsonism: structural changes in cerebral white matter and the association with clinical severity. Arch Neurol. 2012;69(10):1340–1348. doi: 10.1001/archneurol.2012.633. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33(6):1073–1084. doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, et al. Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuro Image. 2010;50(4):1589–1598. doi: 10.1016/j.neuroimage.2009.12.060. [DOI] [PubMed] [Google Scholar]