Abstract
Objectives
The International Prognostic Scoring System (IPSS) is commonly used to predict survival and assign treatment for the myelodysplastic syndromes (MDS). We explored whether self-reported and readily available non-hematologic predictors of survival add independent prognostic information to the IPSS.
Materials and Methods
Retrospective cohort study of consecutive MDS patients ≥age 65 who presented to Dana-Farber Cancer Institute between 2006 and 2011 and completed a baseline quality of life questionnaire. Questions corresponding to functional status and symptoms and extracted clinical-pathologic data from medical records. Kaplan–Meier and Cox proportional hazards models were used to estimate survival.
Results
One hundred fourteen patients consented and were available for analysis. Median age was 73 years, and the majority of patients were White, were male, and had a Charlson comorbidity score of <2. Few patients (24%) had an IPSS score consistent with lower-risk disease and the majority received chemotherapy. In addition to IPSS score and history of prior chemotherapy or radiation, significant univariate predictors of survival included low serum albumin, Charlson score, performance status, ability to take a long walk, and interference of physical symptoms in family life. The multivariate model that best predicted mortality included low serum albumin (HR = 2.3; 95% CI: 1.06–5.14), therapy-related MDS (HR = 2.1; 95% CI: 1.16–4.24), IPSS score (HR = 1.7; 95% CI: 1.14–2.49), and ease taking a long walk (HR = 0.44; 95% CI: 0.23–0.90).
Conclusions
In this study of older adults with MDS, we found that low serum albumin and physical function added important prognostic information to the IPSS score. Self-reported physical function was more predictive than physician-assigned performance status.
Keywords: Myelodysplastic syndromes, Prognosis, Elderly, Prediction, Geriatric, Oncology
1. Introduction
Myelodysplastic syndromes (MDS) are the most common hematologic neoplasms in the elderly, and the incidence of MDS in the U.S. is expected to double by 2030 as the result of population aging.1,2 MDS are clonal bone marrow disorders in which increased intramedullary apoptosis of abnormal progenitor cells leads to ineffective hematopoiesis. Patients suffer the complications of cytopenias and have a substantial risk of transformation to acute myeloid leukemia (AML). Treatment options include supportive care with transfusions and growth factors, immunosuppressive and immunomodulatory drugs, non-intensive chemotherapy including therapy with DNA hypomethylating agents and intensive induction chemotherapy. Non-myeloablative allogeneic bone marrow transplant (BMT) with the intent to cure is now being offered to selected patients up to the age of 75.3 Because MDS primarily affects an older population, care is frequently complicated by the presence of comorbidities and functional impairments which may decrease the tolerability of therapy and shorten survival.
The International Prognostic Scoring System (IPSS) and its revision IPSS-R are widely used tools that predict the risk of transformation to AML and overall survival in MDS based on cytogenetics, percentage of bone marrow blasts, and number of cytopenias.4,5 The original IPSS assigns patients to low-, intermediate-1-, intermediate-2-, or high-risk groups, which, in patients older than 60 years of age, correspond to an average survival of 5.7, 3.5, 1.2 and 0.4 years, respectively. The primary goal of therapy for lower risk disease is to improve symptoms and quality of life and prevent transformation to AML. Patients with higher risk disease have the additional goal of improved survival through anti-neoplastic treatment. Multiple investigators have proposed revision of the IPSS to include other known prognostic factors such as a history of prior chemotherapy or radiation, and new molecular and cytogenetic prognostic information.6–9
A prognostic model cannot perform optimally in an elderly population without considering the impact of comorbidity and functional decline, both of which might be more closely linked to survival than MDS itself.10,11 Over half of older patients with hematologic malignancy have evidence of malnutrition and over a third have impaired physical function.12 These markers of frailty have been associated with increased chemotherapy-related toxicity, poor response to therapy, inability to complete planned course of therapy, and death.13,14 A recent study of geriatric assessment in older patients receiving induction therapy for AML objectively measured deficits in cognition and physical function which conferred worse overall survival in this population.15 However, little is known about the value of non-disease specific predictors of mortality in the prognosis of MDS. In this context, we used clinical and quality of life data from a cohort of older adults with MDS to determine if self-reported and objective geriatric assessment measures might add prognostic value to the IPSS.
2. Experimental/Materials and Methods
2.1. Data Collection
For this analysis, we used data collected on all newly diagnosed patients with hematologic malignancies at the Dana Farber Cancer Institute (DFCI) who participate in a research protocol that includes an EORTC-QLQ-C30 quality of life questionnaire completed at their first visit.16,17 Patients also consented to collection of their clinical and pathological data into the Cancer Research Information System (CRIS) database. CRIS includes information on patient demo-graphics, initial treatment assignment, disease characteristics, pathology tests, hospitalizations, treatments and date and cause of death. We analyzed data on all participants ≥65 years of age who presented between 2006 and 2011 with a new diagnosis of MDS. Consent is approximately 80% for all patients approached.
Patients were eligible for this analysis if they completed the QLQ-C30 questionnaire and had not received previous chemotherapy related to their new diagnosis of MDS. We excluded those who did not return to DFCI after their first evaluation from the database, as we wanted to include information on treatment received and other outcomes. Chart review was performed by a trained medical student (KRF) and verified by a geriatrician-oncologist (JD). We validated all clinical data in the CRIS database. We also collected baseline patient characteristics such as body mass index (BMI), comorbidities, family history of cancer, previous chemotherapy or radiation and relevant laboratory values. This study was approved by the DFCI Institutional Review Board.
2.2. Definition of Predictors
The QLQ-C30 is self-administered and consists of 30 items which measure various domains relevant to function and quality of life in older patients as well as symptoms commonly reported by cancer patients.16 We selected questions that correspond to domains commonly evaluated in older patients (Table 2). We also included measures of fatigue and shortness of breath, the most common symptoms associated with MDS. We did not include questions related to cognitive function in the model as there was no objective cognitive assessment and so few patients reported problems with memory. Patients may answer “not at all,” “a little,” “quite a bit,” or “very much” for all functional and symptom items, and are asked to think about their status during the last week. We categorized responses into two groups: “not at all” vs. all other responses based on the hypothesis that those with the highest levels of function would be the most informative group in our dataset. However, results did not change substantially if other binary categories were used.
Table 2.
EORTC QCL-30 questions by domain (n = 114).
| EORTC QLQ-C30 question | Patients responding N (%)
|
|
|---|---|---|
| “not at all” | “a little,” “quite a bit” or “very much” | |
| Physical function | ||
| 1. Do you have any trouble doing strenuous activities, like carrying a heavy shopping bag or a suitcase? | 43 (38.4) | 69 (61.6) |
| 2. Do you have any trouble taking a long walk? | 31 (27.4) | 82 (72.6) |
| 3. Were you limited in doing either your work or other daily activities? | 41 (37.6) | 68 (62.4) |
| Social function | ||
| 4. Has your physical condition or medical treatment interfered with your family life? | 68 (60.2) | 45 (39.8) |
| 5. Has your physical condition interfered with your social activities? | 61 (55.0) | 50 (45.0) |
| Nutrition/appetite | ||
| 6. Have you lacked appetite? | 81 (71.0) | 32 (29.0) |
| 7. Have you vomited in the last week? | 106 (93.0) | 6 (5.0) |
| MDS-related symptoms | ||
| 8. Were you short of breath? | 41 (36.0) | 73 (64.0) |
| 9. Did you need to rest? | 33 (29.0) | 79 (69.0) |
| 10. Were you tired? | 28 (25.0) | 84 (74.0) |
We divided the IPSS score into lower (low and intermediate-1 risk) and higher risk (intermediate-2 and high risk) categories. We used the Charlson Co-morbidity Index to calculate a baseline comorbidity score for each patient.18 In the model we classified patients as having a Charlson score of <3 or ≥3. We categorized age using the median age of the population (≤72.5 or >72.5 years). We divided race into White vs. other races/ethnicity (American Indian, African American, Hispanic, or other). We also included smoking (ever vs. never), living situation (alone or with others), body mass index (BMI; <25 kg/m2, 25–30 kg/m2, and >30 kg/m2) and serum albumin (<3.5 g/dl vs. ≥3.5 g/dl).
2.3. Definition of Outcomes
We defined survival as the number of months between the first presentation to DFCI with a diagnosis of MDS and the date of last follow-up or the date of patient death. We focused on overall survival (OS) as our outcome since time to progression to AML is influenced by ascertainment bias. We divided treatment type into the following categories: no chemotherapy, non-intensive chemotherapy (Azacitidine or Decitabine), and intensive chemotherapy (induction therapy and/or bone marrow transplant). We were unable to strictly use the standard International Working Group (IWG) criteria for response to therapy,19 which requires complete bone marrow and peripheral hematologic response, as not all patients had a follow-up bone marrow biopsy. Patients were considered to have a complete response if they had a follow-up bone marrow with ≤5% myeloblasts and normal maturation of all cell lines and a complete peripheral blood response (hemoglobin ≥ 11g/dl, platelets ≥ 100 × 109/L neutrophils > 1 × 109/L and blasts 0%) for 2 months or more. If there was no follow-up biopsy, patients with a complete peripheral blood response were considered to have a complete response. We considered those who had less than a complete bone marrow or hematologic response and no evidence of disease progression for at least 2 months to have a partial response. This information was extracted from pathological reports and oncologists’ notes in the medical record.
2.4. Statistical Methods
We used frequencies and percentages to describe the baseline characteristics of the cohort. We used Kaplan–Meier survival curves to determine the relationship between individual predictors (all baseline characteristics listed in Table 1, as well as geriatric predictors from the EORTC QLQ-C30 questionnaire listed in Table 2) and overall survival (OS). We then used Cox proportional-hazards models to estimate hazard ratios (HR) and 95% confidence intervals (95% CI) for survival. Factors predictive on univariate analysis were included in a multivariate Cox proportional hazards model to determine the independent predictors of overall survival. We used a backwards selection method to determine the final multivariable model that best predicts survival. As we had a population that included people with varying levels of disease severity and treatment assignment, we stratified the baseline characteristics and questionnaire responses by treatment type. We considered a p-value less than 0.05 to be statistically significant. All data analysis was performed using SAS software v. 9.0.
Table 1.
Demographic and clinico-pathological characteristics of patients with MDS (n = 114).
| Characteristic | N (%)a |
|---|---|
| Age category | |
| 65–69 | 34 (29.8) |
| 70–74 | 35 (30.7) |
| 75–79 | 22 (19.3) |
| 80+ | 23 (20.2) |
| Male | 85 (74.6) |
| White | 108 (94.7) |
| Lives alone | 13 (11.8) |
| History of tobacco use | 65 (57.0) |
| WHO BMI categories | |
| <25 | 30 (27.1) |
| 25–30 | 48 (43.2) |
| >30 | 33 (29.7) |
| Baseline ECOG Performance Status | |
| 0 | 51 (44.7) |
| 1 | 49 (43.0) |
| ≥2 | 14 (12.3) |
| Baseline Charlson comorbidity score | |
| 0 | 43 (37.7) |
| 1 | 19 (16.7) |
| 2 | 30 (26.3) |
| ≥3 | 22 (19.3) |
| Serum albumin <3.5 g/dl | 11 (9.7) |
| Number of cytopeniasd | |
| 0 | 16 (14.0) |
| 1 | 36 (31.6) |
| 2 | 39 (34.2) |
| 3 | 23 (20.2) |
| Cytogeneticse | |
| Good | 76 (66.6) |
| Intermediate | 19 (16.7) |
| Poor | 19 (16.7) |
| % Bone marrow blastsf | |
| <5% | 74 (66.1) |
| 5–10% | 27 (24.1) |
| >10% | 11 (9.8) |
| IPSS Category | |
| Low risk | 27 (23.7) |
| Intermediate risk-1 | 52 (45.6) |
| Intermediate risk-2 | 30 (26.3) |
| High risk | 5 (4.4) |
| Previous chemotherapy or radiation | 20 (17.5) |
| Treatment type received | |
| No chemotherapy | 53 (46.5) |
| Non-intensive chemotherapyb | 44 (38.6) |
| Intensive chemotherapyc | 17 (14.9) |
May not add up to 100% due to missing variables.
5-Azacytidine or decitabine.
Induction chemotherapy and/or non-myeloablative bone marrow transplant.
Component of the IPSS score. Platelets < 100,000/μl; hemoglobin < 10 g/dl; neutrophils < 1800/μl.
Component of the IPSS score. Good: normal, -Y, del(5q), del(20q); intermediate: other abnormalities; poor: complex (≥3 abnormalities) or chromosome 7 anomalies.
Component of the IPSS score.
3. Results
Between 2006 and 2011, 300 patients 65 and older who presented to DFCI with a diagnosis of MDS were invited to participate. Of these, 92 (30.7%) were excluded because they did not complete the questionnaire at their initial visit and 94 (31.3%) because they either did not return to DFCI after their initial consultation or had received previous chemotherapy for MDS, leaving a total of 114 patients for the analysis.
Baseline characteristics of the cohort are summarized in Table 1. The median age at diagnosis was 72.5 years and the majority of the population was male (74.6%), was White (94.7%), and did not live alone (88.2%). The cohort was relatively healthy; 54.4% of patients had a Charlson score of 0 or 1 and only 19.3% had a score of ≥3. Only three patients (2.6%) reported needing help with activities of daily living, and only five patients (4.4%) reported having any trouble with memory. Eleven (9.7%) patients had a low serum albumin at baseline. Most patients (69.3%) had lower risk MDS at presentation. Twenty patients (17.5%) had a history of treatment with chemotherapy or radiation for prior cancer.
Self-reported variables from the QLQ-C30 that were relevant to geriatric domains are displayed in Table 2. Overall the cohort had a high level of physical function. Forty-three respondents (38.4%) had no trouble doing strenuous activities, 31 (27.4%) respondents reported no trouble taking a long walk, and 41 (37.6%) respondents were not limited in their work or other daily activities. Over half felt that their condition has not interfered with family life or social activities. In contrast, only 7% of respondents had no fatigue and only 36% had no shortness of breath.
About one-half (49.0%) of patients initially received non-intensive chemotherapy and eight patients (7.0%) underwent intensive therapy as initial treatment. Of these, 21.4% eventually received a non-myeloablative bone marrow transplant. A complete or partial hematologic or bone marrow response to therapy was achieved by 36.0% of patients receiving non-intensive chemotherapy, 38.0% of those receiving induction chemotherapy and 39.0% of those receiving non-myeloablative BMT.
Baseline characteristics and questionnaire responses stratified by treatment assignment are shown in Tables 3 and 4. Patients who received no chemotherapy tended to be older, have low-risk IPSS scores, and higher comorbidity. Non-intensive therapy was associated with higher risk disease, more cytopenias, worse self-reported physical and social function, and more symptoms that the other two groups. Those who received intensive therapies were substantially younger, had less comorbidity and reported higher levels of physical and social function.
Table 3.
Baseline characteristics of older adults with MDS by treatment group.
| Characteristic | No chemotherapy (N = 53) |
Non-intensive chemotherapy (N = 44) |
Intensive chemotherapy (N = 17) |
|---|---|---|---|
|
| |||
| N (%)a | |||
| Age category | |||
| 65–69 | 12 (22.6) | 11 (25.0) | 11 (64.7) |
| 70–74 | 10 (18.9) | 19 (43.2) | 6 (35.3) |
| 75–79 | 15 (28.3) | 7 (15.9) | 0 |
| 80+ | 16 (30.2) | 7 (15.9) | 0 |
| Male | 39 (73.6) | 31 (70.5) | 15 (88.2) |
| White | 52 (98.1) | 40 (90.9) | 16 (94.1) |
| Lives alone | 9 (17.0) | 4 (9.1) | 0 |
| History of tobacco use | 31 (58.5) | 22 (50.0) | 5 (29.4) |
| WHO BMI categories | |||
| <25 | 16 (31.4) | 12 (27.9) | 2 (11.8) |
| 25–30 | 23 (45.1) | 16 (37.2) | 9 (52.9) |
| >30 | 12 (23.5) | 15 (34.9) | 6 (35.3) |
| Baseline Charlson comorbidity score | 17 (32.1) | 16 (36.7) | 10 (58.8) |
| 0 | 7 (13.2) | 9 (20.5) | 3 (17.7) |
| 1 | 16 (30.2) | 11 (25.00) | 3 (17.7) |
| 2 | 13 (24.5) | 8 (18.2) | 1 (5.9) |
| ≥3 | |||
| Baseline ECOG PS | |||
| 0 | 20 (37.8) | 22 (50.0) | 9 (52.9) |
| 1 | 28 (52.8) | 17 (38.6) | 4 (23.5) |
| ≥2 | 5 (9.4) | 5 (11.3) | 4 (23.5) |
| Serum albumin <3.5 g/dl | 7 (13.2) | 4 (9.1) | 0 (0.0) |
| Number of cytopenias | |||
| 0 | 10 (18.9) | 4 (1) | 2 (11.8) |
| 1 | 19 (35.9) | 12 (27.3) | 5 (29.4) |
| 2 | 15 (28.3) | 19 (43.2) | 5 (29.4) |
| 3 | 9 (17.0) | 9 (20.5) | 5 (29.4) |
| Bone marrow blasts | |||
| <5% | 44 (86.3) | 21 (47.7) | 9 (52.9) |
| 5–10% | 5 (9.8) | 16 (36.4) | 6 (35.3 |
| >10% | 2 (3.9) | 7 (15.9) | 3 (11.8) |
| IPSS category | |||
| Low risk | 20 (37.7) | 5 (11.4) | 2 (11.8) |
| Intermediate risk-1 | 26 (49.1) | 19 (43.2) | 7 (41.2) |
| Intermediate risk-2 | 5 (9.4) | 17 (38.6) | 8 (47.1) |
| High risk | 2 (3.8) | 3 (6.8) | 0 (0.00) |
| Previous chemotherapy or radiation | 11 (20.8) | 9 (20.5) | 0 (0.00) |
May not add up to 100% due to missing variables.
Table 4.
Questionnaire responses of older adults with MDS by treatment group.
| EORTC QLQ-C30 question | N (%) responding ”not at all”
|
||
|---|---|---|---|
| No chemotherapy (N = 53) |
Non-intensive chemotherapy (N = 44) |
Intensive chemotherapy (N = 17) |
|
| Physical function | |||
| 1. Do you have any trouble doing strenuous activities, like carrying a heavy shopping bag or a suitcase? | 21 (39.6) | 16 (38.1) | 6 (35.3) |
| 2. Do you have any trouble taking a long walk? | 17 (32.1) | 7 (16.2) | 7 (41.2) |
| 3. Were you limited in doing either your work or other daily activities? | 25 (48.1) | 20 (46.5) | 7 (50.0) |
| Social function | |||
| 4. Has your physical condition or medical treatment interfered with your family life? | 33 (63.4) | 24 (54.6) | 11 (64.7) |
| 5. Has your physical condition interfered with your social activities? | 31 (60.78) | 23 (53.5) | 7 (41.2) |
| Nutrition/appetite | |||
| 6. Have you lacked appetite? | 37 (69.8) | 30 (68.8) | 14 (87.5) |
| 7. Have you vomited in the last week? | 100 (0.00) | 38 (88.3) | 16 (94.1) |
| MDS-related symptoms | |||
| 8. Were you short of breath? | 17 (32.1) | 17 (38.6) | 7 (41.2) |
| 9. Did you need to rest? | 14 (27.5) | 11 (25.0) | 8 (47.1) |
| 10. Were you tired? | 15 (28.9) | 8 (18.6) | 5 (29.4) |
The cohort had a median OS of 25 months, as shown in Fig. 1A. As expected, IPSS score was a significant predictor of mortality (Fig. 1B). Patients with lower IPSS scores (low or intermediate-1 risk) had an overall survival of 35 months as compared to 12 months for higher risk patients (intermediate-2 or high risk; p < 0.001). A history of prior treatment with chemotherapy or radiation (i.e., therapy-related MDS) was also associated with substantially poorer survival (9 vs. 26 months, p = 0.02). In addition to these known prognostic factors, a number of non-cancer-related variables were also predictive of survival. A Charlson score of <3 was associated with a median OS of 29 months vs. 14 months for a score of ≥3 (p = 0.04). Serum albumin (<3.5 g/dl) was a powerful predictor, with an associated survival of 4 vs. 26 months for low vs. normal albumin; p < 0.005; Fig. 2A). Of the QOL questions we investigated, we found that no history of vomiting (26 vs. 8.5 months, p = 0.02), no trouble taking a long walk (53 vs. 21 months, p = 0.006; Fig. 2B) and no impact of physical symptoms on family life (36 vs. 14 months, p = 0.024) in the previous week were significantly associated with longer survival. Physician-assigned ECOG performance status < 1 (median OS 35 vs. 21 months; p = 0.096) was not as powerful a predictor as patient reported ease taking a long walk, so we chose to use the patient-reported variable in the model. Median age (<72.5) was not predictive of OS in our cohort (21 vs. 25 months; p = 0.49). Kaplan–Meier survival estimates for the predictors of interest are shown in Table 5. Those who received no chemotherapy, non-intensive, and intensive chemotherapy had a median OS of 29, 14 and >53 months, respectively (p = 0.07).
Fig. 1.
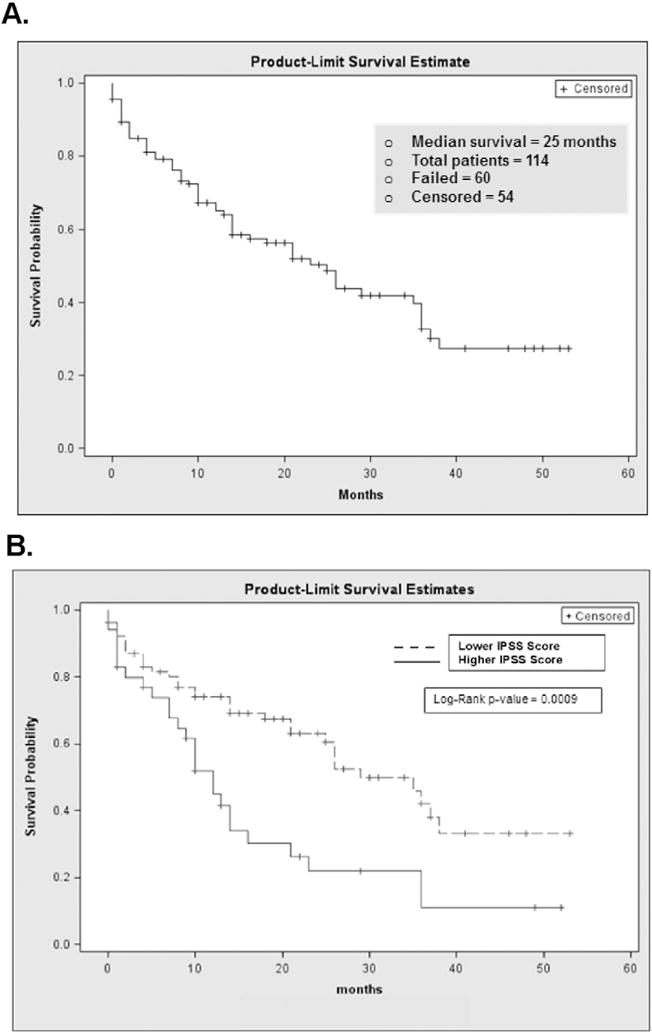
Kaplan–Meier survival curves for overall survival (A) and by IPSS status (B).
Fig. 2.
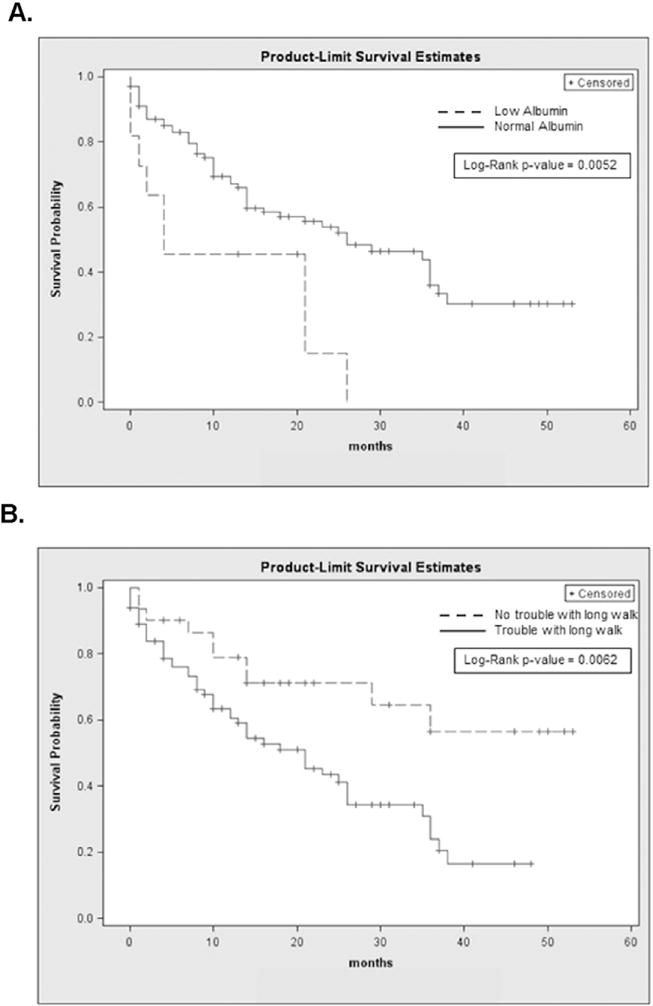
Kaplan-Meier survival curves for albumin concentration (A) and difficulty with taking a long walk (B).
Table 5.
Kaplan–Meier survival estimates based on patient and tumor characteristics.
| Characteristic | Variable | Median OS (months) |
Log-rank p valuea |
1-year OS (%surviving) |
2-year OS (%surviving) |
|---|---|---|---|---|---|
| Age | Age at diagnosis | ||||
| ≤72.5 | 21 ± 1.51 | 0.49 | 62 ± 0.62 | 48 ± 1.78 | |
| >72.5 | 25 ± 1.98 | 68 ± 0.77 | 53 ± 1.80 | ||
| Disease specific factors | IPSS score | 0.001 | |||
| Lower risk (score 0–1) | 35 ± 2.59 | 74 ± 0.61 | 64 ± 1.44 | ||
| Higher risk (score > 1) | 12 ± 1.08 | 45 ± 1.07 | 22 ± 1.90 | ||
| Previous chemo/radiation | |||||
| No | 26 ± 1.56 | 0.020 | 68 ± 0.6 | 55 ± 1.37 | |
| Yes | 9 ± 1.08 | 48 ± 1.45 | 27 ± 2.81 | ||
| Comorbidities/risk factors | Charlson score | ||||
| <3 | 29 ± 2.70 | 0.043 | 78 ± 0.79 | 66 ± 1.87 | |
| ≥3 | 14 ± 0.90 | 57 ± 0.74 | 41 ± 1.61 | ||
| Nutrition | Serum albumin | 0.005 | |||
| ≥3.5 g/dl | 26 ± 2.4 | 67 ± 0.59 | 52 ± 1.32 | ||
| <3.5 g/dl | 4 ± 0.6 | 45 ± 1.80 | 14 ± 3.2 | ||
| Vomiting in the past week | 0.022 | ||||
| None | 26 ± 1.48 | 68 ± 0.56 | 53 ± 1.30 | ||
| Some | 8.5 ± 1.73 | 25 ± 2.45 | 0 | ||
| Physical function | Trouble taking a long walk | 0.006 | |||
| None | 53b | 79 ± 0.92 | 71 ± 2.09 | ||
| Some | 21 ± 1.28 | 61 ± 0.68 | 43 ± 1.49 | ||
| Social function | Effect of physical symptoms on family life | 0.024 | |||
| None | 36 ± 3.10 | 73 ± 0.67 | 57 ± 1.58 | ||
| Some | 14 ± 1.12 | 52 ± 0.95 | 39 ± 1.94 |
Derived from the Log-Rank test.
Median survival not reached by 53 months of follow-up.
We included factors that were statistically significant on univariate analysis in a multivariable Cox proportional hazards model to identify independent predictors of OS. Table 6 displays the model that best predicted OS, which included IPSS score (HR, 1.68; 95% CI 1.14 to 2.49), history of previous chemotherapy and/or radiation (HR, 2.21; 95% CI 1.16 to 4.24), low serum albumin (HR, 2.34; 95% CI 1.06 to 5.14) and no difficulty taking a long walk (HR, 0.45; 95% CI 0.23 to 0.90). Because this is a heterogeneous population and treatment type could be a confounder, we added a treatment variable (chemotherapy vs. no chemotherapy) to the final model. It did not change the selection of the final variables or their hazard ratios substantially.
Table 6.
Final multivariable model of survival.
| Variable | Hazard ratioa (HR) | 95% CI |
|---|---|---|
| IPSS score | 1.68 | 1.14, 2.49 |
| Previous chemotherapy or radiation | 2.21 | 1.16, 4.24 |
| Low serum albumin | 2.34 | 1.06, 5.14 |
| No trouble taking a long walk | 0.45 | 0.23, 0.90 |
Hazard ratios calculated by Cox proportional hazards models.
4. Discussion
In this cohort study of older patients with MDS, non-hematologic factors added important prognostic information to traditional pathologic and clinical predictors of mortality. The model that best predicted survival combined disease-specific factors, history of previous treatment with chemotherapy and/or radiation, serum albumin, and a self-reported measure of strenuous activity. The self-reported measure of function was more accurate in predicting survival than traditional physician-assigned ECOG performance status. Our study represents one of the first to use self-assessment variables to predict survival in patients with MDS, and suggests the utility of developing comprehensive risk assessment tools for older patients with MDS that include geriatric as well as disease-specific domains.
Treatment for older patients must be individualized based on prognosis, preferences and fitness for therapy. However, there is evidence that older MDS patients are less likely than younger patients to undergo risk stratification prior to treatment with chemotherapy in routine clinical practice.20 The National Comprehensive Cancer Network recommends assessment of comorbidities, geriatric syndromes and frailty into the routine care of older cancer patients with any cancer diagnosis.21 Available evidence supports comprehensive geriatric assessment as the most evidence-based method of detecting and quantifying fitness for therapy in cancer care.22–24 It should be emphasized that we did not perform geriatric assessment in this study but used readily available quality of life and clinical data. Predictors obtained through thorough geriatric assessment can be expected to be even more valuable for prognostication. Although the need for such assessment is well recognized,15,25–27 simpler tools that are feasible for use in oncology settings are still under development.
Well-conducted studies in patients with cancer have demonstrated that identification of frailty and functional decline can help guide treatment decisions.28–31 Objectively measured physical performance and self-reported fatigue predict complications, hospital stays and ICU admissions following surgery for pancreas cancer.29 The feasibility of inpatient geriatric assessment for older adults undergoing induction therapy for AML has also been demonstrated.31 A recent study used multidimensional geriatric assessment in 195 older patients with high-risk MDS and AML to assess treatment allocation and overall survival.32 Dependency in ADLs, poor performance status, high comorbidity score and fatigue all remained independent predictors of mortality when adjusted for traditional prognostic factors.
Low serum albumin is a well-established risk factor for mortality in many diseases, and was the most powerful predictor of survival in our study. While hypo-albuminemia is commonly ascribed to poor nutrition, there is growing evidence that it is the underlying inflammation of chronic disease that suppresses albumin production and is responsible for its associated morbidity.19 Only three of the 20 patients with low albumin had a history had a history of prior or concurrent cancer, suggesting that the low albumin was related to non-cancer comorbidities or the MDS itself.
We found that self-reported ability to take a long walk with ease in the week prior to MDS diagnosis was also a powerful prognostic indicator, and more predictive than physician-assigned ECOG performance status in this fairly functional older population. Elderly patients with low physical activity are known to have increased mortality, disability, and nursing home stays.33 Slow gait speed in particular is a strong predictor of chronic disability, and is closely linked with mortality.34 Oncologists have long known that physical function predicts of outcome in patients with cancer.12,35–37 However, commonly used measures of general or overall performance status like the ECOG or Karnofsky scales do not detect the smaller gradations of functional loss that may be relevant in older adults. In a similar cohort of older patients with newly diagnosed AML, we found that questions that asked about higher levels of physical function had powerful prognostic value even in patients with a normal performance status.38
Lack of vomiting in the week prior to assessment was a univariate predictor of survival. None of these patients were receiving treatment for MDS, and only one was receiving treatment for a concurrent cancer, so vomiting in this context may function as a marker of non-cancer comorbidity and nutritional status.
The addition of a comorbidity index to traditional prognostic factors has been shown to improve risk stratification in general MDS populations.39,40 In our cohort, a higher Charlson score increased mortality in univariate analysis only. This is likely because our cohort may have substantially less comorbidity than other populations. Similarly, age was not an independent predictor of mortality, but we did not include younger patients in the analysis.
A number of factors should be considered in the interpretation of our results. The patients who present to our regional cancer center are a select group with fewer comorbidities and functional limitations than expected for their age. By excluding patients who did not complete a baseline questionnaire, we further selected for those who were well enough to avoid immediate admission to the hospital. For all of these reasons, the overall survival of our cohort is likely better than would be seen in a general population of older adults with MDS. The self-reported variables from our validated quality of life survey, while relevant to geriatric domains, were not designed specifically for this purpose. About 20% of our population had a prior history of cancer treatment, suggesting they may have “secondary” MDS. The IPSS was validated in a cohort that did not have any history of prior chemotherapy or radiation. However, although it does not work very well in therapy-related MDS, the score is commonly used in this group of patients, and we adjusted for prior treatment in our multivariable model.
The IPSS assessed predictors at the time of original MDS diagnosis, but we used first presentation to DFCI as the baseline as we did not have a clear date of initial diagnosis on all patients. The majority of cases of MDS presented to DFCI within 3 months of their initial bone marrow biopsy. Hematologic abnormalities may precede the initial biopsy by months to years, and in clinical practice prognostication is generally done from the time the hematologist meets the patient. We did not extract ECOG performance status from the medical record, and thus we were not able to adjust our model for this commonly used measure of functional status. We also did not use strict IWG criteria for response, which would require follow-up bone marrow biopsy; however, in the “real world” observational setting of MDS treatment for the elderly, this is very often not done, especially if counts are improving. Finally, while our sample size is large for a single institution, it limited the number of individual predictors we were able to examine.
While these factors may limit generalizability, the goal of our study was to determine which readily available clinical and self-reported variables would improve prediction of mortality in a population of older patients with MDS who are likely to be candidates for chemotherapy. Oncologists frequently consider concurrent medical conditions and performance status when deciding on fitness for chemotherapy. As we have shown in an older population of patients with AML, geriatric assessment domains can be highly predictive of outcome even in those with the highest performance status and fewest comorbidities.39 We had the advantage of a prospectively collected, high quality database primarily comprised of this category of patient.
In summary, this study of highly selected older adults with MDS, serum albumin and self-reported physical function added important prognostic information to the IPSS, a commonly used tool for risk stratification and treatment decision-making. Our findings suggest the utility of developing comprehensive risk assessment tools for older patients with MDS that include markers of geriatric assessment domains as well as genetic and disease-specific information. These tools could be used to help make treatment decisions, anticipate complications, and provide appropriate support during cancer care. Prospective collection of non-hematologic markers of survival should therefore be incorporated into clinical trials and observational studies for older patients with hematologic malignancies to allow for the development of more comprehensive predictive models.
Acknowledgments
We gratefully acknowledge the Dana Farber patients who made this research possible.
Funding Source
This research was funded by a grant from the Dana Farber Cancer Institute (JAD) and the MSTAR Program (American Federation for Aging Research/NIH Grant #1T35AG038027-02 9); KAS, AES). Dr. Driver gratefully acknowledges support from a Veterans’ Administration Career Development Award).
Footnotes
This paper was presented in abstract form at the AGS Annual Meeting, Grapevine, Texas, May 2–6, 2013.
Disclosures and Conflict of Interest Statement
The authors report no circumstance or competing interest that could be construed or perceived as influencing the interpretation of the results. Dr. Richard M Stone has served in a Consultant or Advisory Role for Genzyme © and has received investigator-initiated research funding from Novartis. Dr. Daniel J. DeAngelo has served in a consultant or advisory role for Novartis ©.
Author Contributions
All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
Conception and design: J. Driver, G. Motyckova, R. Stone, K. Fega.
Data collection: K. Fega, J. Driver, M. Wadleigh.
Analysis and interpretation of data: J. Driver, K. Fega, G.Motyckova, A. Sherman, D. DeAngelo, G. Abel, I. Galinsky, D. Steensma, M. Wadleigh, R. Stone.
Manuscript writing and approval: K. Fega, G. Abel, G. Motyckova, J. Driver, A. Sherman, D. DeAngelo, G. Abel, I. Galinsky, D. Steensma, M. Wadleigh, R. Stone.
Sponsor’s Role
The funders of this project had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
References
- 1.Knickman JR, Snell EK. The 2030 problem: caring for aging baby boomers. Health Serv Res. 2002;37(4):849–884. doi: 10.1034/j.1600-0560.2002.56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stauder R. The challenge of individualised risk assessment and therapy planning in elderly high-risk myelodysplastic syndromes (MDS) patients. Ann Hematol. 2012;1:1. doi: 10.1007/s00277-012-1472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abel GA, Koreth J. Optimal positioning of hematopoietic stem cell transplantation for older patients with myelodysplastic syndromes. Curr Opin Hematol. 2013;20(2):150–156. doi: 10.1097/MOH.0b013e32835d8e8e. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 5.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454–2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejar R, Stevenson KE, Caughey BA, Abdel-Wahab O, Steensma DP, Galili N, et al. Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30(27):3376–3382. doi: 10.1200/JCO.2011.40.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germing U, Hildebrandt B, Pfeilstocker M, Nosslinger T, Valent P, Fonatsch C, et al. Refinement of the international prognostic scoring system (IPSS) by including LDH as an additional prognostic variable to improve risk assessment in patients with primary myelodysplastic syndromes (MDS) Leukemia. 2005;19(12):2223–2231. doi: 10.1038/sj.leu.2403963. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113(6):1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlegelberger B, Gohring G, Thol F, Heuser M. Update on cytogenetic and molecular changes in myelodysplastic syndromes. Leuk Lymphoma. 2012;53(4):525–536. doi: 10.3109/10428194.2011.618235. [DOI] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 11.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamaker ME, Prins MC, Stauder R. The relevance of a geriatric assessment for elderly patients with a haematological malignancy – a systematic review. Leuk Res. 2014;38(3):275–283. doi: 10.1016/j.leukres.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Tucci A, Ferrari S, Bottelli C, Borlenghi E, Drera M, Rossi G. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer. 2009;115(19):4547–4553. doi: 10.1002/cncr.24490. [DOI] [PubMed] [Google Scholar]
- 14.Wildes TM, Ruwe AP, Fournier C, Gao F, Carson KR, Piccirillo JF, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J geriatr oncol. 2013;4(3):227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group . The EORTC QLQ-C30 scoring manual. 3rd. Brussels: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–3674. [PubMed] [Google Scholar]
- 20.Gattermann N, Kundgen A, Kellermann L, Zeffel M, Paessens B, Germing U. The impact of age on the diagnosis and therapy of myelodysplastic syndromes: results from a retrospective multicenter analysis in Germany. Eur J Haematol. 2013;91(6):473–482. doi: 10.1111/ejh.12196. [DOI] [PubMed] [Google Scholar]
- 21.Hurria A, Browner IS, Cohen HJ, Denlinger CS, deShazo M, Extermann M, et al. Senior adult oncology. J Natl Compr Canc Netw. 2012;10(2):162–209. doi: 10.6004/jnccn.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25(14):1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 23.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60(2):120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 24.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25(14):1936–1944. doi: 10.1200/JCO.2006.10.2954. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg PL, Attar E, Bennett JM, Bloomfield CD, De Castro CM, Deeg HJ, et al. NCCN clinical practice guidelines in oncology: myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9(1):30–56. doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decoster L, Kenis C, Van Puyvelde K, Flamaing J, Conings G, De Greve J, et al. The influence of clinical assessment (including age) and geriatric assessment on treatment decisions in older patients with cancer. J Geriatr Oncol. 2013;4(3):235–241. doi: 10.1016/j.jgo.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Freyer G, Geay JF, Touzet S, Provencal J, Weber B, Jacquin JP, et al. Comprehensive geriatric assessment predicts tolerance to chemotherapy and survival in elderly patients with advanced ovarian carcinoma: a GINECO study. Ann Oncol. 2005;16(11):1795–1800. doi: 10.1093/annonc/mdi368. [DOI] [PubMed] [Google Scholar]
- 28.Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29(27):3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 29.Dale W, Hemmerich J, Kamm A, Posner MC, Matthews JB, Rothman R, et al. Geriatric assessment improves prediction of surgical outcomes in older adults undergoing pancreaticoduodenectomy: a prospective cohort study. Ann Surg. 2014;259(5):960–965. doi: 10.1097/SLA.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Extermann M. Basic assessment of the older cancer patient. Curr Treat Options Oncol. 2011;12(3):276–285. doi: 10.1007/s11864-011-0161-5. [DOI] [PubMed] [Google Scholar]
- 31.Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Ellis LR, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc. 2011;59(10):1837–1846. doi: 10.1111/j.1532-5415.2011.03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschler B, Ihorst G, Platzbecker U, Germing U, Marz E, de Figuerido M, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98(2):208–216. doi: 10.3324/haematol.2012.067892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56(12):2211–2216. doi: 10.1111/j.1532-5415.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28(3):380–386. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Using reference data on quality of life—the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3) Eur J Cancer. 1998;34(9):1381–1389. doi: 10.1016/s0959-8049(98)00136-1. [DOI] [PubMed] [Google Scholar]
- 37.Hurria A, Lichtman SM, Gardes J, Li D, Limaye S, Patil S, et al. Identifying vulnerable older adults with cancer: integrating geriatric assessment into oncology practice. J Am Geriatr Soc. 2007;55(10):1604–1608. doi: 10.1111/j.1532-5415.2007.01367.x. [DOI] [PubMed] [Google Scholar]
- 38.Sherman AE, Motyckova G, Fega KR, Deangelo DJ, Abel GA, Steensma D, et al. Geriatric assessment in older patients with acute myeloid leukemia: a retrospective study of associated treatment and outcomes. Leuk Res. 2013;37(9):998–1003. doi: 10.1016/j.leukres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E, et al. Risk stratification based on both disease status and extra-hematologic comorbidities in patients with myelodysplastic syndrome. Haematologica. 2011;96(3):441–449. doi: 10.3324/haematol.2010.033506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperr WR, Wimazal F, Kundi M, Baumgartner C, Nosslinger T, Makrai A, et al. Comorbidity as prognostic variable in MDS: comparative evaluation of the HCT-CI and CCI in a core dataset of 419 patients of the Austrian MDS Study Group. Ann Oncol. 2010;21(1):114–119. doi: 10.1093/annonc/mdp258. [DOI] [PubMed] [Google Scholar]


