Abstract
New methods and strategies for the direct functionalization of C–H bonds are beginning to reshape the fabric of retrosynthetic analysis, impacting the synthesis of natural products, medicines, and even materials1. The oxidation of allylic systems has played a prominent role in this context as possibly the most widely applied C–H functionalization due to the utility of enones and allylic alcohols as versatile intermediates, along with their prevalence in natural and unnatural materials2. Allylic oxidations have been featured in hundreds of syntheses, including some natural product syntheses regarded as “classics”3. Despite many attempts to improve the efficiency and practicality of this powerful transformation, the vast majority of conditions still employ highly toxic reagents (based around toxic elements such as chromium, selenium, etc.) or expensive catalysts (palladium, rhodium, etc.)2. These requirements are highly problematic in industrial settings; currently, no scalable and sustainable solution to allylic oxidation exists. As such, this oxidation strategy is rarely embraced for large-scale synthetic applications, limiting the adoption of this important retrosynthetic strategy by industrial scientists. In this manuscript, we describe an electrochemical solution to this problem that exhibits broad substrate scope, operational simplicity, and high chemoselectivity. This method employs inexpensive and readily available materials, representing the first example of a scalable allylic C–H oxidation (demonstrated on 100 grams), finally opening the door for the adoption of this C–H oxidation strategy in large-scale industrial settings without significant environmental impact.
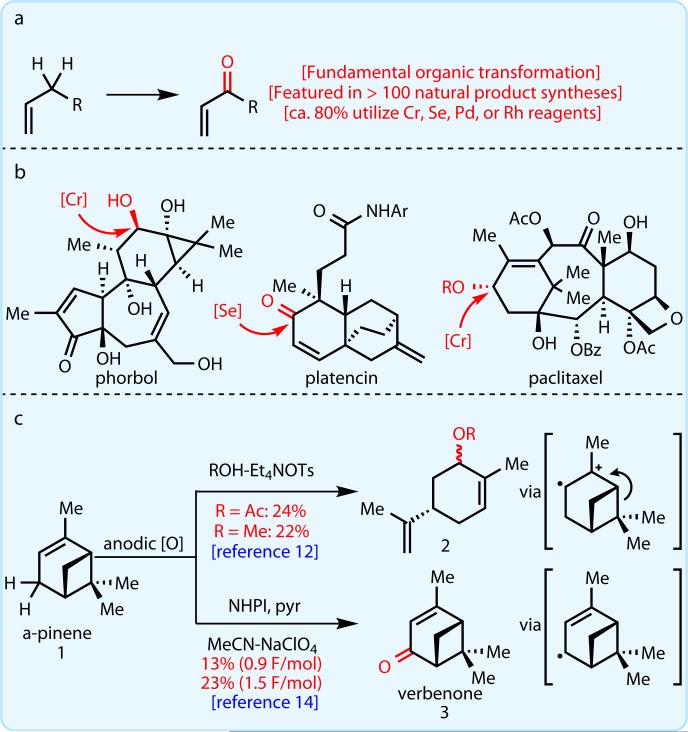
Electrochemical oxidation presents an attractive alternative to traditional chemical reagents for large-scale applications, in large part due to the generation of less toxic waste than that produced by current chemical processes4,5. In addition, electrochemical conditions are compatible with a wide range of functional groups6–10, tend to have higher overall energy efficiency as compared to thermal processes, and, due to their limited use, offer new intellectual property space for small-molecule synthesis4. The first electrochemical allylic oxidation was reported by Shono and coworkers in 196811,12 (Figure 1C). Direct oxidation of α-pinene (1) led to the fragmentation of the cyclobutane ring with incorporation of methanol or acetic acid (depending on the solvent) to give products 2 in 22–24% yield. A major advance in this field came in 1985, when Masui and coworkers reported the indirect oxidation of 1 using N-hydroxyphthalimide (NHPI) as an electrochemical mediator13–15 (Figure 1C). Unfortunately, verbenone (3) was isolated in only 13–23% yield. Although these reactions are not useful in a preparative sense, they were a proof of concept that served as a foundation for our work.
Fig. 1. Widely applied allylic oxidation.
a, Sustainable allylic C–H oxidation is an unsolved problem. b, Case studies from classic total syntheses. c, Electrochemical oxidation represents a potential solution.
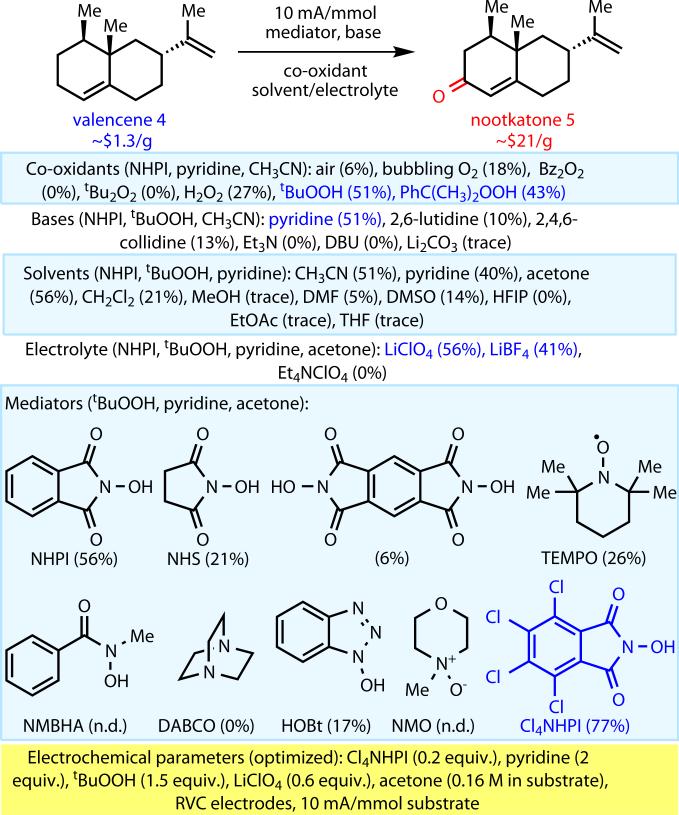
In our own laboratory, systematic and extensive experimentation led to the identification of three modifications of the original Masui precedent which transformed this process into a synthetically useful electrochemical allylic C–H oxidation (Figure 2). As described below, these modifications include the addition of a simple co-oxidant, the identification of a new electrochemical mediator, and the design of a reliable and inexpensive setup.
Fig. 2.
Optimization of a new sustainable allylic C–H oxidation.
From the outset of this work, we avoided the use of expensive electrodes such as precious metals (e.g. Pt or Au), focusing our efforts exclusively on carbon. Initial optimization was undertaken using graphite rods, but despite clean conversion of starting material to desired product, mass recovery was typically low. We considered that this may in part be due to absorption of the substrate onto the graphite. Switching to reticulated vitreous carbon (RVC, 100 ppi available from K.R. Reynolds Co. for ca. $3/electrode) electrodes proved to be far more productive.
In our hands, the original Masui conditions14 applied to valencene (4) led to only 6% isolated yield of nootkatone (5), the principal fragrance component of grapefruit aroma (Figure 2). Our hypothesis was that air was the oxygen-atom source in this transformation, which was qualitatively confirmed by bubbling O2 gas in the reaction – resulting in an improved isolated yield of 18%. However, NHPI/O2 systems16–18 have been explicitly avoided by the pharmaceutical industry, along with other oxygen-mediated reactions, due to the obvious challenges with flammability and other issues arising from reliably and safety in performing oxygen-mediated reactions on large scale19,20; as such, applications of aerobic oxidations in the pharmaceutical and fine chemical industries remain sparse21–23. Indeed, within Bristol-Myers Squibb such processes are explicitly avoided. Thus, a number of co-oxidants were evaluated, using NHPI as a mediator, and tert-butyl hydroperoxide (tBuOOH) led to substantial increases in reaction conversion and reproducibility, delivering 5 in 51% yield. Using tBuOOH without the NHPI mediator led to only 18% isolated yield under otherwise identical conditions.
With a suitable co-oxidant selected, attention turned to the optimization of base, solvent, and electrolyte. A variety of organic and inorganic bases were evaluated, with pyridine proving to be ideal. The use of acetone as solvent led to a slightly increased yield (56% 5 isolated) and was chosen as a general solvent for this reaction due to its ability to solubilize a wide range of organic substrates. Acetonitrile, dichloromethane, pyridine, or mixtures of these four solvents could also be used. The electrolyte LiBF4 could be used in place of LiClO4 with little decrease in yield, but tetraalkylammonium salts were not competent electrolytes.
Although most of the other mediators studied were inferior to the original NHPI, we reasoned that the addition of electron-withdrawing groups to the phthalamide scaffold would improve the reactivity of the catalyst24. Thus, tetrachloro-N-hydroxyphthalimide (Cl4NHPI) was chosen due to its ease of preparation from tetrachlorophthalic anhydride, an industrial non-toxic flame retardant (ca. $30/kg from VWR). The expectation of increased reactivity was supported by cyclic voltammetry data. In the case of NHPI, a reversible redox couple is observed at 0.78 V vs. Ag/AgCl in the presence of excess pyridine, whereas Cl4NHPI shows a redox couple at 0.87 V vs. Ag/AgCl under identical conditions. This slightly increased oxidation potential is consistent with the generation of a higher-energy and more reactive phthalimido-N-oxyl radical, and to our delight, the use of Cl4NHPI as a mediator led to a cleaner reaction profile and an isolated yield of 77%.
The final optimized conditions for oxidation of 4 to 5 is as follows: 20 mol% Cl4NHPI, pyridine (2.0 equiv), tBuOOH (1.5 equiv), and LiClO4 as the supporting electrolyte (0.1 M) in acetone (6 mL/mmol substrate) under constant current conditions in an undivided cell. Notably, no precautions to exclude oxygen or water were undertaken, technical-grade solvents and reagents were employed, and a simple setup of two reticulated vitrous carbon (RVC) electrodes separated by a glass slide was used (see Supporting Information for a photographic guide of the experimental setup).
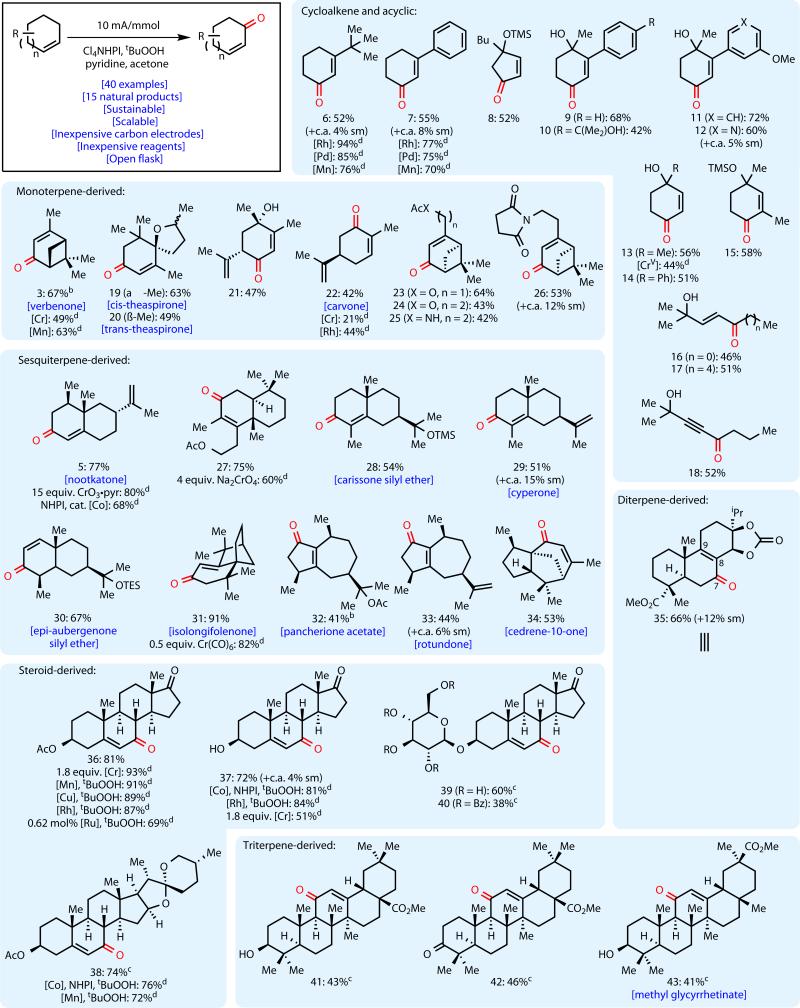
Our initial explorations into the tolerance focused on several cycloalkene-derived substrates of interest to Bristol-Myers Squibb process chemistry as part of ongoing drug discovery efforts (Figure 3). Thus, tert-butyl cyclohexenone 6 could be prepared as a single regioisomer in 52% yield, while phenyl cyclohexenone 7 was prepared in 55% yield. Pleasingly, cyclopentenone 8 could be prepared from TMS-protected cyclopentenol in 52% yield. Unprotected tertiary alcohols 9–12 could be prepared from the corresponding aryl-substituted cyclohexenols in reasonable yields. Most notably, the Lewis basic pyridine-containing enone 12 was prepared in 60% yield. No alcohol elimination and aromatization was observed, and no allylic transposition occurred; this complimentary reactivity to most chromium-based oxidants and the use of unprotected alcohols is particularly notable. Furthermore, the unsubstituted cyclohexenone products 13 and 14 were prepared in 56% and 51% yield, comparing favorably to our previously reported CrV-mediated oxidation for the synthesis of 13. Substitution at the alpha position of the enone was tolerated as well, with 15 being isolated in 58% yield. In addition to the cyclic substrates described above, several acyclic alkenes of various chain lengths were successfully oxidized under the reaction conditions. Enones 16 and 17 were prepared in 46% and 51% yields, respectively. Interestingly, propargylic alcohol 18 was formed in 52% yield from its corresponding alkyne.
Fig. 3. Scope of the electrochemical allylic oxidation.
Yields refer to isolated yields of products after chromatography on SiO2. aStandard conditions: terpenoid substrate (0.5 mmol), Cl4NHPI (0.1 mmol), pyridine (1.0 mmol), tBuOOH (0.75 mmol), LiClO4 (0.3 mmol), and acetone (3 mL). bMeCN (3 mL) replaced acetone as solvent. cterpenoid substrate (0.25 mmol), Cl4NHPI (0.1 mmol), pyridine (1.0 mmol), LiClO4 (0.3 mmol), CH2Cl2 (1.5 mL), and acetone (1.5 mL) were used. dSee Supporting Information for references.
Due to their prevalence in the drug discovery, flavor, and fragrance industries, subsequent efforts focused on a variety of representative terpene classes. The oxidation products of monoterpenes are amongst the most widely used and correspondingly valuable substances not only as fragrances and flavors, but also as building blocks in synthesis. As such, we evaluated the efficiency with which the electrochemical allylic oxidation could be applied to these substances. Verbenone (3), prepared in 13–23% yield in Masui's original report, was prepared in 67% yield. Both isomers of the food additive theaspirane could be oxidized to the natural products cis- and trans-theaspirone 19 and 20 in 63% and 49% yields. Carvone-derived enone 21 was prepared in 47% yield, while carvone (22) was prepared in 42% yield. Myrtenol acetate and nopol acetate were converted to the corresponding oxo-myrtenol and oxo-nopol compounds 23 and 24 in 64% and 43% yield. Furthermore, aza-nopol analogues 25 and 26 were prepared in 42% and 53% yield, further highlighting the tolerance for nitrogen-containing functionalities under these reaction conditions. Notably, in all cases, isolated yields are comparable to those in the literature using other methods, and low product yields were obtained in all cases in our hands under Ishii's NHPI/O2 conditions after prolonged heating16–18. To put these results in context, an extensive comparative survey of literature conditions and yields is included in the Supporting Information.
Sesquiterpenoid and diterpenoid natural products, many of which are components of essential oils, have provided inspiration for countless novel strategies and methods in synthesis primarily due to their complex and dense structures as well as their promising biological activities25. Allylic oxidation of valencene gave nootkatone (5) in 77% yield (vide supra). Sclareolide-derived terpene 27 was synthesized in 75% yield using the electrochemical method. Eudesmane natural products carissone (28, as its TMS ether) and cyperone (29) were prepared in 54% and 51% yields, respectively. The related aubergenone skeleton was successfully oxidized to give enone 30 in 67% yield. Isolongifolenone (31) was prepared from the feedstock chemical isolongifolene in 91% yield. Natural products in the guaiane family were oxidized to afford the natural products pancherione acetate (32) and rotundone (33) in 41% and 44% yields, respectively, and the complex [3.2.1]-bicyclic system in cedrene was oxidized to give cedren-10-one (34) in 53% yield. Under electrochemical conditions, abietic acid derivative 35 could be prepared in 66% isolated yield along with 12% recovered starting material. Interestingly, while the starting material contained the Δ7,8 bond, isomerization to the Δ8,9 olefin occurred, and no Δ7,8-enone was observed. This anomalous result may be due to hydrogen-atom abstraction at the C-9 position followed by by trapping of the allylic radical at the C-7 position.
The oxidation of steroid and triterpene substrates has been shown to improve properties such as solubility and pharmacokinetics, and the development of tools to modify their “oxidation barcodes” are of immediate importance26. Electrochemical oxidation of acetate-protected dehydroepiandrosterone (DHEA) gave enone product 36 in 81% yield. Notably, unprotected DHEA could also be oxidized to give enone 37 in 72% yield. Diosgenin acetate underwent smooth oxidation to give 38 in 74% yield. Encouraged by the tolerance for free alcohols and sensitive acetals, glycosylated derivatives of DHEA were evaluated. The tetrabenzoyl protected glycoside 39 was prepared in 60% yield, and, remarkably, the unprotected glycoside 40 could be prepared in 38% yield, demonstrating chemoselectivity that would be out of reach using classical oxidants such as chromium. Free hydroxyl-containing methyl oleanolate derivative 41 was prepared in 43% isolated yield, while oxidation of the ketone-containing substrate gave the desired enone 42 in 46%. Methyl glycyrrhetinate (43), an important starting material for a variety of oxidized medicinally relevant triterpenes, was also prepared in 41% yield.
In nearly all substrates evaluated, isolated yields compare favorably to literature precedent using traditional reagent-based oxidants. In some cases, such as the known conversion of 4 to 5 using 15 equivalents of CrO3•pyridine in 80% yield, not only is the isolated yield comparable, but our conditions obviate the need for the excessive use of toxic reagents and minimize the use of solvent and aqueous media for extraction/isolation.
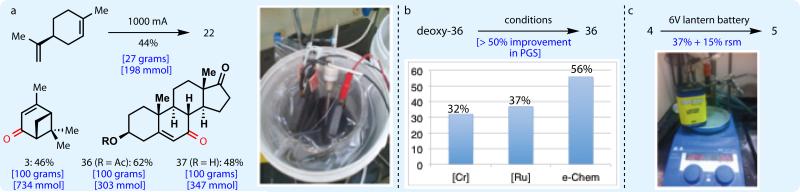
In order to demonstrate the feasibility of adopting this technology in a process setting, the described conditions were applied to substrates of relevance to Asymchem Laboratories. These reactions were conducted using inexpensive graphite plate electrodes in a beaker open to air (Figure 4A, inset photograph), and LiBF4 was used as the supporting electrolyte. Using this modest setup, 22 was prepared on 27-gram scale (198 mmol) from limonene in 44% yield, and 3 was prepared in 55% yield on 27-gram scale. Further scale-up to 100 grams (734 mmol) gave 46% yield of 3. Notably, carrying out the identical transformation with traditional chromium reagents (e.g. toxic chromium hexacarbonyl) would require at least 81 grams of chromium reagent followed by extensive efforts to remove Cr-based contaminants. Sterol 37 and its acetate 36 were produced in 48% yield (100 g, 347 mmol) and 62% yield (100g, 303 mmol), respectively. Highlights of this successful external field test include operational simplicity, safe procedure, simple workup, and ease of product isolation.
Fig. 4. Practicality of electrochemical method.
a. 100-gram scale electrochemical allylic oxidation. b. Calculated Process Greenness Score (PGS) for CrO3-mediated, RuCl3-catalyzed, and electrochemical oxidation of deoxy-36 to 36 shows improvement from 32.1% to 55.8%. Cost and toxicity associated with chromium and ruthenium use and disposal are not included in PGS. c. Use of 6-volt lantern battery as readily-available power source for allylic oxidation.
To verify the improved environmental footprint of the electrochemical allylic oxidation, we compared the Process Greenness Scores (PGS) for the electrochemical preparation of 36 to two known literature methods20,27 (Figure 4B). The PGS is a method often utilized by industrial companies, such as Bristol-Myers Squibb, to evaluate the potential environmental impact of chemical manufacturing processes. The scoring parameters are closely aligned with the 12 principles of green chemistry28, amongst which limiting waste generation and maximizing process efficiency are two main metrics of environmentally friendly processes. A greener reaction exhibits a higher PGS. Oxidation reactions, especially aliphatic C-H oxidations, are generally associated with lower than average PGS due to typically low process yields and the common use of toxic metal mediators in stoichiometric quantities. Unsurprisingly, the CrO3-mediated oxidation of deoxy-36 scored lowest in terms of PGS (32.1%). The RuCl3-catalyzed oxidation had an improved, albeit still modest, PGS of 37.1%. We were pleased to find that the electrochemical allylic oxidation showed a dramatically improved PGS of 55.8% (an improvement of >50%). This difference is significant and shows the step-change in applicability of this new technology. For comparison, the PGS for a typical amide bond formation (EDC, HOBt) ranges from 55–70%, while the PGS of the widely employed Pd-catalyzed cross-coupling of aryl halides with boronic acids falls between 45–60%. As a further testament to its robustness, the electrochemical oxidation was carried out using a 6-volt lantern battery29,30 (Figure 4C), with valencene being converted to nootkatone in 37% yield with 15% recovered starting material.
Although this reaction is useful for the oxidation of numerous natural and unnatural carbon skeletons, it is not without its limitations. For example, while cyclic substrates are all reactive, not all acyclic alkenes give very high conversion to enone products, nor do electron-deficient alkenes. In some cases, allylic alcohol products were isolated alongside enone products, though with prolonged reaction times these products are converted to the desired enones. Yields for some substrate classes were modest (e.g. 32 and 33), in part due to incomplete conversion, substrate decomposition, or adsorption to the electrode surface. In nearly all cases, however, isolated yields are comparable to alternative procedures present in the literature.
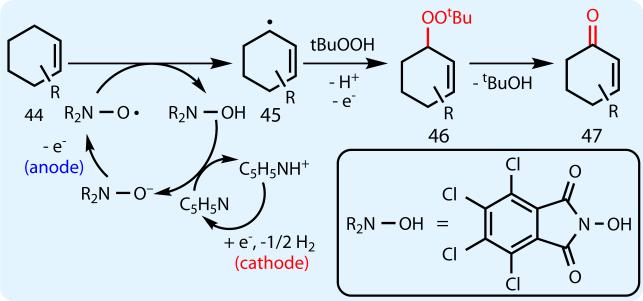
Mechanistic aspects of the initiation step of this new transformation may have parallels to other NHPI-catalyzed oxidations16–18 (Figure 5). Thus, deprotonation of Cl4NHPI by pyridine, followed by anodic oxidation, leads to the tetra-chlorophthalimido N-oxyl radical species. Olefinic substrate 44 would then undergo hydrogen atom abstraction, regenerating Cl4NHPI as well as the relatively stable allylic radical species 45. Reaction with electrochemically-generated tBuOO• would then give allylic peroxide 46, which, upon elimination of tBuOH, affords enone 47 (see Supporting Information for more details).
Fig. 5.
Proposed mechanism for electrochemical allylic oxidation.
Supplementary Material
Acknowledgments
This work was supported by an NSF predoctoral fellowship (B.R.R.), National Institute of General Medical Sciences Grant GM-097444, Asymchem, and Bristol-Myers Squibb. We thank Dr. D.-H. Huang and Dr. L. Pasternack for assistance with NMR spectroscopy; Dr. A. L. Rheingold, Dr. C. E. Moore, and Dr. M. A. Galella for x-ray crystallographic analysis; and Dr. D. G. Blackmond, Dr. O. Luca, Dr. T. Paschkewitz, Dr. Y. Ishihara and Dr. T. Razler for helpful discussions.
Footnotes
Author Contributions E.J.H., B.R.R., and P.S.B. conceived this work; E.J.H., B.R.R., K.C., M.D.E., and P.S.B. designed experiments; E.J.H. and B.R.R. conducted experiments and analyzed data; Y.C. and J.T. performed large-scale experiments; E.J.H., B.R.R., K.C., M.D.E., and P.S.B. wrote the manuscript.
Author Information Metrical parameters for the structure of 24 are available free of charge from the Cambridge Crystallographic Data Center under reference number CCDC-1058554. The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
Supplementary Information is available in the online version of the paper.
References
- 1.Gutekunst WR, Baran PS. C–H Functionalization logic in total synthesis. Chem. Soc. Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- 2.Weidmann V, Maison W. Allylic oxidations of olefins to enones. Synthesis. 2013;45:2201–2221. [Google Scholar]
- 3.Nakamura A, Nakada M. Allylic oxidations in natural product synthesis. Synthesis. 2013;45:1421–1451. [Google Scholar]
- 4.Sequeira CAC, Santos DMF. Electrochemical routes for industrial synthesis. J. Braz. Chem. Soc. 2009;20:387–406. [Google Scholar]
- 5.Degner D. Organic electrosyntheses in industry. In: Steckchan E, editor. Electrochemistry III. Springer; Berlin, Germany: 1988. pp. 1–95. [Google Scholar]
- 6.Moeller KD. Synthetic Applications of Anodic Electrochemistry. Tetrahedron. 2000;56:9527–9554. [Google Scholar]
- 7.Sperry JB, Wright DL. The application of cathodic reductions and anodic oxidations in the synthesis of complex molecules. Chem. Soc. Rev. 2006;35:605–621. doi: 10.1039/b512308a. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida J.-i., Kataoka K, Horcajada R, Nagaki A. Modern strategies in electroorganic synthesis. Chem. Rev. 2008;108:2265–2299. doi: 10.1021/cr0680843. [DOI] [PubMed] [Google Scholar]
- 9.Francke R, Little RD. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 2014;43:2492–2521. doi: 10.1039/c3cs60464k. [DOI] [PubMed] [Google Scholar]
- 10.Gütz C, Bänziger M, Bucher C, Galvao TR, Waldvogel SR. Development and scale-up of the electrochemical dehalogenation for the synthesis of a key intermediate for NS5A inhibitors. Org. Process Res. Dev. Article ASAP. doi: 10.1021/acs.oprd.5b00272. [Google Scholar]
- 11.Shono T, Kosaka T. Organic synthesis by electrolysis III anodic allylic substitution. Tetrahedron Lett. 1968;9:6207–6208. [Google Scholar]
- 12.Shono T, Ikeda A. Electroorganic chemistry X anodic allylic substitution. J. Am. Chem. Soc. 1972;94:7892–7898. [Google Scholar]
- 13.Masui M, Hara S, Ueshima T, Kawaguchi T, Ozaki S. Anodic oxidation of compounds having benzylic or allylic carbon and α-carbon to hetero atom using N-hydroxyphthalimide as a mediator. Chem. Pharm. Bull. 1983;31:4209–4211. [Google Scholar]
- 14.Masui M, Hosomi K, Tsuchida K, Ozaki S. Electrochemical oxidations of olefins using N-hydroxyphthalimide as a mediator. Chem. Pharm. Bull. 1985;33:4798–4802. [Google Scholar]
- 15.Ueda C, Noyama M, Ohmori H, Masui M. Reactivity of phthalimide-N-oxyl: a kinetic study. Chem. Pharm. Bull. 1987;35:1372–1377. [Google Scholar]
- 16.Foricher J, Fürbringer C, Pfoertner K. Process for the catalytic oxidation of isoprenoids having allylic groups. 1991 Jul. U. S. Patent 5,030,739.
- 17.Ishii Y, et al. A novel catalysis of N-hydroxyphthalimide in the oxidation of organic substrates by molecular oxygen. J. Org. Chem. 1995;60:3934–3935. [Google Scholar]
- 18.Recupero F, Punta C. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide. Chem. Rev. 2007;107:3800–3842. doi: 10.1021/cr040170k. [DOI] [PubMed] [Google Scholar]
- 19.Miller RA, Li W, Humphrey GR. A ruthenium catalyzed oxidation of steroidal alkenes to enones. Tetrahedron Lett. 1996;37:3429–3432. [Google Scholar]
- 20.Harre M, et al. Some reaction safety aspects of ruthenium-catalyzed allylic oxidations of δ-5-steroids in the pilot plant. Org. Process Res. Dev. 1998;2:100–104. [Google Scholar]
- 21.Campbell AN, Stahl SS. Overcoming the ‘oxidant problem’: strategies to use O2 as the oxidant in organometallic C–H oxidation reactions catalyzed by Pd (and Cu) Acc. Chem. Res. 2012;45:851–863. doi: 10.1021/ar2002045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osterberg PM, et al. Experimental limiting oxygen concentrations for nine organic solvents at temperatures and pressures relevant to aerobic oxidations in the pharmaceutical industry. Org. Process Res. Dev. Article ASAP. doi: 10.1021/op500328f. doi: 10.1021/op500328f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mudryk B, Zheng B, Chen K, Eastgate MD. Development of a robust process for the preparation of high-quality dicyclopropylamine hydrochloride. Org. Process Res. Dev. 2014;18:520–527. [Google Scholar]
- 24.Cai Y, Koshino N, Saha B, Espenson JH. Kinetics of self-decomposition and hydrogen atom transfer reactions of substitute phthalimide N-oxyl radicals in acetic acid. J. Org. Chem. 2005;70:238–243. doi: 10.1021/jo048418t. [DOI] [PubMed] [Google Scholar]
- 25.Modzelewska A, Sur S, Kumar SK, Khan SR. Sesquiterpenes: natural products that decrease cancer growth. Curr. Med. Chem. –Anti-Cancer Agents. 2005;5:477–499. doi: 10.2174/1568011054866973. [DOI] [PubMed] [Google Scholar]
- 26.Michaudel Q, et al. Improving physical properties via C–H oxidation: chemical and enzymatic approaches. Angew. Chem. Int. Ed. 2014;53:12091–12096. doi: 10.1002/anie.201407016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marwah P, Lardy HA. Process for effecting allylic oxidation using dicarboxylic acid imides and chromium reagents. 2002 May 7; U. S. US6384251 B1.
- 28.Anastas PT, Warner JC. Green Chemistry: Theory and Practice. Oxford University Press; 1998. [Google Scholar]
- 29.Frey DA, Wu N, Moeller KD. Anodic electrochemistry and the use of a 6-volt lantern battery: a simple method for attempting electrochemically based synthetic transformations. Tetrahedron Lett. 1996;37:8317–8320. [Google Scholar]
- 30.Frankowsi KJ, Liu R, Milligan GL, Moeller KD, Aubé J. Practical electrochemical anodic oxidation of polycyclic lactams for late state functionalization. Angew. Chem. Int. Ed. 2015;54:10555–10558. doi: 10.1002/anie.201504775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.