Abstract
Background
Viral infections are a leading fatal complication for patients with primary immunodeficiency (PID) who require hematopoietic stem cell transplantation (HSCT). Use of virus-specific T-lymphocytes (VST) has been successful for treatment and prevention of viral infections after HSCT for malignant and non-malignant conditions. Here, we describe the clinical use of VST in PID at four centers.
Objective
To evaluate the safety and efficacy of VST for treatment of viral infections in patients with PID.
Methods
Patients with PID who have received VST therapy on previous or current protocols were reviewed in aggregate. Clinical information including transplantation details, viral infections, and use of antiviral and immunosuppressive pharmacotherapy were evaluated. Data regarding VST production, infusions, and adverse reactions were compared.
Results
Thirty-six patients with twelve classes of PID diagnoses received 37 VST products before or after HSCT. Twenty-six patients (72%) had been diagnosed with infections with cytomegalovirus, Epstein-Barr virus, adenovirus, BK virus, and/or human herpesvirus 6 (HHV6). Two patients were treated prior to HSCT due to EBV-associated lymphoproliferative disease (LPD). Partial or complete responses against targeted viruses occurred in 81% of patients overall. Time to response varied from two weeks to three months (median 28 days). Overall survival at six months after therapy was 80%. Four patients developed graft versus host disease (GVHD) in the 45 days following VST infusion, which in most cases was therapy-responsive.
Interpretation
VST derived from either stem cell donors or third-party donors are likely safe and effective for treatment of viral infections in patients with PID.
Keywords: Primary Immunodeficiency, immunotherapy, cytotoxic T-lymphocytes, antiviral therapy
Introduction
Primary Immunodeficiencies (PID) are a large group of congenital defects of immunity, and have many heterogeneous features, which can include abnormally frequent or severe illness from common organisms, opportunistic infections, autoimmune phenomena, and allergic disease. Among forms of PID with impaired or absent T-lymphocyte function such as severe combined immunodeficiency (SCID), viral infections are frequent and often devastating.1,2 In children with SCID, cytomegalovirus (CMV), Epstein-Barr Virus (EBV), and adenovirus (ADV) are the most common cases of viral-associated mortality.3 Although antiviral pharmacotherapy exists, it is expensive, and frequently complicated by toxicities and drug resistance.4 Response to pharmacotherapy is often incomplete without accompanying intact cellular immunity.
Definitive treatment of SCID and many other forms of PID requires reconstitution of T-cell immunity, which can be accomplished by hematopoietic stem cell transplantation (HSCT) or gene therapy for certain monogenic disorders.5,6 Active viral infections have been shown to negatively impact survival during HSCT,1,7 and patients with PID remain extremely vulnerable to viral infections during and after HSCT, in part due to the 3-6 months required for T cell engraftment to occur. This is of particular concern in recipients of stem cells from virus-naïve sources such as umbilical cord blood, and in patients receiving HLA-mismatched stem cell products, which require depletion of mature T-cells to prevent graft-versus host disease (GVHD).8 Unfortunately, clearance of many viral infections is difficult or impossible without T-cell reconstitution.
Virus-specific T-lymphocytes (VST) have been used with great success in preventing and treating viral infections following HSCT.9-11 Though methods have varied, the overall goal of VST therapy is to isolate donor T-cells with activity against one or more viruses, while excluding alloreactive T-cells that might cause GVHD. The majority of studies to date have produced VST by ex vivo culture and expansion of T-lymphocytes following stimulation with viral antigens using donor-derived antigen presenting cells. VST production previously required that a donor have existing immunity against the targeted viruses. However new methods using specific cytokines have permitted culture of VST from virus-naïve cord blood and adult donors, and the use of overlapping peptide pools encompassing viral antigens has reduced culture time in virus-exposed donors to 10-12 days.12-14 VST can also be isolated directly from peripheral blood, either by selection of MHC multimer binding T-cells or by selection of T-cells that secrete interferon gamma after stimulation with viral antigens.15,16 Selection technologies allow rapid production of VST (with under a day of processing time), but requires large volumes of blood, and can only be performed from donors that have prior viral exposure.
Most prior studies utilizing VST have focused on their administration following HSCT, and used the stem cell donor as source of VST.9,11 Alternately, partially matched VST that have been previously generated from healthy donors and banked (third-party VST) have been used successfully as “off the shelf” therapy for viral infections or prophylaxis.17,18 This approach eliminates the time and expense required for customized products.
Previous reports detailing the use of VST have not described their safety and efficacy exclusively in the PID setting. Thus an aggregate perspective regarding VST in PIDD is not available to physicians faced with treatment decisions. Here, we present, the largest multicenter series to date of VST use for prevention and treatment of viral diseases in the PID population.
Methods
Retrospective review was performed to collect clinical information regarding patients with PID diagnoses who received VST therapy between 2003 and 2014 at 4 institutions (Table 1). Patients received VST infusions in the context of seven clinical protocols (three closed, and four current), or off-study via compassionate use agreements. Ethical board approval was received at each institution for the administration of these novel T cell therapies (either on existing protocols, or via dedicated emergency protocols for compassionate use), as well as for the collection of the aggregate PID data presented here.
Table 1.
VST Studies and Participating Institutions.
| Study | Specificity | VST Stimulation | Manufacturing Institution | Treating Institution |
|---|---|---|---|---|
| ETNA NCT00058812 | EBV | LCL19 | BCM | BCM |
| VICTA NCT00078533 | EBV/CMV/ADV | Dendritic cells and LCL transduced with Ad5/35 vector encoding pp6520 | BCM | BCM |
| LYPTAIST NCT00590083 | EBV/ADV | Dendritic cells and LCL transduced with Ad5/35 vector21 | BCM | BCM |
| ACTCAT NCT00880789 | EBV/CMV/ADV | Dendritic cells and LCL transduced with Ad5/35 vector encoding pp6512,22 | BCM | BCM |
| ARMS NCT01570283 | EBV/ADV/CMV/BK/H HV6 | PBMC's pulsed with overlapping peptide23 | BCM | BCM |
| GOS EIND | EBV | LCL | BCM | GOSH |
| MUSTAT (NCT01945814 | EBV/CMV/ADV | PBMC's pulsed with overlapping peptide | CNMC | CNMC |
| TREPID | EBV/CMV/ADV | PBMC's pulsed with overlapping peptide | CNMC | CNMC |
| CNMC EIND | EBV/CMV/ADV | PBMC's pulsed with overlapping peptide | CNMC | CNMC |
| NCL EIND | EBV | LCL | Aberdeen | NCL |
BCM: Baylor College of Medicine; GOSH: Great Ormond Street Hospital; CNMC: Children's National Medication Center, NCL: Newcastle Hospital; LCL: lymphoblastoid cell lines; PBMC: peripheral blood mononuclear cells.
Twenty-three of the evaluated patients were previously reported within the larger publication of their study protocol, and details were updated for this study.20,21,23-25 The remaining patients were unpublished. Demographics, immunologic and genetic phenotype (if available), transplantation details, and the specifics of VST production and dosing were recorded, as was information on viral infections both before and after VST therapy. Antiviral responses to VST therapy were classified as complete (resolution of viremia and/or visceral symptoms), partial (≥ 1 log decrease in viral copy number and/or improvement in visceral symptoms without complete resolution, or transient resolution with subsequent return of viremia and/or symptoms within 3 months of infusion), or no response (no discernable change in viral copy number or clinical symptoms).
Results
Patient demographics
Thirty-six patients with PID received one or more infusions of VSTs during the 10-year study period (Table 2). The patients had twelve classes of underlying diagnoses (Figure 1). Severe combined immunodeficiency and X-linked lymphoproliferative disease (XLP)/related disorders were the most common disorder types. Age at transplantation ranged from 2 months to 19 years, whereas age at VST infusion ranged from 5 months to 19 years. HSCT donors included matched related donors (4), mismatched related (haploidentical) donors (5), unrelated donors (22), and cord blood (4).
Table 2. Patients Characteristics and Response to VST Therapy.
| Center | P # | Dx | Age at HSCT |
Donor | Time from HSCT to VST (mo) |
Viral infections prior to VST |
Viral status at time of VST infusion |
Prior antiviral tmts |
VST source and specificity |
Immuno- suppress- ion at time of VST therapy |
VST dose |
Antiviral response |
Adverse Events |
Follow- up time (years post- HSCT) |
Current status |
Study # / Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BCM | 1 | IL7RA-SCID | 6 mo | 6/6 UCB | 4.5 | None | N/A | None | Cord blood, CMV/EBV/ADV | CSA, prednisone | 1 dose of 1.5×10E7/m2 | No detectable EBV, ADV, CMV | None | 2 yrs 7 mo | Alive & well | NCT008 80789 / Unpubl. |
| 2 | IL2RG-SCID | 8 mo | 5/6 UCB | 4.5 | None | N/A | None | Cord blood, CMV/EBV/ADV | CSA/prednisone | 1 dose of 2.5×10E7/m2 | No detectable EBV, ADV CMV | None | 2 yrs 4 mo | Alive & well | NCT008 80789 / Unpubl. | |
| 3 | IL2RG-SCID | 2 mo | 5/6 UCB | 3 | None | N/A | None | Cord blood, CMV/EBV/ADV | MMF/prednisone | 1 dose of 1×10E7/m2 | No detectable EBV, ADV CMV | None | 3 yrs 2 mo | Alive & well | NCT008 80789 / Unpubl. | |
| 4 | IL2RG-SCID | 2 mo | MMRD (Maternal haploidentical) | 9 | None | N/A | None | HSCT Donor, CMV/EBV/ADV | None | 1 dose of 1×10E7/m2 | No detectable EBV, ADV CMV | None | 4 yr 4 mo | Alive & well | NCT000 78533 / Ref 20 | |
| 5 | SCID, unknown gene | 1 yr | 10/10 MUD | 2 | None | N/A | None | HSCT Donor, ADV | CSA, prednisone | 1 dose of 1.35×10E8/m2 | No detectable ADV | None | 11 mo | Alive & well | NCT005 90083 / Ref 21 | |
| 6 | MHC II deficiency | 10 mo | 10/10 MRD | 1.5 | CMV | CMV viremia, pneumonitis | Foscarnet, Ganciclovir, Cytogam | HSCT Donor, CMV/EBV/ADV | CSA | 1 dose of 1×10E7/m2 | CMV - CR | None | 2 yrs 10 mo | Alive & well | NCT000 78533 / Ref 20 | |
| 7 | WAS | 8 mo | 9/10 MMUD | 5 | None | N/A | None | HSCT Donor, CMV/EBV/ADV | None | 1 dose of 1×10e7/m2 | No detectable EBV, ADV CMV | None | 5 yrs 3 mo | Alive & well | NCT000 78533 / Ref 20 | |
| 8 | WAS | 1.2 yrs | 10/10 MUD | 2.3 | EBV | EBV viremia | Rituximab | Donor EBV | CSA | 1 dose of 2×10E7/m2 | EBV – CR | None | 3 yr 9 mo | Alive and well | NCT000 58812 / Ref 24 | |
| 9 | NK defect/ SCAEBV | 12 yrs | 7/10 MMUD | 7 | EBV | EBV viremia | Foscarnet for prior CMV | HSCT Donor, EBV | None | 1 dose of 1×10E8/m2 | EBV - CR | None | 4 yrs | Alive & well | NCT000 58812 / Ref 24 | |
| 10 | SCAEBV | 6.9 yrs | MRD (syngeneic) | 2.5 | EBV | EBV viremia | None | Donor EBV | None | 1 dose of 2×10E7/m2 | EBV – PR | None | 4 yrs 6 mo | Died -progressive T-cell lymphoma | NCT000 58812 / Ref 24 | |
| 11 | SCAEBV | 10.6 yrs | 10/10 MUD | 12 | EBV-LPD | EBV resolved | Rituximab | Donor EBV | None | 1 dose of 2×10E7/m2 | No further EBV reactivation | None | 10 yr 3 mo | Alive and well | NCT000 58812 / Ref 24 | |
| 12 | XLP (SLAM mutation) | 19 mo | 8/8 MUD | 3 | EBV | EBV viremia | None | HSCT Donor, EBV | CSA, prednisone | 1 dose of 2×10E7/m2 | EBV-CR | None | 9 yrs 5 mo | Alive & well | NCT000 58812 / Ref 24 | |
| 13 | XLP and lymphoma | 16 yrs | 9/10 MMUD | 2 | None | N/A | None | HSCT Donor, EBV | CSA | 1 dose of 2×10E7/m2 | No detectable EBV | None | 1 yr 6 mo | Alive & well | NCT000 58812 / Ref 24 | |
| 14 | LAD1 | 6 mo | 10/10 MRD | 2 | ADV | ADV viremia | Cidofovir | HSCT Donor, CMV/EBV/ADV/HHV6/BK | FK | 1 dose of 2×10E7/m2 | ADV - CR | None | 1 yr 7 mo | Alive & well | NCT015 70283 / Ref 23 | |
| 15 | GATA2 | 18 yrs | 9/10 MMUD | 3 | BK, CMV | BK viremia | Foscarnet, ValGanciclovir | HSCT Donor, CMV/EBV/ADV/HHV6/BK | FK/prednisone | 1 dose of 2×10E7/m2 | BK - CR; EBV - CR * | None | 1 yr 11mo | Alive & well | NCT015 70283 / Ref 23 | |
| 16 | HLH | 19 yrs | 10/10 -MUD | 2 | HHV6, BK | HHV6, BK viremia | None | HSCT Donor, CMV/EBV/ADV/HHV6/BK | MMF/prednisone | 1 dose of 1×10E7/m2 | HHV6 - CR; BK- NR, EBV-CR* | None | 1 yr 10 mo | Alive & well | NCT015 70283 / Ref 23 | |
| GOSH | 17 | CID/enterocolitis | 5.9 yrs | 10/10 MUD | 5.3 | EBV-LPD | EBV resolved | COP/Rituxima b | Donor EBV | Prednisolone | 1 dose of 2.5×10E7/m2 | No further EBV reactivation | None | 13 years 1 month | Alive & well | EIND / Ref 24 |
| 18 | CID/enterocolitis | 4.7 yrs | 10/10 MUD | 3.7 | None | N/A | None | Donor EBV | Methylpred | 1 dose of 2.5×10E7/m2 | No detectable EBV | None | 11 years 2 months | Alive & well | EIND / Ref 24 | |
| 19 | WAS | 17.7 yrs | 10/10 MUD | 4.3 | EBV-LPD | EBV resolved | Rituximab | Donor EBV | None | 1 dose of 2.5×10E7/m2 | No further EBV reactivation | None | 13 years 2 months | Alive & well | EIND / Ref 24 | |
| 20 | XLP | 12.4 yrs | 9/10 MMUD | 2.4 | EBV | EBV resolved | Rituximab | Donor EBV | CSA, MMF | 1 dose of 2.0×10E7/m2 | No further EBV reactivation | None | 10 years 6 months | Alive & well | EIND / Ref 24 | |
| 21 | XLP | 11.8 yrs | 9/10 MMUD | 3.3 | EBV | EBV viremia | None | Donor EBV | CSA | 1 dose of 2.0×10E7/m2 | EBV - PR | None | 11 years | Alive & well | EIND / Ref 24 | |
| 22 | XLP | 8.7 yrs | 9/10 MMUD | 2.8 | EBV | EBV resolved | Rituximab | Donor EBV | CSA | 1 dose of 2.0×10E7/m2 | EBV - CR* | None | 10 years 1 month | Alive & well | EIND / Ref 24 | |
| 23 | XLP | 11.8 yrs | 9/10 MMUD | 4 | EBV-LPD | EBV viremia | COP/COP AD | Donor EBV | CSA | 1 dose of 2.0×10E7/m2 | EBV - PR | None | 9 years 2 months | Alive & well | EIND / Ref 24 | |
| 24 | XLP-like | 5.4 yrs | 9/10 MMUD | 2.8 | EBV | EBV resolved | Rituximab | Donor EBV | CSA, Prednisolone | 1 dose of 2.0×10E7/m2 | No further EBV reactivation | None | 10 years 1 month | Alive & well | EIND / Ref 24 | |
| CNMC | 25 | ADA-SCID | N/A | N/A | Pre-transplant (6 yrs old) | EBV, CMV | EBV-LPD | Rituximab, ganciclovir | Third party (MMUD, 5/10 match), CMV/EBV/ADV | None | 1 dose of 5 × 10E6/m2 | EBV - NR | None | N/A | Died -EBV lymphoma, D+110 | NCT025 10404 / Unpubl. |
| 26 | SCID, unknown gene | 5 mo | MMRD (Maternal 7/10) | 1 | CMV | CMV viremia | Ganciclovir, Foscarnet, Cidofovir | HSCT Donor (7/10 match) CMV/EBV/ADV | MMF | 2 doses of 5×10E6/m2 | CMV - PR | None | 1 yr | Alive & well | NCT019 45814 / Unpubl. | |
| 27 | SCID, unknown gene | 4 mo | MMRD (Maternal 7/12) | 1 | CMV | CMV viremia | Foscarnet, Cidofovir, Cytogam | HSCT Donor, CMV/EBV/ADV | None | 1 dose of 1 × 10E7/m2 | CMV-NR | None | N/A | Died, refractory CMV and disseminated BCG, D+92 | NCT019 45814 / Unpubl. | |
| 2.5 | CMV viremia and pneumonitis | Third-party (MMUD, 4/12 match), CMV/EBV/ADV | None | 1 dose of 2 × 10E7/m2 | N/A | N/A | <2 weeks | |||||||||
| 28 | MHC II deficiency | 7 mo | MRD | 2 | None | N/A | Acyclovir (ppx) | HSCT Donor, CMV/EBV/ADV | CSA/prednisone | 1 dose of 5×10E6/m2 | CMV - CR* | aGVHD grade II, pericardial effusion | 10 mo | Alive & well | NCT019 45814 / Unpubl. | |
| 29 | HLH (STXBP2) | 4 yrs | 8/10 MMUD | 1 | CMV, EBV | CMV, EBV viremia | Ganciclovir, Rituximab | HSCT Donor, CMV/EBV/ADV | FK, prednisone | 2 doses of 1×10E7/m2 | CMV-CR, EBV-CR | Grade I cGVHD | 4 mo | Alive, grade I cGVHD | NCT019 45814 / Unpubl | |
| 30 | WAS | 17 months | 9/10 MMUD | 2 | None | N/A | None | HSCT Donor, CMV/EBV/ADV | FK/prednisone | 1 dose of 2 × 10E7/m2 | EBV-CR* | None | 2 months | Alive & well | NCT019 45814 / Unpubl. | |
| 31 | IL-10R Deficiency | 10 mo | MMRD (Maternal 6/8) | 0.7 | CMV, ADV, HBV | ADV viremia /pneumonia | Cidofovir, Foscarnet | Third-party (Paternal, 6/8 match), CMV/EBV/ADV | None | 1 dose of 2×10E7/m2 | ADV - CR, CMV-CR* | Acute GVHD, grade II gut | 5 mo | Alive, ongoing GVHD | EIND / Unpubl. | |
| NCL | 32 | CGD | 12 yrs | 11/12 MMUD | 27 | BK, CMV, EBV (urine) HHV6, ADV (blood), EBV-LPD | EBV-LPD | Acyclovir, rituximab | Third party (MMUD, 3/10 match), EBV | Methylpred | 4 doses of 2×10E6/kg (5.9×10E7/m2) | EBV - NR | None | 0.3 | Died -PTLD | EIND/Ref 25 |
| 33 | CTPS1 | 15 mo | 10/10 UCD | 1 month pre-HSCT | EBV (pre-transplant LPD), RSV, ADV | EBV-LPD | Acyclovir, rituximab | Third party (MMUD, 9/10 match), EBV | None, though received COP prior to VST* | 2 doses of 2×10E6/kg (3.9×10E7/m2) | EBV - CR | None | 2.5 yrs | Alive & well | EIND/Ref 25 | |
| 34 | HLH | 7 yrs | MUD | 1.7 | HHV6, EBV | EBV viremia | Foscarnet, Acyclovir, rituximab | Third party (MMUD, 5/10 match), EBV | CSA/MMF/Methylpred/infliximab | 3 doses of 2×10E6/kg (6.2×10E7/m2) | EBV - PR | Cerebral edema | Died 26 days after first VST dose | Died, EBV-PTLD with cerebral edema, D+76 | EIND/Unpubl. | |
| 35 | CID | 3.7 yrs | 9/10 MMUD (HLA-DQmm) | 5 | ADV HHV6, EBV-LPD | EBV-LPD | Acyclovir, cidofovir, rituximab, COP | Third party (MMUD, 3/10 match) EBV | Methylpred/FK/infliximab/ECP | 4 doses of 2×10E6/kg (4.6×10E7/m2) | EBV - NR | None | N/A | Died -PTLD | EIND/Unpubl. | |
| 36 | CD4 lymphopenia | 13 mo | MMRD (Maternal 5/10) | 8 days | Multi-drug resistant CMV | CMV-viremia | Ganciclovir, Foscarnet, Cidofovir | HSCT Donor, CMV-selected cells | CSA/MMF | 1 dose of 2×10E6/kg (3.8×10E7/m2) | CMV-PR | GVHD, grade III skin | D+280 | Died of fungal pneumonia, D+280 | EIND/Unpubl. |
Abbreviations: P#: Patient #; TMTS: treatments; BCM: Baylor College of Medicine; CNMC: Children's National Medical Center; NCL: Newcastle Hospital; GOSH: Great Ormond Street Hospital; UCB: Umbilical cord blood, MMRD: Mismatched related donor; MRD: matched related donor; MMUD: mismatched unrelated donor; MUD: matched unrelated donor; ADV: adenovirus; LPD: lymphoproliferative disease; COP: cyclophosphamide/ vincristine/ prednisone; COPAD: cyclophosphamide/vincristine/ prednisolone/doxorubicin; CSA: Cyclosporin A; MMF: Mycophenylate mofetil; FK: Tacrolimus; CR: complete response; PR: partial response; NR: no response; BCG: Bacillus-calmette-guerin; PTLD: post-transplantation lymphoproliferative disease; Mo: months; Yrs: years.
Asterisks denote viral infections/reactivations that occurred following VST infusion. Follow-up time () is as of 12/31/2014. Rows corresponding to patients who were prophylactically treated with VST are shown in grey.
Figure 1.
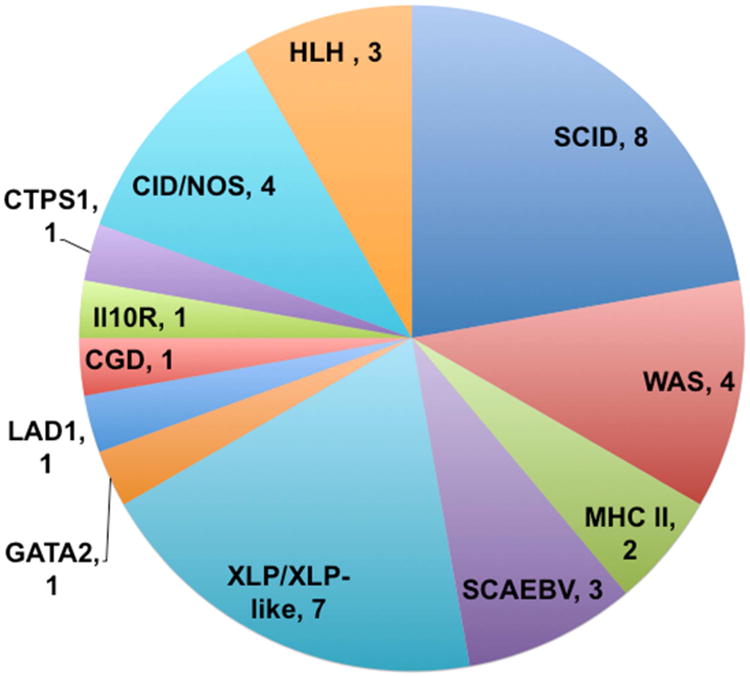
Underlying forms of Primary Immunodeficiency in patients receiving VST therapy.
Two of the patients received VST infusions prior to planned HSCT. For those receiving HSCT, the time between HSCT and VST infusion varied from 8 days to 27 months. Twenty six patients were receiving immunosuppressive medications at the time of VST infusion, either for prophylaxis or treatment for GVHD, including calcineurin inhibitors (20), corticosteroids (16), mycophenylate mofetil (6), infliximab (1), and extracorporeal photopheresis (1). All patients receiving corticosteroids were weaned to ≤ 0.5mg/kg/day (prednisone equivalents) prior to VST infusion.
VST production and infusion
VST were produced by previously described protocols from HSCT donors, cord blood, or third-party donors.12,23-25 Thirty-five patients received VST that were produced via culture methods (Table 1, Supplemental Figure 1), and one patient was treated with streptamer-selected VST.15 VST were used that targeted 1 virus (19 products), 2 viruses (1 product), 3 viruses (14 products), or 5 viruses (3 products). In all cases, VST were tested for sterility, as well viral specificity by INF-γ ELISpot. Most lines (excepting some dedicated third-party VST lines17) were tested for lack of alloreactivity using mismatched PHA blasts or LCL as targets in cytotoxicity assays (using either 51Cr release or flow cytometry-based methods25). Patients received VST by IV infusion at doses ranging from 5×106 to 1.35×108 cells/m2 based on individual protocol details (Table 2).
Viral infections and response
Of the patients evaluated, 26 (72%) had been diagnosed with CMV, EBV, Adenovirus, HHV6, and/or BK virus for which they were treated. Twenty (56%) had active infection with one or more viruses, many of which were resistant to antiviral pharmacotherapy, at the time of VST infusion (Table 2). Two patients had multiple viral infections at the time of infusion. Eight patients had been diagnosed with EBV-LPD, and of these, four had ongoing EBV-LPD at the time of VST infusion. The remaining sixteen patients (44%) were treated prophylactically and had no active viral infections at the time of infusion, but were considered at high risk for infections. Of these patients, thirteen (81%) remained free of detectable CMV, EBV, and ADV following infusion, whereas the remaining three patients (19%) had reactivations of CMV or EBV, all of which resolved.
Complete or partial antiviral responses were seen in 86% of patients with CMV (n=7), 76% of patients with EBV (n=16), and all patients with ADV (n=2), and HHV6 (n=1). Six patients had reactivation of CMV (n=2) or EBV (n=4) after VST infusion. All reactivation infections resolved within 1-2 months, and in 4 patients, no antiviral pharmacotherapy was required after VST infusion. One of the two patients who were treated for multiple viral infections (Patients #16, 29) had clearance of all viruses following VST infusion. Patient #16 had clearance of EBV and HHV6 viremia, but had no improvement in BK viral load, and retrospectively the utilized VST line was shown to lack specificity against BK virus, likely due to lack of donor exposure to the virus.
Decreases in viral copy numbers in blood were seen at 2 weeks to 3 months after infusion (Figure 2), with a median response time of 1 month. There was no correlation between VST dose and likelihood of antiviral response (reduction in viral load), nor timing of response (data not shown). Antiviral efficacy was seen in most cases despite use of immunosuppressive medications including calcineurin inhibitors, MMF, and low-dose corticosteroids (<0.5mg/kg/day prednisone). In some cases, use of immunosuppressive medications during or after initial VST infusion warranted additional doses of VST. In patients #26 and #29, second VST infusions resulted in partial or complete resolution of CMV and EBV.
Figure 2.
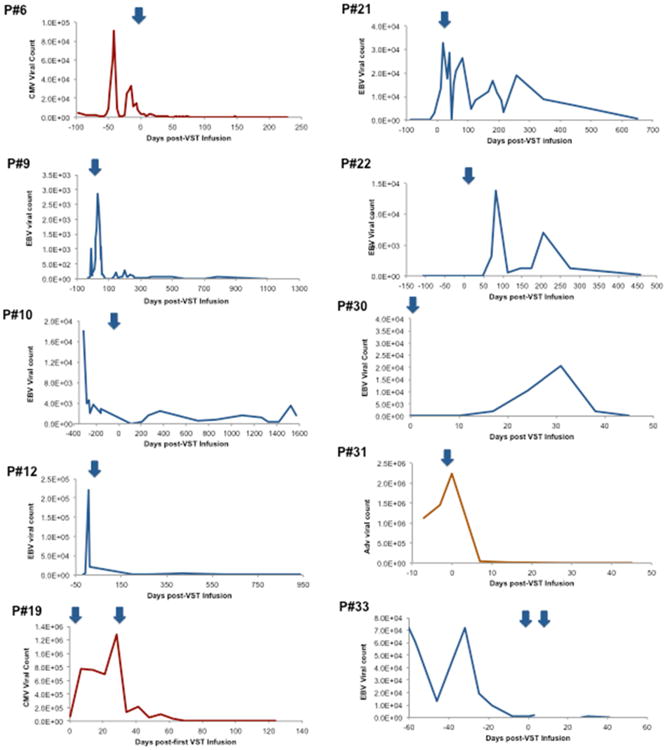
Antiviral responses to VST therapy: Representative plots of Viral copy numbers (Red=CMV, Blue=EBV, Orange=ADV) over time are shown before and after VST infusion(s) (noted as blue arrows).
Of note, two patients (Patients #25 and 33) received third-party VST infusions prior to HSCT, both for EBV-LPD. Patient #33 received rituximab, cyclophosphamide, vincristine, and prednisone for treatment of EBV-LPD. She received two doses of well-matched third-party VSTs at day -21 and day -14 prior to unrelated cord blood transplantation, which was successful without further viral disease (Figure 2). Patient #25 had no response to a single dose of third-party VST for both CMV and EBV, and was found to have monomorphic EBV-associated lymphoma, which was refractory to chemotherapy.
Overall survival and adverse events following VST infusion
Overall survival in treated patients was 80% at 6 months following VST infusion. Most early deaths occurred within 3 months of therapy and were due to existing infections, including four patients who died from progressive EBV-LPD, and one patient who died due to progressive CMV and disseminated BCG. One patient died from CNS complications of EBV-LPD at day +26 following VST infusion.
Adverse reactions following VST infusion were generally mild in all patients. Four patients (11%) developed GVHD following VST infusion, which was often associated with weaning of immunosuppression and suspected to be transplant-associated rather than attributable to VST infusion. In three of the cases, GVHD was therapy responsive without notable viral reactivation. Patient #28 developed a sterile pericardial effusion at 1 month following VST infusion, which resolved after placement of a pericardial window.
Discussion
Viral infections and reactivations are a significant cause of morbidity and mortality in patients with moderate to severe forms of PID, and account for as much as one-third of transplant-related mortality.2,3,7 Adoptive immunotherapy with VST has been highly successful for prophylaxis and treatment of CMV, EBV, and ADV infections following HSCT in over 300 patients with both malignant and non-malignant conditions treated in Europe, Australasia and the US. Though previously limited by the costs and specialization required for production, new protocols have reduced these barriers to VST production, while widening the available donor poor for this therapy. While early diagnosis of SCID and related T-cell immunodeficiencies through newborn screening has been very successful in permitting earlier HSCT and definitive treatment prior to development of many infections,26 the impact of viral infections on survival even after HSCT remains substantial. Further, newborn screening is not available universally and does not detect forms of PID that lack T-cell lymphocytopenia.
Here, we describe the first and largest series of patients with PID who were treated with VST for prevention or treatment of viral infections. Viral reduction or clearance was seen in the majority of patients treated, in spite of failure of antiviral pharmacotherapy in many cases. Clinical benefit was seen in spite of low VST doses, as the cells have been shown in these and other studies to be capable of expansion in vivo in the setting of viral infections, which has been demonstrable in prior studies by IFN-γ ELISpot analysis of the peripheral blood of patients following VST infusion.20,27,28 Time to effect varied from 2 weeks to 3 months, with most responses seen within 1 month. Though this study was inadequately powered to evaluate the impact of cell dose on the likelihood or timing of antiviral efficacy, prior phase I trials have established that a VST dose of 2×10E7/m2 is typically well tolerated and effective.17,28,29 Clinical benefit was seen with VST derived from a variety of donors, including HSCT donors, cord blood, and third-party donors. VST therapy was successful in a patient with CTPS1 deficiency and EBV-LPD prior to transplant (patient #33), who had clearance of disease after third-party VST therapy following which he underwent successful umbilical cord blood transplantation. One other patient has been previously reported who was similarly treated successfully in the pre-transplant period,30 suggesting that this may be a viable bridge therapy for PID patients with refractory viral infections prior to HSCT. Recent studies of third-party derived VST following HSCT have demonstrated that even partially matched VST lines are clinically effective, as long as antiviral activity is mediated through a shared MHC allele.17,18,31
Most VST treatment failures occurred in patients with advanced viral disease., particularly in patients with treatment-refractory EBV LPD. Previous studies have shown that in vivo expansion is required for antiviral efficacy, which likely lessens the potential impact of VST in patients who are critically ill. Nonetheless, patient #31 had a rapid response to VST therapy in spite of advanced adenoviral lung disease requiring intensive care. Patient #27 failed to improve after maternal-derived VST therapy in spite of in vitro confirmation of antiviral restriction through shared MHC alleles. Viral escape in this case may have been due to variability within viral epitopes, graft rejection through non-shared haplotype or epitope recognition through non-shared MHC alleles.
Previous studies have demonstrated that different classes of immunosuppressive agents differentially impact T-cell proliferation and cytotoxicity. Calcineurin inhibitors have been shown to reduce proliferation but not cytoxicity in vitro.32 This has been confirmed in clinical studies, as VST have been successfully used to treat EBV-LPD following solid organ transplantation in spite of the use of calcineurin inhibitors.33 Conversely, corticosteroids do impact T-cell function, and most VST protocols to date have excluded patients on moderate to high doses of steroids.
Adverse reactions to VST infusion were generally mild, which is in agreement with most prior studies. Four cases of GVHD were described, of which three were therapy responsive and more likely due to HSCT than VST therapy. A recent review of adverse reactions to VST therapy showed that most toxicity was attributable to premedications (acetaminophen and diphenhydramine) rather than the cellular therapy itself.34 The CNS complication in patient #34, while highly concerning, was not definitively tied to VST therapy by histology or CSF cytology.
One prior case of systemic inflammatory response syndrome was described in a cancer patient with bulky tumor burden treated with EBV-VST.35 One report of grade III “Bystander” GVHD has been described after third-party VST therapy in a patient with adenoviremia,36 but no similar events have occurred in many subsequent trials, including those utilizing only partially HLA matched VST.17,18 Nonetheless, caution is prudent when considering VST therapy for patients with advanced viral disease.
This study is subject to the many limitations of retrospective analyses. Though it represents the largest collection to date of patients with PID treated with VST therapy, it remains underpowered and heterogeneous, making it difficult to draw conclusions on optimal VST dosing, or on the relative effectiveness of VST therapy versus antiviral pharmacotherapy. Additionally, studies of VST therapy have not so far utilized control groups to evaluate the impact on overall survival, though given the clear benefits of this therapy in prior trials, withholding therapy in an eligible patient would be ethically questionable.
VST represent a novel therapy for the prevention and treatment of viral infections for patients with PID before and after HSCT. Rapid advancements in production techniques promise to increase the availability of this treatment option in the coming years, while preclinical studies to target additional viruses, including RSV and influenza, may further extend the usefulness of VST therapy.37 Given the results of this study, a phase II trial of VST therapy for PID would be invaluable to determine whether this treatment impacts long term survival and antiviral pharmacotherapy needs. In time, both HSCT donor-derived and “off the shelf” VST therapy may become the standard of care alongside antiviral pharmacotherapy for patients with PID.
Supplementary Material
Supplemental Figure 1: Manufacturing methods of virus-directed T-cells. 1. EBV-specific T-cell were produced by serial stimulation of peripheral blood mononuclear cells (PBMC) with donor-derived, irradiated EBV-lymphoblastoid cells (EBV-LCL) over 5-6 stimulations. 2. Multivirus-specific T-cells were produced using EBV-LCL that were virally transduced with a Ad5f35-pp65 vector for three stimulations. 3. Multivirus-specific T-cells were produced from cord blood mononuclear cells (CBMC) using donor-derived dendritic cells (DC) that were virally transduced with a Ad5f35-pp65 vector, followed by two stimulations with Ad5f35-pp65 tranduced, irradiated EBV-LCL. 4. Multivirus-specific T-cells were produced using a rapid protocol with stimulation of PBMC with peptide pools encompassing viral epitopes (against CMV, EBV, adenovirus, +/- HHV6 and BK virus) in a single stimulation, followed by 10-12 days of culture in a Grex-10 culture device.
Clinical Implications.
Therapy with VST is associated with control or prevention of targeted viruses and has minimal associated toxicity in patients with PID, and could become a standard of care in patients with viral complications before or after HSCT.
Acknowledgments
The authors would like to thank our patients and families, referring colleagues, and Dr. Malcolm Brenner (Baylor College of Medicine), Dr. David Jacobsohn, Dr. Brett Loechelt, Dr. Cecilia Barese, Fahmida Hoq, Neha Joshi (Children's National Medical Center), the National Heart, Lung and Blood Institute, the National Cancer Institute (PO1 CA148600), the Leukemia and Lymphoma Society, The Production Assistance for Cellular Therapies (PACT) program, The Clinical Research Center at Texas Children's Hospital, the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine, the Clinical Immunology Society, the American Academy of Allergy, Asthma, and Immunology ARTrust, the American College of Allergy, Asthma and Immunology, the Amy Strelzer Manasevit Scholar Award, the Clinical and Translational Science Institute at Children's National Medical Center, the Clinical Immunology Society, the staff of the Center for Cell and Gene Therapy at Baylor College of Medicine, the staff of the Division of Blood and Marrow Transplantation and the Program for Cell Enhancement and Technologies for Immunotherapy at Children's National Medical Center, and the Jeffrey Modell Foundation for their support of this research.
Funding: This research was funded by grants from the National Institutes of Health: RO1CA061384 (to CMR), P50CA126752 (to HEH), U54 HL081007 (to CMR and HEH), National Cancer Institute PO1 CA148600e02 (to CMB), a SCOR from the Leukemia and Lymphoma Society (HEH), The Production Assistance for Cellular Therapies (PACT) program [NHLBI contract #HHSN268201000007C], the Clinical Research Center at Texas Children's Hospital, the Dan L. Duncan Institute for Clinical and Translational Research at Baylor College of Medicine, the Amy Strelzer Manasevit Scholar Award (to CMB and AML), the Clinical Immunology Society (to SKN), A Jeffrey Modell Diagnostic and Research Center Grant (to JSO), American Academy of Allergy, Asthma, and Immunology (to SKN), American College of Allergy, Asthma, and Immunology (to MDK), the Clinical and Translational Science Institute at Children's National (to MDK), and a Translational Research Grant from the Jeffrey Modell Foundation (to MDK)
Abbreviations
- ADV
adenovirus
- BCG
Bacillus calmette-guerin
- CMV
cytomegalovirus
- EBV
Epstein-Barr virus
- GVHD
graft versus host disease
- HHV6
human herpesvirus 6
- HSCT
hematopoietic stem cell transplantation
- LCL
lymphoblastoid cell lines
- LPD
lymphoproliferative disease
- PBMC
peripheral blood mononuclear cells
- PID
primary immunodeficiency
- SCID
severe combined immunodeficiency
- VST
virus-specific T-lymphocytes
- XLP
X-linked lymphoproliferative disease
Footnotes
COI Statement: The authors have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371(5):434–46. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odek C, Kendirli T, Dogu F, et al. Patients with primary immunodeficiencies in pediatric intensive care unit: outcomes and mortality-related risk factors. J Clin Immunol. 2014;34(3):309–15. doi: 10.1007/s10875-014-9994-6. [DOI] [PubMed] [Google Scholar]
- 3.Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49(1-3):25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellar RS, Peggs KS. Management of multidrug-resistant viruses in the immunocompromised host. Br J Haematol. 2012;156(5):559–72. doi: 10.1111/j.1365-2141.2011.08988.x. [DOI] [PubMed] [Google Scholar]
- 5.Worth AJ, Booth C, Veys P. Stem cell transplantation for primary immune deficiency. Current opinion in hematology. 2013;20(6):501–8. doi: 10.1097/MOH.0b013e328365a13b. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh S, Thrasher AJ, Gaspar HB. Gene therapy for monogenic disorders of the bone marrow. Br J Haematol. 2015 doi: 10.1111/bjh.13520. [DOI] [PubMed] [Google Scholar]
- 7.Gennery AR, Slatter MA, Grandin L, et al. Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602–10e1-11. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Leen AM, Tripic T, Rooney CM. Challenges of T cell therapies for virus-associated diseases after hematopoietic stem cell transplantation. Expert Opin Biol Ther. 2010;10(3):337–51. doi: 10.1517/14712590903456003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leen AM, Heslop HE, Brenner MK. Antiviral T-cell therapy. Immunol Rev. 2014;258(1):12–29. doi: 10.1111/imr.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644–56. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson E, Peggs KS. Cytomegalovirus-specific T-cell therapies: current status and future prospects. Immunotherapy. 2015;7(2):135–46. doi: 10.2217/imt.14.99. [DOI] [PubMed] [Google Scholar]
- 12.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley PJ, Melenhorst JJ, Nikiforow S, et al. CMV-specific T cells generated from naive T cells recognize atypical epitopes and may be protective in vivo. Science translational medicine. 2015;7(285):285ra63. doi: 10.1126/scitranslmed.aaa2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vera JF, Brenner LJ, Gerdemann U, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010;33(3):305–15. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neudorfer J, Schmidt B, Huster KM, et al. Reversible HLA multimers (Streptamers) for the isolation of human cytotoxic T lymphocytes functionally active against tumor- and virus-derived antigens. J Immunol Methods. 2007;320(1-2):119–31. doi: 10.1016/j.jim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–7. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 17.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–23. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116(23):5045–9. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CA, Ng CY, Heslop HE, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. Journal of hematotherapy. 1995;4(2):73–9. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 20.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 21.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–92. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanley PJ, Lam S, Shpall EJ, Bollard CM. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. J Vis Exp. 2012;63:e3627. doi: 10.3791/3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Science translational medicine. 2014;6(242):242ra83. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–35. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers MA, Wilkie GM, Robinson N, et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br J Haematol. 2014;167(3):402–10. doi: 10.1111/bjh.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwan A, Abraham RS, Currier R, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. Jama. 2014;312(7):729–38. doi: 10.1001/jama.2014.9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micklethwaite KP, Savoldo B, Hanley PJ, et al. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010;115(13):2695–703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013 doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–58. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 30.Wynn RF, Arkwright PD, Haque T, et al. Treatment of Epstein-Barr-virus-associated primary CNS B cell lymphoma with allogeneic T-cell immunotherapy and stem-cell transplantation. The lancet oncology. 2005;6(5):344–6. doi: 10.1016/S1470-2045(05)70171-6. [DOI] [PubMed] [Google Scholar]
- 31.Haque T, Taylor C, Wilkie GM, et al. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation. 2001;72(8):1399–402. doi: 10.1097/00007890-200110270-00012. [DOI] [PubMed] [Google Scholar]
- 32.Savoldo B, Goss J, Liu Z, et al. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72(6):1078–86. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]
- 33.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–31. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 34.Cruz CR, Hanley PJ, Liu H, et al. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy. 2010;12(6):743–9. doi: 10.3109/14653241003709686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadopoulou A, Krance RA, Allen CE, et al. Systemic inflammatory response syndrome after administration of unmodified T lymphocytes. Mol Ther. 2014;22(6):1134–8. doi: 10.1038/mt.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qasim W, Derniame S, Gilmour K, et al. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. Br J Haematol. 2011;154(1):150–3. doi: 10.1111/j.1365-2141.2011.08579.x. [DOI] [PubMed] [Google Scholar]
- 37.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20(8):1622–32. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Manufacturing methods of virus-directed T-cells. 1. EBV-specific T-cell were produced by serial stimulation of peripheral blood mononuclear cells (PBMC) with donor-derived, irradiated EBV-lymphoblastoid cells (EBV-LCL) over 5-6 stimulations. 2. Multivirus-specific T-cells were produced using EBV-LCL that were virally transduced with a Ad5f35-pp65 vector for three stimulations. 3. Multivirus-specific T-cells were produced from cord blood mononuclear cells (CBMC) using donor-derived dendritic cells (DC) that were virally transduced with a Ad5f35-pp65 vector, followed by two stimulations with Ad5f35-pp65 tranduced, irradiated EBV-LCL. 4. Multivirus-specific T-cells were produced using a rapid protocol with stimulation of PBMC with peptide pools encompassing viral epitopes (against CMV, EBV, adenovirus, +/- HHV6 and BK virus) in a single stimulation, followed by 10-12 days of culture in a Grex-10 culture device.


