Abstract
The dopaminergic innervation of the striatum has been implicated in learning processes and in the development of human speech and language. Several lines of evidence suggest that evolutionary changes in dopaminergic afferents of the striatum may be associated with uniquely human cognitive and behavioral abilities, including the association of the human-specific sequence of the FOXP2 gene with decreased dopamine in the dorsomedial striatum of mice. To examine this possibility, we quantified the density of tyrosine hydroxylase-immunoreactive (TH-ir) axons as a measure of dopaminergic innervation within five basal ganglia regions in humans, great apes, and New and Old World monkeys. Our results indicate that humans differ from nonhuman primate species in having a significant increase in dopaminergic innervation selectively localized to the medial caudate nucleus. This region of the striatum is highly interconnected, receiving afferents from multiple neocortical regions, and supports behavioral and cognitive flexibility. The medial caudate nucleus also shows hyperactivity in humans lacking a functional FOXP2 allele and exhibits altered dopamine concentrations in humanized Foxp2 mice. Additionally, striatal dopaminergic input was not altered in chimpanzees that used socially learned attention-getting sounds versus those that did not. This evidence indicates that the increase in dopamine innervation of the medial caudate nucleus in humans is a species-typical characteristic not associated with experience-dependent plasticity. The specificity of this increase may be related to the degree of convergence from cortical areas within this region of the striatum and may also be involved in human speech and language.
Keywords: caudate nucleus, putamen, striatum, dopamine, tyrosine hydroxylase, evolution, FOXP2, RRID AB_390204, RRID SciRes_000114
Graphical abstract
Humans are unique among primates in having increased dopaminergic innervation of the medial caudate nucleus, a region that is highly interconnected with the neocortex and that has been implicated in speech and language production. These results indicate that striatal dopamine has played an important role in the evolution of the human brain.
Introduction
Midbrain dopaminergic (DAergic) neurons innervate the basal ganglia and play a critical role in the modulation and integration of both motor and cognitive functions (Haber, 2014). The basal ganglia are involved in several independent closed-loop systems (i.e., cortico basal ganglia) with a topographical organization that is evident in the cortical area, input region, and output structure (Haber, 2003; Haber et al., 2006; Haber et al., 1995; Middleton, 2000; Middleton and Strick, 2000; Middleton and Strick, 2002; Nambu, 2011; Postuma and Dagher, 2006). Although topographically organized, several lines of evidence indicate discrete areas of convergence within the striatum that receive inputs from diverse cortical regions (Averbeck et al., 2014; Draganski et al., 2008; Haber et al., 2006). In contrast to specificity of relationships, these findings suggest greater integration and synchronization of separate information streams within the striatum than previously thought (Averbeck et al., 2014; Draganski et al., 2008). The basal ganglia have not been extensively studied as a target in the evolution of higher cognitive function because many of its fundamental components do not appear to vary among species (Ericsson et al., 2011; Smeets et al., 2000; Stephenson-Jones et al., 2011) in spite of some notable differences. A dramatic increase in connectivity characterizes the non-mammalian to mammalian transition (Smeets et al., 2000), and this increase in connectivity has been expanded in human and nonhuman primates as the neocortex increased in size and became more differentiated (Averbeck et al., 2014; Fudge et al., 2004; Postuma and Dagher, 2006; Wise et al., 1996). Because DA is considered to be the major neuromodulator of basal ganglia functions (Haber, 2014; Parent et al., 1995), it is reasonable to expect that the increased connectivity of cortico-basal ganglia circuits would require reorganization of DAergic innervation in the primate brain, with further differences expected between human and nonhuman primates to support the evolution of human-specific cognitive abilities.
Dopamine (DA) plays a modulatory role in the executive cortico-basal ganglia loop and is involved in human language as well as theory of mind, working memory, learning, and the ability to recognize causal relationships between an action and its consequences (Dominey and Inui, 2009; Giraud et al., 2008; Lex and Hauber, 2010; Poletti et al., 2011). In human language, the basal ganglia are active during speech production, sentence comprehension, and in the processing of grammar and syntax (Prat et al., 2007; Teichmann et al., 2008; Teichmann et al., 2006; Ullman et al., 1997). The only gene that has been definitively and consistently linked to central aspects of speech and language production in humans is FOXP2 (Enard, 2011; Enard et al., 2002; Lai et al., 2001; Lai et al., 2003). It appears that FOXP2 contributed to the evolution of human language through its effects on executive corticostriatal circuits, with a possible specific effect of altering the density of DAergic innervation (Enard, 2011; Enard et al., 2009; Reimers-Kipping et al., 2011; Schreiweis et al., 2014).
For humans, reduced DA in the left caudate nucleus increases the accuracy of phonological processing, as indicated by positron emission tomography (PET) (Tettamanti et al., 2005). Genetically engineered mice expressing the human variant of FOXP2 exhibit altered vocalizations and a decrease in DA concentrations in the striatum, nucleus accumbens, globus pallidus, frontal cortex, and cerebellum (Enard et al., 2009). A subsequent analysis revealed that DA levels are reduced in the left dorsomedial, but not dorsolateral, striatum (Schreiweis et al., 2014). These effects are accompanied by increased dendritic branching of striatal neurons (Enard et al., 2009) and enhanced procedural forms of learning that are modulated by DA and critical to language and speech (Schreiweis et al., 2014). In humans lacking a functional copy of the FOXP2 gene, there are altered activity patterns within the medial caudate nucleus of the left hemisphere as revealed by fMRI and PET studies (Liégeois et al., 2003; Vargha-Khadem et al., 1998). Specifically, hyperactivation of the medial caudate nucleus was observed in affected individuals of the KE family, who display verbal dyspraxia including impairment in word repetition, nonword repetition, and sequential orofacial movements (Vargha-Khadem et al., 1998).
Foxp2 includes 3 functional regions: a poly-Q region (exons 5–6), a zinc-finger domain (exon 8), and a forkhead DNA binding domain (exons 12–14; Bruce and Margolis, 2002). Foxp2 is often assumed to be relatively conserved among mammals (Enard et al., 2002), but comparative studies have shown otherwise. For example, sequence variation has been reported in echolocating bats (Li et al., 2007), lesser apes, and great apes (Ely et al., 2002; Erwin et al., 2002). It also appears that evolutionary changes in FOXP2 transcription factor binding sites may have occurred in the lineage leading to modern humans, based on comparisons with Neanderthals (Maricic et al., 2013). Two residues differentiate human from chimpanzee FoxP2 protein (Enard et al., 2002), however it is not known if the fixed human/chimpanzee sequence difference in the forkhead DNA binding region of exons 12–13 results in a change in DAergic innervation within regions of the human basal ganglia compared to other closely related primate species. Based on studies of transgenic mice, humans would be expected to have fewer DAergic inputs specific to the dorsomedial striatum. The dorsomedial region of the striatum in the mouse presumably corresponds to the primate medial caudate nucleus, while the dorsolateral striatum of the mouse may correspond to the primate putamen (Balleine et al., 2007; Liégeois et al., 2003; Schreiweis et al., 2014; Vargha-Khadem et al., 1998; Woolley et al., 2013). The homology of the dorsomedial striatum and primate medial caudate nucleus is based on both connectivity with prefrontal and orbitofrontal cortex and involvement in behavioral flexibility and the homology of the motor regions is based on function (Clarke et al., 2008; Öngür and Price, 2000; Ragozzino, 2007; Ragozzino et al., 2002; Roberts et al., 2007; Schilman et al., 2008; Thorn et al., 2010; Woolley et al., 2013; Yin and Knowlton, 2006).
Earlier reports suggest that the human striatum has a more complex neurochemical organization relative to other mammals, with greater heterogeneity in the levels of immunostaining for neurochemical markers in different regions rather than the discrete, chemically-distinct compartments described in other species (Holt et al., 1997; Holt et al., 1996). However, rigorous quantitative comparisons between human and nonhuman primates are lacking. In the current study, we conducted a stereologic analysis of the DAergic innervation within regions of the basal ganglia (dorsal and medial caudate nucleus, putamen, and globus pallidus) to determine if reorganization of the striatal DAergic innervation is evident between humans and other primates possibly due to the evolution of uniquely human abilities.
Materials and Methods
Specimens
Brain samples from the left hemisphere of 49 individuals representing six primate species were used in the present study. They included New World monkeys (tufted capuchins), Old World monkeys (pig-tailed macaques and olive baboons), African great apes (western lowland gorillas and common chimpanzees), and humans (see Table 1 for details). All individuals were adults and free of neuropathology. Sexes were balanced within species as much as possible, based on the opportunistic nature of collecting these brains. Human brain samples were provided by the El Paso County Coroner in Colorado (as approved by the Colorado College Institutional Review Board, #011311-1), the Northwestern University Alzheimer’s Disease Center Brain Bank, and the National Disease Research Interchange (NDRI). The human subjects exhibited no evidence of cognitive changes before death, and all received a score of zero for the CERAD senile plaque grade (Mirra et al., 1991) and the Braak and Braak neurofibrillary tangle stage (Braak and Braak, 1991). The nonhuman primate brains were acquired from American Zoo and Aquarium-accredited zoos or research institutions and maintained in accordance with federally recognized standards, guidelines, and principles (National Research Council, 2011; NIH/OLAW, 2002; USDA, 2013) and accrediting bodies (AAALAC-I), with full approval and oversight by each institution’s animal care and use committee. Postmortem interval was less than 17 hours for all individuals. All brains were immersion-fixed in 10% buffered formalin for a minimum of 7 days, transferred to a 0.1 M buffered saline solution with 0.1% sodium azide, and stored at 4° C. While all five sampling regions were available for the majority of specimens, some areas were missing in the dissected blocks that were provided from a few individuals (see Table 1).
Table 1.
Study sample.
| Species | Common Name | Sex | Age | ALv/Nv |
|---|---|---|---|---|
| Homo sapiens | Human | M | 24 | ‡ |
| Homo sapiens | Human | M | 44 | * |
| Homo sapiens | Human | M | 44 | †§ |
| Homo sapiens | Human | M | 54 | ‡ |
| Homo sapiens | Human | M | 56 | ‡ |
| Homo sapiens | Human | M | 59 | †§ |
| Homo sapiens | Human | F | 22 | †§ |
| Homo sapiens | Human | F | 25 | †§ |
| Homo sapiens | Human | F | 30 | ‡ |
| Homo sapiens | Human | F | 35 | †§ |
| Homo sapiens | Human | F | 43 | ‡ |
| Homo sapiens | Human | F | 45 | ‡ |
|
| ||||
| Pan troglodytes | Chimpanzee (AG+) | M | 17 | † |
| Pan troglodytes | Chimpanzee (AG+) | M | 19 | * |
| Pan troglodytes | Chimpanzee (AG−) | M | 24 | ‡ |
| Pan troglodytes | Chimpanzee (AG−) | M | 40 | ‡§ |
| Pan troglodytes | Chimpanzee | M | 40 | * |
| Pan troglodytes | Chimpanzee | M | 46 | * |
| Pan troglodytes | Chimpanzee (AG−) | F | 35 | † |
| Pan troglodytes | Chimpanzee (AG+) | F | 44 | |
| Pan troglodytes | Chimpanzee (AG−) | F | 12 | |
| Pan troglodytes | Chimpanzee | F | 39 | * |
| Pan troglodytes | Chimpanzee (AG+) | F | 41 | § |
|
| ||||
| Gorilla gorilla | Western lowland gorilla | M | 11 | * |
| Gorilla gorilla | Western lowland gorilla | M | 16 | * |
| Gorilla gorilla | Western lowland gorilla | M | 21 | ‡|| |
| Gorilla gorilla | Western lowland gorilla | M | 34 | ठ|
| Gorilla gorilla | Western lowland gorilla | M | 40 | * |
|
| ||||
| Macaca nemestrina | Pig-tailed macaque | M | 2 | * |
| Macaca nemestrina | Pig-tailed macaque | M | 4 | * |
| Macaca nemestrina | Pig-tailed macaque | M | 7 | † |
| Macaca nemestrina | Pig-tailed macaque | M | 15 | * |
| Macaca nemestrina | Pig-tailed macaque | F | 6 | * |
| Macaca nemestrina | Pig-tailed macaque | F | 9 | * |
| Macaca nemestrina | Pig-tailed macaque | F | 15 | ‡ |
| Macaca nemestrina | Pig-tailed macaque | F | 15 | † |
|
| ||||
| Papio anubis | Olive baboon | M | 6 | * |
| Papio anubis | Olive baboon | M | 7 | * |
| Papio anubis | Olive baboon | M | 9 | * |
| Papio anubis | Olive baboon | M | 10 | * |
| Papio anubis | Olive baboon | F | 5 | † |
| Papio anubis | Olive baboon | F | 9 | * |
| Papio anubis | Olive baboon | F | 12 | * |
|
| ||||
| Cebus apella | Tufted capuchin | M | 3 | * |
| Cebus apella | Tufted capuchin | M | 16 | * |
| Cebus apella | Tufted capuchin | M | 17 | * |
| Cebus apella | Tufted capuchin | F | 13 | * |
| Cebus apella | Tufted capuchin | F | 17 | * |
| Cebus apella | Tufted capuchin | F | 18 | * |
Age is in years, M, male; F, female. ALv/Nv = TH-ir axon length density/total neuron density.
Included in among-species analyses for all five regions;
Included in among-species analyses for striatum;
Included in among-species analyses for globus pallidus;
Included in among-species analyses for dorsal caudate nucleus and putamen only;
Included in among-species analyses for medial caudate nucleus only. Chimpanzees that used attention-getting vocalizations are indicated by AG+ and those that did not are indicated by AG−.
Sample Processing
All samples were from the left hemisphere. Because human and nonhuman primate brains (especially great apes) are a limited resource, it was not possible to obtain both hemispheres for every individual because many brain collection protocols freeze one hemisphere and immersion-fix the other. Although there are differences in connectivity between the left versus right basal ganglia components (Postuma and Dagher, 2006), these differences were not considered to be a factor in the present analysis of executive and motor cortico-basal ganglia loops. Furthermore, the striatal regions implicated in human language are exclusive to the left hemisphere (Schreiweis et al., 2014), making it the hemisphere of interest for the present analyses.
Prior to sectioning, specimens were cryoprotected in a series of sucrose solutions (10, 20, and 30%) until saturated. Brains were frozen in dry ice and cut into 40 μm-thick sections using a Leica SM2000R freezing sliding microtome (Leica, Buffalo Grove, IL). Sections were placed into individual microcentrifuge tubes containing freezer storage solution (30% each of distilled water, ethylene glycol, and glycerol, and 10% 0.244 M phosphate buffered saline) and numbered sequentially. Sections were stored at −20°C until further processing. Every tenth section was stained for Nissl substance with a solution of 0.5% cresyl violet. Nissl-stained sections were used to identify anatomical regions of interest and to quantify neuron densities.
Identification of Sampling Regions
All regions in the present study were readily delineated using Nissl-stained sections (Graybiel, 2005). Sampled regions for this analysis are shown in Figure 1 and were selected based on the topographical organizations of both motor and executive cortico-basal ganglia loops that have been described in humans and monkeys (Haber, 2003; Haber and Behrens, 2014; Haber et al., 2006; Haber and Knutson, 2010; Middleton, 2000; Middleton and Strick, 2002; Nambu, 2011; Parent, 1990; Parent et al., 1995; Postuma and Dagher, 2006). Regions involved in the executive loop that were analyzed were the head of the caudate nucleus (dorsal and medial regions) and the anteromedial GPi. Regions representative of the motor loop included the dorsal putamen and intermediate GPi.
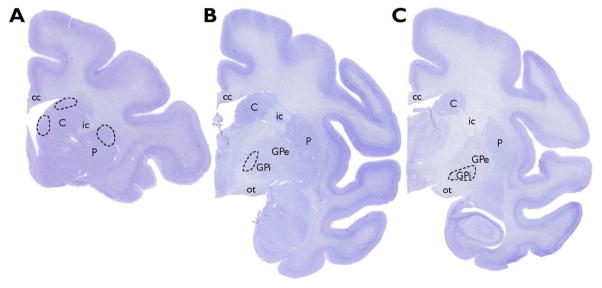
Figure 1.
Nissl-stained sections from a pig-tailed macaque showing the regions sampled for this study. Each sampling region is indicated by a dashed line, with the dorsal and medial caudate nucleus and putamen represented in (A). The anteromedial dorsal GPi is indicated in (B) and the intermediate ventral GPi is illustrated in (C). Abbreviations: C = caudate nucleus; cc = corpus callosum; GPe = external globus pallidus; GPi = internal globus pallidus; ic = internal capsule; ot = optic tract; P = putamen.
In the executive loop, focal projections from the dorsolateral prefrontal cortex (e.g., areas 9 and 46) are received by the dorsal region of the head of the caudate nucleus (Calzavara et al., 2007; Haber et al., 2006; Robinson et al., 2012). Areas 9 and 46 are involved in working memory and the perception and inference of mental states (Frith and Frith, 2006; Levy and Goldman-Rakic, 2000). Specific functions attributed to the head of the caudate nucleus include working memory and strategic planning processes (Barbas, 2000), theory of mind, emotion recognition (Kemp et al., 2013; Kemp et al., 2012), and representation of action-outcome contingencies that support flexibility in goal-directed behaviors (Grahn et al., 2008).
The medial caudate nucleus corresponds to the DA-deficient dorsomedial striatal region in humanized Foxp2 mice (Liégeois et al., 2003; Schreiweis et al., 2014; Vargha-Khadem et al., 1998). In primates, the medial caudate nucleus receives projections from the orbitofrontal and anterior cingulate cortices, areas associated with decision-making, motivation, reward, and vocalization (Allman et al., 2001; Haber and Behrens, 2014).
The head of the caudate nucleus relays information to the dorsal anteromedial GPi. Damage to this area of the globus pallidus results in cognitive deficits in category and letter fluency (word generation tasks that test aspects of language and executive retrieval), proactive interference (impaired learning as a consequence of previously learned information), and impaired performance on the paced auditory serial addition test (auditory processing speed and flexibility and calculation ability) (Lombardi et al., 2000).
The motor loop involves somatosensory cortical areas projecting to the putamen. The dorsal putamen, which receives information from the leg and foot region (Künzle, 1975), was selected for comparisons among primates because alternative regions (i.e., orofacial and hand) may be expected to exhibit differences between human and nonhuman primates. Information from the dorsal putamen is then relayed to the ventral intermediate GPi, and lesions in this region do not result in cognitive deficits (Lombardi et al., 2000).
Immunohistochemistry
Every tenth section from each subject and sampling area was immunohistochemically processed for tyrosine hydroxylase (TH; the rate-limiting enzyme in DA synthesis) using the avidin-biotin-peroxidase method as described previously (Raghanti et al., 2009; Raghanti et al., 2008b). Briefly, sections were pretreated for antigen retrieval by incubation in 0.05% citraconic acid (pH 7.4) at 85–90°C for 30 minutes. Endogenous peroxidase was quenched and sections were preblocked, pretreated, and incubated in a rabbit anti-TH polyclonal antibody (Millipore, AB152, RRID AB_390204) at a dilution of 1:1,000 for 24 hours at room temperature followed by 24 hours at 4°C. This antibody is derived from rat pheochromocytoma and labels a band at approximately 60 kDa by Western blot in human and nonhuman primates (Lewis et al., 1994; Wolf et al., 1991) and has been validated for use in a wide range of applications, including immunohistochemistry (Hunter et al., 2012; Raghanti et al., 2008b; Sharma et al., 2010). Sections were then incubated in a biotinylated secondary antibody (1:200) followed by the avidin-peroxidase complex (PK-6100, Vector Laboratories, Burlingame, CA). A 3,3′-diaminobenzidine-peroxidase (DAB) substrate with nickel enhancement was used as the chromogen (SK-4100, Vector Laboratories).
Data Acquisition
Quantitative analyses were performed using computer-assisted stereology with an Olympus BX-51 photomicroscope system equipped with a digital camera and StereoInvestigator software version 10 (MBF Bioscience, Williston, VT, RRID SciRes_000114). Subsampling techniques were performed for each species to determine appropriate sampling parameters (Slomianka and West, 2005).
The regional length of TH-ir axons was calculated using the SpaceBalls probe at 100x (N.A. 1.4) under Koehler illumination (Calhoun et al., 2004; Calhoun and Mouton, 2000; Kreczmanski et al., 2005; Mouton et al., 2002). Beginning from a randomly selected section within the sampling region, 3 to 4 equidistant sections were sampled. Mounted section thickness was measured at every fifth sampling site. Axons were marked where they intersected the outline of the hemisphere. As described previously, the axonal length density, ALv, was calculated as the total fiber length divided by the planimetric measurement of the reference volume sampled (Raghanti et al., 2008a; Raghanti et al., 2008b; Raghanti et al., 2008c).
The regional neuron density, Nv, was assessed in adjacent Nissl-stained sections using an optical disector combined with a fractionator sampling scheme. The optical disector probe was performed using a 40x objective (N.A. 0.75). Neurons were counted when the nucleolus was in focus within the counting frame and displayed the presence of a large, lightly stained nucleus, a distinct nucleolus, and lightly stained proximal portions of dendritic processes. Nv was calculated as the sum of neurons counted within the sum of optical disectors divided by the product and volume of the disector (Sherwood et al., 2005). To correct for tissue shrinkage in the z axis, the height of the disector was multiplied by the ratio of section thickness to the actual weighted mean thickness after mounting and dehydration. No correction was necessary for the x and y dimensions because shrinkage in section surface area is minimal (Dorph-Petersen et al., 2001).
The ratio of ALv/Nv was used for comparative analyses among species rather than the absolute total axon length to avoid several confounding factors. For example, cell density per unit volume varies with brain size (Gabi et al., 2010; Haug, 1987; Sherwood et al., 2007). Thus, the ratio of ALv/Nv allows for evaluation of fiber density in the context of species differences in neuron density. Furthermore, postmortem interval, method of fixation, and amount of time in fixative are factors that contribute to preprocessing tissue shrinkage. Additional tissue shrinkage may also occur with histological and immunohistochemical procedures. These confounding factors are unavoidable, however, the ALv/Nv ratio serves to standardize data for differential tissue shrinkage among species and individuals.
Statistical Analyses
The ratio of TH-ir ALv/Nv was analyzed among species using a mixed model ANOVA with repeated-measures. Area (dorsal or medial caudate nucleus, putamen, dorsal anteromedial GPi, and ventral intermediate GPi) was the within-subjects factor and species was the between-subjects factor. Fisher’s least significant difference (LSD) post hoc tests were performed to evaluate significant effects. Statistical analyses were performed using SPSS (version 13.0) and Statistica (version 6.0), and the level of significance (α) was set at 0.05 for all statistical tests.
An additional analysis that included chimpanzees that used socially learned attention-getting vocalizations (AG+; n = 4, 2 males, 2 females) was compared with chimpanzees that did not use these vocalizations (AG−; n = 4, 2 males, 2 females) to evaluate potential experience-dependent plasticity of striatal DAergic expression. Among captive chimpanzees, some individuals use vocalizations to get the attention of humans and this is a socially transmitted skill that appears to be passed from mother to offspring (Russell et al., 2013; Taglialatela et al., 2012). Student’s t-tests were used to compare ALv/Nv in the dorsal and medial caudate nucleus and putamen between chimpanzee groups.
Results
Figure 2 provides examples of TH immunostaining in the striatum and globus pallidus for each species. The patterns of immunostaining were comparable to previous studies in humans and nonhuman primates (Graybiel, 1990; Hedreen, 1999; Holt et al., 1997; Holt et al., 1996). Within the striatum, we observed fine varicose axons organized into zones of weaker immunoreactivity (striosomes) embedded in regions of intense staining (matrix), with a denser immunoreactivity present in the medial and ventral caudate nucleus. Axons were more sparsely distributed in the GPi overall, with many being of a larger diameter.
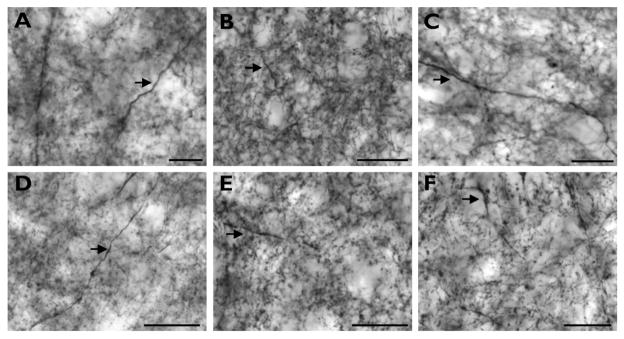
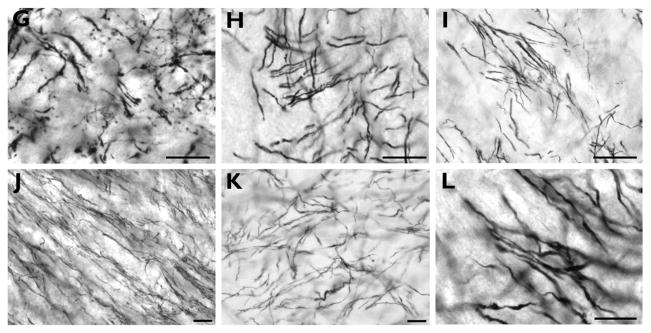
Figure 2.
High-powered photomicrographs showing TH immunostaining in the medial caudate nucleus (A–F) and globus pallidus (G–L) of capuchin (A, G), pig-tailed macaque (B, H), baboon (C, I), gorilla (D, J), chimpanzee (E, K), and human (F, L). These images are meant to demonstrate the intensity of the immunostaining rather than the axon density in each species. Arrows in A–F indicate medium and large diameter axons that were occasionally observed within striatal regions. Scale bars = 250 μm.
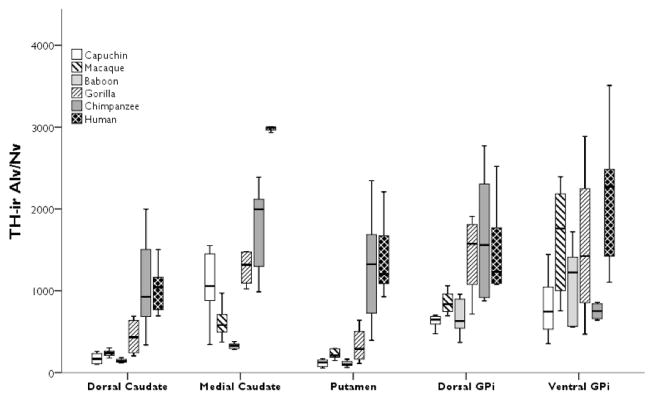
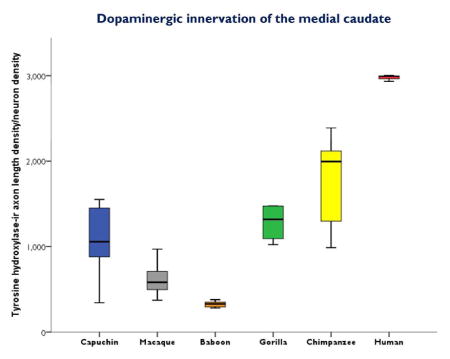
The repeated-measures ANOVA for ALv/Nv yielded significant main effects of area (F(4,104) = 29.02, p < 0.01) and species (F(5,26) = 22.27, p < 0.01), with a significant interaction (F(20,104) = 3.12, p < 0.01; Fig. 3). Post hoc analyses revealed that humans had the highest TH-ir ALv/Nv in the medial caudate nucleus (all p’s < 0.01). Among the nonhuman primates, gorillas and capuchins had higher TH-ir ALv/Nv in the medial caudate nucleus compared to baboons (all p’s < 0.02; see Fig. 3).
Figure 3.
Boxplots showing TH-ir ALv/Nv for each species in the dorsal and medial caudate nucleus, putamen, dorsal anteromedial GPi, and ventral intermediate GPi. The TH-ir ALv/Nv values in the human medial caudate nucleus are uniformly higher with no overlap with other species’ values and show very little interindividual variation. Whiskers indicate 1 SD.
Humans and chimpanzees both possessed higher ALv/Nv in the dorsal caudate nucleus and putamen relative to capuchins, pig-tailed macaques, baboons, and gorillas (all p’s < 0.05; see Fig. 3). In the globus pallidus, humans and chimpanzees had significantly higher ALv/Nv than capuchins, pig-tailed macaques, and baboons in the dorsal anteromedial GPi (all p’s < 0.02), while gorillas had increased ALv/Nv relative to capuchins and baboons (all p’s < 0.02). In the ventral intermediate GPi, humans had significantly higher ALv/Nv than baboons and chimpanzees (all p’s < 0.01) and capuchins had significantly lower ALv/Nv compared to pig-tailed macaques, gorillas, and humans (all p’s < 0.02).
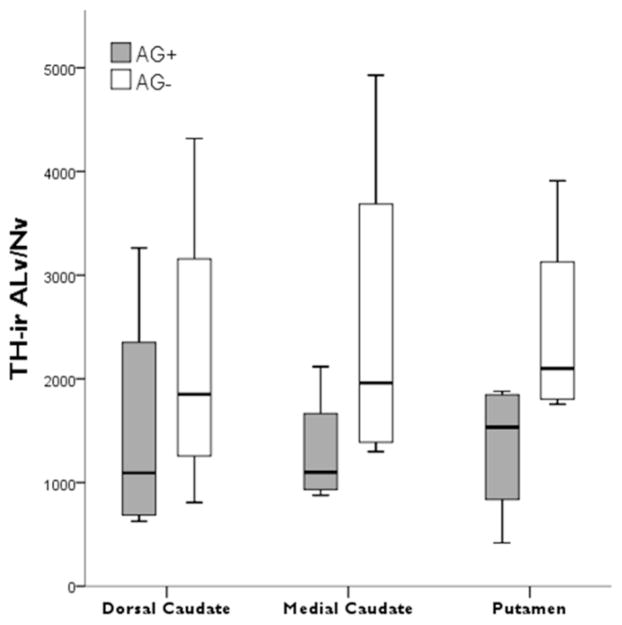
Among chimpanzees, no significant differences were detected between those that used learned attention-getting vocalizations compared to those that did not (dorsal caudate nucleus t = −0.71, p = 0.50; medial caudate nucleus t = −1.40, p = 0.21; putamen t = −1.87, p = 0.11; Fig. 4).
Figure 4.
Boxplots showing TH-ir ALv/Nv in the striatal regions of the chimpanzees that used learned attention-getting vocalizations (AG+) versus those that did not (AG−).
Discussion
Primates have increased the size and number of association areas of the cortex, resulting in a tremendous amount of information being channeled through basal ganglia circuits (Smeets et al., 2000). It has been proposed that reorganization of the basal ganglia would have been required to support this increase in cortico-basal ganglia circuitry, and such an alteration would require an increase in modulatory components to maintain the flow of information. DA is the major modulator of cortico-basal ganglia circuits and functions in the synthesis of information across motor, limbic, and executive processing streams (Haber, 2014), and thus might have been a target in the evolution of the basal ganglia. Previous research demonstrated significant differences between rodents and primates in DAergic input to thalamic circuits that seems to coincide with a dramatic increase in the number of interneurons, the targets of that increased innervation, in the primate thalamus (García-Cabezas et al., 2009). García-Cabezas and colleagues reported that dopamine transporter-ir axons were denser throughout the thalamus of the pigtailed macaque monkey relative to that of the rat, with a particularly dense innervation present in the mediodorsal nucleus and motor ventral nuclei. Their ultrastructural analyses revealed that the dopamine transporter axons largely targeted thalamic interneurons in the monkey thalamus, and there is a distinct lack of interneuron populations within the rat thalamus (García-Cabezas et al., 2009).
Here we present an extensive comparative analysis of DAergic innervation in the human and nonhuman primate basal ganglia. Our findings show that, remarkably, humans are distinguished from other primate species in having higher density of DAergic innervation in the medial caudate nucleus. It is notable that the human striatum is significantly smaller in overall size than would be predicted based on nonhuman primate brain scaling trends (Barger et al., 2014; Yin et al., 2009). Thus, our findings highlight the potential for evolutionary reorganization of a human brain structures in the absence of overt volumetric enlargement. Such region-selective increase in DAergic innervation may mediate the increased complexity and integration of information required for the processing of computational aspects of human and speech and language..
The role of striatal DA in the processing of human speech and language has generated tremendous interest with recent research in transgenic mouse models using the human FOXP2 gene (Enard et al., 2009; Reimers-Kipping et al., 2011; Schreiweis et al., 2014). The medial caudate nucleus shows hyperactivation in humans that lack a functional FOXP2 gene using fMRI and PET imaging techniques (Liégeois et al., 2003; Vargha-Khadem et al., 1998) and is associated with dysphasia and impaired orofacial motor control (Lai et al., 2001; Liégeois et al., 2003; Vargha-Khadem et al., 1998). Our finding of a relatively greater DAergic input in the human medial caudate nucleus differs from expectations based on data from mice expressing the human FOXP2 gene. In mice, the insertion of the human FOXP2 gene resulted in a 70% reduction in DA concentrations within the dorsomedial striatum compared to wild type littermates, with no differences in the dorsolateral striatum (Schreiweis et al., 2014). Enhanced procedural, but not declarative, memory was coincident with decreased DA in humanized Foxp2 mice. Procedural memory processes are preferentially invoked during speech and language and it was suggested that the decrease in DA levels within the dorsomedial striatum contributed to the switch from declarative to procedural memory during behavioral experiments (Schreiweis et al., 2014). The association of reduced DA in the human left caudate nucleus with increased accuracy in phonological processing (Tettamanti et al., 2005) further suggested that lower levels of DA are functionally important for human language. However, based on the results presented here, humans possess higher baseline DA concentrations in the medial caudate nucleus relative to other species.
The human medial caudate nucleus, which is the putative homologue of the dorsomedial region of the mouse striatum, shows increased activity on PET and MRI in humans who lack a functional FOXP2 gene and exhibit severe deficits in speech and language (Vargha-Khadem et al., 1998). Of note, this same region, the medial caudate nucleus, uniquely displays a convergence of inputs from different prefrontal cortical areas (dorsal anterior cingulate cortex, ventral medial prefrontal cortex, orbitofrontal cortex, dorsolateral prefrontal cortex) and premotor cortex in the rhesus macaque monkey, indicating that integration of information from different functional domains may be especially pronounced in this part of the striatum (Averbeck et al., 2014). Interestingly, humans and chimpanzees diverged from the other species included in the present analysis by having increased TH-ir ALv/Nv in the dorsal caudate nucleus and dorsal putamen. These findings indicate that a significant reorganization of the striatal DAergic innervation occurred after the panin-hominin clade branched from the gorilla lineage, approximately 10 million years ago (Scally et al., 2012).
We were also able to demonstrate that using learned attention-getting vocalizations did not alter DAergic input to the striatum in chimpanzees. This result suggests that such a form of experience-dependent plasticity in DAergic afferents is unlikely to account for the differences observed between humans and nonhuman primates. In conclusion, our results show that humans possess significantly higher DAergic innervation within the medial caudate nucleus, a region implicated in the computational aspects of language and speech (i.e., speech recognition and generation), relative to nonhuman primate species. Also, humans and chimpanzees together showed an increased DAergic innervation for other regions of the striatum. Although the previous research on mice engineered to express the human FOXP2 gene showed decreased DA levels within the dorsomedial striatum, it is important to note that the gene was expressed within the context of a mouse genome. The positive selection of two amino acids in the human FOXP2 gene is functionally relevant, with the human and chimpanzee sequences having differential effects on downstream transcription when expressed in human cell lines (Konopka et al., 2009). It is possible that the FOXP2 mutation in humans that results in language deficits affects DA concentrations in the medial caudate nucleus, and this could contribute to the abnormally high levels of activity on MRI (Liégeois et al., 2003; Vargha-Khadem et al., 1998). Hyperactivation of the caudate nucleus is also observed in patients with Parkinson’s disease when in a hypodopaminergic state (Tinaz et al., 2008). This is attributed to a decreased efficiency of processing that resulted in a compensatory hyperactivation. The two diseases most often associated with basal ganglia dysfunction are Huntington’s disease and Parkinson’s disease. Both diseases affect the production of aspects of speech and language (Benítez-Burraco, 2009; Gordon and Illes, 1987; Huber et al., 2012; Sambin et al., 2012; Teichmann et al., 2008; Tinaz et al., 2008), and both are characterized by decreased striatal DAergic input (Bédard et al., 2011; Cachope and Cheer, 2014). It is possible that the increased DAergic innervation that characterizes the human medial caudate nucleus was necessary for the evolution of speech and language, but became vulnerable in neurodegenerative disorders affecting the basal ganglia. Future work may reveal whether the human medial caudate nucleus possesses unique connectivity patterns and how increased DAergic modulation contributed to the evolution of human language acquisition.
Acknowledgments
We would like to thank Cheryl Stimpson, Shunya Yagi, and Bridget Wicinski for expert technical assistance. We are grateful to each of the following for the use of brain materials for this study: The National Chimpanzee Brain Resource (NIH grant NS092988), The Great Ape Aging Project supported by NIH grant AG014308, the National Primate Research Center at the University of Washington (NIH grant RR000166), the Northwestern University Alzheimer’s Disease Center Brain Bank (supported by Alzheimer’s Disease Core Center grant AG013854, from the National Institute on Aging to Northwestern University, Chicago, IL), the National Disease Research Interchange (NDRI) with support from NIH grant OD011158, and the El Paso County Coroner in Colorado.
Grant sponsor: The National Science Foundation; Grant number: BCS-1316829 (M.A.R. and SMA-1542848 to C.C.S. and W.D.H.). Grant sponsor: The National Institutes of Health; Grant numbers: NS042867 and NS073134 (W.D.H. and C.C.S), and AG014308 (J.M.E.).
Footnotes
Conflict of interest statement
The authors do not have any conflicts of interest.
Role of authors
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: MAR. Acquisition of data: MAR, MKE, ARS, and LJW. Analysis and interpretation of data: MAR and MKE. Drafting of the manuscript: MAR and MKE. Critical revision of the manuscript for important intellectual content: ARS, LJW, JJE, WDH, JME, BJ, PRH, and CCS. Statistical analysis: MAR and MKE. Obtained funding: MAR. Administrative, technical, and material support: MKE, ARS, LJW, JJE, WDH, JME, BJ, PRH, and CCS. Study supervision: MAR.
Literature cited
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- Averbeck BB, Lehman J, Jacobson M, Haber SN. Estimates of projection overlap and zones of convergence within frontal-striatal circuits. J Neurosci. 2014;34:9497–9505. doi: 10.1523/JNEUROSCI.5806-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barger N, Hanson KL, Teffer K, Schenker-Ahmed NM, Semendeferi K. Evidence for evolutionary specialization in human limbic structures. Front Hum Neurosci. 2014;8:Article 277. doi: 10.3389/fnhum.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard C, Wallman MJ, Pourcher E, Gould PV, Parent A, Parent M. Serotonin and dopamine striatal innervation in Parkinson’s disease and Huntington’s chorea. Parkinsonism Relat Disord. 2011;17:593–598. doi: 10.1016/j.parkreldis.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Benítez-Burraco A. Huntington’s disease: molecular foundations and implications in the characterization of the neuronal mechanisms responsible for linguistic processing. Rev Neurol. 2009;48:75–84. [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bruce HA, Margolis RL. FOXP2: novel exons, splice variants and CAG repeat length stability. Hum Genet. 2002;111:136–144. doi: 10.1007/s00439-002-0768-5. [DOI] [PubMed] [Google Scholar]
- Cachope R, Cheer JF. Local control of striatal dopamine release. Front Behav Neurosci. 2014;8:Article 188. doi: 10.3389/fnbeh.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Mao Y, Roberts JA, Rapp PR. Reduction in hippocampal cholinergic innervation is unrelated to recognition memory impairment in aged rhesus monkeys. J Comp Neurol. 2004;475:238–246. doi: 10.1002/cne.20181. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Mouton PR. Length measurement: new developments in neurostereology and 3D imagery. J Chem Neuroanat. 2000;20:61–69. doi: 10.1016/s0891-0618(00)00074-0. [DOI] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur J Neurosci. 2007;26:2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortexe. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council NR. Guide for the Care and Use of Laboratory Animals. 8. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Dominey PF, Inui T. Cortico-striatal function in sentence comprehension: insights from neurophysiology and modeling. Cortex. 2009;45:1012–1018. doi: 10.1016/j.cortex.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Nyengaard JR, Gundersen HJ. Tissue shrinkage and unbiased stereological estimation of particle number and size. J Microsc. 2001;204:232–246. doi: 10.1046/j.1365-2818.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ. Evidence for segregated and integrative connectivity patterns in human basal ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely J, Khun HH, Boysen ST, Erwin JM, Frels WI, Hof PR. FOXP2 language gene exhibits coding DNA sequence variation among humans and apes. Invited oral and poster presentation, COE-2 International Symposium, Evolution of the Apes and the Origin of the Human Beings; Inuyama, Japan. 14–17 November 2002.2002. [Google Scholar]
- Enard W. FOXP2 and the role of cortico-basal ganglia circuits in speech and language evolution. Curr Opin Neurobiol. 2011;21:1–10. doi: 10.1016/j.conb.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Enard W, Gehre S, Hammerschmidt K, Holter SM, Blass T, Somel M, Bruckner MK, Schreiweis C, Winter C, Sohr R, Becker L, Wiebe V, Nickel B, Giger T, Muller U, Groszer M, Aldler T, Aguilar A, Bolle I, Calzada-Wack J, Dalke C, Ehrhardt N, Favor J, Fuchs H, Gailus-Durner V, Hans W, Holzlwimmer G, Javaheri A, Kalaydjiev S, Kallnik M, Kling E, Kunder S, Mossbrugger I, Naton B, Racz I, Rathkolb B, Rozman J, Schrewe A, Busch DH, Graw J, Ivandic B, Klingenspor M, Klopstock T, Ollert M, Quintanilla-Martinez L, Schulz H, Wolf E, Wurst W, Zimmer A, Fisher SE, Morgenstern R, Arendt T, de Angelis MH, Fischer J, Schwarz J, Pääbo S. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell. 2009;137:961–971. doi: 10.1016/j.cell.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Paabo S. Molecular evolution of FOXP2, a gene involved in speech and language. Nature. 2002;418:869–872. doi: 10.1038/nature01025. [DOI] [PubMed] [Google Scholar]
- Ericsson J, Silderberg G, Robertson B, Wikstrom MA, Grillner S. Striatal cellular properties conserved from lampreys to mammals. J Physiol. 2011;589:2979–2992. doi: 10.1113/jphysiol.2011.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin JM, Hof PR, Ely JJ, Perl DP. One genontology: advancing understanding of aging through studies of great apes and other primates. In: Erwin JM, Hof PR, editors. Aging in Nonhuman Primates Interdisc Top Geront. Vol. 31. 2002. pp. 1–21. [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, McClain C. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476:330–347. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabi M, Collins CE, Wong P, Torres LB, Kaas JH, Herculano-Houzel S. Cellular scaling rules for the brains of an extended number of primate species. Brain Behav Evol. 2010;76:32–44. doi: 10.1159/000319872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cabezas MA, Martínez-Sánchez P, Sánchez-González MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A-L, Neumann K, Bachoud-Levi A-C, von Gudenberg AW, Euler HA, Lanfermann H, Preibisch C. Severity of dysfluency correlates with basal ganglia activty in persistent developmental stuttering. Brain Lang. 2008;104:190–199. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Gordon WP, Illes J. Neurolinguistic characteristics of language production in Huntington’s disease: a preliminary report. Brain Lang. 1987;31:1–10. doi: 10.1016/0093-934x(87)90056-3. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience. 2014;282:248–257. doi: 10.1016/j.neuroscience.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Behrens TEJ. The neural network underlying incentive-based learning: Implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region ini primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy to human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kunisho K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant) Am J Anat. 1987;180:126–142. doi: 10.1002/aja.1001800203. [DOI] [PubMed] [Google Scholar]
- Hedreen JC. Tyrosine hydroxylase-immunoreactive elements in the human globus pallidus and subthalamic nucleus. J Comp Neurol. 1999;409:400–410. doi: 10.1002/(sici)1096-9861(19990705)409:3<400::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. J Comp Neurol. 1997;384:1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Hersh L, Saper CB. Cholinergic innervation in the human striatum: a three compartment model. Neuroscience. 1996;74:67–87. doi: 10.1016/0306-4522(96)00094-2. [DOI] [PubMed] [Google Scholar]
- Huber JE, Darling M, Francis EJ, Zhang D. Impact of typical aging and Parksinson’s disease on the relationship among breath pausing, syntax, and punctuation. Am J Speech Lang Pathol. 2012;21:368–379. doi: 10.1044/1058-0360(2012/11-0059). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn T, Belden C, Sabbagh MN, Beach TG, Roher AE. Morphological and pathological evolution of brain microcirculation and aging in Alzheimer’s disease. PLoS ONE. 2012;7:e36893. doi: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J, Berthel MC, Henry A, Namer IJ, Musacchio M, Dufour A, Sellal F. Caudate nucleus and social cognition: neuropsychological and SPECT evidence from a patient with focal caudate lesion. Cortex. 2013;49:559–571. doi: 10.1016/j.cortex.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Kemp J, Despres O, DSellal F, Dufour A. Theory of Mind in normal ageing and neurodegenerative pathologies. Ageing Res Rev. 2012;11:199–219. doi: 10.1016/j.arr.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Konopka G, Bomar JM, Winden K, Coppola G, Jonsson ZO, Gao F, Peng S, Preuss TM, Wohlschlegel JA, Geschwind DH. Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature. 2009;462:213–217. doi: 10.1038/nature08549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczmanski P, Schmidt-Kastner R, Heinsen H, Steinbusch HWM, Hof PR, Schmitz C. Stereological studies of capillary length density in the frontal cortex of schizophrenics. Acta Neuropathologica. 2005;109:510–518. doi: 10.1007/s00401-005-1003-y. [DOI] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Lai CSD, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413:519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Lai CSD, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126:455–462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Haycock JW. Expression and distribution of two isoforms of tyrosine hydroxylase in macaque monkey brain. Brain Res. 1994;656:1–13. doi: 10.1016/0006-8993(94)91360-9. [DOI] [PubMed] [Google Scholar]
- Lex B, Hauber W. The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb Cortex. 2010;20:873–883. doi: 10.1093/cercor/bhp151. [DOI] [PubMed] [Google Scholar]
- Li G, Wang J, Rossiter SJ, Jones G, Zhang S. Accelerated FoxP2 evolution in echolocating bats. PLoS ONE. 2007;2:e900. doi: 10.1371/journal.pone.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liégeois F, Baldeweg T, Connelly A, Gadian DG, Mishkin M, Vargha-Khadem F. Language fMRI abnormalities associated with FOXP2 gene mutation. Nat Neurosci. 2003;6:1230–1237. doi: 10.1038/nn1138. [DOI] [PubMed] [Google Scholar]
- Lombardi WJ, Gross RE, Trepanier LL, Lang AE, Lonzano AM, Saint-Cyr JA. Relationship of lesion location to cognitive outcome following microelectrode-guided pallidotomy for Parksinson’s disease: support for the existence of cognitive circuits in the human pallidum. Brain. 2000;123:746–758. doi: 10.1093/brain/123.4.746. [DOI] [PubMed] [Google Scholar]
- Maricic T, Günther V, Georgiev O, Gehre S, Ćurlin M, Scgreiweis C, Naumann R, Burbano HA, Meyer M, Lalueza-Fox C, de la Rasilla M, Rosas A, Gajović S, Kelso J, Enard W, Schaffner W, Pääbo S. A recent evolutionary change affects a regulatory element in the human FOXP2 gene. Mol Biol Evol. 2013;30:844–852. doi: 10.1093/molbev/mss271. [DOI] [PubMed] [Google Scholar]
- Middleton FA. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000;42:183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cereb Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel S, Sumi S, Crain B, Brownlee L, Vogel F, Hughes J, van Belle G, Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD) Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microscopy. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Nambu A. Somatotopic organization of the primate basal ganglia. Front Neuroanat. 2011;5:Article 26. doi: 10.3389/fnana.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH/OLAW. US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training. 2002 http://grants.nih.gov/grants/olaw/references/phspol.htm#PublicHealthServicePolicyonHumaneCareandUseofLaboratory.
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Parent A, Cote P-Y, Lavoie B. Chemical anatomy of primate basal ganglia. Prog Neurobiol. 1995;46:131–197. [PubMed] [Google Scholar]
- Poletti M, Enrici I, Bonuccelli U, Adenzato M. Theory of mind in Parkinson’s disease. Behav Brain Res. 2011;219:342–350. doi: 10.1016/j.bbr.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Prat CS, Keller TA, Just MA. Individual differences in sentence comprehension: a functional magnetic resonance imaging investigation of syntactic and lexical processing demands. J Cog Neurosci. 2007;19:1950–1963. doi: 10.1162/jocn.2007.19.12.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Spocter MA, Stimpson CD, Erwin JM, Bonar CJ, Allman JM, Hof PR, Sherwood CC. Species-specific distributions of tyrosine hydroxylase-immunoreactive neurons within the prefrontal cortex of anthropoid primates. Neuroscience. 2009;158:1551–1559. doi: 10.1016/j.neuroscience.2008.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cholinergic innervation of the frontal cortex: Differences among humans, chimpanzees, and macaque monkeys. J Comp Neurol. 2008a;506:409–424. doi: 10.1002/cne.21546. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Neuroscience. 2008b;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cereb Cortex. 2008c;18:584–597. doi: 10.1093/cercor/bhm089. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann NY Acad Sci. 2007;1121:355–375. doi: 10.1196/annals.1401.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJY, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers-Kipping S, Hevers W, Pääbo S, Enard W. Humanized Foxp2 specifically affects cortico-basal ganglia circuits. Neuoscience. 2011;175:75–84. doi: 10.1016/j.neuroscience.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Tomic DL, Parkinson CH, Roeling TA, Cutter DJ, Robbins TW, Everitt BJ. Forebrain connectivity of the prefrontal cortex in the marmoset monkey (Callithrix jacchus): an anterograde and retrograde tract-tracing study. J Comp Neurol. 2007;502:86–112. doi: 10.1002/cne.21300. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Blangero J, Sanghera MK, Pessoa L, Fox PM, Uecker A, Friehs G, Young KA, Griffin JL, Lovallo WR, Fox PT. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage. 2012;60:117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JL, McIntyre J, Hopkins WD, Taglialatela JP. Vocal learning of a communicative signal in captive chimpanzees (Pan troglodytes) Brain Lang. 2013;127:520–526. doi: 10.1016/j.bandl.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambin S, Teichmann M, de Diego Balaguer R, Giavazzi M, Sportiche D, Schlenker P, Bachoud-Lévi AC. The role of the striatum in sentence processing: disentangling syntax from working memory in Huntington’s disease. Neuropsychologia. 2012;50:2625–2635. doi: 10.1016/j.neuropsychologia.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Scally A, Dutheil JY, Hillier LW, Jordan GE, Goodhead I, Herrero J, Hobolth A, Lappalainen T, Mailund T, Marques-Bonet T, McCarthy S, Montgomery SH, Schwalie PC, Tang YA, Ward MC, Xue Y, Yngvadottir B, Alkan C, Andersen LN, Ayub Q, Ball EV, Beal K, Bradley BJ, Chen Y, Clee CM, Fitzgerald S, Graves TA, Gu Y, Heath P, Heger A, Karakoc E, Kolb-Kokocinski A, Laird GK, Lunter G, Meader S, Mort M, Mullikin JC, Munch K, O’Connor TD, Phillips AD, Prado-Martinez J, Rogers AS, Sajjadian S, Schmidt D, Shaw K, Simpson JT, Stenson PD, Turner DJ, Vigilant L, Vilella AJ, Whitener W, Zhu B, Cooper DN, de Jong P, Dermitzakis ET, Eichler EE, Flicek P, Goldman N, Mundy NI, Ning Z, Odom DT, Ponting CP, Quail MA, Ryder OA, Searle SM, Warren WC, Wilson RK, Schierup MH, Rogers J, Tyler-Smith C, Durbin R. Insights into hominid evolution from the gorilla genome sequence. Nature. 2012;483:169–175. doi: 10.1038/nature10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to cebtral parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Schreiweis C, Bornschein U, Burgeuiére E, Kerimoglu C, Schreiter S, Dannemann M, Goyal S, Rea E, French CA, Puliyadi R, Groszer M, Fisher SE, Mundry R, Winter C, Hevers W, Pääbo S, Enard W, Graybiel AM. Humanized Foxp2 accelerates learning by enhancing transitions from declarative to procedural performance. Proc Nat Acad Sci USA. 2014;111:14253–14258. doi: 10.1073/pnas.1414542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y, Xu T, Graf WM, Fobbs A, Sherwood CC, Hof PR, Allman JM, Manaye KF. Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J Comp Neurol. 2010;518:963–971. doi: 10.1002/cne.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Stimpson CD, Bonar CJ, de Sousa AJ, Preuss TM, Hof PR. Scaling of inhibitory interneurons in areas V1 and V2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain Behav Evol. 2007;69:176–195. doi: 10.1159/000096986. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Raghanti MA, Wenstrup JJ. Is humanlike cytoarchitectural asymmetry present in another species with complex social vocalization? A stereologic analysis of mustached bat auditory cortex. Brain Res. 2005;1045:164–174. doi: 10.1016/j.brainres.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Smeets WJAJ, Marin O, Gonzalez A. Evolution of the basal ganglia: new perspectives through a comparative approach. J Anat. 2000;196:501–517. doi: 10.1046/j.1469-7580.2000.19640501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Taglialatela JP, Reamer L, Schapiro SJ, Hopkins WD. Social learning of a communicative signal in captive chimpanzees (Pan troglodytes) Biol Lett. 2012;8:498–501. doi: 10.1098/rsbl.2012.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann M, Dupoux E, Cesaro P, Bachoud-Lévi A-C. The role of the striatum in sentence processing: Evidence from a priming study in the early stages of Huntington’s disease. Neuropsychologia. 2008;46:174–185. doi: 10.1016/j.neuropsychologia.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Teichmann M, Dupoux E, Kouider S, Bachould-Levi AC. The role of the striatum in processing language rules: evidence from word perception in Huntington’s disease. J Cogn Neurosci. 2006;18:1555–1569. doi: 10.1162/jocn.2006.18.9.1555. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Fazio F, Perani D. Basal ganglia and language: phonology modulates dopaminergic release. Neuroreport. 2005;16:397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron. 2010;66:781–795. doi: 10.1016/j.neuron.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinaz S, Schendan HE, Stern C. Fronto-striatal deficit in Parkinson’s disease during semantic event sequencing. Neurobiol Aging. 2008;29:397–407. doi: 10.1016/j.neurobiolaging.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, Pinker S. A neural dissociation within language: evidence that the mental dictionary is part tof declarative memory, and that grammatical rules are processed by the procedural system. J Cogn Neurosci. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- USDA. Animal Welfare Act and Animal Welfare Regulations. Washington, D.C: U.S. Goverment Printing Office; 2013. (7 USC, Title 7-AGRICULTURE. CHAPTER 54 - TRANSPORTATION, SALE, AND HANDLING OF CERTAIN ANIMALS. http://awic.nal.usda.gov/government-and-professional-resources/federal-laws/animal-welfare-act. [Google Scholar]
- Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A. 1998;95:12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Murray EA, Gerfen CR. The frontal cortex-basal ganglia system in primates. Crit Rev Neurobiol. 1996;10:317–356. doi: 10.1615/critrevneurobiol.v10.i3-4.30. [DOI] [PubMed] [Google Scholar]
- Wolf ME, LeWitt PA, Bannon MJ, Dragovic LJ, Kapatos G. Effect of aging on tyrosine hydroxylase protein content and the relative number of dopamine nerve terminals in human caudate. J Neurochem. 1991;56:1191–1200. doi: 10.1111/j.1471-4159.1991.tb11410.x. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Laeremans A, Gantois I, Mantini D, Vermaercke B, Op de Beeck HP, Swinnen SP, Wenderoth N, Arckens L, D’Hooge R. Homologous involvement of striatum and prefrontal cortex in rodent and human water maze learning. Proc Nat Acad Sci USA. 2013;110:3131–3136. doi: 10.1073/pnas.1217832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin D, Valles FE, Fiandaca MS, Forsayeth J, Larson P, Starr P, Bankiewicz KS. Striatal volume differences between non-human and human primates. J Neurosci Methods. 2009;176:200–205. doi: 10.1016/j.jneumeth.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]