Abstract
Background
The months immediately following completion of treatment for childhood acute lymphoblastic leukemia (ALL) are often regarded as a stressful time for children and families. In this prospective, longitudinal study, the prevalence and predictors of anxiety and depressive symptoms after completion of treatment were examined.
Methods
Participants included 160 children (ages 2-9 years) with standard-risk ALL enrolled on Children's Oncology Group protocol AALL0331. Parents completed standardized rating scales of children's emotional-behavioral functioning, and measures of coping and family functioning at ∼1, 6, and 12 months after diagnosis, and again 3 months following completion of chemotherapy.
Results
Three months off-therapy, 24% of survivors had at-risk/clinically elevated anxiety scores and 28% had elevated depression scores, significantly higher than the expected 15% in the general population (p=0.028 and 0.001, respectively). Patients with elevated anxiety one-month post-diagnosis were at greater risk for off–therapy anxiety (OR=4.1; 95% CI, 1.31-12.73, p=0.022), and those with elevated depressive symptoms 6-months post-diagnosis were at greater risk for off-therapy depression (OR=7.88, 95% CI, 2.61-23.81, p=0.0002). In adjusted longitudinal analyses, unhealthy family functioning (p=0.008), and less reliance on social support coping (p=0.009) were associated with risk for emotional distress. Children from Spanish-speaking families (p=0.05) were also at greater risk for distress.
Conclusions
A significant proportion of children experience emotional distress during and after therapy for ALL. These data provide a compelling rationale for targeted early screening, and psychosocial interventions to support family functioning and coping skills.
Keywords: Childhood, acute lymphoblastic leukemia, anxiety, depression, survivorship
Introduction
As the percentage of children surviving standard-risk acute lymphoblastic leukemia (ALL) now exceeds 90%1, attention must be paid to how best to support survivors' emotional wellbeing during and after completion of therapy. Chemotherapy treatment for ALL may directly impact children's emotional functioning, as systemic corticosteroids, essential components of successful treatment, have been linked to behavioral side effects2,3. During active treatment with corticosteroids, concerns regarding frequent mood swings, irritability, depression and anxiety, as well as problems with behavioral control and attention have been widely reported in childhood cancer patients3-5. Children may also experience changes in emotional-behavioral functioning due to painful procedures and frequent clinic visits that disrupt typical age-appropriate activities6.
Debate exists regarding the prevalence of emotional-behavioral difficulties in children after treatment for leukemia. Some studies have concluded that childhood ALL survivors are similar to peers in their emotional-behavioral functioning7,8, whereas others observed that at least a subset of survivors continues to experience distress long after treatment has ended9,10. Differences in findings may be due to heterogeneous leukemia subtypes, varying risk of relapse, differences in time elapsed since diagnosis, in the intensity of treatment protocols and outcome measures used, and a paucity of longitudinal studies.
Understanding risk factors for children's emotional-behavioral functioning after cancer treatment is important in order to improve identification of those at greatest risk for future difficulties, and to elucidate possible avenues for intervention. In a previous longitudinal investigation of the first year of therapy, we reported that depression and anxiety are significant problems in the immediate post-diagnosis period for children with standard-risk ALL11. While the prevalence of anxiety lessened after the first month of therapy, depressive symptoms persisted throughout the first year. It is unknown whether this pattern continues into the early off-therapy period. With extended follow-up of this same cohort, we are now able to examine emotional functioning post-treatment.
The months immediately following completion of treatment have been described as one of the most difficult and anxiety-producing periods for cancer patients and families,12 presumably due to fear of relapse and loss of clinical support. The possibility of persistent neurobehavioral side-effects from corticosteroids as well as posttraumatic stress may also contribute to the emotional-behavioral functioning of survivors post-treatment, yet the incidence of such difficulties in young children during this important time has not been well documented. This study was designed to determine the prevalence and predictors of anxiety and depressive symptoms in childhood ALL survivors at three months after completion of treatment.
Methods
Participants
Participants were drawn from a large, representative sample of children with standard-risk ALL (i.e., initial white blood cell count < 50,000/uL and ages 1.0-9.9 years, no evidence of central nervous system or testicular leukemia, rapid early response to therapy based on bone marrow morphology, and minimal residual disease at the end of induction) enrolled on a Children's Oncology Group (COG) therapeutic study (AALL0331; NCT00103285; http://www.cancer.gov/clinicaltrials) between 2005 and 2009. This chemotherapy-only protocol involved a three-drug (vincristine, pegaspargase, and dexamethasone) four-week induction, and intrathecal chemotherapy. The current study consisted only of patients categorized and treated as average-risk. Therapeutic randomization consisted of standard consolidation vs. an intensified consolidation that added two doses of cyclophosphamide and pegaspargase. A second randomization, comparing standard interim maintenance with oral methotrexate vs. augmented interim maintenance with escalating intravenous methotrexate, was halted in 2008 based upon results of the CCG 1991 SR-ALL trial13 that showed better outcomes with intravenous methotrexate.
Emotional-behavioral outcomes were collected as part of an ancillary study at thirty-one COG sites that included community-based and tertiary care centers. Additional eligibility for this ancillary study included: age≥2 years and at least one parent with reading comprehension in English or Spanish (to ensure survey comprehension). Of 195 individuals who enrolled on AALL0331 and met eligibility criteria for this study, 24 declined and 170 consented to participate. Of those who consented, four withdrew from AALL0331 before the first survey evaluations; six were not given surveys because of errors at study sites.
Procedures
The institutional review board of each participating center approved the protocol and study documents. Informed consent, and assent if indicated, was obtained from all participants in accordance with the Declaration of Helsinki. The identified primary caregiver (the child's mother in 84% of instances) was asked to complete all survey measures at four specified timepoints: day one of consolidation (∼one month after diagnosis, T1), the end of the delayed intensification (∼six months after diagnosis, T2), six months after starting maintenance (∼twelve months after diagnosis, T3), and three months after completion of therapy (∼thirty-three months after diagnosis for females, and forty-five months after diagnosis for males, T4).
Measures
Anxiety and Depression were assessed using the Behavioral Assessment System for Children, Second Edition: Parent Report Scale (BASC-2), a valid and reliable instrument that has been frequently used in pediatric oncology studies14,15. The BASC-2 scales are reported as standardized T-scores (mean=50, standard deviation=10), based on the age and sex of the child. Scores of 60-69 represent the at-risk range and scores ≥70 represent the clinically significant range. The BASC-2 has been standardized on normative data obtained from a large, representative sample of children living in the United States (n=12,350), including Spanish-speaking children and parents who completed the Spanish BASC-2 form16. From this standardization sample, expected frequencies of elevated scores are available. While anxiety and depression scales were the primary outcomes of interest, aggression and hyperactivity scales were also examined.
Family Functioning was measured using the General Functioning scale of the Family Assessment Device (FAD-GF)17,18. Parents were asked to indicate the degree to which each statement described their family (e.g. “We are able to make decisions about how to solve problems”) on a 1-4 scale. Scores ≥2 reflect unhealthy family functioning17.
Parental Coping Behaviors were assessed by the Coping Health Inventory for Parents (CHIP)19. This checklist comprises three scales: (1) Maintaining family integration/optimism, (2) Maintaining social support/self-esteem, and (3) Understanding the medical situation, and has been validated for children with chronic illnesses. Parents rated how helpful specific coping behaviors were on a 4-point scale ranging from “not helpful” to “extremely helpful.” Higher scores reflect greater reliance on that particular coping pattern.
Physical Symptoms of pain and hurt and nausea were assessed by subscales of the Pediatric Quality of Life Inventory (PedsQL) 3.0 Cancer Module20. Parents rated how much of a problem each symptom has been in the past month. Scores were transformed on a 0 (worst health) to100 (best health) scale.
Sociodemographic Characteristics were obtained via parent survey, including questions about ethnicity, primary language spoken at home, household income, marital status, maternal education and family size.
Statistical Analysis
Data were analyzed using SAS software, version 9.3. Descriptive statistics were calculated to characterize sample composition. Of 160 participants, 140, 127, 134, and 96 completed evaluations at each respective timepoint. The Chi-square exact tests and Kruskal-Wallis tests were used to compare participants with complete evaluations at 3-months after completion of treatment (T4) with those who did not complete T4 measurement.
Primary outcomes were BASC-2 anxiety and depression scales at T4. The proportions of patients with elevated scores (i.e., those in the “at least at-risk” and “clinical” ranges) were compared to the corresponding proportions expected in the normative population of healthy children, using a two-sided binomial exact test. Logistic regression was used to examine elevations in anxiety and depression on therapy (i.e., at T1, T2, and T3) as predictors of elevated anxiety and depression scores after completion of treatment (T4).
To examine the contribution of demographic, treatment, and family factors to distress after treatment (i.e., anxiety, depression, and mixed anxiety and depression symptoms), univariate and multivariate longitudinal analyses were conducted. Multinomial logistic regression models with four categories of the dependent variable BASC-2 elevated T-score (i.e., “only anxiety elevated”, “only depression elevated” and “both anxiety and depression elevated” compared to the reference group “no elevations; neither depression nor anxiety elevated”) were used, taking into consideration the dependence of repeated measurements at the 4 timepoints for each participant. The following independent variables were analyzed: age at diagnosis, gender, race and ethnicity, caregiver's age, household income, maternal education, marital status, the presence of a neurological event on therapy, pain and hurt subscales, as well as repeated measures of general family functioning and coping behaviors. Child and family factors that were at least marginally associated with elevated anxiety and depression scores (i.e. p≤0.1) in univariate analysis were included in corresponding multivariate analysis.
Results
Demographic characteristics
Sociodemographic and treatment characteristics are presented in Table 1. At the final assessment (T4, 3-months after completing chemotherapy), 60% of initial participants completed survey measurements. When participants with complete data at T4 were compared to those with missing data, there were no differences in key sociodemographic or treatment factors, with the exception of sex. Specifically, boys were less likely to have complete emotional-behavioral measures at T4 than girls. This may be due to differences in treatment duration between males and females, with males requiring a longer treatment period. There were no significant differences in T1 levels of anxiety, depression, pain and nausea, or coping behaviors between participants with complete data at the final assessment and those with missing data (p>.05).
Table 1. Sample characteristics.
| Participants T12 (n=159) |
Participants T42 (n=96) |
Missing T42 (n=64) |
P-value | |
|---|---|---|---|---|
| Age at diagnosis, n(%) | 1.000 | |||
| Pre-school (ages 2-4) | 86 (54.1%) | 52 (54.17%) | 35 (54.69%) | |
| School-age (ages 5-9) | 73 (45.9%) | 44 (45.83%) | 29 (45.31%) | |
|
| ||||
| Sex, n(%) | <0.01 | |||
| Female | 76 (47.8%) | 59 (61.46%) | 18 (28.13%) | |
| Male | 83 (52.2%) | 37 (38.54%) | 46 (71.88%) | |
|
| ||||
| Child ethnicity, n(%) | 0.75 | |||
| White, non-Hispanic | 108 (67.9%) | 65 (67.71%) | 43 (67.19%) | |
| Black, non-Hispanic | 11 (6.9%) | 5 (5.21%) | 6 (9.38%) | |
| Hispanic | 26 (16.4%) | 17 (17.71%) | 10 (15.63%) | |
| Other | 14 (8.8%) | 9 (9.38%) | 5 (7.81%) | |
|
| ||||
| Marital status, n(%) | 0.40 | |||
| Married | 105 (66.0%) | 77 (80.21%) | 44 (68.75%) | |
| Not Married | 45 (28.3%) | 16 (16.67%) | 14 (21.88%) | |
| Missing | 9 (5.7%) | 3 (3.12%) | 6 (9.4%) | |
|
| ||||
| Treatment randomization, n(%) | 0.23 | |||
| Standard CS/standard IM-DI1 | 42 (26.42%) | 26 (27.08%) | 17 (26.56%) | |
| Intensified CS/standard IM-DI1 | 51 (32.08%) | 26 (27.08%) | 25 (39.06%) | |
| Standard CS/augmented IM-DI1 | 37 (23.27%) | 27 (28.13%) | 10 (15.63%) | |
| Intensified CS/augmented IM-DI1 | 29 (18.24%) | 17 (17.71%) | 12 (18.75%) | |
CS=Consolidation, IM=Interim Maintenance, DI=Delayed Intensification
T1=start of consolidation, T4=3 months after completion of therapy
p-value denotes exact chi-squared test comparison between T4 participants and missing T4 data
Prevalence of emotional-behavioral problems
Mean scores for anxiety and depression were within the average range across all timepoints. However, after completion of chemotherapy, the frequency of at-risk or clinically significant anxiety and depression scores was greater than the frequency expected based on the normative population of children (15%). Specifically, 24% of survivors showed at-risk or clinically significant elevations in anxiety (p=0.028) and 29% displayed elevations in depression (p=0.001) at T4. Longitudinal patterns of the proportion of children with elevated anxiety and depression scores are displayed in Figure 1. As shown, rates of at-risk or clinically significant anxiety scores appeared to decrease over the first year (25.2% at T1, 17.5% at T2, 14.2% at T3), but increased at T4 to a rate similar with that observed early in treatment (24%). The frequency of anxiety scores in the more severe category of “clinically significant” also decreased over the first year of treatment, and appeared to increase slightly at T4. However, the prevalence of anxiety scores in the “clinically significant” range at T4 (6.3%) was not statistically different from expectations based on population normative data (4%, p=0.37).
Figure 1.
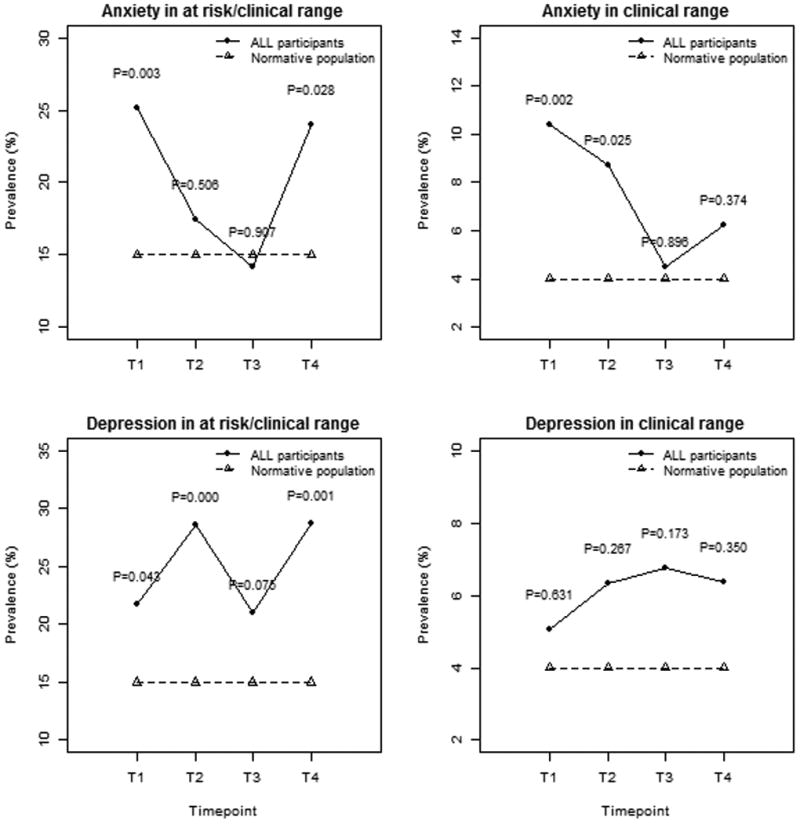
Prevalence of elevated anxiety and depression scores during and after therapy. Note: T1=start of consolidation; T2=end of delayed intensification; T3=six months after starting maintenance; T4=three months after completion of treatment
The prevalence of elevated depression scores remained fairly consistent across cancer treatment (21.7% at T1, 28.6% at T2, 21.1% at T3) and into the post-treatment period (28.7% at T4), significantly higher than expected compared to the normative population (all p<0.05). The frequency of more severe depression scores (i.e. in the “clinically significant” range) was not statistically different from expected levels at any timepoint during or after therapy.
As distress in children commonly presents as comorbid anxiety-depression symptoms21, the proportion of children with elevations on both anxiety and depression scales was examined at each of the four timepoints. The prevalence of those with at-risk/clinically-significant elevations on both anxiety and depression scales decreased by 12-months post diagnosis (13% at T1, 15% at T2, 8% at T3), and increased again after completion of therapy (12% at T4). The frequency of elevations on scales sampling aggression and hyperactivity were consistent with rates expected in the general population across all 4 timepoints (data not shown).
Longitudinal patterns of emotional-behavioral functioning
Anxiety
In comparison to children who had anxiety scores in the average range, those with anxiety scores in the at-risk/clinically significant range at the start of consolidation were 4.1 times more likely to have elevated anxiety scores at T4 (95%CI, 1.31-12.73, p=0.022). Children with at-risk/clinically elevated scores at 6-months post diagnosis were 5.5 times more likely to have continued elevations at T4 (95%CI, 1.58-19.11, p=0.009), and children with at-risk/clinically elevated scores at 12-months post diagnosis were 6.2 times more likely to have elevations at T4 (95%CI, 1.78-21.78, p=0.005). Risk estimates were even greater for children with clinically significant scores at 6- and 12-months post diagnosis. Specifically, children with clinically significant scores at 6-months (OR=26.3, 95%CI, 3.37-204.6, p=0.004) or 12-months post diagnosis (OR=18.0, 95%CI, 2.14-151.19, p=0.023) were at greatly increased risk for persistent distress after treatment compared to those with average and at-risk scores.
Depression
Children with depression scores in the at-risk/clinically-significant range 6-months after diagnosis were almost 8 times more likely than children with depression scores in the average range, to have at least at-risk depression scores at T4 (OR=7.88, 95%CI, 2.61-23.81, p=0.0002). Children with at-risk/clinically significant elevations at 12-months post diagnosis were 3.7 times more likely to have elevations at T4 (95%CI, 1.33-10.50, p=0.014). Importantly, children with depression scores in the clinically significant range at the start of consolidation were 17 times more likely to have scores in the clinically significant range at T4 than children with average or at-risk scores (95%CI, 2.06-145.65, p=0.025).
Predictors of depression, anxiety, and combined depression-anxiety symptoms
Longitudinal univariate multinomial regression analyses adjusted for time elapsed since diagnosis are presented in Table 2. Spanish-speaking families were at increased risk of mixed anxiety and depression symptoms (i.e., elevations on both anxiety and depression scales; p=0.047). Unhealthy family functioning was associated with mixed anxiety and depression symptoms (p=0.030), and elevated depression only (p=0.024), but not elevated anxiety (p=0.225). A parental style characterized by less reliance on social support coping behaviors was associated with mixed anxiety and depression symptoms (p=0.027). Having a history of a neurological event on therapy was associated with elevations in depression only (p=0.042). There were no differences in risk for anxiety or depression among the four chemotherapy treatment arms.
Table 2. Univariate associations with anxiety, depression, and combined symptoms.
| Anxiety | Depression | Both Anx & Dep | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Caregiver's age | 0.96(0.86,1.07) | 0.370 | 0.97(0.90,1.05) | 0.353 | 0.91(0.81,1.03) | 0.110 |
|
| ||||||
| Age at diagnosis | ||||||
| Pre-school (2-4) | Reference group | Reference group | Reference group | |||
| School-age (5-12) | 0.30(0.03,3.08) | 0.157 | 0.68(0.15,3.16) | 0.395 | 0.68(0.06,7.81) | 0.564 |
|
| ||||||
| Child's sex | ||||||
| Male | 1.42(0.15,13.23) | 0.568 | 0.89(0.20,4.10) | 0.779 | 1.39(0.12,15.52) | 0.617 |
| Female | ||||||
|
| ||||||
| Child Race/Ethnicity | ||||||
| White, non- Hispanic | Reference group | Reference group | Reference group | |||
| Hispanic | 3.96(0.29,50.81) | 0.153 | 0.93(0.10,8.39) | 0.897 | 4.34(0.21,87.15) | 0.170 |
| Black, non-Hispanic | 0.49(0.01,126.4) | 0.634 | 1.91(0.11,31.96) | 0.426 | 0.77(0.01,162.2) | 0.853 |
| Other | 0.68(0.01,50.17) | 0.733 | 0.92(0.06,13.90) | 0.904 | 4.12(0.09,186.2) | 0.249 |
|
| ||||||
| Primary language | ||||||
| English | Reference group | Reference group | Reference group | |||
| Spanish | 4.09(0.79,21.64) | 0.083 | 0.54(0.12,2.52) | 0.374 | 6.28(1.03,38.4) | 0.047 |
|
| ||||||
| Annual family income | ||||||
| ≥$50,000 | Reference group | Reference group | Reference group | |||
| <$50,000 | 0.55(0.16,1.93) | 0.284 | 0.93(0.36,2.38) | 0.854 | 1.74(0.39,7.69) | 0.397 |
|
| ||||||
| Maternal education | ||||||
| Some college | Reference group | Reference group | Reference group | |||
| Less than college | 1.39(0.42,4.64) | 0.550 | 0.91(0.38,2.15) | 0.802 | 2.24(0.65,7.68) | 0.174 |
|
| ||||||
| Marital status | ||||||
| Married | Reference group | Reference group | Reference group | |||
| Not married | 0.53(0.10,2.99) | 0.41 | 0.87(0.28,2.68) | 0.78 | 0.99(0.18,5.43) | 0.99 |
|
| ||||||
| General Family Functioning (FAD) | ||||||
| Healthy function | Reference group | Reference group | Reference group | |||
| Unhealthy function | 1.98(0.61,6.43) | 0.225 | 2.62(1.61,5.92) | 0.024 | 4.43(1.19,16.43) | 0.030 |
|
| ||||||
| Family size | 1.37(0.93,2.04) | 0.099 | 1.04(0.75,1.44) | 0.792 | 1.17(0.74,1.88) | 0.436 |
|
| ||||||
| Maintaining family integration (CHIP) | 0.99(0.94,1.05) | 0.779 | 0.96(0.92,1.01) | 0.081 | 0.96(0.90,1.03) | 0.205 |
|
| ||||||
| Maintaining social support (CHIP) | 1.00(0.95,1.06) | 0.890 | 0.97(0.93,1.01) | 0.152 | 0.93(0.88,0.99) | 0.027 |
|
| ||||||
| Understanding medical situation (CHIP) | 1.04(0.93, 1.17) | 0.473 | 0.97(0.89, 1.05) | 0.384 | 0.96(0.85, 1.09) | 0.484 |
|
| ||||||
| Pain and hurt (PedsQL) | 0.99(0.96, 1.01) | 0.193 | 0.99(0.97, 1.01) | 0.156 | 0.98(0.96, 1.01) | 0.113 |
|
| ||||||
| Nausea (PedsQL) | 0.98(0.95, 1.02) | 0.319 | 0.99(0.97, 1.02) | 0.450 | 0.98(0.94, 1.02) | 0.205 |
|
| ||||||
| Therapeutic arm1 | ||||||
| SC/SIM-SDI | Reference group | Reference group | Reference group | |||
| IC/SIM-SDI | 3.99(0.18,89.97) | 0.196 | 1.25(0.15,10.37) | 0.692 | 0.77(0.03,18.77) | 0.757 |
| SC/AIM-ADI | 1.67(0.05,51.71) | 0.587 | 1.38(0.15,12.44) | 0.597 | 1.16(0.04,32.65) | 0.869 |
| IC/AIM-ADI | 2.60(0.08,86.75) | 0.361 | 2.20(0.22,21.74) | 0.276 | 0.72(0.02,31.86) | 0.752 |
|
| ||||||
| Neurological event on therapy | ||||||
| No | Reference group | Reference group | Reference group | |||
| Yes | 0.67(0.11,4.16) | 0.661 | 2.99(1.04,8.58) | 0.042 | 2.72(0.38,19.39) | 0.309 |
SC=Standard Consolidation, IC=Intensified Consolidation, SIM-SDI=Standard Interim Maintenance and Standard Delayed Intensification, AIM-ADI=Augmented Interim Maintenance and Augmented Delayed Intensification
In longitudinal multivariate multinomial regression models (Table 3), less reliance on maintaining social support coping behaviors (p=0.009) and primary language spoken at home (p=0.047) remained significant predictors of mixed symptoms of anxiety and depression such that children from Spanish-speaking homes were more likely to have at-risk/clinically significant elevations on both anxiety and depression scales. Significant predictors of at-risk/clinically elevated depression only from multivariate analyses included unhealthy family functioning (p=0.008).
Table 3. Multivariate associations with anxiety, depression, and combined symptoms.
| Anxiety | Depression | Both Anx & Dep | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Timepoint | ||||||
| 1 | 0.72(0.21,2.41) | 0.579 | 0.55(0.17,1.82) | 0.317 | 1.26(0.34,4.70) | 0.724 |
| 2 | 0.21(0.04,1.19) | 0.076 | 1.27(0.43,3.76) | 0.655 | 1.46(0.37,5.79) | 0.583 |
| 3 | 0.47(0.13,1.74) | 0.250 | 1.21(0.44,3.38) | 0.701 | 0.39(0.08,1.78) | 0.215 |
|
| ||||||
| Family Size | 1.55(0.91,2.66) | 0.110 | 1.30(0.85,1.97) | 0.214 | 0.86(0.44,1.66) | 0.638 |
|
| ||||||
| Maintaining family integration (CHIP) | 1.01(0.91,1.13) | 0.813 | 0.97(0.91,1.04) | 0.394 | 1.09 (0.97,1.22) | 0.158 |
|
| ||||||
| Maintaining social support (CHIP) | 1.05(0.96,1.15) | 0.288 | 0.99(0.93,1.04) | 0.622 | 0.88(0.79,0.96) | 0.009 |
|
| ||||||
| General Family Functioning (FAD) | ||||||
| Healthy function | Reference group | Reference group | Reference group | |||
| Unhealthy function | 0.90(0.20,4,02) | 0.884 | 3.78(1.45,9.87) | 0.008 | 4.64(0.90,23.9) | 0.066 |
|
| ||||||
| Primary language | ||||||
| English | Reference group | Reference group | Reference group | |||
| Spanish | 2.46(0.38,15.95) | 0.336 | 0.29(0.03,2.42) | 0.244 | 10.9(1.03,115.1) | 0.047 |
| Neurological event on therapy | ||||||
| No | Reference group | 0.648 | Reference group | 0.273 | Reference group | 0.899 |
| Yes | 0.66(0.10,4.21) | 1.76(0.63,4.90) | 1.13(0.17,7.26) | |||
Discussion
In this multi-site, prospective, longitudinal study of children with standard-risk ALL, we sought to determine the prevalence of anxiety and depressive symptoms at three months after completion of chemotherapy. While the majority of parents described their child's emotional-behavioral functioning as consistent with population norms, a significant portion of the cohort reported elevated depression and anxiety in the at-risk/clinically significant range at frequencies higher than the 15% expected in the general population (28% and 24%, respectively). When data were examined longitudinally, the prevalence of elevated anxiety appeared to decrease over the first year, but increased again after completion of therapy, to a level similar with that observed near the time of diagnosis. Frequency of elevated depressive symptoms was significant across all four time-points, when compared to expectations in the general population.
Few studies have examined the emotional-behavioral functioning of ALL survivors throughout chemotherapy and into the off-treatment period. The results of the current investigation are in contrast to some prior studies, which found internalizing symptoms to normalize after completion of therapy22 and some cross-sectional studies which suggest that survivor's emotional-behavioral functioning after treatment is similar to their peers8,23. Other studies like ours have found that emotional distress continues to be an important issue for a subset of survivors at the end of therapy24. Our longitudinal findings suggest that a substantial proportion of childhood ALL survivors experience persistent mood difficulties throughout cancer treatment and into survivorship, and heightened anxiety at diagnosis and again during the initial off-treatment phase.
An important finding in the current study was that children with elevated depression and anxiety early in therapy were at significantly increased risk for persistent distress after completion of treatment. That is, children with elevated anxiety scores at the start of consolidation were 4 times more likely to have elevated anxiety scores after treatment, and children with elevated depression scores at 6 months after diagnosis were 8 times more likely to have elevated depression after treatment. Given this, rapid identification of anxiety and depressive symptoms and effective interventions directed towards children who exhibit distress (e.g., mindfulness-based stress reduction, cognitive-behavioral therapeutic approaches) early in the course of treatment may help mitigate long-term emotional distress. Psychologists/social workers are well-versed in such techniques, and at some hospitals these therapies can be delivered alongside medical care, particularly when psychosocial team members are embedded within oncology, and introduced early in treatment as part of the standard of care.
In adjusted analyses, children of Spanish-speaking families were almost 11 times more likely to have elevations on both anxiety and depression scales than children from homes where English is the primary language. While the Spanish BASC-2 is a validated and widely accepted tool for measuring mood/behavior of children from Spanish-speaking families, it is possible that this finding may reflect subtle differences in measurement of these constructs due to translation of items and/or cultural differences in the way items were perceived. Few studies have examined the emotional functioning of minority cancer survivors from Spanish-speaking families. One cross-sectional study of childhood cancer survivors in a long-term follow-up clinic25 found poorer psychosocial quality of life among Hispanic children after completion of treatment, compared to non-Hispanic survivors. Another study found a higher prevalence of posttraumatic stress and depression in Hispanic parents of childhood cancer survivors, even after adjusting for other sociodemographic differences25. Generally, mental health care utilization is lower among Hispanics, and differences in access and attitudes towards mental health care may contribute to this26,. Language and acculturation issues may be barriers to seeking out and receiving psychological support during and after cancer treatment and contribute to anxiety27.
Risk factors for distress (i.e., combined depression-anxiety symptoms) also included less reliance on social support coping behaviors. Unhealthy family functioning was strongly associated with risk for elevated depression symptoms, and marginally associated with risk for combined anxiety-depression symptoms. Family functioning has been previously linked to children's emotional well-being in healthy children28 and in children with chronic medical illness29 and injury30. Interventions to support family functioning and improve coping skills (e.g., maternal problem-solving skills training31, family systems approaches) also have the potential to improve mood.32 Age, gender, socioeconomic status, and therapeutic randomization did not predict depression or anxiety symptoms. As in other studies, the prevalence of aggression and hyperactivity did not differ from expectations based on population norms.
Strengths of this study include a high participation rate (82% of those eligible) and a representative Hispanic/Spanish-speaking sample that has been under-studied. The treatment protocols examined represent current regimens for ALL, and the longitudinal study design allowed us to examine early predictors of distress after completion of therapy. There are also limitations. Given participants' young age, only parent proxy reports were used. While this is an accepted way to measure emotional functioning in young children, parent ratings may be influenced by their own stress and worry, which was not specifically examined. Attrition was also noted over the duration of the study. However, when participants with complete T4 data were compared to those with missing data, they differed only with respect to gender (i.e., fewer boys with complete T4 data), likely due to the longer treatment period required of males. There were no differences in T1 anxiety, depression, pain and nausea, or coping behaviors between participants with complete and missing T4 data.
Our analysis found a statistically higher prevalence of elevated anxiety and depression scores in the at least “at-risk” range, but not in the more severe “clinically significant” range after completion of treatment. This may reflect that emotional distress was present in our sample, but at the more moderate end of the spectrum in terms of severity. An alternative explanation could be inadequate sample size. Higher frequencies of “clinically significant” anxiety and depression scores were observed at all timepoints in comparison to expectations in the general population, though these did not often reach statistical significance. While the BASC-2 is appropriate for screening, it is not sufficient for diagnosis, and it is likely that a smaller proportion of our sample would have met diagnostic criteria for anxiety/depression upon clinical interview. The level of functional impairment associated with symptoms reported here is also not known. Nonetheless, previous studies have found distress to be associated with suffering and poor health outcomes, leading many to call for the inclusion of screening/interventions to mitigate distress into the standard of care33,34. Early identification of children at-risk for distress after cancer may allow for more rapid intervention to alleviate psychological symptoms, foster adaptive family functioning/coping, and prevent symptom escalation or persistent distress.
In conclusion, a significant portion of childhood ALL survivors experience persistent anxiety and depression symptoms after completion of treatment. Routine screening for distress, and assessment of parental coping styles and general family functioning, are important ways to identify children and families who may need a higher level of psychosocial care including comprehensive evaluation and targeted support during/after treatment. Brief surveys such as the Psychosocial Assessment Tool 2.035(which taps family functioning/coping) or the Distress Thermometer36 (modeled after pain scales) may be useful in identifying children at risk, and can be administered in <5 minutes by a social worker, nurse, or other professional during clinic visits. Timing of screening should, at minimum, occur when patients are at greatest risk for distress (i.e., near the start of treatment, at transitions during treatment, and soon after completing treatment). Children from Spanish-speaking households are also at risk for distress after completion of treatment and represent a group that warrants culturally sensitive screening and intervention. While there are promising interventions to mitigate distress in children with chronic illness, few have been empirically tested in childhood cancer survivors and little information exists regarding when in the course of cancer treatment is the best time to intervene. The efficacy and timing of psychological interventions for children to reduce risk for anxiety and depression symptoms after ALL is an important topic, and further research is necessary to determine the best pathways to mitigate distress and support the emotional well-being of children and families as they enter they enter the off-treatment period.
Acknowledgments
Funding Sources: This research was supported by grants from the National Institutes of Health (NIH) to the Children's Oncology Group (COG) including CA13539, CA98543 and CA180886 (COG Chair's grant), U10 CA98413 and CA180899 (COG Statistical Center), and a Community Cancer Oncology Program grant U10CA095861from the National Cancer Institute Division of Cancer Prevention to COG. This publication was also made possible by CTSA grant UL1TR000142 from the National Center for Advancing Translational Science, a component of NIH. Dr. Kunin-Batson is supported in part by the Pine Tree Apple Tennis Classic Cancer Research Fund. Dr. Kadan-Lottick is supported in part by American Cancer Society Scholar Grant 119700-RSGHP-10-107-01-CPHPS.
Footnotes
Conflicts of Interest: None
Author Contributions: Alicia S. Kunin-Batson: Conceptualization, methodology, writing – original draft, and writing – review and editing. Xiaomin Lu: Methodology, formal analysis, data curation, writing – original draft, and writing – review and editing. Lyn Balsamo: Writing – review and editing. Kelsey Graber: Conceptualization, resources, data curation, and writing – review and editing. Meenakshi Devidas: Conceptualization, methodology, formal analysis, data curation, writing – original draft, and writing – review and editing. Stephen P. Hunger: Conceptualization, resources, writing – review and editing, and supervision. William L. Carroll: Conceptualization, methodology, investigation, resources, and writing – review and editing. Naomi J. Winick: Conceptualization, resources, writing – review and editing, and supervision. Leonard A. Mattano Jr.: Conceptualization, investigation, and writing – review and editing. Kelly W. Maloney: Conceptualization, investigation, writing – review and editing, and supervision. Nina S. Kadan-Lottick: Conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review and editing, visualization, supervision, project administration, and funding acquisition.
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pound CM, Clark C, Ni A, Athale U, Lewis V, Halton JM. Corticosteroids, behavior, and quality of life in children treated for acute lymphoblastic leukemia; a multicentered trial. Journal of pediatric hematology/oncology. 2012;34(7):517–523. doi: 10.1097/MPH.0b013e318257fdac. [DOI] [PubMed] [Google Scholar]
- 3.Drigan RSA, Gelber RD. Behavioral effects of corticosteroids in children with acute lymphoblastic leukemia. Medical and pediatric oncology. 1992;20:13–21. doi: 10.1002/mpo.2950200104. [DOI] [PubMed] [Google Scholar]
- 4.Kayani S, Shannon DC. Adverse behavioral effects of treatment for acute exacerbation of asthma in children: a comparison of two doses of oral steroids. CHEST Journal. 2002;122(2):624–628. doi: 10.1378/chest.122.2.624. [DOI] [PubMed] [Google Scholar]
- 5.Mrakotsky CM, Silverman LB, Dahlberg SE, et al. Neurobehavioral side effects of corticosteroids during active treatment for acute lymphoblastic leukemia in children are age-dependent: Report from Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Pediatric blood & cancer. 2011;57(3):492–498. doi: 10.1002/pbc.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landier W, Alice MT. Use of complementary and alternative medical interventions for the management of procedure-related pain, anxiety, and distress in pediatric oncology: an integrative review. Journal of pediatric nursing. 2010;25(6):566–579. doi: 10.1016/j.pedn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenzel SL, Krull KR, Hockenberry M, et al. Oxidative stress and neurobehavioral problems in pediatric acute lymphoblastic leukemia patients undergoing chemotherapy. Journal of pediatric hematology/oncology. 2010;32(2):113. doi: 10.1097/MPH.0b013e3181c9af84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell LK, Scaduto M, Van Slyke D, Niarhos F, Whitlock JA, Compas BE. Executive function, coping, and behavior in survivors of childhood acute lymphocytic leukemia. Journal of pediatric psychology. 2009;34(3):317–327. doi: 10.1093/jpepsy/jsn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz KAP, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. Journal of Clinical Oncology. 2007;25(24):3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- 10.Kazak AE. Evidence-based interventions for survivors of childhood cancer and their families. Journal of pediatric psychology. 2005;30(1):29–39. doi: 10.1093/jpepsy/jsi013. [DOI] [PubMed] [Google Scholar]
- 11.Myers RM, Balsamo L, Lu X, et al. A prospective study of anxiety, depression, and behavioral changes in the first year after a diagnosis of childhood acute lymphoblastic leukemia. Cancer. 2014;120(9):1417–1425. doi: 10.1002/cncr.28578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbie WL, Ogle SK, Reilly M, et al. Identifying the educational needs of parents at the completion of their child's cancer therapy. Journal of Pediatric Oncology Nursing. 2010;27(4):190–195. doi: 10.1177/1043454209360778. [DOI] [PubMed] [Google Scholar]
- 13.Matloub Y, Bostrom BC, Hunger SP, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood. 2011;118(2):243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe-Christensen C, Mullins LL, Stinnett TA, Carpentier MY, Fedele DA. Use of the Behavioral Assessment System for Children 2nd edition: Parent report scale in pediatric cancer populations. Journal of clinical psychology in medical settings. 2009;16(4):322–330. doi: 10.1007/s10880-009-9174-7. [DOI] [PubMed] [Google Scholar]
- 15.Carpentieri SC, Meyer EA, Delaney BL, et al. Psychosocial and behavioral functioning among pediatric brain tumor survivors. Journal of neuro-oncology. 2003;63(3):279–287. doi: 10.1023/a:1024203323830. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children, Second Edition: Manual. Bloomington, MN: NCS Pearson, Inc; 2004. [Google Scholar]
- 17.Miller IW, Epstein NB, Bishop DS, Keitner GI. The McMaster family assessment device: reliability and validity. Journal of Marital and Family Therapy. 1985;11(4):345–356. [Google Scholar]
- 18.Kabacoff RI, Miller IW, Bishop DS, Epstein NB, Keitner GI. A psychometric study of the McMaster Family Assessment Device in psychiatric, medical, and nonclinical samples. Journal of family psychology. 1990;3(4):431. [Google Scholar]
- 19.McCubbin HI, McCubbin MA, Patterson JM, Cauble AE, Wilson LR, Warwick W CHIP. Coping health inventory for parents: An assessment of parental coping patterns in the care of the chronically ill child. Journal of Marriage and the Family. 1983:359–370. [Google Scholar]
- 20.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 21.Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological bulletin. 2014;140(3):816. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcoux S, Robaey P, Krajinovic M, Moghrabi A, Laverdière C. Predictive factors of internalized and externalized behavioral problems in children treated for acute lymphoblastic leukemia. Pediatric blood & cancer. 2012;58(6):971–977. doi: 10.1002/pbc.24079. [DOI] [PubMed] [Google Scholar]
- 23.Noll RB, MachLean WE, Whitt JK, et al. Behavioral Adkjustmnet and Social Functioning of Long-Term, Survivors of Childhood Leukemia: Parent and Teacher Reports. Journal of pediatric psychology. 1997;22(6):827–841. doi: 10.1093/jpepsy/22.6.827. [DOI] [PubMed] [Google Scholar]
- 24.Furlong W, Rae C, Feeny D, et al. Health-related quality of life among children with acute lymphoblastic leukemia. Pediatric blood & cancer. 2012;59(4):717–724. doi: 10.1002/pbc.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meeske KA, Sherman-Bien S, Hamilton AS, et al. Mental health disparities between hispanic and non-hispanic parents of childhood cancer survivors. Pediatric blood & cancer. 2013;60(9):1470–1477. doi: 10.1002/pbc.24527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alegria M, Canino G, Ríos R, et al. Mental health care for Latinos: Inequalities in use of specialty mental health services among Latinos, African Americans, and non-Latino Whites. Psychiatric Services. 2014 doi: 10.1176/appi.ps.53.12.1547. [DOI] [PubMed] [Google Scholar]
- 27.Johns A, Oland A, Katz E, et al. Qualitative analysis of the role of culture in coping themes of Latina and European American mothers of children with cancer. Journal of Pediatric Oncology Nursing. 2009 doi: 10.1177/1043454209334416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheidow AJ, Henry DB, Tolan PH, Strachan MK. The role of stress exposure and family functioning in internalizing outcomes of urban families. Journal of Child and Family Studies. 2014;23(8):1351–1365. doi: 10.1007/s10826-013-9793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferro MA, Boyle MH. The Impact of Chronic Physical Illness, Maternal Depressive Symptoms, Family Functioning, and Self-esteem on Symptoms of Anxiety and Depression in Children. Journal of abnormal child psychology. 2015;43(1):177–187. doi: 10.1007/s10802-014-9893-6. [DOI] [PubMed] [Google Scholar]
- 30.Lax Pericall MT, Taylor E. Family function and its relationship to injury severity and psychiatric outcome in children with acquired brain injury: a systematized review. Developmental Medicine & Child Neurology. 2014;56(1):19–30. doi: 10.1111/dmcn.12237. [DOI] [PubMed] [Google Scholar]
- 31.Sahler OJ, Fairclough DL, Phipps S, et al. Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: report of a multisite randomized trial. Journal of consulting and clinical psychology. 2005;73(2):272–283. doi: 10.1037/0022-006X.73.2.272. [DOI] [PubMed] [Google Scholar]
- 32.Kaslow NJ, Broth MR, Smith CO, Collins MH. Family-Based Interventions for Child and Adolescent Disorders. Journal of Marital and Family Therapy. 2012;38(1):82–100. doi: 10.1111/j.1752-0606.2011.00257.x. [DOI] [PubMed] [Google Scholar]
- 33.Holland J, Weiss T. The new standard of quality cancer care: integrating the psychosocial aspects in routine cancer from diagnosis through survivorship. The Cancer Journal. 2008;14(6):425–428. doi: 10.1097/PPO.0b013e31818d8934. [DOI] [PubMed] [Google Scholar]
- 34.Pirl WF, Fann JR, Greer JA, et al. Recommendations for the implementation of distress screening programs in cancer centers: Report from the American Psychosocial Oncology Society (APOS), Association of Oncology Social Work (AOSW), and Oncology Nursing Society (ONS) joint task force. Cancer. 2014;120(19):2946–2954. doi: 10.1002/cncr.28750. [DOI] [PubMed] [Google Scholar]
- 35.Kazak AE, Barakat LP, Ditaranto S, et al. Screening for psychosocial risk at pediatric cancer diagnosis: the psychosocial assessment tool. Journal of pediatric hematology/oncology. 2011;33(4):289–294. doi: 10.1097/MPH.0b013e31820c3b52. [DOI] [PubMed] [Google Scholar]
- 36.Patel SK, Mullins W, Turk A, Dekel N, Kinjo C, Sato JK. Distress screening, rater agreement, and services in pediatric oncology. Psycho-Oncology. 2011;20(12):1324–1333. doi: 10.1002/pon.1859. [DOI] [PubMed] [Google Scholar]


