Abstract
The literature shows evidence for long-lasting effects of low birth weight (LBW) on many health outcomes, but little is known about effects on self-perceived health. Findings are mixed and studies are small, mostly focusing on LBW effects on health outcomes before adulthood. Further, as LBW and most health conditions including self-perceived health are partly heritable, associations between birth weight (BW) and adverse health outcomes may also be due to shared genetic as well as other (pre- and postnatal) unmeasured environmental influences. We explored LBW effects on self-perceived health in early and later adulthood using a very large and genetically informative sample of more than 50,000 Swedish twins. In addition, analyses within twin pairs (the co-twin control design) were used to examine potential associations between BW and the offspring’s risk for poor self-perceived health independent of shared environmental or genetic factors, evidence which is critical for the understanding of underlying mechanisms. Results showed that lower BW was significantly associated with poorer self-perceived health during adulthood, although the effect size was small. Co-twin control analyses suggested that this increased risk may be due to shared underlying liability (environmental or genetic) rather than a direct effect of BW, but findings were not conclusive.
Keywords: Birth weight, fetal growth, subjective health, self-rated health, twin design
Introduction
Low birth weight (LBW) has been shown to have long-term adverse effects on health outcomes throughout the lifespan (e.g. Alexander, Henry Dasinger, & Intapad, 2014; Kramer, 2013; Mu et al., 2014). In particular, LBW is associated with increased risks of chronic disease in later life, such as asthma, high blood pressure, cardiovascular disease, type 2 diabetes, impaired glucose homeostasis, osteoporosis, impaired bone mass and early reproductive aging (e.g. Alexander et al., 2014; Law & Shiell, 1996; Martinez-Mesa et al., 2013; Mu et al., 2014; Whincup et al., 2008) as well as mortality (D’Onofrio, Class, et al., 2013). Although LBW may indicate poor fetal growth and/or pre-term birth, associations between LBW and long-term risks have primarily been attributed to fetal growth restriction (Risnes et al., 2011).
In recent years, much progress has been made to incorporate a patient’s self-perceived health into medical research, as it provides a summary across various health conditions and an estimate of an individual’s overall sense of well-being and impact of disease (Sprangers et al., 2009). Interestingly, it has been shown that self-perceived health (even if based on a single item) is often superior to more objective clinical assessments for predicting patients’ survival (Gotay, Kawamoto, Bottomley, & Efficace, 2008; Idler & Benyamini, 1997). A possible explanation for this is that an individual’s perspective on his or her own health may capture not only physical well-being but also various psychosocial states influencing health outcomes. Thus, self-ratings of perceived health are direct, simple, and global measures that reflect the respondent’s experience of healthiness. The self-perceived health questions used in past studies vary: while some ask about global health in general, others are more comparative asking about present health compared to others of the same age, or to one’s own health in the past (adding an element of time to the question). The latter framing of the question is most commonly used in gerontological research as health is expected to decline most in old age. However, the predictive value for morbidity and health outcomes has been fairly consistent regardless of the question used (for a detailed review see Idler & Benyamini, 1997).
To date little is known about the self-perceived health status of LBW infants in adulthood and throughout life (Natalucci et al., 2013). The few studies exploring LBW effects on self-perceived health report contradictory results, with some studies finding negative effects of LBW (e.g. Saigal et al., 1996), while others find no effect (e.g. Saigal et al., 2006), or even a positive effect of LBW on self-perceived health (e.g. Natalucci et al., 2013). These contradictory findings may be due to small sample sizes (all but one study have N < 200), or to the different age ranges or different health outcome measures used (for a systematic review, including study sizes and measures, see Zwicker & Harris, 2008).
Research on birth weight (BW) effects on self-perceived health is limited to the mid-twenties and below, with no study to date exploring potential effects beyond that age (Zwicker & Harris, 2008). This is especially important as the variance in self-perceived health measures in adulthood has been shown to be very small in younger age groups and to increase with age (e.g. Svedberg, Gatz, Lichtenstein, Sandin, & Pedersen, 2005).
Further, most studies examining links between LBW and self-perceived health do not control for genetic and other unmeasured pre- and postnatal environmental risk factors, which is only possible with twin or other genetically informative designs (D’Onofrio, Lahey, Turkheimer, & Lichtenstein, 2013). Some of the factors that increase the likelihood of LBW (e.g. maternal health or hostile environments; Kramer, 2013) may also cause adverse health outcomes in the offspring. In other words, the precursors to LBW may be the causal mechanisms for adverse health outcomes rather than LBW being directly causal. For example, a recent study showed that offspring of mothers reporting worse health during pregnancy have a higher risk themselves to deliver a LBW infant (Teoli, Zullig, & Hendryx, 2013). Most health conditions, including self-perceived health, have been shown to be at least partly heritable (Mosing, Pedersen, Martin, & Wright, 2010; Mosing, Zietsch, Shekar, Wright, & Martin, 2009; Sorace et al., 2015) and similar findings have been reported for BW (Magnus, 1984), gestational age (Weinberg & Shi, 2009), and fetal growth (Svensson, Pawitan, Cnattingius, Reilly, & Lichtenstein, 2006). Thus, the same genetic influences causing poor health in the mother may also lead to LBW or fetal growth restriction, and subsequently cause poorer health in her offspring. This could explain some of the associations found between LBW and adverse health effects in offspring (D’Onofrio, Class, Lahey, & Larsson, 2014; Oberg, Cnattingius, Sandin, Lichtenstein, & Iliadou, 2011).
Here we explore BW effects on self-perceived health in early and later adulthood using a very large and genetically informative sample of more than 50,000 Swedish twins. First, we aim to establish whether there is an overall significant effect of BW on three different measures of self-perceived health in adulthood (after adjusting for gestational age and other covariates). Next, we explore whether these potential associations differ by sex or by age (i.e., age 45 and below versus age 46 and above). Finally, co-twin control analyses allow us to determine whether potential associations between LBW and self-perceived health are due to shared genetic or environmental effects (shared underlying liability) or whether they are truly causal in nature, i.e. the effects of fetal growth on self-perceived health are direct environmental (D’Onofrio et al., 2014; McGue, Osler, & Christensen, 2010). As twin siblings have identical gestational age, BW differences within twin pairs reflect differences in fetal growth.
Methods
Participants
The data were derived from two surveys based on two large cohorts of twins registered with the Swedish Twin Registry (STR; Lichtenstein et al., 2002; Magnusson et al., 2013). The first survey, assessing health related issues, was based on 39,000 twins in the Screening Across the Lifespan of Twins (SALT) study, born between 1926 to 1958, and was conducted between 1998 and 2002 through a computer assisted phone interview (Lichtenstein et al., 2002). The second survey, based on the Study of Twin Adults: Genes and Environment (STAGE) cohort, was conducted via the web between 2005 and 2006 and contained, among others, a whole range of health-related questions on approximately 32,000 twin individuals born between 1959 and 1985 (Lichtenstein et al., 2006). The two samples were merged based on the relevant measures and the final sample contained a total of 51,558 twin individuals (22,425 from STAGE and 29,133 from SALT) who had birth information as well as self-perceived health ratings available. The sample consisted of 2,517 monozygotic (MZ) male, 3,592 MZ female, 2,687 dizygotic (DZ) male, 3,508 DZ female and 6,654 DZ opposite-sex pairs as well as 6,302 male and 6,952 female single twins without the co-twin participating (86 male and 108 female pairs with missing zygosity). Single twin individuals and those with missing zygosity were retained for estimation of the assocations between BW and self-perceived health in the cohort. Zygosity of the twins was determined using questions about intrapair similarities and subsequently confirmed in a subset of the twins in the STR using genotyping, which showed that the questionnaire-based zygosity determination was correct for more than 98% of twin pairs (Lichtenstein et al., 2002). For further details on the SALT and STAGE cohorts and zygosity determination in the STR, see Lichtenstein et al. (2002; 2006). The sample age ranged between 19 to 74 years (mean = 45.9, sd = 13.1, median = 46). To split the sample into two equally sized age groups the median was used as a cutoff, i.e. everyone aged 45 and younger versus everyone aged over 45 years.
Measures
Birth weight (BW)
BW for the twins as well as other relevant birth related covariates (see Covariates below) were derived from official birth records. For those born between 1926 and 1972, birth information was based on a nation-wide collection of information from original birth records recorded by midwives and/or doctors at the time of birth, while for those born after 1973 it was based on records from the Swedish Medical Birth Register. Birth record data were then linked to the collected twin data using the unique personal identification number assigned to each Swedish citizen. For details on the BW measures and matching procedure see Hogberg et al. (2013). BW effects were explored as continuous (birthweight in grams divided by 250 for ease in interpreting statistical results) as well as dichotomized coded as low (≤ 2500g) versus normal (> 2500g) based on commonly used classifications and past findings (Kramer, 2013). In addition, as there was some indication for a potential non-linear relationship between self-perceived health and BW, analyses were repeated with quadratic (continuous) BW.
Self-perceived health outcomes
Here we used three different self-perceived health outcome variables which were dichotomized to differentiate between healthy and less healthy individuals. The first self-perceived health question was ‘How do you estimate your general health?’ rated as excellent, good, versus average, not so good, and bad. This variable will subsequently be referred to as self-rated health (SrH). The second question was ‘How do you estimate your health compared to five years ago?’ rated as better, the same, versus worse and will be referred to as SrH-5years. Third, we used the question: ‘Do you think your health status prevents you from doing things you want to do?’ rated as not at all, versus to some extent, or a great deal. Subsequently, we will refer to this variable as Activity. Tetrachoric correlations between the three self-perceived health measures ranged between 0.59 and 0.74.
Covariates
A number of factors that may also influence both BW and self-reported health were included as demographic covariates: sex of participant (if the sexes were analyzed together), age of participant at time health was rated; and birth information covariates: parity (the number of children previously born to the same mother), age of mother at birth, and gestational age (days of pregnancy since the first day of the last menstrual period until birth). To make sure we did not lose power in the corrected analyses (compared to uncorrected) due to missing values in covariates, missing values were replaced with the mean per zygosity (as the occurrence of DZ twins increases with maternal age and parity) for parity, age of mother, and gestational age.
Cohort analyses
To obtain an odds ratio (OR) for the full cohort, logistic regressions using maximum likelihood estimation were conducted in StataIC 13 (StataCorp, 2013), including BW (either coded as LBW vs. normal BW (NBW), or as a continuous variable) as the predictor with each of the three self-perceived health measures as outcome variables. To account for possible associations between BW of related individuals (i.e. twins), robust estimators of variance were computed using the cluster option in Stata (StataCorp, 2013). Initially, a baseline model was fitted with BW and each of the three outcome variables separately including all data (the full age range and males and females combined) without correcting for any of the covariates. The analyses were then repeated including all covariates (age, sex, parity, age of mother, and gestational age) using the full sample, as well as for males and females and the younger and older age groups separately. If the association between BW and self-perceived health was significant in the full cohort, co-twin control analyses were conducted for that specific health measure.
Co-twin control design
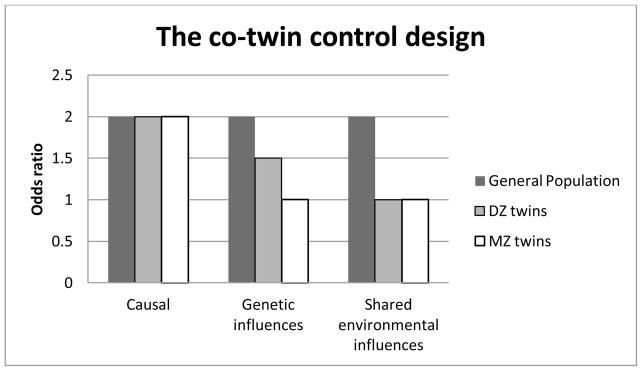
A discordant co-twin control design (Kendler et al., 1993; McGue et al., 2010) was used to explore potential genetic and shared environmental mediation of existing associations between BW and any of the self-perceived health outcomes. With this design, the risk of poorer self-perceived health of individuals discordant for BW (LBW versus NBW) is compared between unrelated individuals (population risk), within MZ, and within DZ pairs. This design essentially assumes that identical twins (MZ) share all their genes and nonidentical twins (DZ) on average only share 50% of their segregating genes, while the two members of a twin pair (regardless of MZ or DZ) share aspects of their environment, such as the intrauterine environment, exposure to maternal factors (e.g. pre-natal maternal health conditions or medication use), early life experience (e.g. post-natal maternal depression), and shared family environment (e.g., family climate) – all so called shared environmental influences. Importantly, BW frequently differs between twins although they share gestational age – both twins of a pair are generally born within a very short time frame (Bagchi & Salihu, 2006). Thus, BW differences within twin pairs represent variability in fetal growth (Bagchi & Salihu, 2006). By using the co-twin control design we control for shared environmental effects such as gestational age, prenatal environment, type of birth (C-section vs. natural), parental age, parental physical and mental health (e.g. PTSD, depression, and SES), and rearing environment, as well as for half (within DZ) or even all (within MZ) genetic influences. This way we can disentangle the direct effects of BW from shared environmental and genetic effects. If restricted fetal growth per se causes poor self-perceived health, we would not only find the association on the population level, i.e. individuals with LBW are more likely to report poor self-perceived health (odds ratio [OR] > 1), but also within MZ and DZ twins discordant for BW. Thus, MZ or DZ LBW twins would rate their health worse on average than their NBW co-twins and the strength of the association would not be attenuated by their degree of relatedness (McGue et al., 2010).
However, if the relationship was not directly causal, but due to overlapping underlying factors influencing both BW and self-perceived health outcome, we would expect different patterns of ORs for the discordant twins (compared to the population risk) depending on the source of covariation, i.e. shared environmental or shared genetic influences. If overlapping genetic influences explained the entire association, we would expect no elevated risk for poor self-perceived health in the LBW twin compared to the NBW twin within MZ pairs discordant for BW (OR=1). This is because both twins would have inherited the same risk genes for LBW and poor (self-perceived) health. The OR for DZ twins (sharing half their genes on average) would be expected to be intermediate between the one for the general population and the one for MZ twins (McGue et al., 2010).
Finally, if the relationship between the BW and self-perceived health were entirely due to overlapping shared environmental influences, we expect ORs within both MZ and DZ twins to be similar in magnitude and close to 1.0 as the LBW twin would not have an elevated risk for poor objective health compared to the NBW co-twin regardless of zygosity (as MZ and DZ twins of a pair share all their family environment). Figure 1 summarizes the different patterns of expected ORs for the different underlying mechanisms – note, though, that associations between BW and self-perceived health may in reality result from a combination of these underling mechanisms.
Figure 1.
Expected patterns of odds ratios if the relationship between birth weight and objective health were causal, due to overlapping genetic influences, or due to shared environmental influences in the general population, and DZ and MZ twins discordant for birth weight (adapted from McGue et al. 2010).
For the co-twin control analyses using the dichotomized BW measures all same-sex pairs with twins discordant for LBW (i.e. one twin > 2500g and the co-twin ≤ 2500g) were included resulting in 1,512 MZ pairs and 1,543 same-sex DZ pairs (approximately 24% of same-sex pairs) of which 857 – 913 twin pairs were also discordant for the respective self-perceived health measure. See Supplementary Figure 1 for the distribution of BW differences in pairs classified as discordant based on the LBW versus NBW dichotomy. For the continuous BW measure all same-sex pairs with twins discordant for the respective health variable were included in the co-twin control analyses resulting in 1,526 – 1,608 MZ pairs and 1,847 – 2,505 DZ pairs (depending on the health measure). ORs were then calculated separately among MZ and DZ twins for comparison with the cohort risk using the conditional logistic regression option for matched case-control groups in StataIC 13 (StataCorp, 2013). Note that the co-twin control analyses do not require correction for covariates as each twin is matched to its co-twin who shares the same age, sex, parity, age of mother (at birth), and gestational age (in addition to many other environmental influences). To account for the number of analyses conducted (i.e. two different BW classifications to predict three self-perceived health outcomes in the full cohort as well as in discordant MZ and DZ twins), a p-value of 0.01 was used as significance level in all within-group analyses.
Finally, as an additional test for shared underlying liability (genetic or environmental) we calculated whether the risk for low self-perceived health of NBW twins with a LBW co-twin was elevated compared to the risk of a NBW twin of a concordant pair. As explained above, if shared underlying liability causes both a decrease in BW as well as in self-perceived health, we would expect that in discordant pairs the twin with the NBW would also have been exposed to the same influences causing a decrease in health, resulting in a lower health rating in those twins compared to NBW twins with a NBW co-twin. If LBW causes the worse self-perceived health outcome we would not expect to see a significant difference between the two types of NBW twins – they both would rate their health as similarly good. Based on the same concept using the continuous BW, we tested whether the twins with good health from pairs discordant for health rating were more likely to have lower BW compared to healthy twins with a healthy co-twin.
Results
Preliminary analyses
The mean BW was 2,653g (sd = 513) and the sample contained 31,637 (61.4%) individuals with NBW and 19,921 (38.6%) with LBW – expected rates of LBW in a twin sample (Blickstein, 2002). Table 1 shows descriptive statistics for the three self-perceived health measures in the full sample, by age group, sex and BW. The majority of individuals rated their health as good and did not feel compromised by their health status.
Table 1.
Percentage of individuals who rated their health as good or did not feel compromised in their health status in the total sample, by age group, sex, and birth weight.
| N | SrH | SrH-5years | Activity | |
|---|---|---|---|---|
| Total sample | 51,558 | 75.9% | 79.2% | 75.8% |
| Age group | ||||
| < 46 years | 24,351 | 80.5% | 82.5% | 79.4% |
| ≥ 46 years | 26,207 | 71.5% | 75.9% | 71.9% |
| Sex | ||||
| Male | 23,536 | 79.1% | 79.6% | 78.6% |
| Female | 28,022 | 73.2% | 78.8% | 73.4% |
| BW | ||||
| Low BW | 19,921 | 74.5% | 78.8% | 74.9% |
| Normal BW | 31,637 | 76.8% | 79.4% | 76.3% |
| BW in g | ||||
| <1500 | 644 | 70.1% | 77.8% | 72.1% |
| 1500–1999 | 4,388 | 73.3% | 79.3% | 73.9% |
| 2000–2499 | 14,017 | 75.2% | 78.8% | 75.4% |
| 2500–2999 | 18,920 | 76.6% | 79.1% | 76.2% |
| ≥3000 | 13,589 | 76.7% | 79.7% | 76.3% |
Note. BW = birth weight; g = grams; N = Number; SrH = self-rated health; SrH-5years = self-rated health compared to 5 years ago.
Cohort analyses
Table 2 shows associations between BW (conceptualized as low vs. normal and as a continuous variable) and the self-perceived health variables for the full cohort from analyses both uncorrected and subsequently corrected for covariates as well as for different age groups and males and females separately. The inclusion of covariates did not substantially change the ORs for BW effects on the health variables. In the full sample, ORs suggested that LBW significantly increased the risk for poor SrH and for reduced Activity, but had little effect on SrH-5years, while BW as a continuous variable (250g lower BW) was significantly negatively associated with all three self-perceived health outcomes (Table 2). Further, ORs were similar between the sexes and across the two age groups with overlapping confidence intervals. Results based on the quadratic continuous BW were very similar to the continuous (linear) BW analyses in terms of significance; however, effect sizes were smaller (ORs < 1.01), suggesting no underlying non-linear relationships. Full results available upon request.
Table 2.
Odds ratios (95% confidence intervals) for the self-perceived health variables in relation to birth weight low versus normal in the upper half and birth weight measured continuously in the lower half of the table. ORs are shown uncorrected and corrected for age, sex, age of mother, parity, and gestational age for the full sample as well as different age and sex sub-samples.
| Low versus normal birth weight
| |||||
|---|---|---|---|---|---|
| Unadjusted | Full Sample | < 46 years | ≥46 years | Male | Female |
| SrH | 1.13* (1.08–1.18) | 1.16* (1.10–1.25) | 1.13* (1.06–1.19) | 1.07 (1.00–1.14) | 1.12* (1.06–1.19) |
| SrH-5years | 1.04 (0.99–1.08) | 1.08 (1.01–1.16) | 1.01 (0.95–1.07) | 1.02 (0.95–1.09) | 1.04 (0.98–1.10) |
| Activity | 1.08* (1.04–1.13) | 1.10* (1.03–1.18) | 1.08* (1.02–1.14) | 1.01 (0.95–1.08) | 1.09* (1.03–1.15) |
|
| |||||
| With Covariates –age, sex, age of mother, parity, and gestational age
| |||||
| SrH | 1.12* (1.07–1.17) | 1.13* (1.05–1.21) | 1.12* (1.05–1.19) | 1.11* (1.03–1.19) | 1.14* (1.07–1.21) |
| SrH-5years | 1.04 (0.99–1.09) | 1.09 (1.01–1.17) | 1.00 (0.94–1.07) | 1.02 (0.95–1.10) | 1.05 (0.98–1.12) |
| Activity | 1.09* (1.04–1.14) | 1.10* (1.02–1.18) | 1.08 (1.01–1.15) | 1.05 (0.98–1.13) | 1.11* (1.05–1.18) |
|
| |||||
| Continuous birthweight (effects of 250g lower birthweight)
| |||||
| Unadjusted | Full Sample | < 46 years | ≥46 years | Male | Female |
|
| |||||
| SrH | 1.03* (1.02–1.04) | 1.04* (1.03–1.06) | 1.03* (1.02–1.05) | 1.01 (1.00–1.03) | 1.03* (1.02–1.04) |
| SrH-5years | 1.01 (1.00–1.02) | 1.03* (1.01–1.05) | 1.00 (0.99–1.02) | 1.01 (1.00–1.03) | 1.00 (0.99–1.01) |
| Activity | 1.02* (1.01–1.03) | 1.03* (1.01–1.04) | 1.02* (1.01–1.04) | 1.00 (0.99–1.02) | 1.02* (1.01–1.04) |
|
| |||||
| Adjusted for age, sex, age of mother, parity, and gestational age
| |||||
| SrH | 1.04* (1.03–1.05) | 1.04* (1.02–1.06) | 1.04* (1.02–1.06) | 1.03* (1.01–1.05) | 1.04* (1.03–1.06) |
| SrH-5years | 1.02* (1.00–1.03) | 1.03* (1.02–1.05) | 1.00 (0.99–1.02) | 1.02 (1.01–1.04) | 1.01 (0.99–1.03) |
| Activity | 1.03* (1.02–1.04) | 1.03* (1.01–1.05) | 1.03* (1.01–1.05) | 1.02 (1.00–1.04) | 1.04* (1.02–1.05) |
Note. All ORs have been corrected for relatedness of the sample.
p < 0.01
All covariates except gestational age showed a significant effect on the three health measures (p < 0.01). Individuals had significantly increased odds to report poor health with increasing age (ORs: 1.02 – 1.07), increasing parity (ORs: 1.06 – 1.11), and younger age of the mother (ORs: 0.98 – 0.99), as well as when they were female (ORs: 1.06 – 1.50) (results not shown).
Within-pair analyses
We then conducted co-twin control analyses for the self-perceived health measures that were significantly associated with BW in the cohort analyses. Co-twin control analyses were only conducted in the full sample of discordant pairs, as the age and sex sub-groups showed similar ORs with overlapping confidence intervals. Results of the co-twin control analyses for LBW versus NBW (SrH and Activity) and for the continuous birthweight variable (SrH, SrH-5years, and Activity) are shown in Figure 2 and 3, respectively.
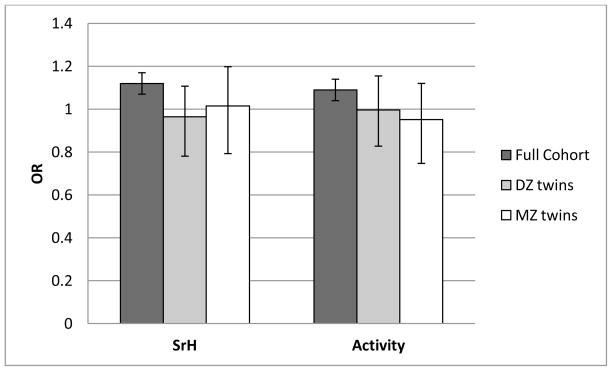
Figure 2.
Observed odds ratios (OR) for self-rated health (SrH) and activity as compared between individuals with low and normal birth weight. Bars show results for the full cohort (corrected for relatedness and all covariates) as well as same-sex DZ and MZ twins discordant for birth weight.
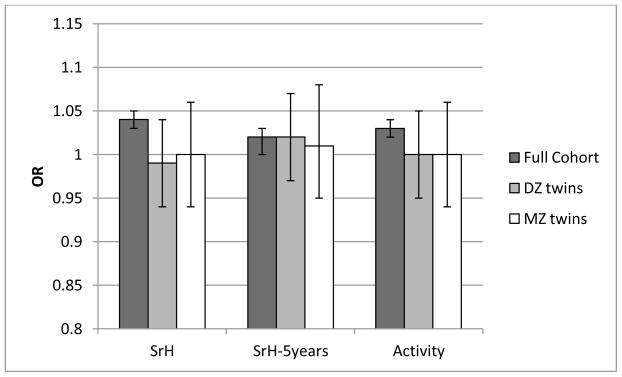
Figure 3.
Observed odds ratios (OR) for the self-perceived health outcomes as predicted by continuous birth weight (250g lower weight). Bars show results for the full cohort (corrected for relatedness and covariates) as well as for discordant same-sex DZ and MZ twins.
While the majority of ORs were significant in the full cohort, ORs in the co-twin control analyses (for both MZ and DZ twins) were generally lower and not significantly different from 1.0 (Figure 2 and 3). However, confidence intervals for the co-twin control analyses were wide (compared to the cohort level analyses) probably due to the reduced sample size as only discordant twins were included (see methods co-twin control design). Thus the effects of BW on self-perceived health in MZ and DZ twin pairs were not significantly different from corresponding associations in the full cohort. In line with that, between-group analyses showed that, compared to the NBW twins from concordant pairs, NBW twins from discordant pairs only had significantly elevated odds for low SrH, but not for Activity or SrH-5years. And similarly, twins perceiving their health as good had comparable birthweights regardless of whether they belonged to a concordant or a discordant pair, suggesting no shared underlying liability (genetic or environmental).
To estimate the minimum sample size needed to find an OR of 1.13 as significantly different from 1.0 (close to what was found for the dichotomous BW measure), a power analyses was performed. With an alpha of 0.05 and 80% power, the projected sample size needed is approximately N = 7,000. Similarly, to find an OR of 1.03 to be significantly different from 1.0 (using the continuous BW measure) the projected sample size needed is approximately N = 10,000. This suggests that although we had ample power to find a significant effect on the cohort level, we lacked power to find a significant OR in the discordant pairs, making comparison of the ORs between the groups difficult.
Discussion
The present study aimed to explore long-term effects of BW (conceptualized both as LBW versus NBW and as a continuous variable) on three self-perceived health measures during early and later adulthood using a large and genetically informative sample of more than 50,000 twins. Results suggested that those with lower BW had elevated odds to report poor health (SrH and SrH-5years) and to feel that their health status was preventing them from their daily activities (Activity). Although the associations seemed somewhat more pronounced in females and in the younger age group, the confidence intervals were wide and overlapping, suggesting no significant differences between the groups.
The present findings are in line with previous studies suggesting that LBW may be a risk factor for poorer self-perceived health outcomes, and that this effect may diminish with increasing age (Zwicker & Harris, 2008), although it is important to note that the present study is the first to include individuals beyond the age of 26 years. Nevertheless, risks were small – around 10% increased risk of poor self-perceived health for LBW individuals and 2% to 4% increased risk of poor self-perceived health for 250g lower weight – and could only be detected because of the large sample size used in this study. This could explain the mixed findings in the previous literature, suggesting that BW effects on self-perceived health may be small and partly measurement dependent.
The finding of only a small difference in self-perceived health ratings in LBW adults compared to NBW adults is surprising given the higher prevalence of chronic illness and regular medication need in this group in childhood as well as during adulthood reported in the literature (Sydsjo, 2011). The discrepancy between objective and self-perceived health outcomes in LBW adults has been explained as a psychological adaptation in chronically ill patients (Schwartz, Andresen, Nosek, & Krahn, 2007), resulting from a shift of internal standard of conceptualization of quality of life over the course of time as a buffer to the impact of adverse physical conditions. This explanation would also be in line with an even smaller LBW effect on self-perceived health in the older subgroup.
The inclusion of the covariates had little effect on the results, although all covariates (i.e. sex, age, age of mother, and parity) had a significant effect on self-rated health, with the exception of gestational age. Our findings are in line with past research showing that female sex (Deeg & Kriegsman, 2003; Svedberg, Bardage, Sandin, & Pedersen, 2006) and increased age are risk factors for poorer self-perceived health (Mosing et al., 2010; Svedberg et al., 2005) and that parity, apart from influencing BW (Kramer, 1987), is associated with other negative health outcomes (Sunyer et al., 2001). Somewhat surprising was the finding that lower age of mother was associated with worse self-perceived health. However, it has been suggested that the association between mother’s age and offspring health may be u-shaped with an increased risk for poor offspring health of very young and very old (i.e. above 35 years) mothers (Myrskyla & Fenelon, 2012). Given that in 87% of the present sample the mother’s age at birth was below the age of 36 (mean = 29.5, sd = 5.7), the upper end of the distribution may have been underrepresented, resulting in a negative association of mother’s age and self-perceived health.
Using the co-twin control design, we tested whether the association between LBW and poor self-perceived health is indeed causal, or better explained by shared genetic or unmeasured environmental influences accounting for both LBW and poor self-perceived health. ORs for MZ and DZ twins discordant for BW were generally lower than the ORs for the full cohort, and close and not significantly different from 1.0 for either self-perceived health item, which is in line with shared underlying liability. Unfortunately, confidence intervals were large resulting in non-significant differences between the groups (full cohort, MZ and DZ ORs) making the results hard to interpret in terms of causality versus shared underlying liability (shared genetic or shared environmental influences). This is likely due to the small effect of BW on self-perceived health (large power was needed to identify the effect on the cohort level) and the limited number of discordant twins included in the co-twin control analyses. Further, analyses between the discordant and concordant NBW/healthy twins showed no significantly elevated risk with exception for SrH (with dichotomous BW variable).
In summary, the present study suggests that BW effects on self-perceived health may be small and partly measurement dependent. Despite the large sample size, this made it difficult to determine whether the existing associations are causal or due to shared underlying liability. Future studies would either need an even larger sample or self-perceived health measures scored on a continuous response scale. However, as mentioned above, using a continuous response scale may also result in highly skewed responses.
We would like to close with some important caveats and limitations inherent to twin analyses, especially in regards to BW. First, generalizability of findings in twins to the general population may be a concern, especially given that twins may generally be more growth restricted in utero compared to singletons (for a detailed review and discussion see Muhlhausler, Hancock, Bloomfield, & Harding, 2011). However, twins do not differ from the general population in morbidity and all-cause mortality across the life-span (Christensen et al., 2001; Oberg et al., 2012), suggesting that twinning does not lead to adverse long-term health outcomes. Further, research has repeatedly shown that also in other domains such as personality, cognitive ability, and risk for mental disease, twins are comparable to the general population (e.g. Christensen et al., 2006; Johnson, Krueger, Bouchard, & McGue, 2002; Kendler, Pedersen, Farahmand, & Persson, 1996). Second, MZ twins are more likely to be mono-chorionic and share one placenta compared to DZ twins, allowing for vascular interconnections between the fetal circulations. If an adverse environment affected the weight of a twin, and if the causal pathway between that adverse environment and later health was mediated through some circulating factor during fetal life, then this circulating factor may also influence the health of the co-twin without affecting its weight. This could potentially result in lower within-pair associations between BW and health outcomes in MZ twins, which in turn may then wrongly be interpreted as suggesting underlying genetic influences (Morley & Dwyer, 2005). Unfortunately, we had no information on chorionicity and number of placentas of our twin pairs and therefore could not correct for this potential confounder. However, a recent study by van Beijsterveldt and coworkers (van Beijsterveldt et al., 2015) found that the influence of chorion type on the MZ correlations is very limited, suggesting that the assumption of equal prenatal environment of mono- and dichorionic MZ twins is tenable. Finally, as mentioned in the method section, although we can theoretically determine the best explanation for an association between two traits – causality versus shared underlying liability (pleiotropy/shared environment), we cannot exclude a combination of different influences solely based on the co-twin control design.
In conclusion, the present study is the first to explore BW effects on self-perceived health in early and later adulthood using a large and genetically informative sample. The findings for the full cohort controlling for relatedness showed that lower BW increased the odds for poor self-perceived health throughout adulthood, although the effect was small. This increased risk may be due to shared underlying liability (environmental or genetic) for both BW and poor self-perceived health rather than to effects of restricted fetal growth as such on subsequent self-perceived health; however, findings of the co-twin control analyses were not conclusive.
Supplementary Material
Acknowledgments
The present work was supported by in part from NIH grant AG037985, the Swedish Research Council 521-2013-8689, the Swedish Research Council for Health, Working Life and Welfare 2013-2292, and the European Union’s Seventh Framework Program (FP7/2011-2015 under grant agreement 259679). The Swedish Twin Registry is supported by the Swedish Ministry of Higher Education. We would like to thank the Swedish twins for their participation.
References
- Alexander BT, Henry Dasinger J, Intapad S. Effect of low birth weight on women’s health. Clinical Therapeutics. 2014 doi: 10.1016/j.clinthera.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi S, Salihu HM. Birth weight discordance in multiple gestations: occurrence and outcomes. J Obstet Gynaecol. 2006;26(4):291–296. doi: 10.1080/01443610600594724. [DOI] [PubMed] [Google Scholar]
- Blickstein I. Normal and abnormal growth of multiples. Semin Neonatol. 2002;7(3):177–185. doi: 10.1053/siny.2002.0105. [DOI] [PubMed] [Google Scholar]
- Christensen K, Petersen I, Skytthe A, Herskind AM, McGue M, Bingley P. Comparison of academic performance of twins and singletons in adolescence: follow-up study. Bmj. 2006;333(7578):1095. doi: 10.1136/bmj.38959.650903.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, Wienke A, Skytthe A, Holm NV, Vaupel JW, Yashin AI. Cardiovascular mortality in twins and the fetal origins hypothesis. Twin Res. 2001;4(5):344–349. doi: 10.1375/1369052012506. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Lahey BB, Larsson H. Testing the developmental origins of health and disease hypothesis for psychopathology using family-based quasi-experimental designs. Child Dev Perspect. 2014;8(3):151–157. doi: 10.1111/cdep.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry. 2013;70(11):1231–1240. doi: 10.1001/jamapsychiatry.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health. 2013;103(1):8. doi: 10.2105/AJPH.2013.301252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeg DJ, Kriegsman DM. Concepts of self-rated health: specifying the gender difference in mortality risk. Gerontologist. 2003;43(3):376–386. doi: 10.1093/geront/43.3.376. [DOI] [PubMed] [Google Scholar]
- Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- Hogberg L, Lundholm C, Cnattingius S, Oberg S, Iliadou AN. Birthweight discordant female twins and their offspring: is the intergenerational influence on birthweight due to genes or environment? Hum Reprod. 2013;28(2):480–487. doi: 10.1093/humrep/des380. [DOI] [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38(1):21–37. [PubMed] [Google Scholar]
- Johnson W, Krueger RF, Bouchard TJ, Jr, McGue M. The personalities of twins: just ordinary folks. Twin Res. 2002;5(2):125–131. doi: 10.1375/1369052022992. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression. A causal analysis. Arch Gen Psychiatry. 1993;50(1):36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Pedersen NL, Farahmand BY, Persson PG. The treated incidence of psychotic and affective illness in twins compared with population expectation: a study in the Swedish Twin and Psychiatric Registries. Psychol Med. 1996;26(6):1135–1144. doi: 10.1017/s0033291700035856. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65(5):663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS. The epidemiology of low birthweight. Nestle Nutr Inst Workshop Ser. 2013;74:1–10. doi: 10.1159/000348382. [DOI] [PubMed] [Google Scholar]
- Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14(8):935–941. [PubMed] [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: A unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252(3):184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlström E, …Pedersen NL. The Swedish Twin Registry in the third millenium: an update. Twin Research and Human Genetics. 2006;9(6):875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- Magnus P. Causes of variation in birth weight: a study of offspring of twins. Clin Genet. 1984;25(1):15–24. doi: 10.1111/j.1399-0004.1984.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Magnusson PK, Almqvist C, Rahman I, Ganna A, Viktorin A, Walum H, …Lichtenstein P. The Swedish Twin Registry: establishment of a biobank and other recent developments. Twin Research and Human Genetics. 2013;16(1):317–329. doi: 10.1017/thg.2012.104. [DOI] [PubMed] [Google Scholar]
- Martinez-Mesa J, Restrepo-Mendez MC, Gonzalez DA, Wehrmeister FC, Horta BL, Domingues MR, Menezes AM. Life-course evidence of birth weight effects on bone mass: systematic review and meta-analysis. Osteoporos Int. 2013;24(1):7–18. doi: 10.1007/s00198-012-2114-7. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci. 2010;5(5):546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley R, Dwyer T. Studies of twins: what can they tell us about the fetal origins of adult disease? Paediatr Perinat Epidemiol. 2005;19(Suppl 1):2–7. doi: 10.1111/j.1365-3016.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Pedersen NL, Martin NG, Wright MJ. Sex differences in the genetic architecture of optimism and health and their interrelation: a study of Australian and Swedish twins. Twin Research and Human Genetics. 2010;13(4):322–329. doi: 10.1375/twin.13.4.322. [DOI] [PubMed] [Google Scholar]
- Mosing MA, Zietsch BP, Shekar SN, Wright MJ, Martin NG. Genetic and environmental influences on optimism and its relationship to mental and self-rated health: a study of aging twins. Behav Genet. 2009;39(6):597–604. doi: 10.1007/s10519-009-9287-7. [DOI] [PubMed] [Google Scholar]
- Mu M, Ye S, Bai MJ, Liu GL, Tong Y, Wang SF, Sheng J. Birth weight and subsequent risk of asthma: a systematic review and meta-analysis. Heart Lung Circ. 2014;23(6):511–519. doi: 10.1016/j.hlc.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Hancock SN, Bloomfield FH, Harding R. Are Twins Growth Restricted? Pediatr Res. 2011;70(2):117–122. doi: 10.1203/PDR.0b013e31821f6cfd. [DOI] [PubMed] [Google Scholar]
- Myrskyla M, Fenelon A. Maternal age and offspring adult health: evidence from the health and retirement study. Demography. 2012;49(4):1231–1257. doi: 10.1007/s13524-012-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalucci G, Becker J, Becher K, Bickle GM, Landolt MA, Bucher HU. Self-perceived health status and mental health outcomes in young adults born with less than 1000 g. Acta Paediatr. 2013;102(3):294–299. doi: 10.1111/apa.12102. [DOI] [PubMed] [Google Scholar]
- Oberg S, Cnattingius S, Sandin S, Lichtenstein P, Iliadou AN. Birth weight predicts risk of cardiovascular disease within dizygotic but not monozygotic twin pairs: a large population-based co-twin-control study. Circulation. 2011;123(24):2792–2798. doi: 10.1161/CIRCULATIONAHA.110.987339. [DOI] [PubMed] [Google Scholar]
- Oberg S, Cnattingius S, Sandin S, Lichtenstein P, Morley R, Iliadou AN. Twinship influence on morbidity and mortality across the lifespan. Int J Epidemiol. 2012;41(4):1002–1009. doi: 10.1093/ije/dys067. [DOI] [PubMed] [Google Scholar]
- Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, …Bracken MB. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):647–661. doi: 10.1093/ije/dyq267. [DOI] [PubMed] [Google Scholar]
- Saigal S, Feeny D, Rosenbaum P, Furlong W, Burrows E, Stoskopf B. Self-perceived health status and health-related quality of life of extremely low-birth-weight infants at adolescence. Jama. 1996;276(6):453–459. doi: 10.1001/jama.1996.03540060029031. [DOI] [PubMed] [Google Scholar]
- Saigal S, Stoskopf B, Pinelli J, Streiner D, Hoult L, Paneth N, Goddeeris J. Self-perceived health-related quality of life of former extremely low birth weight infants at young adulthood. Pediatrics. 2006;118(3):1140–1148. doi: 10.1542/peds.2006-0119. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Andresen EM, Nosek MA, Krahn GL. Response shift theory: important implications for measuring quality of life in people with disability. Arch Phys Med Rehabil. 2007;88(4):529–536. doi: 10.1016/j.apmr.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Sorace J, Rogers M, Millman M, Rogers D, Price K, Queen S, …Kelman J. A comparison of disease burden between twins and control pairs in medicare: quantification of heredity’s role in human health. Popul Health Manag. 2015;6:6. doi: 10.1089/pop.2014.0145. [DOI] [PubMed] [Google Scholar]
- Sprangers MA, Sloan JA, Veenhoven R, Cleeland CS, Halyard MY, Abertnethy AP, …Zwinderman AH. The establishment of the GENEQOL consortium to investigate the genetic disposition of patient-reported quality-of-life outcomes. Twin Res Hum Genet. 2009;12(3):301–311. doi: 10.1375/twin.12.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata: Release 13. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- Sunyer J, Anto JM, Harris J, Torrent M, Vall O, Cullinan P, Newman-Taylor A. Maternal atopy and parity. Clin Exp Allergy. 2001;31(9):1352–1355. doi: 10.1046/j.1365-2222.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Bardage C, Sandin S, Pedersen NL. A prospective study of health, life-style and psychosocial predictors of self-rated health. Eur J Epidemiol. 2006;21(10):767–776. doi: 10.1007/s10654-006-9064-3. [DOI] [PubMed] [Google Scholar]
- Svedberg P, Gatz M, Lichtenstein P, Sandin S, Pedersen NL. Self-rated health in a longitudinal perspective: a 9-year follow-up twin study. J Gerontol B Psychol Sci Soc Sci. 2005;60(6):S331–S340. doi: 10.1093/geronb/60.6.s331. [DOI] [PubMed] [Google Scholar]
- Svensson AC, Pawitan Y, Cnattingius S, Reilly M, Lichtenstein P. Familial aggregation of small-for-gestational-age births: the importance of fetal genetic effects. Am J Obstet Gynecol. 2006;194(2):475–479. doi: 10.1016/j.ajog.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Sydsjo G. Long-term consequences of non-optimal birth characteristics. Am J Reprod Immunol. 2011;1:81–87. doi: 10.1111/j.1600-0897.2011.01035.x. [DOI] [PubMed] [Google Scholar]
- Teoli DA, Zullig KJ, Hendryx MS. Maternal fair/poor self-rated health and adverse infant birth outcomes. Health Care Women Int. 2013;18:18. doi: 10.1080/07399332.2013.862796. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Overbeek LI, Rozendaal L, McMaster MT, Glasner TJ, Bartels M, … Boomsma DI. Chorionicity and Heritability Estimates from Twin Studies: The Prenatal Environment of Twins and Their Resemblance Across a Large Number of Traits. Behav Genet. 2015 doi: 10.1007/s10519-015-9745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Shi M. The genetics of preterm birth: using what we know to design better association studies. Am J Epidemiol. 2009;170(11):1373–1381. doi: 10.1093/aje/kwp325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, …Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. Jama. 2008;300(24):2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- Zwicker JG, Harris SR. Quality of life of formerly preterm and very low birth weight infants from preschool age to adulthood: a systematic review. Pediatrics. 2008;121(2):e366–e376. doi: 10.1542/peds.2007-0169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.