Abstract
The pathogenic process in Alzheimer’s disease (AD) appears to be closely linked to the neurotoxic action of amyloid-β (Aβ) oligomers. Recent studies have shown that these oligomers bind with high affinity to the membrane-anchored cellular prion protein (PrPC). It has also been proposed that this binding might mediate some of the toxic effects of the oligomers. Here, we show that the soluble (membrane anchor-free) recombinant human prion protein (rPrP) and its N-terminal fragment N1 block Aβ oligomers-induced inhibition of long-term potentiation (LTP) in hippocampal slices, an important surrogate marker of cognitive deficit associated with AD. rPrP and N1 are also strikingly potent inhibitors of Aβ cytotoxicity in primary hippocampal neurons. Furthermore, experiments using hippocampal slices and neurons from wild-type and PrPC null mice (as well as rat neurons in which PrPC expression was greatly reduced by gene silencing) indicate that, in contrast to the impairment of synaptic plasticity by Aβ oligomers, the cytotoxic effects of these oligomers, and the inhibition of these effects by rPrP and N1, are independent of the presence of endogenous PrPC. This suggests fundamentally different mechanisms by which soluble rPrP and its fragments inhibit these two toxic responses to Aβ. Overall, these findings provide strong support to recent suggestions that PrP-based compounds may offer new avenues for pharmacological intervention in AD.
Keywords: Alzheimer’s disease, Amyloid-beta (Aβ), Prion protein, Synaptic plasticity, Long-term potentiation, cytotoxicity
Introduction
Alzheimer’s disease (AD) is associated with the accumulation of extracellular amyloid plaques in the brain (Hardy and Selkoe, 2002; Selkoe, 2001). The main component of these plaques is a 40-43 residue amyloid β (Aβ) peptide, a product of proteolytic processing of amyloid precursor protein. Even though Aβ plaques composed of amyloid fibrils are the neuropathological hallmark of the disease, a large body of evidence indicates that the main neurotoxic species are not mature plaques but smaller assemblies of Aβ, often referred to as soluble oligomers (Benilova et al., 2012; Hardy and Selkoe, 2002; Klein et al., 2001; Selkoe, 2001; Small et al., 2001; Walsh and Selkoe, 2007). The precise molecular structure of these oligomers and the mechanism by which they trigger pathogenic processes in AD are, however, largely unknown, hindering efforts to develop effective therapeutic strategies.
A recent important development was the finding that the cellular prion protein (PrPC), an ubiquitous glycoprotein attached to the plasma membrane via the glycosylphosphatidylinositol (GPI) anchor can act as a receptor of Aβ oligomers (Lauren et al., 2009). It was also proposed that this receptor plays a major role in AD pathogenesis, mediating neurotoxic effects of Aβ oligomers such as inhibition of long-term potentiation (LTP), neuronal cell death, and memory impairment on some mouse models of AD (Barry et al., 2011; Freir et al., 2011; Gimbel et al., 2010; Kostylev et al., 2015; Kudo et al., 2012; Lauren et al., 2009; Rushworth et al., 2013; Um et al., 2012). Even though the notion of a critical role of PrPC as a mediator of Aβ toxicity in AD has been challenged in some other studies (Balducci et al., 2010; Calella et al., 2010; Cisse et al., 2011; Kessels et al., 2010), there is a consensus that Aβ oligomers bind with high affinity to both cell-surface PrPC (Dohler et al., 2014; Freir et al., 2011; Lauren et al., 2009; Nicoll et al., 2013; Zou et al., 2011) and soluble, GPI-free recombinant human prion protein (Balducci et al., 2010; Chen et al., 2010; Fluharty et al., 2013; Nieznanski et al., 2014).
These developments prompted studies to explore whether recombinant human prion protein (rPrP) or its fragments could be used as inhibitors of Aβ oligomers toxicity. Even though limited in scope, initial observations in this regard were encouraging, showing that rPrP and/or its fragments inhibit Aβ assembly into amyloid fibrils (Fluharty et al., 2013; Nieznanski et al., 2012; Younan et al., 2013), as well as some of the toxic actions of different forms of Aβ oligomers (Fluharty et al., 2013; Nieznanski et al., 2012; Williams et al., 2015). However, these proteins have not yet been assessed in terms of their ability to prevent critical functional responses to Aβ, such as the impairment of synaptic plasticity. Here, we report that both full-length rPrP as well as the N1 fragment corresponding to residues 23-110 are potent suppressors of Aβ-induced inhibition of long-term potentiation (LTP) in hippocampal slices. This finding is of particular relevance to potential therapeutic applications of PrP-based compounds in AD, as LTP is a widely used electrophysiological correlate of learning and memory (Malenka and Bear, 2004). We also show that both proteins are strikingly effective inhibitors of Aβ cytotoxicity in primary neurons. However, in contrast to the impairment of synaptic plasticity by Aβ oligomers, the cytotoxic effects of these oligomers (and the inhibition of these effects by rPrP and N1) appear to be independent of the presence of endogenous PrPC. Altogether, these observations strongly suggest that PrP-based compounds can potentially lead to new approaches for pharmacological intervention in AD.
Materials and methods
Preparation of Prion Protein and Its Fragments
Bacterial expression and purification of rPrP and its truncated variants were performed as described previously (Morillas et al., 1999). Protein concentration was determined by measuring absorbance at 276 nm using the appropriate extinction coefficients.
Preparation of Aβ Oligomers
Human Aβ1-42 peptide (Aβ) was purchased from American Peptide Company (Sunnyvale, CA). Lyophilized peptide was solubilized in hexafluoro-2-propanol, divided into aliquots and, after solvent evaporation, stored at −80 °C. Immediately before use, the peptide was dissolved in 10 mM NaOH to a concentration of 400 μM and subjected to 10 cycles of 10-s sonication on ice to remove any residual aggregates. Disaggregated Aβ was diluted in 10 mM sodium phosphate, pH 7.4, to a concentration of 100 μM. To prepare soluble Aβ oligomers, the peptide (20 μM) was incubated in 50 mM sodium phosphate buffer, pH 7.4, for 3 h at 37 °C. Once every 20 min, the plates were subjected to shaking for 10 s. As shown previously, these experimental conditions result in Aβ preparations that are highly enriched in soluble oligomers (see Fig. 1A). Aβ mixtures with rPrP or its fragments were prepared by two different procedures. The first type of preparation (denoted Preparation A) was obtained by adding rPrP or its fragments at a desired concentration to freshly disaggregated Aβ and incubating these mixtures as described above for the peptide alone. The second type (Preparation B) was prepared by adding rPrP or its fragments to Aβ oligomers that were preformed in the absence of these proteins.
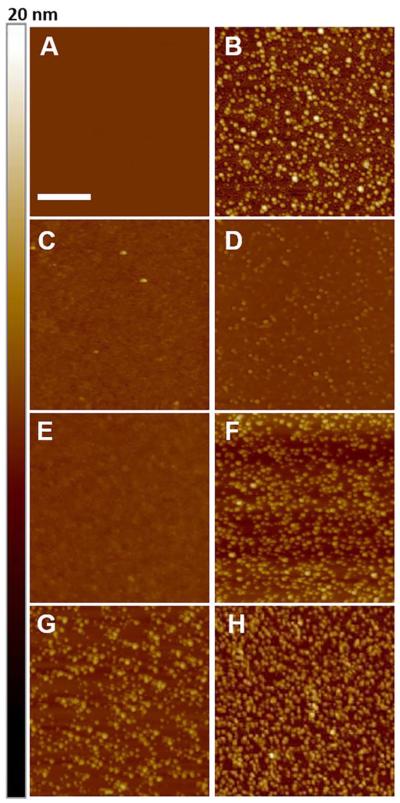
Fig. 1.
Representative AFM images (height mode) of samples used in toxicity experiments. (A) Control image of mica substrate. (B) Oligomers of Aβ alone. (C) Mixture of Aβ and rPrP at a molar ratio of 5:1. (D) Mixture of Aβ and N1 at a molar ratio of 5:1. (E) Mixture of Aβ and rPrP at a molar ratio of 20:1. (F) Mixture of Aβ and N1 at a molar ratio of 20:1. Samples shown in panels B-F were prepared by adding to freshly disaggregated Aβ buffer alone or buffer containing rPrP or N1 and incubating the mixtures for 3 h at 37 °C as described in Preparation of Aβ Oligomers for Preparation A. (G and H) Mixtures of Aβ and rPrP (G) or Aβ and N1 (H) prepared by adding rPrP or N1 to Aβ oligomers that were preformed in the absence of these proteins (Preparation B as described in Preparation of Aβ Oligomers); Aβ to rPrP or N1 molar ratio of 5:1. Scale bar represents 200 nm. Color Z-scale on the right side represent height from 0 to 20 nm.
Atomic force microscopy (AFM)
Morphology of different preparations of Aβ oligomers was analyzed by AFM using a MultiMode AFM (Digital Instruments, Santa Barbara, CA) equipped with a NanoScope IV controller (Jones and Surewicz, 2005; Nieznanski et al., 2012).
Animal Protocols
All procedures involving animals in this study were approved by the Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University.
Primary Neuronal Culture
Primary hippocampal neurons were prepared from embryonic d 18-20 (E18-E20) Sprague Dawley rat (Charles River Laboratories) and wild type (WT) or PrPC knockout (PrP0/0) mouse fetuses by a standard procedure (Bouyer et al., 2004; Kaech and Banker, 2006). In brief, the dissected hippocampi were digested at room temperature with 0.05% trypsin type XI, washed with Hank’s Balanced Salt Solution (Invitrogen), triturated and resuspended in B27/neurobasal medium (Life Technologies) supplemented with 0.5 mM glutamine and 5% deactivated fetal bovine serum (GIBCO BRL). Hippocampal neurons were plated on poly-L-lysine coated coverslips (~3×104 cells/coverslip). After 24 h, the medium was replaced with one without fetal bovine serum. All neurons were maintained by changing half of the culture medium every 3-4 days. For PrPC knockdown, Accell SMART pool siRNA containing a mixture of four siRNA sequences designed to target rat PrP (E-098489) was used according to the manufacturer’s instruction (Dharmacon/Thermo Fisher Scientific). A nontargeting scrambled sequence (D-001910) was used as a negative control. The efficiency of the knockdown of PrPC was evaluated after 72 h by Western blot analysis using monoclonal antiprion 3F10 antibody (epitope PrP residues 135-150).
Cytotoxicity Measurements
The primary neurons medium was replaced with serum-free Neurobasal medium supplemented with 2% (v/v) B27, and neurons were treated for 24 h with Aβ oligomers alone or Aβ mixtures with rPrP or its fragments prepared as described above. Cell viability following this treatment was assessed using a lactate dehydrogenase (LDH) assay kit (Takara Bio Inc., Shiga, Japan). Total LDH was determined by lysing all cells with 2% Triton X-100, and experimental data were expressed (after correction for small spontaneous LDH release in the absence of any additives) as percentage of total LDH released under given experimental conditions.
Brain Slice Preparation and Electrophysiology
In all LTP experiments, 400-μm thick transverse hippocampal slices were prepared from 1-2 months old male mice (wild-type mice on a C57BL/6JEiJ or FVB/N background, or PrP-null mutant mice on a FVB/N background) as described previously (Costa and Grybko, 2005). Experiments were performed at room temperature (24 ± 0.5 °C) in a submersion recording chamber containing artificial cerebral spinal fluid (aCSF) (in mM: 120 NaCl, 3.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, and 10 D-glucose, saturated with 95% O2 and 5% CO2). A customized electrophysiology setup was designed to allow for simultaneous recordings of treated and untreated hippocampal slices from the same animal. Field evoked postsynaptic potentials (fEPSPs) recordings were performed through glass micropipettes (Ag-AgCl recording electrodes, 3-5 MΩ resistance) filled with aCSF inserted into the apical dendritic layer of the CA1 region of the hippocampus. Stimulation of Schaffer collateral/commissural fibers in the CA1 region was applied through Pl/Ir electrodes (FHC Inc., Bowdoin, ME), and the intensity of stimuli was adjusted to 40-50% of the amplitude required to produce population spikes. A stable baseline of synaptic transmission was established for 10 minutes prior to bath application of Aβ oligomers alone or Aβ mixtures with rPrP or N1. Then, while slices were superfused with these test molecules, a second stable synaptic transmission baseline was established for 10 minutes prior to induction of LTP. Bath perfusion of the test molecules continued until 10 min after LTP induction. To reduce the use of Aβ oligomers, aCSF was recirculated during this time. LTP was induced by 4, 1-sec duration, 100-Hz trains of stimuli (HFS), with a 5 min inter-train interval (Hoeffer et al., 2007). Signals from the recording electrode were amplified 2000-5000 times (Brownlee Precision Electrophysiology Amplifier with signal conditioner, Model 440), low-pass filtered (8-pole Bessel) at 2 kHz, and digitized at 20 kHz by a Digidata digitizer (1322A, Molecular Devices, Sunnyvale, CA) into a Microsoft Windows based microcomputer. The PCLAMP software (version 8, Molecular Devices) was used for data acquisition and off-line data analysis.
Statistical analysis
Synaptic efficacy was determined by the slope of fEPSPs, normalized to the mean value of fEPSP slopes recorded prior to the induction of LTP. Statistical significance was determined by one or two-way Analysis of Variance (ANOVA) (Statistica, version 12, Dell Inc., Tulsa, OK) of the results for the mean levels of LTP followed, when appropriate, by a post hoc analysis using the Fisher's Least Significant Difference (LSD).
Results
Protective effect of rPrP and the N1 fragment against inhibition of LTP by Aβ oligomers
Previous studies have shown that soluble Aβ oligomers are potent synaptotoxins, inhibiting LTP of the Schaffer collateral pathway between hippocampal CA3 and CA1 pyramidal cells (Benilova et al., 2012; Freir et al., 2011; Lambert et al., 1998; Lauren et al., 2009). Aβ oligomers used in the present study were prepared by incubating freshly disaggregated Aβ peptide for 3 h at 37 °C (see Materials and Method). Atomic force microscopy images of these preparations show large concentration of mostly spherical oligomers with heights between approximately 2 and 6 nm (Fig. 1A). As expected, exposure of hippocampal slices to preparations of these oligomers (500 nM) resulted in a large suppression of LTP (Fig. 2A, B). Remarkably, when slices were exposed to an Aβ mixture with rPrP (prepared by adding rPrP to freshly disaggregated Aβ and incubating the sample under the same conditions as used for generation of Aβ oligomers alone), there was little reduction of LTP compared to the control slices (Fig. 2A, B). This clearly indicates that a soluble recombinant prion protein has a major protective effect against inhibition of LTP by Aβ.
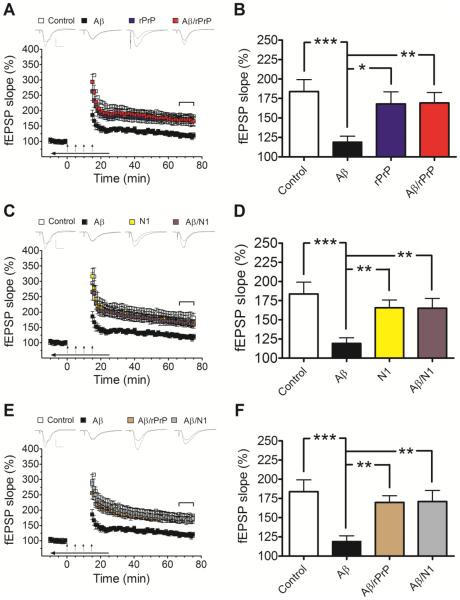
Fig. 2.
rPrP and the N1 fragment prevent Aβ-induced inhibition of LTP in mouse hippocampal slices. (A and C) Field potentials were recorded from the CA1 region of hippocampal slices in the presence or absence of Aβ oligomers alone or Aβ mixtures with rPrP (A) or N1 (C). These mixtures were prepared by adding rPrP or N1 to freshly disaggregated Aβ and incubating the samples for 3 h at 37 °C as described in Preparation of Aβ Oligomers for Preparation A. The concentration of Aβ in each case was 500 nM, and the concentration of rPrP or N1 was 100 nM (Aβ to rPrP or N1 molar ratio of 5:1). Controls are also included for rPrP and N1 alone. (B and D) Average fEPSP slopes during the last 10 minutes of field potential recordings derived from data shown in panels A and C, respectively. These data demonstrate strong protective effect of both rPrP and N1 against inhibition of LTP by Aβ oligomers (F(3, 39)= 5.112, p=0.0044; F(3, 39)=6.078, p=0.0017 for rPrP and N1, respectively). (E and F) A similar protective effect was observed when rPrP or N1 (both at a molar ratio to Aβ of 1:5) were added to Aβ oligomers that were preformed in the absence of these proteins (Preparation B described in Preparation of Aβ Oligomers) (F(3, 39)= 6.352, p=0.0013). The additives were bath applied for 10 minutes before establishing baseline; during tetanic stimulation (arrows); and for an additional 10 minutes after LTP induction (horizontal arrow bar). Representative traces of individual fEPSPs before and after LTP stimulation are shown on the top of panels A, C, and E (vertical bar: 1 mV; horizontal bar: 10 ms). The mean values are based on 10-12 independent experiments (one slice per animal per treatment), and error bars represent SEM. Statistical significance is expressed as *, **, and *** for p<0.05, p<0.01, and p<0.001, respectively. In control experiments it was verified that rPrP or N1 alone have no significant effect on LTP (blue and yellow bars in panels B and D, respectively).
Next, we tested in the same assay N1, a fragment of human prion protein that retains high binding affinity to Aβ oligomers (Fluharty et al., 2013). Importantly, akin to full-length rPrP, N1 almost completely suppressed the inhibitory effect of Aβ on hippocampal LTP (Fig. 2C, D).
The experiments described above were performed at 100 nM concentration of rPrP or N1 (i.e., at 1:5 molar ratio of these proteins to Aβ). The effect of these proteins as a function of their concentration (or molar ratio to Aβ) has not yet been systematically studied. However, in a limited number of experiments using 25 nM rPrP (1:20 molar ratio to Aβ), we did not observe consistent protection against inhibition of LTP (data not shown). In control experiments, it was confirmed that neither rPrP nor N1 alone have measurable effect on the induction or maintenance of LTP (Fig. 2A-D).
As described above, samples used in the LTP experiments shown in Fig. 2 A and B were prepared by incubating the mixtures of freshly disaggregated Aβ with rPrP or N1 for 3 h at 37 °C. Consistent with a previous report (Nieznanski et al., 2012), AFM images show that such preparations of Aβ with rPrP are almost completely devoid of large spherical oligomers that are abundant in identically prepared samples of Aβ alone (Fig. 1B, C). The population of large spherical oligomers is also greatly reduced in samples containing the mixture of Aβ with N1, with most of the remaining particles having heights below ~2 nm (Fig. 1D). These morphological differences suggest that the protective action of rPrP and N1 could be due to their inhibitory effect on the formation of specific oligomeric species of Aβ that are responsible for synaptotoxicity (even though it is unknown presently which of the species populating highly heterogeneous samples of synthetic Aβ oligomers cause inhibition of LTP). Another possibility is that the protective action of rPrP and N1 is due to their ability to “neutralize” preexisting toxic oligomers of Aβ (e.g., by blocking their binding to PrPC and/or other cell-surface receptors). Both of these scenarios are relevant in the context of potential pharmacological interventions in AD using PrP-based compounds. To further explore this issue, we repeated LTP measurements using Aβ/rPrP and Aβ/N1 mixtures prepared by adding rPrP or N1 to Aβ oligomers that were preformed in the absence of these additives. Samples prepared by the latter procedure appear morphologically similar to those of Aβ oligomers alone, displaying heterogeneous populations of large, mostly spherical oligomers (Fig. 1G, H). However, also in this case both rPrP and N1 almost completely suppressed the inhibition of LTP by Aβ (Fig. 2E, F). Altogether, these data clearly demonstrate that the full-length rPrP as well as the N1 fragment almost completely block the inhibitory effect of Aβ on hippocampal LTP. This protective effect is observed both when the proteins are added before oligomerization of Aβ as well as to the preformed Aβ oligomers.
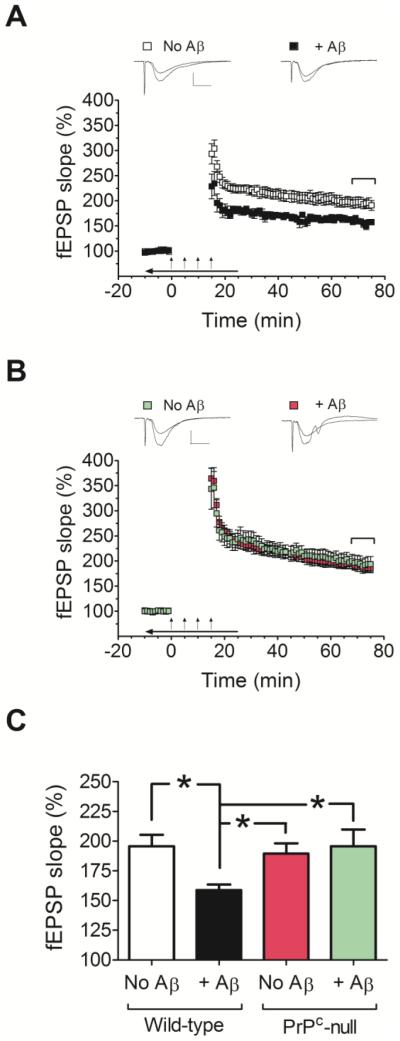
There have been contradictory reports regarding the role of endogenous PrPC in the synaptotoxicity of Aβ oligomers. While some studies indicate that membrane anchored PrPC is crucial for the inhibition of LTP by Aβ oligomers (Barry et al., 2011; Freir et al., 2011; Lauren et al., 2009), other studies appear to be inconsistent with this claim (Calella et al., 2010; Kessels et al., 2010). Therefore, we extended the present electrophysiological experiments to hippocampal slices derived from PrPC null mice. Consistent with previous studies employing two different PrPC null mouse lines (Freir et al., 2011; Lauren et al., 2009), the Aβ oligomers used in the present study failed to impair LTP in the hippocampi of PrPC null mice (Fig. 3B, C). Because our PrPC knockout mice are on a different genetic background than the wild-type mice used in the experiments shown in Fig. 2 (FVB/N and C57BL/6JEiJ, respectively), we repeated the electrophysiological experiments using hippocampal slices from wild-type FVB/N mice. Similar to what we observed for wild-type C57BL/6JEiJ mice, also in this case we found strong inhibitory effect of Aβ oligomers on LTP (Fig. 3A, C). Given the lack of LTP inhibition by Aβ in hippocampal slices from PrPC null mice, no electrophysiological experiments were performed with these slices using rPrP or N1.
Fig. 3.
Comparison of the effects of Aβ oligomers on LTP in hippocampal slices from wild-type and PrP-null mice. (A and B) Field potentials were recorded from the CA1 region of hippocampal slices from FVB/N wild-type mice (A) or PrPC-null mice (FVB/N background) (B), in the absence or presence of Aβ oligomers (500 nM). (C) Average fEPSP slopes during the last 10 minutes of field potential recordings derived from data shown in panels A and B. Note that hippocampal slices from PrPC null mice perfused with Aβ oligomers did not show impaired LTP. A two-way ANOVA showed a significant interaction between genotype and treatment (Aβ) (F(1, 39)= 4.705, p= 0.036). The oligomers were bath applied for 10 minutes before establishing baseline; during tetanic stimulation (arrows); and for an additional 10 minutes after LTP induction (horizontal arrow bar). Representative traces of individual fEPSPs before and after LTP stimulation are shown on the top of panels A and B (vertical bar: 1 mV; horizontal bar: 10 ms). The mean values are based on 10-11 independent experiments (one slice per animal per treatment), and error bars represent SEM. Statistical significance is expressed as * for p<0.05.
Effect of rPrP and its fragments on Aβ cytotoxicity in primary neurons
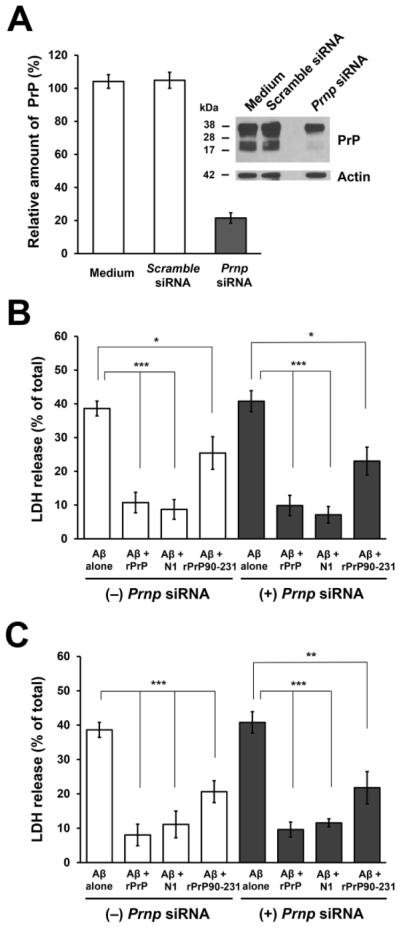
Previously, we showed that rPrP inhibits cytotoxicity of Aβ oligomers in M17 neuroblastoma cells (Nieznanski et al., 2012). To further assess the therapeutic potential of rPrP and its fragments and explore the role of endogenous PrPC in Aβ cytotoxicity, here, we used a more physiologically relevant system of rat primary hippocampal neurons. The levels of PrPC in these neurons were controlled using Prnp siRNA, with Prnp siRNA treated cells showing ~80% reduction of PrPC expression (Fig. 4A). The viability of neurons was assessed by measuring the release of LDH into the medium, an assay frequently used in studies of Aβ cytotoxicity.
Fig. 4.
The effect of rPrP and its fragments on the cytotoxicity of Aβ oligomers in rat primary hippocampal neurons. (A) The efficiency of the knockdown of PrPC in primary neurons after 72 h treatment with Prnp siRNA as evaluated by Western blot analysis using 3F10 antibody (for prion protein) and C4 antibody (for actin). Neurons treated with scrambled siRNA were used as controls. (B and C) Primary neurons (pretreated in the presence or absence of Prnp siRNA) were treated with Aβ oligomers alone and two different preparations of Aβ mixtures with rPrP or its fragments N1 and rPrP90-231. Data shown in panel B were obtained using Aβ mixtures with rPrP or its fragments prepared by adding these proteins to freshly disaggregated Aβ and incubating the samples for 3 h at 37 °C , as described in Preparation of Aβ Oligomers for Preparation A. Data shown in panel C represent experiments using Aβ mixtures with rPrP and its fragments prepared by adding rPrP, N1 or rPrP90-231 to Aβ oligomers that were preformed in the absence of these proteins (Preparation B described in Preparation of Aβ Oligomers). The final concentration of Aβ in each case was 500 nM, and the molar ratio of rPrP or its fragments to Aβ was 1:20. The cytotoxic effect was assessed by measuring LDH release. Mean values are based on at least five independent experiments; error bars represent SEM. ***, p<0.001; **, p<0.01; *, p<0.05.
Preparations of soluble Aβ oligomers used in our study are highly cytotoxic to rat primary neurons. Under the present experimental conditions, ~ 40% of LDH release was observed in the presence of Aβ at a concentration of 500 nM (Fig. 4). However, when cells were treated with Aβ mixtures with rPrP (prepared by adding rPrP to freshly disaggregated Aβ and incubating the sample under identical conditions as those used to obtain Aβ oligomers alone), this cytotoxic effect was dramatically reduced (Fig. 4B). The cytotoxic effect of Aβ alone as well as the inhibition of this effect by rPrP appear to be independent of cellular PrPC, as similar results were obtained for neurons expressing normal levels of PrPC and those pretreated with Prnp siRNA (Fig. 4B).
Next, we tested in this assay N1 and the C-terminal fragment corresponding to human prion protein residues 90-231 (rPrP90-231). Importantly, N1 was equally effective in preventing cytotoxicity of Aβ as the full-length rPrP (Fig. 4B). Inhibitory action was also observed for rPrP90-231, but in this case the effect was substantially weaker. Furthermore, akin to the observations for the full-length rPrP, the inhibition of Aβ toxicity by these two fragments was independent of the level of cellular PrPC (Fig. 4B). Interestingly, at the concentration of rPrP and the fragments used (molar ratio to Aβ of 1:20), samples for rPrP/Aβ and N1/Aβ mixtures prepared by the procedure described above were morphologically different. In the presence of N1, Aβ formed spherical oligomers that in AFM appeared similar to those formed by the peptide alone (Fig. 1E), whereas in the presence of the full-length rPrP the peptide formed much smaller particles, with heights in AFM images below ~1 nm (Fig. 1D). Yet, despite these differences, at the morphological level, the inhibition of Aβ toxicity by rPrP and N1 was very similar (Fig. 4B).
In the next set of experiments, we tested the ability of rPrP and its fragments to inhibit cytotoxicity of Aβ when these proteins where added to already preformed Aβ oligomers. In this case, neither rPrP nor the fragments tested had any detectable effect on Aβ oligomer morphology (Fig. 1G, H). However, also in these experiments, both rPrP and N1 were potent inhibitors of Aβ cytotoxicity (Fig. 4C). Similar to the experiments in which rPrP or its fragments were added to freshly disaggregated Aβ, rPrP90-231 was less effective in suppressing the toxicity of Aβ oligomers. Again, the inhibitory effect of all proteins tested was independent of the levels of cellular PrPC.
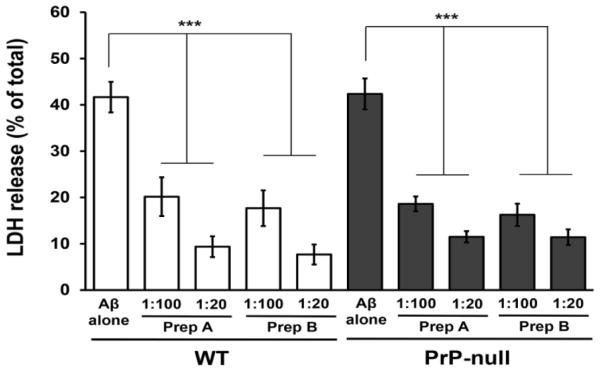
Similar data obtained in experiments using rat neurons expressing normal levels of PrPC and those pretreated with Prnp siRNA suggest that, in contrast to synaptotoxicity of Aβ oligomers, the cytotoxic effects of Aβ as measured by LDH release from primary neurons (and the inhibition of these effects by rPrP and its fragments) are not mediated by the endogenous PrPC. However, the caveat here is the presence of residual PrPC in the knockdown cells (~20% in our study). Therefore, to further explore this issue, we extended our cytotoxicity experiments to primary neurons derived from wild-type and PrPC null mice. No statistically significant differences between these two neuron types were found with regard to Aβ oligomers-induced release of LDH (Fig. 5), providing further evidence that endogenous PrPC is not required for the cytotoxic action of Aβ oligomers.
Fig. 5.
The effects of the N1 fragment of PrP on the cytotoxicity of Aβ oligomers in primary hippocampal neurons from wild-type and PrPC null mice. Neurons were treated with Aβ oligomers alone and mixtures of Aβ with N1 prepared either by adding N1 to freshly disaggregated Aβ and incubating the samples for 3 h at 37 °C (bars labeled “Prep A”) or adding N1 to oligomers that were preformed in the absence of any additives (bars labeled “Prep B”). The final concentration of Aβ in each case was 500 nM; the numbers under the bars indicate molar ratios of N1 to Aβ. The cytotoxic effect was assessed by measuring LDH release. Mean values are based on at least 5-7 independent experiments; error bars represent SEM. ***, p<0.001.
The murine neuron model was also used to further test the effect of one of rPrP fragments on Aβ cytotoxicity. We focused here on N1, the fragment that in rat neurons has similar protective effect against Aβ cytotoxicity as the full-length rPrP. Akin to the observations made using rat neurons, also in this case N1 greatly inhibited Aβ-induced release of LDH, and this protective effect was observed both when N1 was added to Aβ before its oligomerization as well as when it was added to the preformed Aβ oligomers (Fig. 5). Significant protection against Aβ cytotoxicity was found in both cases at concentrations of N1 as low as those corresponding to N1:Aβ molar ratio of 1:100.
Discussion
Previous studies have reported conflicting findings regarding the role of cellular PrPC in the toxic actions of Aβ at the levels of synaptotoxicity, non-specific cytotoxic effects, and memory impairments in AD mouse models (Calella et al., 2010; Cisse et al., 2011; Freir et al., 2011; Kessels et al., 2010; Kudo et al., 2012; Lauren et al., 2009; Nicoll et al., 2013; Nieznanski et al., 2012; Rushworth et al., 2013; Um et al., 2012). As discussed in the literature (Freir et al., 2011; Kostylev et al., 2015; Lauren et al., 2009), factors that could contribute to these discrepancies include differences in Aβ preparations used, different concentrations of active Aβ species present in these preparations and, in case of studies in vivo, differences in animal models. Regarding synaptotoxicity, our present data are fully consistent with the original observation of Lauren et al. (2009), and the subsequent report of Freir et al. (Freir et al., 2011), which have shown that inhibition of LTP in hippocampal slices by synthetic Aβ oligomers requires the presence of endogenous PrPC. A similar PrPC-dependent impairment of LTP was observed upon treatment of hippocampal slices with AD brain-derived Aβ (Freir et al., 2011), which strongly suggested that the synthetic Aβ preparations used in the above mentioned studies, as well as in our experiments, contain active species that mimic synaptotoxic effects of Aβ in AD. It should be noted, however, that one study (Kessels et al., 2010) failed to reproduce the finding that inhibition of LTP in hippocampal slices by Aβ oligomers is PrPC-dependent. In an attempt to explain this apparent discrepancy, it was pointed out in the literature (Lauren et al., 2009) that the effects of Aβ oligomers on LTP reported in the latter study are qualitatively different from those typically observed for synthetic or brain-derived Aβ, suggesting that these atypical effects may be a property of the particular synthetic Aβ preparation used.
As for cytotoxicity, in our present study using rat primary hippocampal neurons, we could not detect any difference in the cytotoxic response to Aβ oligomers (as measured by the LDH release assay) between the control neurons and neurons pretreated with siRNA targeted against endogenous PrPC. Furthermore, no significant differences were found in this regard between primary neurons from wild-type and PrPC null mice. Collectively, these data argue that, in contrast to specific synaptotoxic effects as measured by LTP, the cytotoxic action of Aβ is not mediated by PrPC. This conclusion is consistent with previous studies by Balducci et al. (2010) and Nicoll et al. (2013), which employed different neuronal cell death assays. However, some other studies indicated that, similar to the impairment of synaptic plasticity, cytotoxicity of Aβ oligomers is also dependent on the presence of endogenous PrPC (Um et al., 2012). One can only speculate about the reasons for this discrepancy. It could, for example, potentially result from factors such as differences in Aβ oligomer preparations and/or differences in cytotoxicity assay protocols.
Regardless of any controversy about the specific role of cell-surface PrPC as a mediator of different toxic effects of Aβ, the present data clearly demonstrate that rPrP and its N1 fragment not only suppress cytotoxicity of Aβ oligomers in primary neurons but also prevent Aβ-induced inhibition of LTP in hippocampal slices. The latter finding is especially important from the perspective of therapeutic approaches to AD, as abnormalities of LTP are commonly used as a surrogate marker of the cognitive deficit in the disease. Our present electrophysiological findings on the protective action of rPrP and N1 against Aβ-induced impairment of synaptic plasticity are generally in line with the recent report that N1 suppresses Aβ-induced loss of certain postsynaptic marker proteins in cultured neurons (Fluharty et al., 2013). The relationship between these marker proteins and synaptic plasticity is, however, not well established yet. It should also be noted that expression of an artificial (GPI-free) secreted form of the full-length prion protein was reported to suppress impairment of synaptic plasticity in a transgenic mice model of AD (Calella et al., 2010).
The toxic oligomers of synthetic Aβ used in this and other studies are inherently highly heterogeneous, consisting of a mixture of species ranging from dimers up to large particles composed of tens or even hundreds of Aβ monomers. Which of these species correspond to the toxic oligomers that, in vivo, contribute to the pathogenic process in AD remains unclear (Benilova et al., 2012). The picture is further complicated by the possibility that different toxic effects of Aβ may be associated with distinct oligomeric species. Because the cytotoxic effects of Aβ oligomers appear to be independent of endogenous PrPC, inhibition of these effects by rPrP and its fragments cannot be explained by factors such as competitive blocking of Aβ binding to cell surface PrPC. Full elucidation of the mechanism by which Aβ oligomers exert cytotoxic effects, and the mechanism by which rPrP or N1 inhibit these effects will require further studies. However, one frequently discussed mechanism of Aβ cytotoxicity involves direct interaction with membrane lipids, resulting in cell membrane leakage through defects and/or pores in the lipid bilayer (Demuro et al., 2005; Kagan and Thundimadathil, 2010; Williams and Serpell, 2011). rPrP and N1 could interfere with this process by binding to Aβ oligomeric species, or to the membrane surface, in a manner that prevents toxic Aβ-membrane interactions. The above possibilities are supported by a recent study with chemically crosslinked small oligomeric species of Aβ, in which it was found that rPrP inhibits both the cytotoxicity of these species in neurons as well as their membrane permeation effects in liposomes (Williams et al., 2015). It was also suggested that rPrP or N1 may alter the oligomerization pathway of Aβ, resulting in formation of noncytotoxic (or less cytotoxic) species (Beland et al., 2014; Nieznanski et al., 2012; Williams et al., 2015). By contrast, the mechanism of the protective action of rPrP and N1 against PrPC-dependent impairment of LTP by Aβ oligomers likely involves competitive inhibition of oligomers binding to the PrPC-receptor on the membrane surface. The involvement of distinct mechanisms by which rPrP and N1 inhibit different toxic effect of Aβ oligomers is consistent with our present observation that protection against LTP impairment occurs at higher concentrations of rPrP or N1 than those required to prevent less specific cytotoxic effects. It is also possible that different toxic effects are associated with distinct species present in heterogeneous preparations of Aβ oligomers. In this context, it should be noted that cytotoxic effects are observed for both large Aβ oligomers as well as small species such as dimers or tetramers (Benilova et al., 2012; Williams et al., 2015), whereas a recent report suggests that inhibition of LTP is caused only by larger, protofibrillar structures of Aβ (Nicoll et al., 2013). This suggestion is consistent with the report that the subpopulation of water-soluble Aβ oligomers in AD brain with highest affinity for PrPC consists of species with relatively high molecular weight, and that brain levels of these PrPC-interacting Aβ species correlate with memory impairment in AD mouse models (Kostylev et al., 2015).
Mechanistic questions notwithstanding, the present findings, together with previous observations (Fluharty et al., 2013; Nieznanski et al., 2012), indicate that synthetic PrP fragments (or compounds containing these fragments or their analogues) may offer new avenues for pharmacological intervention in AD. It should be noted, however, that the present study is limited to experiments in vitro using synthetic Aβ oligomers. Even though recent data indicate that PrPC-dependent synaptotoxic effects of synthetic Aβ preparations closely mimic those of AD brain-derived Aβ oligomers (Freir et al., 2011), the usefulness of PrP-based compounds as potential therapeutic agents needs to be further verified using AD mouse models. The present data suggest N1 as the lead PrP fragment for these future studies, as this fragment contains the critical binding sites for Aβ oligomers and is as active as the full-length rPrP in suppressing toxic effects of these oligomers in vitro. Structurally, the region of PrP corresponding to N1 is highly flexible and largely disordered. Because the high affinity binding of N1 to Aβ oligomers requires both the N-terminal residues 23-27 as well as the C-terminal segment ~95-110 (Chen et al., 2010; Fluharty et al., 2013), one possible strategy toward the development of pharmacological agents could be to design peptides with appropriate linkers connecting these two critical segments.
Highlights.
Soluble prion protein and the N1 fragment prevent inhibition of LTP by Aβ oligomers
Soluble prion protein and N1 are also potent inhibitors of Aβ cytotoxicity
Only some neurotoxic effects of Aβ oligomers are mediated by endogenous PrPC
PrP-based compounds may be useful as therapeutic agents in AD
ACKNOWLEDGMENTS
We thank Dr. Thomas Williams for help with figure preparation. This work was supported by NIH Grants NS074317 and NS083687, the Spitz Brain Health Innovation Pilot Grant, and the ALANA Foundation USA. K.N. was supported by grant 2013/10/M/NZ4/00311 from the Polish National Science Centre.
Abbreviations
- PrPC
membrane-anchored cellular prion protein
- rPrP
recombinant human prion protein
- Aβ
amyloid β
- GPI
glycosylphosphatidylinositol
- LTP
long-term potentiation
- LDH
lactate dehydrogenase
- fEPSPs
Field evoked postsynaptic potentials
- aCSF
artificial cerebral spinal fluid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTION
JJS-M performed and analyzed LTP experiments and contributed to writing the paper. KS prepared recombinant prion proteins and Aβ oligomers, analyzed samples by AFM, performed cytotoxicity experiments in primary neurons and analyzed data. J-KC prepared primary neurons and contributed to cytotoxicity experiments. VAR and AIS prepared rat primary neurons used in initial experiments. KN contributed to data interpretation and critically revised the manuscript. ACSC analyzed data and wrote the paper. WKS conceived and coordinated the study and wrote the paper.
References
- Balducci C, Beeg M, Stravalaci M, Bastone A, Sclip A, Biasini E, Tapella L, Colombo L, Manzoni C, Borsello T, Chiesa R, Gobbi M, Salmona M, Forloni G. Synthetic amyloid-beta oligomers impair long-term memory independently of cellular prion protein. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2295–2300. doi: 10.1073/pnas.0911829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J. Neurosci. 2011;31:7259–7263. doi: 10.1523/JNEUROSCI.6500-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland M, Bedard M, Tremblay G, Lavigne P, Roucou X. Abeta induces its own prion protein N-terminal fragment (PrPN1)-mediated neutralization in amorphous aggregates. Neurobiol. Aging. 2014;35:1537–1548. doi: 10.1016/j.neurobiolaging.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat. Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- Bouyer P, Bradley SR, Zhao J, Wang W, Richerson GB, Boron WF. Effect of extracellular acid-base disturbances on the intracellular pH of neurones cultured from rat medullary raphe or hippocampus. J. Physiol. 2004;559:85–101. doi: 10.1113/jphysiol.2004.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calella AM, Farinelli M, Nuvolone M, Mirante O, Moos R, Falsig J, Mansuy IM, Aguzzi A. Prion protein and Abeta-related synaptic toxicity impairment. EMBO Mol. Med. 2010;2:306–314. doi: 10.1002/emmm.201000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yadav SP, Surewicz WK. Interaction between human prion protein and amyloid-beta (Abeta) oligomers: role OF N-terminal residues. J. Biol. Chem. 2010;285:26377–26383. doi: 10.1074/jbc.M110.145516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisse M, Sanchez PE, Kim DH, Ho K, Yu GQ, Mucke L. Ablation of cellular prion protein does not ameliorate abnormal neural network activity or cognitive dysfunction in the J20 line of human amyloid precursor protein transgenic mice. J. Neurosci. 2011;31:10427–10431. doi: 10.1523/JNEUROSCI.1459-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa AC, Grybko MJ. Deficits in hippocampal CA1 LTP induced by TBS but not HFS in the Ts65Dn mouse: a model of Down syndrome. Neurosci. Lett. 2005;382:317–322. doi: 10.1016/j.neulet.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- Dohler F, Sepulveda-Falla D, Krasemann S, Altmeppen H, Schluter H, Hildebrand D, Zerr I, Matschke J, Glatzel M. High molecular mass assemblies of amyloid-beta oligomers bind prion protein in patients with Alzheimer's disease. Brain. 2014;137:873–886. doi: 10.1093/brain/awt375. [DOI] [PubMed] [Google Scholar]
- Fluharty BR, Biasini E, Stravalaci M, Sclip A, Diomede L, Balducci C, La Vitola P, Messa M, Colombo L, Forloni G, Borsello T, Gobbi M, Harris DA. An N-terminal fragment of the prion protein binds to amyloid-beta oligomers and inhibits their neurotoxicity in vivo. J. Biol. Chem. 2013;288:7857–7866. doi: 10.1074/jbc.M112.423954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freir DB, Nicoll AJ, Klyubin I, Panico S, Mc Donald JM, Risse E, Asante EA, Farrow MA, Sessions RB, Saibil HR, Clarke AR, Rowan MJ, Walsh DM, Collinge J. Interaction between prion protein and toxic amyloid beta assemblies can be therapeutically targeted at multiple sites. Nat. Commun. 2011;2:336. doi: 10.1038/ncomms1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, Gunther EC, Lauren J, Gimbel ZA, Strittmatter SM. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J. Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Dey A, Sachan N, Wong H, Patterson RJ, Shelton JM, Richardson JA, Klann E, Rothermel BA. The Down syndrome critical region protein RCAN1 regulates long-term potentiation and memory via inhibition of phosphatase signaling. J. Neurosci. 2007;27:13161–13172. doi: 10.1523/JNEUROSCI.3974-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Surewicz WK. Fibril conformation as the basis of species- and strain-dependent seeding specificity of mammalian prion amyloids. Cell. 2005;121:63–72. doi: 10.1016/j.cell.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nat. Protoc. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kagan BL, Thundimadathil J. Amyloid peptide pores and the beta sheet conformation. Adv. Exp. Med. Biol. 2010;677:150–167. doi: 10.1007/978-1-4419-6327-7_13. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Nguyen LN, Nabavi S, Malinow R. The prion protein as a receptor for amyloid-beta. Nature. 2010;466:E3–4. doi: 10.1038/nature09217. discussion E4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends. Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, Vortmeyer A, Strittmatter SM. Prion-Protein-interacting Amyloid-beta Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models. J. Biol. Chem. 2015;290:17415–17438. doi: 10.1074/jbc.M115.643577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo W, Lee HP, Zou WQ, Wang X, Perry G, Zhu X, Smith MA, Petersen RB, Lee HG. Cellular prion protein is essential for oligomeric amyloid-beta-induced neuronal cell death. Hum. Mol. Genet. 2012;21:1138–1144. doi: 10.1093/hmg/ddr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Morillas M, Swietnicki W, Gambetti P, Surewicz WK. Membrane environment alters the conformational structure of the recombinant human prion protein. J. Biol. Chem. 1999;274:36859–36865. doi: 10.1074/jbc.274.52.36859. [DOI] [PubMed] [Google Scholar]
- Nicoll AJ, Panico S, Freir DB, Wright D, Terry C, Risse E, Herron CE, O'Malley T, Wadsworth JD, Farrow MA, Walsh DM, Saibil HR, Collinge J. Amyloid-beta nanotubes are associated with prion protein-dependent synaptotoxicity. Nat. Commun. 2013;4:2416. doi: 10.1038/ncomms3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieznanski K, Choi JK, Chen S, Surewicz K, Surewicz WK. Soluble prion protein inhibits amyloid-beta (Abeta) fibrillization and toxicity. J. Biol. Chem. 2012;287:33104–33108. doi: 10.1074/jbc.C112.400614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieznanski K, Surewicz K, Chen S, Nieznanska H, Surewicz WK. Interaction between prion protein and Abeta amyloid fibrils revisited. ACS Chem. Neurosci. 2014;5:340–345. doi: 10.1021/cn500019c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth JV, Griffiths HH, Watt NT, Hooper NM. Prion protein-mediated toxicity of amyloid-beta oligomers requires lipid rafts and the transmembrane LRP1. J. Biol. Chem. 2013;288:8935–8951. doi: 10.1074/jbc.M112.400358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Small DH, Mok SS, Bornstein JC. Alzheimer's disease and Abeta toxicity: from top to bottom. Nat. Rev. Neurosci. 2001;2:595–598. doi: 10.1038/35086072. [DOI] [PubMed] [Google Scholar]
- Um JW, Nygaard HB, Heiss JK, Kostylev MA, Stagi M, Vortmeyer A, Wisniewski T, Gunther EC, Strittmatter SM. Alzheimer amyloid-beta oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012;15:1227–1235. doi: 10.1038/nn.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J. Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- Williams TL, Choi JK, Surewicz K, Surewicz WK. Soluble Prion Protein Binds Isolated Low Molecular Weight Amyloid-beta Oligomers Causing Cytotoxicity Inhibition. ACS Chem. Neurosci. 2015;6:1972–1980. doi: 10.1021/acschemneuro.5b00229. [DOI] [PubMed] [Google Scholar]
- Williams TL, Serpell LC. Membrane and surface interactions of Alzheimer's Abeta peptide--insights into the mechanism of cytotoxicity. FEBS J. 2011;278:3905–3917. doi: 10.1111/j.1742-4658.2011.08228.x. [DOI] [PubMed] [Google Scholar]
- Younan ND, Sarell CJ, Davies P, Brown DR, Viles JH. The cellular prion protein traps Alzheimer's Abeta in an oligomeric form and disassembles amyloid fibers. FASEB J. 2013;27:1847–1858. doi: 10.1096/fj.12-222588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou WQ, Xiao X, Yuan J, Puoti G, Fujioka H, Wang X, Richardson S, Zhou X, Zou R, Li S, Zhu X, McGeer PL, McGeehan J, Kneale G, Rincon-Limas DE, Fernandez-Funez P, Lee HG, Smith MA, Petersen RB, Guo JP. Amyloid-beta42 interacts mainly with insoluble prion protein in the Alzheimer brain. J. Biol. Chem. 2011;286:15095–15105. doi: 10.1074/jbc.M110.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]