Abstract
Background
In tumors carrying BRCA mutations, DNA damage caused by standard cytotoxic chemotherapy can be potentiated by poly [ADP-ribose] polymerase (PARP) inhibitors, leading to increased cell death through synthetic lethality. Individuals carrying mutations in BRCA have an increased incidence of triple negative breast cancer (TNBC). In order to assess the role of PARP inhibition in the treatment of TNBC, we conducted a randomized phase II trial of the combination of veliparib, a small molecule PARP inhibitor, with the cytotoxic agent cyclophosphamide versus cyclophosphamide alone in patients with refractory TNBC.
Methods
Adult patients with TNBC were randomized to receive oral cyclophosphamide 50 mg once daily with or without oral veliparib at 60 mg daily in 21-day cycles. Patients on the cyclophosphamide arm could crossover to the combination arm at disease progression.
Results
Forty-five patients were enrolled; 18 received cyclophosphamide alone and 21 received the combination as their initial treatment regimen. Lymphopenia was the most common grade 3/4 toxicity noted in both arms. One patient in the cyclophosphamide alone arm, and 2 in the combination arm had objective responses. Response rates and median progression free survival did not significantly differ between both treatment arms.
Conclusion
The addition of veliparib to cyclophosphamide, at the dose and schedule evaluated, did not improve the response rate over cyclophosphamide treatment alone in patients with heavily pre-treated triple-negative breast cancer.
Keywords: metronomic cyclophosphamide, PARP inhibition, DNA damage repair, BRCA, HR defect
INTRODUCTION
Lacking significant estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 expression, triple-negative breast cancers (TNBC) are deficient in the major molecular targets for breast cancer against which treatments are available, leaving conventional chemotherapy as the only standard treatment option. TNBC is characterized by aggressive growth, high proliferation rate, and an overall poor prognosis [1]. Patients carrying germline BRCA1 mutations are more likely to develop TNBC [2], and patients under 50 years of age with TNBC were found to have a >10% likelihood of carrying a BRCA1 mutation [3]. TNBC, however, is now an entity increasingly recognized as encompassing a spectrum of distinct tumors with highly diverse molecular characteristics including BRCA1 inactivation, a high frequency of TP53 mutations, and widespread genomic instability associated with a basal-like gene expression signature on hierarchical clustering analysis [4,5].
BRCA1/2 genes are involved in the homologous recombination pathway of DNA damage repair. The presence of deleterious BRCA mutations confers sensitivity to inhibition of poly [ADP-ribose] polymerase (PARP), an enzyme in the base excision repair pathway—clinical responses have been observed in patients with BRCA mutant ovarian and breast cancers treated with small molecule PARP inhibitors [6,7]. Olaparib, one such PARP inhibitor, was approved by the US Food and Drug Administration for the treatment of women with advanced BRCA mutant ovarian cancer whose disease has progressed following three or more prior chemotherapy regimens. In preclinical models, co-administration of PARP inhibitors with cytotoxic chemotherapies, including cyclophosphamide, has been shown to increase DNA damage, resulting in improved antitumor effects. Because patients with TNBC have a high probability of carrying DNA repair defects, including BRCA mutations, and we had previously observed clinical benefit from the combination of the PARP inhibitor veliparib with low-dose oral cyclophosphamide in patients with ovarian and breast cancer carrying deleterious BRCA mutations [8], we set out to evaluate the activity of this combination in patients with TNBC [9–11]. To characterize the relative contribution of the PARP inhibitor to the activity of the drug combination, we conducted a randomized, multicenter phase II trial of oral cyclophosphamide with and without veliparib in patients with TNBC, and compared the response rates (partial and complete responses, PR+CR). Secondary objectives included measuring PAR levels in peripheral blood mononuclear cells (PBMCs), and levels of phosphorylated histone H2AX (γH2AX), a marker of DNA damage response, in circulating tumor cells (CTCs) [12,13].
PATIENTS AND METHODS
Eligibility criteria
Patients 18 or older with metastatic TNBC (documented ER negative, PR negative, and Her2/neu negative from the original pathology report per ASCO/CAP guidelines) [14,15] whose disease had progressed following at least one line of standard therapy were eligible. An ECOG performance status ≤ 1 and adequate liver, kidney, and marrow function defined as an absolute neutrophil count ≥ 1,500/μL, platelets ≥ 100,000/μL, total bilirubin < 1.5 x the upper limit of normal (ULN), aspartate aminotransferase and alanine aminotransferase ≤ 2.5 x ULN, creatinine < 1.5 x ULN were required. Prior exposure to PARP inhibitors or cyclophosphamide was allowed, unless administered previously in combination.
Prior anticancer therapy or surgery must have been completed at least 4 weeks prior to enrollment, and evidence of disease progression by staging scans was required. Patients unable to swallow pills or those with uncontrolled intercurrent illness, women who were pregnant or breastfeeding, and patients with gastrointestinal conditions that might predispose to drug intolerability or poor drug absorption were excluded. Patients with treated brain metastases stable for greater than 4 weeks off steroids were eligible. This trial was conducted under a National Cancer Institute (NCI)-sponsored IND with institutional review board approval at each participating site. Informed consent was obtained by the investigators from each participant, and protocol design and conduct followed all applicable regulations, guidances, and local policies [ClinicalTrials.gov Identifier: NCT01306032].
Trial design
This was an open-label, multicenter, randomized phase II study of the combination of veliparib and oral cyclophosphamide compared to oral cyclophosphamide alone in patients with triple-negative, refractory breast cancer. AbbVie supplied veliparib (ABT-888) to the Division of Cancer Treatment and Diagnosis, NCI, under a Collaborative Research and Development Agreement. Cyclophosphamide was obtained from commercial sources.
Based on the maximum tolerated dose established for the combination in a prior phase I trial, cyclophosphamide was administered orally at 50 mg once daily, along with oral veliparib at 60 mg once daily throughout a 21-day cycle. There were no restrictions on food consumption, and patients maintained study diaries documenting when drugs were taken and any associated side effects. Adverse events were graded according to NCI Common Toxicity Criteria version 4.0. Doses of both drugs were to be reduced for grade 2 non-hematologic and grade 4 hematologic toxicities. Non-hematologic toxicities had to resolve to ≤ Grade 1 (except electrolyte abnormalities, which had to resolve to ≤ Grade 2) and hematologic toxicities to ≤ Grade 2 (except lymphopenia) prior to starting the next cycle. Radiographic evaluation was performed at baseline and every three cycles to assess tumor response based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16].
Statistics
The trial was randomized and used a phase 2.5 design with a 0.10 alpha level one-sided test to compare clinical responses for the combination therapy to those of single-agent cyclophosphamide [17]. A total of 44 patients in each arm would permit 80% power to detect a difference between a 10% response rate for the single-agent arm and 30% for the combination of the two agents. In addition, median progression free survival (PFS) probabilities were compared between the two arms, using a 0.10 alpha level one-sided test. An early stopping rule was included to end accrual after approximately 44 total patients were enrolled if the response rate on the combination arm was very low.
Correlative Studies
CTC and PBMC correlative studies were performed only for patients enrolled at the NIH Clinical Center. Blood samples for CTC separation were collected into 7.5 mL CellSave tubes (Veridex) prior to study drug administration (baseline), 24 hours after the first dose of drugs, before the first dose on cycle 2, and immediately before each restaging (every 3 cycles); CTC γH2AX levels were determined for each sample as previously described [13]. PBMCs were collected in 8 mL Cell Prep tubes (Becton Dickinson) on cycle 1 day 1 at baseline and at 4 and 24 hours after both drugs, on cycle 2 day 1 before dosing and 4 hours after both drugs, and just prior to each restaging; PAR levels were measured. PBMC and CTC sampling were repeated after patient crossover.
RESULTS
Demographics
Of the 45 patients who enrolled on this study, 39 were evaluable for tumor response (Table 1). All patients who received either drug regimen on study were evaluable for assessment of toxicity. Of the six patients who were not evaluable for response, two chose to withdraw before treatment, two withdrew after beginning treatment, one had disease progression necessitating palliative intervention before beginning treatment, and one patient contracted a grade 3 lung infection and was taken off study before completion of the first cycle. For their initial treatment regimen, 18 of the 39 evaluable patients received cyclophosphamide alone, and 21 received the study drug combination (cyclophosphamide + veliparib); 16 patients from the cyclophosphamide-only arm crossed over to the combination regimen after disease progression. Information regarding BRCA mutation status was collected from patients if previously determined per standard of care guidelines; otherwise, BRCA testing was not performed as part of the study protocol. Seven patients had known deleterious BRCA1/2 mutations (2 in the combination arm and 5 in the cyclophosphamide-only arm), and four were known to be BRCA wild type (3 in the combination arm and 1 in the cyclophosphamide-only arm). Patients were heavily pretreated with a median of 3 lines of prior therapy— including one who had received prior therapy with the PARP inhibitor olaparib.
Table 1.
Patient Characteristics
| Characteristic | No. of patients |
|---|---|
| Number of patients enrolled/evaluable | 45/39 |
| Median age, years (range) | 54 (34 – 77) |
| ECOG performance status | |
| 0 | 34 |
| 1 | 11 |
| ≥ 2 | 0 |
| BRCA status | |
| Positive | 7 |
| Negative | 4 |
| Unknown | 34 |
| Median number of prior therapies (range) | 3 (1 – 11) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Toxicity
Study drugs were generally well tolerated (Table 2). Lymphopenia was the most common adverse event in both arms. Grade 3 leucopenia, neutropenia, and activated partial thromboplastin time (APTT) prolongation were each reported for one patient (less than 5%). Grade 3/4 non-hematological toxicities included a thromboembolic event and hyponatremia, both on the combination arm (less than 5%). A death on study from heart failure occurred in the follow up period off treatment in one patient receiving the combination (Table 2).
Table 2.
Adverse events by patient, grade 2 or greater and at least possibly related to study drugs (N = 39 evaluable patients).
| Adverse Event | C Alone | V+C at Crossover | V+C Combination | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Grade 2 | Grade 3 | Grade 2 | Grade 3 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Anemia | 2 | 1 | 1 | 1 | 3 | - | - | - |
| ALP increased | - | - | - | - | 1 | - | - | - |
| AST increased | - | - | - | - | 1 | - | - | - |
| Anorexia | - | - | - | 1 | - | - | - | - |
| Dyspnea | - | - | - | - | 1 | - | - | - |
| Fatigue | 2 | - | 2 | 1 | 1 | - | - | - |
| Generalized muscle weakness | - | - | - | - | 1 | - | - | - |
| Heart Failure | - | - | - | - | - | - | - | 1 |
| Hyperglycemia | - | - | - | - | 2 | - | - | - |
| Hypoalbuminemia | - | - | - | - | 1 | - | - | - |
| Hypokalemia | - | - | - | - | - | 1 | - | - |
| Hypophosphatemia | - | - | - | - | 2 | - | - | - |
| Hyponatremia | - | - | - | - | - | 1 | - | - |
| Leucopenia | - | - | - | - | 3 | 1 | - | - |
| Lung infection | - | - | - | - | - | 1 | - | - |
| Lymphopenia | - | 2 | 2 | 2 | 5 | 2 | 2 | - |
| Neutropenia | - | - | - | - | - | 1 | - | - |
| Oral mucositis | 1 | - | - | - | - | - | - | - |
| APTT prolonged | - | - | - | - | - | 1 | - | - |
| Sinus tachycardia | - | - | - | - | 1 | - | - | - |
| Thrombo-embolic event | - | - | - | - | 1 | - | - | |
| Thrombocytopenia | - | - | - | - | - | 1 | - | - |
| Ventricular tachycardia | - | - | - | - | - | - | 1 | - |
| Vomiting | - | - | 1 | - | - | - | - | - |
| Weight Loss | - | - | - | 1 | - | - | - | - |
Worst grade for each patient. N= 18 for C alone, 16 for V + C at crossover, and 21 for V + C combination. Abbreviations: V, veliparib; C, cyclophosphamide.
Efficacy
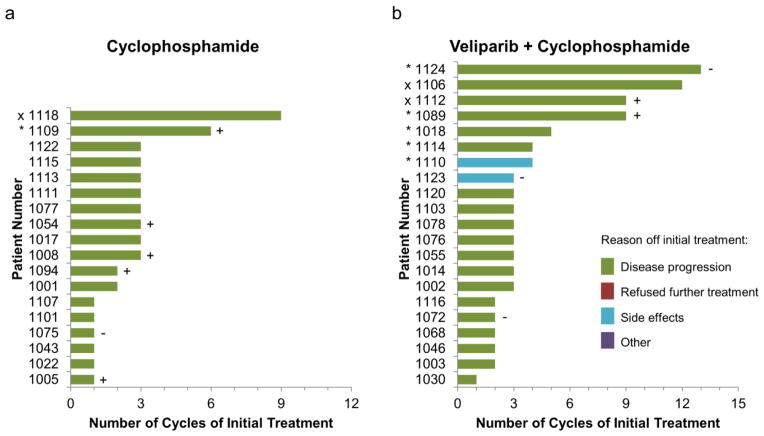
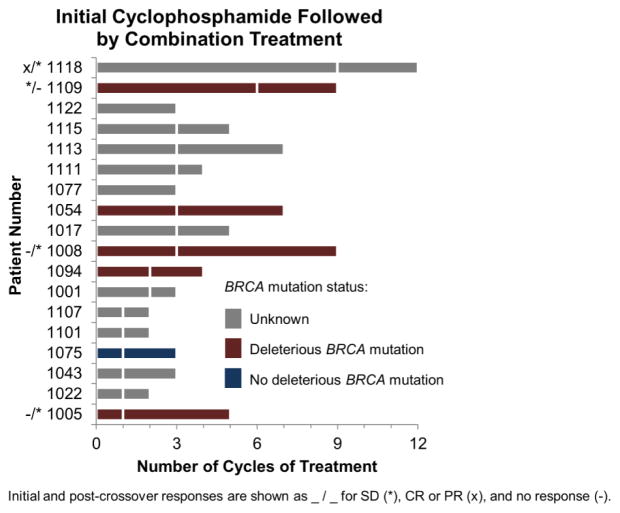
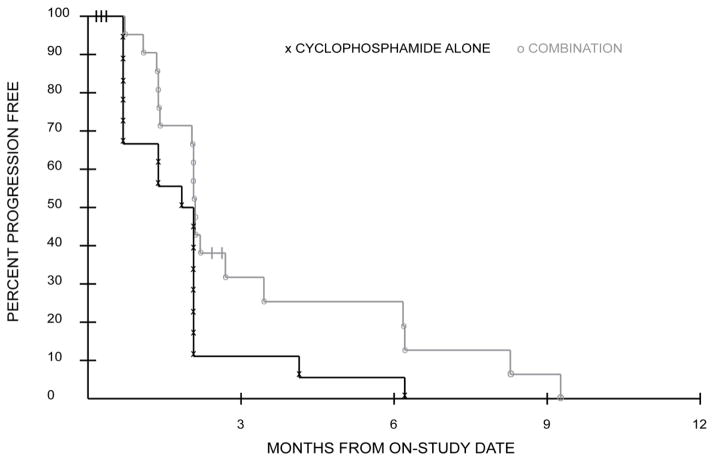
The addition of veliparib to cyclophosphamide did not improve the response rate (CR+PR) over cyclophosphamide treatment alone. One patient in the cyclophosphamide arm and two patients in the combination arm had partial responses. The number of cycles of initial and crossover treatment per patient are shown in Fig. 1 and 2, respectively. Based on 42 patients who were included in the PFS analysis, there was a statistical trend toward a difference in PFS favoring the combination treatment (P=0.034; Fig. 3). Median PFS was not different between the treatment arms—1.9 and 2.1 months; however, the curves became more separated after the median, accounting for much of the observed difference, with 4 patients surviving to 6 months progression free on the combination arm compared to only 1 patient on the cyclophosphamide-only arm.
Fig. 1.
Patient response during initial treatment. Best response of stable disease (SD, *) or complete or partial responses (CR or PR, x) are shown. Deleterious BRCA mutation (BRCA–positive) are indicated with (+); absence of BRCA mutation are indicated with (−). Patients with neither symbol have unknown BRCA status. (A.) Eighteen patients began on the cyclophosphamide-only treatment arm (1 SD and 1 PR) and (B.) Twenty-one patients began on the combination treatment arm (5 SD and 2 PR).
Fig. 2.
Patient response in cyclophosphamide-only arm after crossover to cyclophosphamide+veliparib treatment arm. Best response at any point during initial treatment and after crossover is indicated for SD (*) and CR or PR (x) [e.g. */*]. Bars are fractured at the point of crossover from cyclophosphamide-only to the combination. Deleterious BRCA mutation (BRCA-positive) are indicated in dark red; absence of BRCA mutation is indicated blue. Unknown BRCA status is shown in grey.
Fig. 3.
Progression Free Survival curves during initial treatment for patients on cyclophosphamide-alone and combination treatments show a trend towards increased progression free survival for patients on combination treatment; P=0.034
Correlative Studies
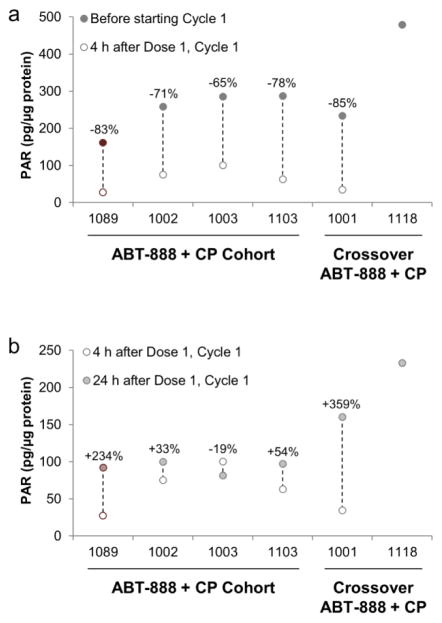
PBMC samples collected 4 hours following administration of veliparib from 4 patients on the combination arm, and 2 patients who had crossed over to the combination arm showed a 65% to 85% reduction in PAR levels from baseline (Fig. 4a). PAR levels in four of these patients had partially rebounded by the 24-hour collection time point (Fig. 4b).
Fig. 4.
PAR levels in patient PBMC samples. Data shown in red are for patients with deleterious BRCA mutations, and data in grey are from patients with unknown BRCA status. a PAR levels decrease after the first treatment with veliparib and cyclophosphamide. No PBMC data were available for Pt#1118 4 hours after Dose 1, Cycle 1. b PAR levels partially rebound in four patients by 24 hours after the first treatment with veliparib and cyclophosphamide. No PBMC data were available for Pt#1118 4 hours after Dose 1, Cycle 1.
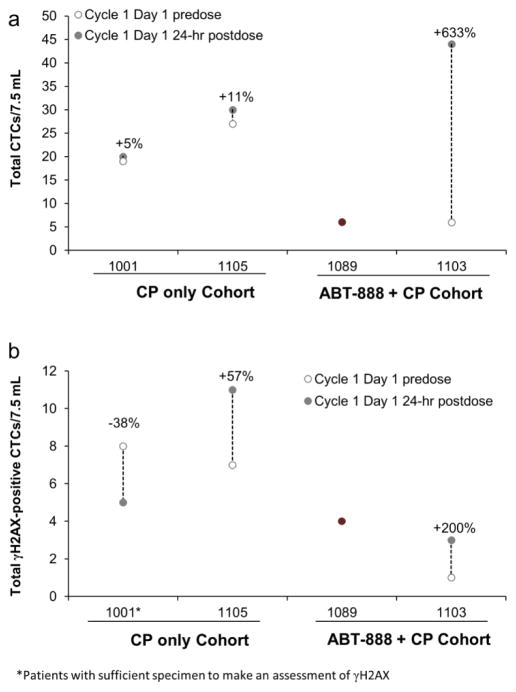
Five patients had CTC counts high enough to be evaluable (defined as ≥6 CTCs), 3 from the cyclophosphamide-only arm and 2 from the combination arm, with counts ranging from 6 to 59 per 7.5 mL whole blood during the course of treatment; total CTC numbers increased for each of these patients over the collection period. No significant increase in the ratio of the γH2AX-positive CTCs over baseline levels was measured in any of the patients (Fig. 5).
Fig. 5.
Changes in a total CTCs and b total γH2AX-positive CTCs in 7.5 mL blood samples collected at baseline and 24-hour postdose during cycle 1 day1. Percent changes from baseline are indicated for each evaluable patient. Data shown in red for patients with deleterious BRCA mutations, and in grey for patients with unknown BRCA status. Although patient #1118 was evaluable for CTCs, she did not have samples collected predose and 24 hours postdose on cycle 1 day 1. No baseline CTC data was available for patient #1089.
DISCUSSION
The cytotoxic agent cyclophosphamide causes extensive DNA damage and activates DNA damage response (DDR) elements such as the PARP enzymes and the tumor suppressor genes BRCA1/2. Inhibiting PARP-mediated DDR with small molecule inhibitors is known to confer increased sensitivity to cyclophosphamide [9–11]. The strategy to improve the efficacy of cytotoxic chemotherapy by co-administration of a PARP inhibitor has been limited by the associated increase in toxicity, necessitating reduction in the doses of the chemotherapeutic agent(s). The combination of veliparib with oral cyclophosphamide was well tolerated in the phase I trial, thus allowing for sustained and chronic low-dose administration of both agents, and subsequent continuous PARP inhibition [8]. This trial was designed to further investigate the combination treatment, and to address whether the addition of the PARP inhibitor veliparib to oral cyclophosphamide would increase the efficacy of a low, well-tolerated oral dose of cyclophosphamide in patients with advanced TNBC.
While we did not observe an increase in the objective response rate (CR+PR) to oral cyclophosphamide with the addition of veliparib as the initial therapy in this trial, there was some indication of increased PFS, as well as an increase in the number of patients with prolonged disease stabilization in the combination group. The treatment regimen described here was also administered to patients with BRCA-mutant ovarian cancer or pretreated primary peritoneal, fallopian tube, or high-grade serous ovarian cancers (HGSOC) on a separate cohort of this clinical trial. Although there were clinical responses in these patients, similarly there was no statistically significant difference in the response rate between the combination and cyclophosphamide-only arms [18].
The limited instances of patient response in this study may be explained in part by the low dosage of veliparib (60 mg daily) administered. Although this dose was determined to be the MTD in the phase I trial of this combination and shown to inhibit tumor levels of PAR [8], additional studies have suggested that higher doses of veliparib are more clinically active either alone or in combination with different cytotoxic backbone therapies. There has been a wide range of doses tested clinically to date for this agent, with the recommended phase II dose of single agent veliparib being established at 400mg twice daily, and 150mg twice daily in combination with standard chemotherapy in TNBC [19,20]. Additionally, the presence of deleterious BRCA mutations in a tumor increases sensitivity to PARP inhibitors; however, in this study most patients had not been tested for BRCA mutations. Furthermore, most study participants had undergone multiple therapies previously, including one who had received another PARP inhibitor, olaparib. Therefore the possibility that the low response rate observed here is due to resistance acquired through prior exposure to anticancer treatments cannot be excluded.
Pharmacodynamic biomarker assessment in the phase I trial of this combination had noted a significant decrease in PAR levels in both PBMCs and paired pre- and post-dose tumor biopsies across all tested dose levels [8]. In the present study, PAR levels also strongly decreased in PBMCs at the assayed once daily combination dose; however PAR levels had partially recovered at the 24 hour time point, potentially due to the known short half-life of veliparib. Although this is expected to contribute to intermittent reversal of PARP inhibition, the ideal duration and intensity of inhibition in PARP-mediated DDR is unclear, and may not necessarily correlate with the lack of clinical efficacy noted for this combination. The present study did not show notable increases in γH2AX-positive CTCs, but the small sample number and low CTC yield obtained in patient blood samples were limiting. Additionally, the relatively low dose of metronomic cyclophosphamide may not have been sufficient to trigger an increase in γH2AX levels.
PARP and its enzymatic product PAR are postulated to play a role in DDR regulation at multiple stages; PARylation has been implicated in DNA repair, chromatin modulation, and mitosis [21]. The best reported clinical efficacy of PARP inhibitors has been in tumors with BRCA deficiency, through synthetic lethality; however, other homologous recombination pathway defects (including defects in DNA damage sensors such as ATM, ATR, CHK1/2, or RAD51) confer sensitivity to PARP inhibition preclinically, and this effect may partially explain why a subset of non-BRCA mutated patients show sensitivity to PARP inhibitors [22]. In addition, the known complexity of the DNA repair mechanisms also makes it unlikely that a single biomarker panel can completely predict individual responsiveness to PARP inhibition; other factors such as epigenetic mechanisms, platinum susceptibility, or drug efflux proteins may contribute to PARP inhibitor resistance [23]. The interest in exploring the importance of these different pathways in contributing to clinical benefit from PARP inhibition is evident from ongoing trials. Approaches such as whole exome and transcriptome analysis, planned for the veliparib and irinotecan combination trial in BRCA mutated and non-mutated TNBC, may help define a biomarker signature of response to veliparib and PARP inhibitors in general [24]; trials such as the phase I/II of veliparib in combination with carboplatin and etoposide in treatment-naïve extensive stage small cell lung cancer (NCT02289690), or the phase II trial of veliparib in combination with carboplatin and paclitaxel in squamous cell lung cancer (NCT02106546) will also clarify the role of platinum-sensitivity as a surrogate marker for PARP responsiveness in non-gynaecological malignancies.
Recently, immune modulation of the tumor microenvironment through checkpoint inhibitor blockade has reported clinical benefits in TNBC [18,25]. The presence of tumor infiltrating lymphocytes (TILs) [26], as well as the amount of TILs found in the resected surgical specimens post neoadjuvant therapy [27], has been reported to correlate with prognosis in this disease. It is postulated that the high mutational load, functioning as tumor-associated antigens, is implicated in the mechanism of response to checkpoint blockage [28]. These findings would suggest the possibility of new synergistic therapeutic partners, such as PARP inhibitors combined with immune checkpoint blockade agents, particularly for TNBC.
In this trial, we were able to administer the combination of PARP inhibitor with cytotoxic chemotherapy without significant toxicity, and similar approaches continue to be actively tested in TNBC as well as a variety of other tumor types [29]. Nevertheless, careful consideration will be needed for future trial designs involving veliparib, as well as other PARP inhibitors, to better clarify outstanding questions such as the ideal sequencing of these agents in relation to conventional chemotherapy, (whether platinum-based or not), dosing, their role as maintenance therapy, and the efficacy of one inhibitor versus another. Various ongoing trials will further inform these questions, and will maximize the therapeutic benefit from these agents.
Acknowledgments
We thank Drs. Andrea Regier Voth, Leidos Biomedical Research, Inc., and Mariam Konaté, Capital Consulting Corporation, for editorial assistance in the preparation of this manuscript. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contracts No. HHSN261200800001E, N01-CM-2011-00071, N01-CM-2011-00032, N01-CM-2011-00100, and N01-CM-2011-00038. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(36):5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 3.Robertson L, Hanson H, Seal S, Warren-Perry M, Hughes D, Howell I, Turnbull C, Houlston R, Shanley S, Butler S, Evans DG, Ross G, Eccles D, Tutt A, Rahman N TMG TNTT Bcsc. BRCA1 testing should be offered to individuals with triple-negative breast cancer diagnosed below 50 years. British journal of cancer. 2012;106(6):1234–1238. doi: 10.1038/bjc.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, Rugo HS, Liu MC, Stearns V, Come SE, Timms KM, Hartman AR, Borger DR, Finkelstein DM, Garber JE, Ryan PD, Winer EP, Goss PE, Ellisen LW. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 2015;33(17):1902–1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Varhol R, Tam A, Dhalla N, Zeng T, Ma K, Chan SK, Griffith M, Moradian A, Cheng SW, Morin GB, Watson P, Gelmon K, Chia S, Chin SF, Curtis C, Rueda OM, Pharoah PD, Damaraju S, Mackey J, Hoon K, Harkins T, Tadigotla V, Sigaroudinia M, Gascard P, Tlsty T, Costello JF, Meyer IM, Eaves CJ, Wasserman WW, Jones S, Huntsman D, Hirst M, Caldas C, Marra MA, Aparicio S. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 7.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 8.Kummar S, Ji J, Morgan R, Lenz H-J, Puhalla SL, Belani CP, Gandara DR, Allen D, Kiesel B, Beumer JH, Newman EM, Rubinstein L, Chen A, Zhang Y, Wang L, Kinders RJ, Parchment RE, Tomaszewski JE, Doroshow JH. A Phase I Study of Veliparib in Combination with Metronomic Cyclophosphamide in Adults with Refractory Solid Tumors and Lymphomas. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18(6):1726–1734. doi: 10.1158/1078-0432.ccr-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kummar S, Chen A, Parchment R, Kinders R, Ji J, Tomaszewski J, Doroshow J. Advances in using PARP inhibitors to treat cancer. BMC Med. 2012;10(1):25. doi: 10.1186/1741-7015-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandhu SK, Yap TA, de Bono JS. Poly(ADP-ribose) polymerase inhibitors in cancer treatment: A clinical perspective. European journal of cancer. 2010;46(1):9–20. doi: 10.1016/j.ejca.2009.10.021. doi: http://dx.doi.org/10.1016/j.ejca.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Curtin NJ, Szabo C. Therapeutic applications of PARP inhibitors: Anticancer therapy and beyond. Mol Aspects Med. 2013;34(6):1217–1256. doi: 10.1016/j.mam.2013.01.006. doi: http://dx.doi.org/10.1016/j.mam.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redon CE, Nakamura AJ, Zhang Y-W, Ji J, Bonner WM, Kinders RJ, Parchment RE, Doroshow JH, Pommier Y. Histone γH2AX and Poly(ADP-Ribose) as Clinical Pharmacodynamic Biomarkers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(18):4532–4542. doi: 10.1158/1078-0432.ccr-10-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang LH, Pfister TD, Parchment RE, Kummar S, Rubinstein L, Evrard YA, Gutierrez ME, Murgo AJ, Tomaszewski JE, Doroshow JH, Kinders RJ. Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16(3):1073–1084. doi: 10.1158/1078-0432.CCR-09-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. Journal of Clinical Oncology. 2010;28(16):2784–2795. doi: 10.1200/jco.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Archives of Pathology & Laboratory Medicine. 2007;131(1):18–43. doi: 10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) European journal of cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. S0959-8049(08)00873-3 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Simon RM, Steinberg SM, Hamilton M, Hildesheim A, Khleif S, Kwak LW, Mackall CL, Schlom J, Topalian SL, Berzofsky JA. Clinical Trial Designs for the Early Clinical Development of Therapeutic Cancer Vaccines. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(6):1848–1854. doi: 10.1200/JCO.2001.19.6.1848. [DOI] [PubMed] [Google Scholar]
- 18.Investigational PD-L1–targeted Immunotherapy Safe for Patients With Triple-negative Breast Cancer, Effective in Some. 2015 http://www.aacr.org/Newsroom/pages/News-Release-Detail.aspx?ItemID=707#.Vk8-WZ0o6pq.
- 19.Puhalla S, Beumer JH, Pahuja S, Appleman LJ, Tawbi HA-H, Stoller RG, Lee JJ, Lin Y, Kiesel B, Yu J, Tan AR, Belani CP, Chew HK, Garcia AA, Morgan R, Giranda VL, Shepherd SP, Chen AP, Chu E. Final results of a phase 1 study of single-agent veliparib in patients with either BRCA1/2-mutated cancer, platinum-refractory ovarian, or basal-like breast cancer. J Clin Oncol. 2014;32(suppl 5s) abstr 2570. [Google Scholar]
- 20.Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, Rubin SC, Rutherford TJ, Chan JK, Chen A, Swisher EM. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecologic oncology. 2015;137(3):386–391. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langelier MF, Planck JL, Roy S, Pascal JM. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336(6082):728–732. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michels J, Vitale I, Saparbaev M, Castedo M, Kroemer G. Predictive biomarkers for cancer therapy with PARP inhibitors. Oncogene. 2014;33(30):3894–3907. doi: 10.1038/onc.2013.352. [DOI] [PubMed] [Google Scholar]
- 23.Wagner LM. Profile of veliparib and its potential in the treatment of solid tumors. OncoTargets and therapy. 2015;8:1931–1939. doi: 10.2147/OTT.S69935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LoRusso P, Ji JJ, Li J, Heilbrun K, Shapiro G, Sausville EA, Boerner SA, Smith DW, Pilat MJ, Zhang J, Chen AP, Nechiporchik N, Parchment RE. Phase I study of the safety, pharmacokinetics (PK), and pharmacodynamics (PD) of the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888; V) in combination with irinotecan (CPT-11; Ir) in patients (pts) with advanced solid tumors. J Clin Oncol. 2011;29(15) doi: 10.1158/1078-0432.CCR-15-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cimino-Mathews A, Foote JB, Emens LA. Immune targeting in breast cancer. Oncology (Williston Park) 2015;29(5):375–385. [PubMed] [Google Scholar]
- 26.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G, Andre F. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2014;25(3):611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loi S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol. 2014;32(27):2935–2937. doi: 10.1200/JCO.2014.56.7677. [DOI] [PubMed] [Google Scholar]
- 29.Appleman LJ, Beumer JH, Jiang YX, Puhalla S, Lin Y, Owonikoko TK, Harvey RD, Stoller R, Petro DP, Tawbi HAH, Argiris A, Strychor S, Kiesel B, Chu E, Shepherd SP, Giranda VL, Chen AP, Belani CP, Ramalingam SS. A phase I study of veliparib (ABT-888) in combination with carboplatin and paclitaxel in advanced solid malignancies. J Clin Oncol. 2012;30(15) [Google Scholar]