Abstract
The main limitations to the success of transplantation are the anti-graft response developed by the recipient immune system, and the adverse side effects of chronic immunosuppression. Graft-versus-host disease (GVHD) triggered by donor-derived T lymphocytes against the recipient tissues is another serious obstacle in the field of hematopoietic stem cell transplantation. Several laboratories have tested the possibility of promoting antigen (Ag)-specific tolerance for therapy of graft rejection, GVHD, and autoimmune disorders, by developing methodologies that mimic the mechanisms by which the immune system maintains peripheral tolerance in the steady state. It has been long recognized that the silent clearance of cells undergoing apoptosis exerts potent immune-regulatory effects and provides apoptotic cell-derived Ags to those Ag-presenting cells (APCs) that internalize them, in particular macrophages and dendritic cells. Therefore, in situ-targeting of recipient APCs by systemic administration of leukocytes in early apoptosis and bearing donor Ags represents a relatively simple approach to control the anti-donor response against allografts. Here, we review the mechanisms by which apoptotic cells are silently cleared by phagocytes, and how such phenomenon leads to down-regulation of the innate and adaptive immunity. We discuss the evolution of apoptotic cell-based therapies from murine models of organ/tissue transplantation and GVHD, to clinical trials. We make emphasis on potential limitations and areas of concern of apoptotic cell-based therapies, and on how other immune-suppressive therapies used in the clinics or tested experimentally likely also function through the silent clearance of apoptotic cells by the immune system.
Keywords: Apoptotic cells, Dendritic cells, Transplantation, Graft versus host disease, Immunological tolerance
Introduction
In steady-state conditions, programmed cell death by apoptosis and apoptotic cell clearance by motile professional phagocytes and neighboring tissue cells are critical during embryogenesis, tissue remodeling and homeostasis, and deletion of autoreactive lymphocytes 1–3. Recognition and phagocytosis of cells undergoing apoptosis, termed efferocytosis, exert immune-regulatory effects on leukocytes, in particular antigen (Ag)-presenting cells (APCs). Numerous laboratories have attempted to harness the immune-suppressive potential of cells undergoing early apoptosis for therapeutic purposes in transplant rejection, autoimmune diseases, and chronic inflammatory disorders. This review describes the mechanisms by which apoptotic cell clearance regulates the immune response, and how these principles are being applied for the development of apoptotic cell-based therapies for treatment of transplant rejection and graft-versus-host disease (GVHD). It also explains how other therapies already in use may also function through the silent removal of apoptotic cells.
Apoptotic cells as regulators of the immune response
It was initially considered that the rapid removal of apoptotic cells by phagocytes -before the escape of intracellular toxic mediators due to apoptotic cell membrane leakage, was the main reason cells undergoing apoptosis do not elicit inflammatory or immune responses. In 1997, Voll and colleagues demonstrated that phagocytosis of apoptotic cells by monocytes stimulates secretion of interleukin (IL)-10 and decrease release of pro-inflammatory cytokines including Tumor Necrosis Factor (TNF)-α, IL-1β and IL-12 4. Since then, numerous studies have shown that apoptotic cells control the function not only of macrophages 5, but also dendritic cells (DCs), the latter the most potent APCs. Interaction of apoptotic cells with immature DCs restricts up-regulation of DC-activation markers (i.e. MHC molecules, CD40, CD80, CD86 and CD83), secretion of pro-inflammatory mediators, and its T cell stimulatory ability, all effects resistant to challenge with DC-activating mediators 6–15 (Figure 1). Other effects associated with apoptotic cell uptake include enhanced capability of the target DCs of inducing CD4+ FoxP3+ regulatory T cells (Treg), increased secretion of TGF-β and IL-10, and IFN-γ-induced release of indoleamine 2,3-dioxygenase (IDO), the latter an enzyme that catabolizes tryptophan generating metabolites that inhibit T cell function 11, 13, 16–19. Macrophages that recognize apoptotic cells down-regulate innate and acquire immunity by releasing of IL-10, TGF-β, and PGE2 4, 5. Following interaction with apoptotic cells, macrophages and DCs secrete an inactive (latent) form of TGF-β. Interestingly, the αvβ6 and αvβ8 integrin expressed by DCs activates locally latent TGF-β released in areas of phagocytosis of apoptotic cells 20, 21. Efferocytosis-induced TGF-β secretion, acting in an autocrine/paracrine fashion, down-regulates synthesis of pro-inflammatory eicosanoids and nitric oxide in activated macrophages, the latter by down-regulating expression of inducible nitric oxide synthase 22. Molecules involved in binding and internalization of apoptotic cells also down-regulate inducible nitric oxide synthase in microglial cells, which is critical during silent removal of apoptotic neurons during development and aging 23, 24. By contrast, others have shown that following efferocytosis, macrophages and activated DCs increase production of nitric oxide, which suppresses inflammation and T cell immunity 25, 26.
Figure 1. Silent clearance of apoptotic cells.

In the steady-state, cells undergoing apoptosis release soluble factors, which include immuno-regulatory mediators, “keep out” signals (that prevent migration of neutrophils), and “find me” signals (that attract professional phagocytes). Apoptotic cells expose PtdSer and other ACAMPs. Externalized PtdSer binds PtdSer receptors directly or through bridge molecules. ACAMPs are recognized directly by PRRs expressed on the phagocyte membrane. Internalization of apoptotic cells exerts immuno-regulatory effects on target APCs, by down-regulating expression of Ag-presenting (MHC class-I and –II) and T cell co-stimulatory molecules (CD80, CD86), augmenting IDO, reducing secretion of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-12p70, IL-23), and increasing release of immunosuppressive mediators (IL-10, TGF-β). ACAMPs, Apoptotic-Cell-Associated Molecular Patterns; DC, dendritic cell; MØ, macrophage; PRRs, pattern recognition receptors; PtdSer, phosphatidylserine.
DCs that internalize apoptotic cells restrain the function of CD8 T cells by cross-presenting apoptotic cell-derived peptides loaded MHC-I molecules 27. The inhibitory effects are restricted to those DCs that phagocytose the apoptotic cells, whereas the bystander DCs are unaffected 9. Importantly, DCs that internalize apoptotic cells down-regulate the chemokine receptor CCR5, and upregulate or maintain CCR7 expression, resulting in DC migration from periphery to secondary lymphoid organs, where DCs down-regulate T cell immunity and maintain T cell peripheral tolerance 10, 28. Unlike conventional DCs and in vitro-generated myeloid DCs, plasmacytoid DCs (pDCs) are not well equipped to internalize apoptotic cells 14, 29. Although pDCs do not seem to interact directly with apoptotic cells, soluble factors released by macrophages previously incubated with apoptotic cells promote immune-regulatory functions on pDCs 30.
Increasing evidence indicates that apoptotic cells induce a regulatory B-cell (Breg) phenotype. Mouse splenic marginal zone (CD1dhi) B cells, and to a less extent follicular B cells, increase IL-10 secretion when exposed to apoptotic cells 31, 32. This result has been confirmed in human B cells 32. The increase in IL-10 production by B cells requires the interaction of CpG DNA motifs -contained in chromatin complexes translocated to the apoptotic cell surface, with Toll-like receptor (TLR)-9 expressed in the phagosome membrane of B cells. In a mouse transgenic T cell receptor (TCR) system against OVA, the IL-10 released by B cells exposed to apoptotic cells promoted differentiation of IL-10-secreting CD4 T cells 31. Thus, the B cell - apoptotic cell interaction leads to differentiation of Breg-like cells that, as APCs, stimulate differentiation of IL-10-secreting CD4 T cells.
Interaction with apoptotic cells not always suppresses immunity. Phagocytosis of stressed, activated, tumor, or infected cells undergoing apoptosis promotes DC maturation and immunity 6, 7, 17, 33–38, and pro-inflammatory apoptotic cells have been tested to boost immunity for vaccination against cancer and pathogens 39–41. Besides the intrinsic properties of the apoptotic cells, the rate of apoptotic cell clearance, the microenvironment where it occurs, and the opsonins deposited on the apoptotic cells are factors that influence the impact of apoptotic cells on the target APCs and therefore, the consequent ability of the APCs to stimulate or suppress immunity 42–44.
Apoptotic cell clearance and the immune response
Cells undergoing apoptosis release “find me” signals that act as chemotactic factors for phagocytes 45–49. Once the phagocytes reach the apoptotic cells, the latter are recognized through its Apoptotic-Cell-Associated Molecular Patterns (ACAMPs) that function as “eat me” signals for Pattern-Recognition Receptors (PRRs) expressed by the phagocytes (Figure 1). During apoptosis, phosphatidylserine (PtdSer), calreticulin, the annexins A5 and A13, and DNA are some of the intracellular molecules that become exposed on the apoptotic cell surface as ACAMPs. PtdSer, and the annexins A5 and A13 externalized on the apoptotic cell membrane exert potent immune-regulatory effects on target leukocytes 50–53. Apoptotic cells also suppress the immune response through the release of soluble mediators, including IL-10, TGF-β, and annexin A1 54–56 (Figure 1).
On the phagocyte side, T cell immunoglobulin domain and mucin domain protein (TIM) 1, 3 and 4, the membrane receptor kinases Mer and Axl, the αvβ3 and αvβ5 integrins, lectins, scavenger receptors (i.e. CD14, CD36, CD68, SR-A and Lox-1), brain-specific angiogenesis inhibitor 1 (BAI-1), stabilin-2, and receptors for α2 macroglobulin (CD91) and β2-glycoprotein I, participate in apoptotic cell binding by phagocytes 49, 57–59. Soluble proteins including the C1q and iC3b complement fragments, milk fat globule protein-E8 or lactadherin, growth arrest specific gene-6 (Gas-6), protein S, collectins (mannose-binding lectin, surfactant proteins A and D), pentraxins (C-reactive protein, serum amyloid P, long pentaxin 3), thrombospondin-1, and serum β2-glycoprotein opsonize apoptotic cells, forming molecular bridges between ACAMPs on apoptotic cells and PRR on phagocytic cells 49, 57–59 (Figure 1). Externalized PtdSer is recognized by the phagocytes directly by TIM-1, 3 and 4, CD300a, BAI-1, or stabilin-2, and indirectly through bridge molecules like lactadherin that binds to αv integrins, or via soluble Gas-6 and protein S, which bind to the membrane receptor kinases Mer and Axl expressed by phagocytes. Different phagocytic cells, in particular macrophages and DCs, use distinct receptors, and the mechanisms employed for phagocytosis of apoptotic cells differ according to the tissues.
Some of these contacts only “tether” apoptotic cells to phagocytes, whereas others also trigger efferocytosis –“tether and tickle” effect. The multiple signaling pathways by which apoptotic cells down-regulate the pro-inflammatory and –immune functions of APCs have been reviewed elsewhere 60. The redundancy in the mechanisms of apoptotic cell recognition makes apoptotic cell clearance a very efficient process. Elegant studies in mice with defects in molecules involved in recognition, internalization, and lysosomal degradation of apoptotic cells, have shown that accumulation of apoptotic cells in peripheral tissues generates danger signals that cause inflammation, autoimmunity, or chronic inflammatory disorders 2, 3, 49, 59–68. In the clinics, genetic defects in complement factors that opsonize apoptotic cells, and in apoptotic cell clearance by phagocytes have been detected in patients with systemic lupus erythematous 2, 69–75.
The discovery that, under certain conditions, cells undergoing apoptosis down-regulate the pro-inflammatory /-immunogenic properties of APCs, plus the fact that apoptotic cells are a natural source of foreign or autologous Ags for APCs, led to the idea of testing apoptotic cell-based therapies for induction of Ag-specific immuno-suppression in T cell-mediated disorders in the fields of transplantation 76, 77 and autoimmunity 31, 32, 78–82.
Apoptotic cell-based therapies in organ/tissue transplantation
In murine transplant models, systemic administration of donor splenocytes undergoing early apoptosis -induced by UV-B- or γ-irradiation, has been proven to promote donor-specific immunosuppression, and to prolong survival of heterotopic (abdomen) heart allografts 76, 77. Once injected i.v., the blood-borne donor apoptotic leukocytes are rapidly phagocytosed by macrophages and DCs in the spleen, liver and lung 11, 14, 77. In mice, the subset of CD8α+ CD103+ DCs of the splenic marginal zone selectively internalize blood-borne apoptotic cells, and then present the apoptotic cell-associated Ags in a pro-tolerogenic fashion to T cells 83 (Figure 2). Importantly, systemic injection of apoptotic leukocytes induces TGF-β and IL-10 expression by splenic phagocytes 84, 85. Internalization of the donor splenocytes in early apoptosis before transplantation restricts activation of the acceptor APCs in vivo 77. As a consequence, presentation of apoptotic cell-derived donor allo-Ags by recipient quiescent APCs -expressing a low ratio of T cell co-stimulatory vs. regulatory signals, promotes deficient activation followed by transient proliferation and deletion of anti-donor T cells, increasing the percentage of donor-specific CD4 Treg 14, 77, 86, 87 (Figure 2). This inhibitory effect of apoptotic cell clearance on CD80 and CD86 expression by recipient APCs could enhance CTLA4-Ig (betalacept) therapy, which blocks CD80 and CD86 already externalized on the APC surface. I.v. infused apoptotic leukocytes also down-regulate the T cell response by promoting T cell anergy, and inducing “CD4 T cell helpless” CD8 T cells that secrete the pro-apoptotic molecule TRAIL 88.
Figure 2. Immuno-suppressive effects of apoptotic cell-based therapies.
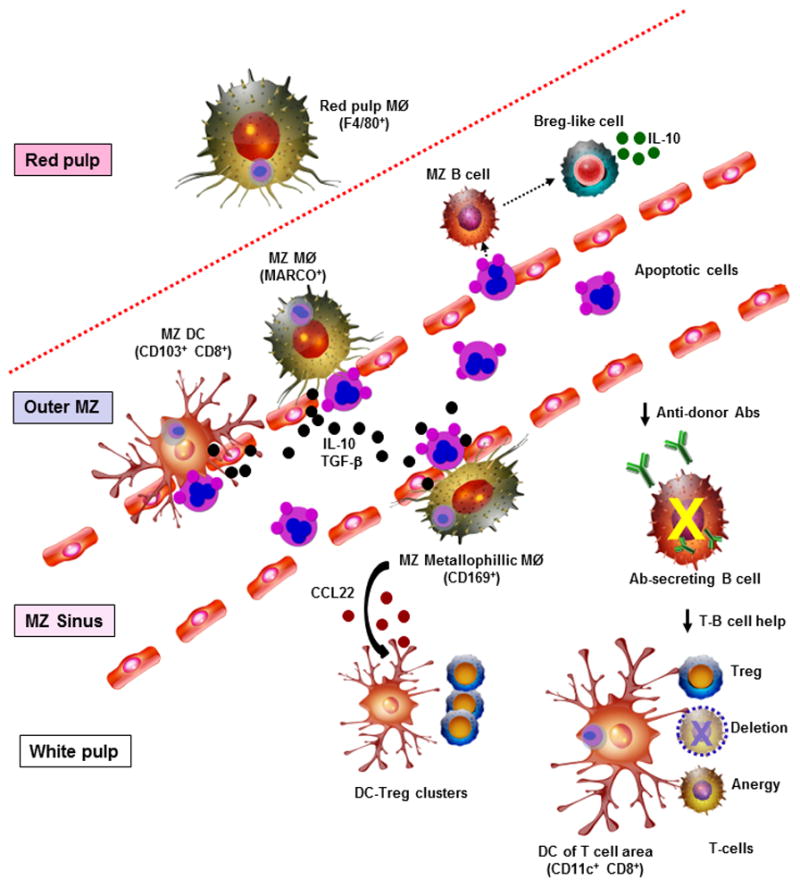
In mice, once apoptotic leukocytes are injected i.v., they are rapidly ingested by marginal zone (MZ) DCs, MZ macrophages (MØ), MZ metallophillic MØ, and red pulp MØ in the spleen. MZ MØ and MZ metallophillic MØ regulate the phagocytosis of circulating apoptotic cells by MZ DCs. MZ metallophillic MØ that have phagocytosed apoptotic cells secrete CCL22, a chemokine that attracts Treg and DCs 84. Following contact with apoptotic cells, MZ B cells and to a less extent follicular B cells acquire a Breg-like phenotype and secrete IL-10. The splenic phagocytes that engulf apoptotic cells release IL-10 and TGF-β into the systemic circulation. In the splenic white pulp, those DCs that have interacted with apoptotic cells present apoptotic cell-derived Ag to T cells in a pro-tolerogenic manner, by promoting CD4 Treg expansion, T cell deletion, and T cell anergy. The consequent reduction in the T-B cell help needed for differentiation of Ab-secreting B cells is likely one of the reasons donor apoptotic cell-based therapies decrease the titer of donor-specific Abs in serum.
Systemic injection of donor apoptotic splenocytes before heart transplantation also decreases the titer of donor-specific antibodies (Abs) in serum, likely due deficient T-B cell help caused by the immune-regulatory effect of the apoptotic cell-therapy on donor-specific CD4 T cells 77 (Figure 2). Alternatively the donor apoptotic cells could regulate directly the function of donor-reactive B cells 31.
The immune-regulatory effect of donor apoptotic cells on the anti-donor T cell response is mediated through macrophages and conventional CD11chigh CD8α+ DCs of the recipient 76, 77, 86, 89. Indeed, CD169+ metalophillic macrophages and MARCO+ macrophages of the splenic marginal zone are critical for the immuno-suppressive effect of i.v. injected apoptotic cells 78, 90. Both subsets of specialized macrophages regulate engulfment by DCs of blood-borne apoptotic cells entering the spleen 78, 90. Following systemic challenge with apoptotic leukocytes in mice, metalophillic macrophages secrete CCL22, a chemokine that promotes accumulation of FoxP3+ Tregs and DCs in the splenic follicles 84 (Figure 2). I.v. infusion of apoptotic splenocytes up-regulates expression of the immune-regulatory molecule PD-L1/2 by splenic macrophages and DCs in mice 14, 85, 86. Although apoptotic cells injected i.v. exert multiple regulatory effects on target APCs, splenic DCs present allopeptides derived from i.v. injected donor splenocytes for a limited time-span, which reaches a plateau 3 days after apoptotic cell infusion 14. This could explain why, in the absence of pharmacological immunosuppression, a single dose of donor apoptotic splenocytes although effective, only prolongs transiently cardiac allograft survival in murine models.
The beneficial effects of donor apoptotic splenocytes on heart allograft survival are donor-specific, take place in different murine strain combinations, and depend on the intrinsic properties of the donor leukocytes in early apoptosis 76, 77. The therapeutic effect of donor apoptotic splenocytes on heart allograft survival depends to a great extent on the interaction of externalized PtdSer with PRRs expressed by recipient APCs 76. The beneficial effect also relies on the timing of administration of the donor apoptotic splenocytes, with optimal results when the apoptotic cells are injected i.v. 7 days before transplantation 76, 77. This 7-day time-window represents a problem for the potential implementation of donor apoptotic cell-based therapies with deceased donors, as occurs in cardiac transplantation. Administration of donor apoptotic splenocytes in combination with a suboptimal dose of anti-CD154 (CD40 ligand) blocking Ab (to inhibit the stimulatory effect of CD40-signaling on recipient APCs), prolonged indefinitely heart allograft survival in mice 77. The accepted allografts showed minimal parenchymal damage, and were infiltrated by CD4+ FoxP3+ Tregs containing IL-10 and TGF-β 77. Thus, apoptotic cell-based therapies could reduce the doses of pharmacological immune-suppressants and co-stimulation blockade currently used and therefore, ameliorates its harmful side-effects.
Donor splenocytes made apoptotic by γ-radiation, when administered i.v. as a single dose (7 days before transplantation), prolonged survival of pancreatic islet allografts in streptozotocin-treated diabetic mice, with a broad range of allograft survival depending on the study 91, 92. By contrast, repeated infusions (7 days before and 1 day after transplantation) of donor splenocytes rendered apoptotic by incubation with the cross-linker ethylene carbodiimide (ECDI), induced donor-specific tolerance and long-term survival of islet allografts in a different donor-recipient mouse strain combination 93. In such studies, the effect of the donor apoptotic leukocytes on islet allograft survival was prevented by depletion of conventional DCs, it was mediated via PD-1/PD-L1-dependent effector T cell down-regulation and Treg expansion, and required the presence of Tregs during tolerance induction 91–93. In another non-vascularized allograft, male apoptotic thymocytes or female apoptotic leukocytes ECDI-coupled with the male CD4 T cell epitope Dby, injected i.v. 7 days before transplant, prolonged survival of minor-mismatch male skin grafts in female mice 84, 94.
The initial studies on apoptotic cell-based therapies in transplantation models were focused on prevention, delay or regulation of the immune response that leads to acute rejection 76, 77. Nevertheless, with the existing success rate of current immunosuppressive therapies, chronic rejection represents a major clinical problem that present-day immuno-suppressants fail to avert. One of the features of chronic rejection is the development of chronic allograft vasculopathy (CAV), a pathology that results from immune and non-immune mechanisms, and that consists of intimal thickening, endothelialitis, elastic fiber disruption, adventitial fibrosis, and leukocyte infiltration of arteries in the organ graft. CAV leads to progressive reduction of the lumen of graft vessels, which become prone to thrombosis. The T cell response against donor-derived peptides presented by self (recipient) MHC molecules –also known as indirect allo-recognition-, and the generation of donor-specific Abs are considered the immunological factors behind CAV. Interestingly, systemic administration of donor apoptotic splenocytes, 7 days before transplantation, reduced CAV in mouse aortic allografts –a model of CAV 14.
Apoptotic cell-based therapies in hematopoietic engraftment and graft-versus-host disease (GVHD)
As in any other allograft, the level of engraftment of bone marrow (BM) or hematopoietic stem cell (HSC) allografts depends to a great extent on the quantity, allo-specificity and function of the remaining recipient T and NK cells that resisted the conditioning regimen, and capable of rejecting the transplanted BM cells. But for those allografts that contain donor-derived passenger T cells like the BM, HSC, liver or intestine, the donor T cells adoptively transferred with the grafts can attack the recipient (host) tissues (i.e. skin, gut, liver), eliciting acute or chronic GVHD 95. Activation of the host innate immune system by the conditioning regimen followed by differentiation of donor-derived effector T cells against the host tissues causes GVHD 96. Thus, targeting host APCs and down-regulating its immunogenic functions by i.v. injected apoptotic cells may control GVHD 96. Indeed, donor apoptotic cells have been proven to decrease BM rejection -therefore increasing BM engraftment-, and to ameliorate GVHD in mice.
In a restrictive BM engraftment model, co-administration (i.v.) of a single bolus of donor apoptotic splenocytes together with the BM allograft facilitated BM engraftment and prevented GVHD in different host mouse strains 97, 98. The beneficial effect of the apoptotic cells did not depend on the stimulus used to trigger cell death, since apoptotic splenocytes generated by γ- or UVB- irradiation, or by Ab-mediated CD95-signaling exerted similar effects 97. Interestingly, administration donor-, host-, or third-party-derived, and even xenogeneic (human) apoptotic leukocytes promoted BM engraftment without GVHD in mice 97, 98. This seemingly MHC-independent effect of the injected apoptotic cells can be attributed to the co-administration of the BM allograft and apoptotic leukocytes, a delivery strategy that simultaneously provided the recipient APCs with donor-Ag from the BM inoculum, and immune-regulatory signals from the apoptotic leukocytes regardless its MHC haplotype.
In mice, the beneficial effect of apoptotic cell-based therapy on BM engraftment correlated with increased numbers FoxP3+ Treg of donor origin, which inhibited proliferation of donor-reactive T cells from the host via a cell contact-mediated mechanism, independent of IL-10 or TGF-β 99. Expansion of the donor-derived Treg required TGF-β, likely secreted by the recipient phagocytes that internalized the apoptotic cells injected i.v. 99. Apoptotic splenocyte infusion along with the BM allograft prevented, via a TGF-β-dependent mechanism, development of cytotoxic Abs against the donor BM, an effect that also favored BM engraftment 98. In the same mouse model, co-administration of donor apoptotic splenocytes with the BM allograft correlated with induction of donor-derived Treg that restrained the anti-host response, which may explain the preventive effect of the apoptotic cell-based therapy on GVHD 99.
Donor-derived pDC precursors seem to constitute a percentage of the “CD8+ TCRneg facilitating cells” that promote HSC engraftment and prevent GVHD 100. Interestingly, i.v. injection of donor pDCs, in combination with donor apoptotic splenocytes and the BM allograft, enhanced the beneficial effect of the apoptotic cells on BM engraftment in mice 30. By contrast, depletion of pDCs from BM allografts prevented the increase in BM engraftment induced by co-administration of donor apoptotic splenocytes in the same model 30. In this scenario, the immune-suppressive effect of the donor pDCs contained in the BM inoculum was induced by soluble factors released by those splenic phagocytes that interacted with the apoptotic cells injected i.v. 30.
Before apoptotic cell-based therapies can be implemented in HSC transplantation in humans, it is key to understand how the current conditioning and maintenance immunosuppressive regimens interfere with the regulatory effects of the systemically administered apoptotic leukocytes. In mice, although cyclosporine A antagonized the beneficial effect of i.v. injected apoptotic splenocytes on BM engraftment, rapamycin worked in a synergistic fashion with the apoptotic cell-based therapy 101. In a recently completed phase I/IIa clinical trial, 13 patients receiving myeloablative HLA-matched allogeneic HSC transplants were treated 1 day before the transplant with a single i.v. infusion of donor leukocytes in early apoptosis, as prophylaxis for GVHD 102. In such study, no adverse effects associated to the exogenous apoptotic cells were detected, and although the apoptotic cell-based therapy did not enhance BM engraftment, the incidence of acute grade II to IV GVHD was lower than the one previously reported and was zero in the 6 patients that received the higher doses of apoptotic cells 102.
Therapies dependent on apoptotic cell clearance
Other cell- or Ab-based approaches used to promote immune-suppression for therapy of transplant rejection, GVHD, or auto-immune disorders also function through generation of apoptotic leukocytes. The apoptotic cells generated in vivo are rapidly internalized by host APCs, which receive immune-regulatory signals and in some cases the pathogenic Ag(s), via the apoptotic leukocytes 60.
In extracorporeal photopheresis (ECP), mononuclear cell-enriched plasma separated by apheresis is exposed to 8-methoxypsoralen and UV-A radiation, which prime the leukocytes for apoptosis. The leukocytes are then reinfused into the patient. Supplementation of pharmacological immunosuppression with ECP has been proven to decrease the risk of heart, kidney, lung, and liver transplant rejection 103–106. In combination with standard therapy, ECP is also indicated for chronic GVHD and steroid-refractory acute GVHD, although the latter requires treatment over a long period of time 107, 108. Importantly, not all GVHD patients respond to ECP and frequent ECP cycles are required, which is a burden for patients with severe acute GVHD.
The effect of ECP on GVHD is probably not caused by induction of apoptosis of host-reactive T cells in peripheral blood, since less than 10% of peripheral T cells are eliminated by a single ECP procedure. Several studies have found that the effects of ECP are associated with induction of host/recipient immunosuppressive DCs by the clearance of the ECP-treated apoptotic cells, and with increased number or function of CD4 Tregs 109–115. Interestingly, ECP does not cause generalized immunosuppression, and the presence of host-reactive T cells in the ECP-treated inoculum seems to correlate directly with the ECP efficacy in GVHD 116–118. Based on these observations, D. Hannani 119 has raised the hypothesis that during ECP, reinfusion of host-reactive T cells undergoing immunogenic cell death provides Ags (host-reactive TCR-derived peptides) plus APC-activating signals to host APCs, which then generate anti-clonotypic CD8 T cells that specifically eliminate the pathogenic T cells responsible for GVHD. However, in patients with chronic GVHD, French et al 117 did not detect reduction of the previously expanded T cell clones in peripheral blood after successful ECP treatment.
In the blood transfusion field, allo-immunity against red blood cells is an adverse effect in less than 10% of blood recipients. In mice, Vallion et al 120 has demonstrated that leukocytes undergoing apoptosis in short-term stored blood, release TGF-β that down-regulates the risk of red blood cell alloimmunization. By contrast, in long-term stored blood, leukocytes become predominately necrotic, increasing the probability of red blood cell alloimmunization 120.
Randomly selected, haplotype-shared, or donor-specific transfusion (DST) plus standard immunosuppression has been employed to promote donor-specific immunosuppression. The use of DST has been discontinued due to the risk of allo-sensitization and the arrival of new immunosuppressants. More recently, donor (or recipient) - derived DCs, rendered tolerogenic by pharmacological or genetic manipulation, have been used to control alloimmunity. The prevailing idea on the mechanism of action of DST and tolerogenic DC-based therapies is that the i.v. injected leukocytes and DCs rapidly undergo apoptosis (due to short life spam or attack by recipient NK and cytotoxic T cells), and therefore serve as a source of donor peptides and immune-regulatory signals for recipient quiescent APCs, which in turn restrain the anti-donor response 121–123.
Administration of T cell-depleting Abs (i.e. CD3 Ab) has been used to treat autoimmunity and transplant rejection. The immune-suppression mediated by CD3 Abs is caused indirectly through release of TGF-β by macrophages and DCs that internalize the apoptotic T cells 124.
Summary
In the clinics, apoptotic cell-based therapies through administration of donor apoptotic leukocytes or apoptotic cell mimics could reduce the use of pharmacological immunosuppression and therefore, its harmful side effects. Different laboratories have proved in murine models that optimal immunosuppression by apoptotic cell administration is achieved with the use of non-stressed leukocytes undergoing early apoptosis, administered i.v. days before or by the time of transplantation, and in quantities that do not overload the mechanisms of apoptotic cell clearance. However, (i) the capacity of the injected apoptotic cells to modulate alloimmunity in recipients with allo-reactive memory T cells, (ii) the shelf-life of apoptotic cells generated in vitro for therapeutic applications, (iii) the stability of the immunosuppression induced by short-lived apoptotic cells -administered once or in multiple doses, and (iv) the risk of allo-sensitization after apoptotic cell infusion, represent critical issues to be addressed in the laboratory or clinical trials, before embarking in the implementation of apoptotic cell-based therapies in transplantation in humans.
Acknowledgments
We apologize for those papers not cited in this concise review due to space limitations. Supported by grants from the American Heart Association 14GRNT19810000 (A.E.M), and the National Institutes of Health R01 AR068249 (A.T.L.), and funds from the T.E. Starzl Transplantation Institute (A.E.M.).
Footnotes
Author contributions
A.E.M and A.T.L. contribute equally to the conception and writing of the manuscript.
Disclosure of potential conflicts of interest
The authors declare no competing financial interests.
References
- 1.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Munoz LE, Lauber K, Schiller M, et al. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Dransfield I, Gregory C, et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 4.Voll RE, Herrmann M, Roth EA, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 5.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 7.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban BC, Willcox N, Roberts DJ. A role for CD36 in the regulation of dendritic cell function. Proc Natl Acad Sci U S A. 2001;98:8750–8755. doi: 10.1073/pnas.151028698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart LM, Lucas M, Simpson C, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 10.Verbovetski I, Bychkov H, Trahtemberg U, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood. 2003;101:611–620. doi: 10.1182/blood-2002-06-1769. [DOI] [PubMed] [Google Scholar]
- 12.Stark MA, Huo Y, Burcin TL, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Kushwah R, Oliver JR, Zhang J, et al. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J Immunol. 2009;183:7104–7118. doi: 10.4049/jimmunol.0900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Shufesky WJ, Montecalvo A, et al. In situ-targeting of dendritic cells with donor-derived apoptotic cells restrains indirect allorecognition and ameliorates allograft vasculopathy. PLoS One. 2009;4:e4940. doi: 10.1371/journal.pone.0004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon KO, O’Flynn J, van der Kooij SW, et al. Phagocytosis of apoptotic or necrotic cells differentially regulates the transcriptional expression of IL-12 family members in dendritic cells. J Leukoc Biol. 2014;96:313–324. doi: 10.1189/jlb.3A1013-538RR. [DOI] [PubMed] [Google Scholar]
- 16.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell- mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notley CA, Brown MA, McGovern JL, et al. Engulfment of activated apoptotic cells abolishes TGF-beta-mediated immunoregulation via the induction of IL-6. J Immunol. 2015;194:1621–1627. doi: 10.4049/jimmunol.1401256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kushwah R, Wu J, Oliver JR, et al. Uptake of apoptotic DC converts immature DC into tolerogenic DC that induce differentiation of Foxp3+ Treg. Eur J Immunol. 2010;40:1022–1035. doi: 10.1002/eji.200939782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Costa TB, Sardinha LR, Larocca R, et al. Allogeneic apoptotic thymocyte- stimulated dendritic cells expand functional regulatory T cells. Immunology. 2011;133:123–132. doi: 10.1111/j.1365-2567.2011.03420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travis MA, Reizis B, Melton AC, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paidassi H, Acharya M, Lacy-Hulbert A. Alpha (v) integrins license regulatory T cells to apoptotic cells and self-associated antigens. Ann N Y Acad Sci. 2010;1209:68–76. doi: 10.1111/j.1749-6632.2010.05783.x. [DOI] [PubMed] [Google Scholar]
- 22.Freire-de-Lima CG, Xiao YQ, Gardai SJ, et al. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grommes C, Lee CY, Wilkinson BL, et al. Regulation of microglial phagocytosis and inflammatory gene expression by Gas6 acting on the Axl/Mer family of tyrosine kinases. J Neuroimmune Pharmacol. 2008;3:130–140. doi: 10.1007/s11481-007-9090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata T, Nagata K, Kobayashi Y. Cutting edge: a critical role of nitric [corrected] oxide in preventing inflammation upon apoptotic cell clearance. J Immunol. 2007;179:3407–3411. doi: 10.4049/jimmunol.179.6.3407. [DOI] [PubMed] [Google Scholar]
- 26.Ren G, Su J, Zhao X, et al. Apoptotic cells induce immunosuppression through dendritic cells: critical roles of IFN-gamma and nitric oxide. J Immunol. 2008;181:3277–3284. doi: 10.4049/jimmunol.181.5.3277. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson TA, Herndon J, Elzey B, et al. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 28.Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte-derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol. 2004;173:189–196. doi: 10.4049/jimmunol.173.1.189. [DOI] [PubMed] [Google Scholar]
- 29.Dalgaard J, Beckstrom KJ, Jahnsen FL, et al. Differential capability for phagocytosis of apoptotic and necrotic leukemia cells by human peripheral blood dendritic cell subsets. J Leukoc Biol. 2005;77:689–698. doi: 10.1189/jlb.1204711. [DOI] [PubMed] [Google Scholar]
- 30.Bonnefoy F, Perruche S, Couturier M, et al. Plasmacytoid dendritic cells play a major role in apoptotic leukocyte-induced immune modulation. J Immunol. 2011;186:5696–5705. doi: 10.4049/jimmunol.1001523. [DOI] [PubMed] [Google Scholar]
- 31.Gray M, Miles K, Salter D, et al. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miles K, Heaney J, Sibinska Z, et al. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci U S A. 2012;109:887–892. doi: 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson U, Walther-Jallow L, Smed-Sorensen A, et al. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 34.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 35.Feng H, Zeng Y, Graner MW, et al. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–4115. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 36.Torchinsky MB, Garaude J, Martin AP, et al. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 37.Green DR, Ferguson T, Zitvogel L, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gurung P, Kucaba TA, Ferguson TA, et al. Activation-induced CD154 expression abrogates tolerance induced by apoptotic cells. J Immunol. 2009;183:6114–6123. doi: 10.4049/jimmunol.0901676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fry TJ, Shand JL, Milliron M, et al. Antigen loading of DCs with irradiated apoptotic tumor cells induces improved anti-tumor immunity compared to other approaches. Cancer Immunol Immunother. 2009;58:1257–1264. doi: 10.1007/s00262-008-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank MO, Kaufman J, Tian S, et al. Harnessing naturally occurring tumor immunity: a clinical vaccine trial in prostate cancer. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winau F, Weber S, Sad S, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Griffith TS, Ferguson TA. Cell death in the maintenance and abrogation of tolerance: the five Ws of dying cells. Immunity. 2011;35:456–466. doi: 10.1016/j.immuni.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yatim N, Jusforgues-Saklani H, Orozco S, et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzelepis F, Verway M, Daoud J, et al. Annexin1 regulates DC efferocytosis and cross-presentation during Mycobacterium tuberculosis infection. J Clin Invest. 2015;125:752–768. doi: 10.1172/JCI77014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauber K, Bohn E, Krober SM, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 46.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gude DR, Alvarez SE, Paugh SW, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. FASEB J. 2008;22:2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truman LA, Ford CA, Pasikowska M, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112:5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 49.Poon IK, Lucas CD, Rossi AG, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14:166–180. doi: 10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bondanza A, Zimmermann VS, Rovere-Querini P, et al. Inhibition of phosphatidylserine recognition heightens the immunogenicity of irradiated lymphoma cells in vivo. J Exp Med. 2004;200:1157–1165. doi: 10.1084/jem.20040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Doffek K, Sugg SL, et al. Phosphatidylserine regulates the maturation of human dendritic cells. J Immunol. 2004;173:2985–2994. doi: 10.4049/jimmunol.173.5.2985. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann PR, Kench JA, Vondracek A, et al. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 53.Linke B, Abeler-Dorner L, Jahndel V, et al. The tolerogenic function of annexins on apoptotic cells is mediated by the annexin core domain. J Immunol. 2015;194:5233–5242. doi: 10.4049/jimmunol.1401299. [DOI] [PubMed] [Google Scholar]
- 54.Gao Y, Herndon JM, Zhang H, et al. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Frank ME, Jin W, et al. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 56.Pupjalis D, Goetsch J, Kottas DJ, et al. Annexin A1 released from apoptotic cells acts through formyl peptide receptors to dampen inflammatory monocyte activation via JAK/STAT/SOCS signalling. EMBO Mol Med. 2011;3:102–114. doi: 10.1002/emmm.201000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 58.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 59.Nagata S, Hanayama R, Kawane K. Autoimmunity and the clearance of dead cells. Cell. 2010;140:619–630. doi: 10.1016/j.cell.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Morelli AE, Larregina AT. Apoptotic cell-based therapies against transplant rejection: role of recipient’s dendritic cells. Apoptosis. 2010;15:1083–1097. doi: 10.1007/s10495-010-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lacy-Hulbert A, Smith AM, Tissire H, et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jinushi M, Nakazaki Y, Dougan M, et al. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117:1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luckashenak N, Schroeder S, Endt K, et al. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008;28:521–532. doi: 10.1016/j.immuni.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 64.Wallet MA, Sen P, Flores RR, et al. MerTK is required for apoptotic cell-induced T cell tolerance. J Exp Med. 2008;205:219–232. doi: 10.1084/jem.20062293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukundan L, Odegaard JI, Morel CR, et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15:1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roszer T, Menendez-Gutierrez MP, Lefterova MI, et al. Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J Immunol. 2011;186:621–631. doi: 10.4049/jimmunol.1002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uderhardt S, Herrmann M, Oskolkova OV, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36:834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Khan TN, Wong EB, Soni C, et al. Prolonged apoptotic cell accumulation in germinal centers of Mer-deficient mice causes elevated B cell and CD4+ Th cell responses leading to autoantibody production. J Immunol. 2013;190:1433–1446. doi: 10.4049/jimmunol.1200824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herrmann M, Voll RE, Zoller OM, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 70.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shoshan Y, Shapira I, Toubi E, et al. Accelerated Fas-mediated apoptosis of monocytes and maturing macrophages from patients with systemic lupus erythematosus: relevance to in vitro impairment of interaction with iC3b-opsonized apoptotic cells. J Immunol. 2001;167:5963–5969. doi: 10.4049/jimmunol.167.10.5963. [DOI] [PubMed] [Google Scholar]
- 72.Baumann I, Kolowos W, Voll RE, et al. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 73.Tas SW, Quartier P, Botto M, et al. Macrophages from patients with SLE and rheumatoid arthritis have defective adhesion in vitro, while only SLE macrophages have impaired uptake of apoptotic cells. Ann Rheum Dis. 2006;65:216–221. doi: 10.1136/ard.2005.037143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donnelly S, Roake W, Brown S, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum. 2006;54:1543–1556. doi: 10.1002/art.21783. [DOI] [PubMed] [Google Scholar]
- 75.Truedsson L, Bengtsson AA, Sturfelt G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity. 2007;40:560–566. doi: 10.1080/08916930701510673. [DOI] [PubMed] [Google Scholar]
- 76.Sun E, Gao Y, Chen J, et al. Allograft tolerance induced by donor apoptotic lymphocytes requires phagocytosis in the recipient. Cell Death Differ. 2004;11:1258–1264. doi: 10.1038/sj.cdd.4401500. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Larregina AT, Shufesky WJ, et al. Use of the inhibitory effect of apoptotic cells on dendritic cells for graft survival via T-cell deletion and regulatory T cells. Am J Transplant. 2006;6:1297–1311. doi: 10.1111/j.1600-6143.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 78.Miyake Y, Asano K, Kaise H, et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia CQ, Peng R, Qiu Y, et al. Transfusion of apoptotic beta-cells induces immune tolerance to beta-cell antigens and prevents type 1 diabetes in NOD mice. Diabetes. 2007;56:2116–2123. doi: 10.2337/db06-0825. [DOI] [PubMed] [Google Scholar]
- 80.Xia CQ, Qiu Y, Peng RH, et al. Infusion of UVB-treated splenic stromal cells induces suppression of beta cell antigen-specific T cell responses in NOD mice. J Autoimmun. 2008;30:283–292. doi: 10.1016/j.jaut.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Notley CA, Brown MA, Wright GP, et al. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol. 2011;186:4967–4972. doi: 10.4049/jimmunol.1003021. [DOI] [PubMed] [Google Scholar]
- 82.Saas P, Kaminski S, Perruche S. Prospects of apoptotic cell-based therapies for transplantation and inflammatory diseases. Immunotherapy. 2013;5:1055–1073. doi: 10.2217/imt.13.103. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka M, Asano K, Qiu CH. Immune regulation by apoptotic cell clearance. Ann N Y Acad Sci. 2010;1209:37–42. doi: 10.1111/j.1749-6632.2010.05746.x. [DOI] [PubMed] [Google Scholar]
- 84.Ravishankar B, Shinde R, Liu H, et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A. 2014;111:4215–4220. doi: 10.1073/pnas.1320924111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Getts DR, Turley DM, Smith CE, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kheradmand T, Wang S, Bryant J, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189:804–812. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Redmond WL, Wei CH, Kreuwel HT, et al. The apoptotic pathway contributing to the deletion of naive CD8 T cells during the induction of peripheral tolerance to a cross-presented self-antigen. J Immunol. 2008;180:5275–5282. doi: 10.4049/jimmunol.180.8.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Griffith TS, Kazama H, VanOosten RL, et al. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178:2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 89.Qiu CH, Miyake Y, Kaise H, et al. Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol. 2009;182:4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 90.McGaha TL, Chen Y, Ravishankar B, et al. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 91.Mougel F, Bonnefoy F, Kury-Paulin S, et al. Intravenous infusion of donor apoptotic leukocytes before transplantation delays allogeneic islet graft rejection through regulatory T cells. Diabetes Metab. 2012;38:531–537. doi: 10.1016/j.diabet.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 92.Wu C, Zhang Y, Jiang Y, et al. Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells. Cell Mol Immunol. 2013;10:393–402. doi: 10.1038/cmi.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo X, Pothoven KL, McCarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105:14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martin AJ, McCarthy D, Waltenbaugh C, et al. Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation. J Immunol. 2010;185:3326–3336. doi: 10.4049/jimmunol.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 96.Saas P, Gaugler B, Perruche S. Intravenous apoptotic cell infusion as a cell-based therapy toward improving hematopoietic cell transplantation outcome. Ann N Y Acad Sci. 2010;1209:118–126. doi: 10.1111/j.1749-6632.2010.05741.x. [DOI] [PubMed] [Google Scholar]
- 97.Bittencourt MC, Perruche S, Contassot E, et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98:224–230. doi: 10.1182/blood.v98.1.224. [DOI] [PubMed] [Google Scholar]
- 98.Perruche S, Kleinclauss F, de Bittencourt MC, et al. Intravenous infusion of apoptotic cells simultaneously with allogeneic hematopoietic grafts alters anti-donor humoral immune responses. Am J Transplant. 2004;4:1361–1365. doi: 10.1111/j.1600-6143.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 99.Kleinclauss F, Perruche S, Masson E, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ. 2006;13:41–52. doi: 10.1038/sj.cdd.4401699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fugier-Vivier IJ, Rezzoug F, Huang Y, et al. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201:373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bonnefoy F, Masson E, Perruche S, et al. Sirolimus enhances the effect of apoptotic cell infusion on hematopoietic engraftment and tolerance induction. Leukemia. 2008;22:1430–1434. doi: 10.1038/sj.leu.2405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mevorach D, Zuckerman T, Reiner I, et al. Single infusion of donor mononuclear early apoptotic cells as prophylaxis for graft-versus-host disease in myeloablative HLA-matched allogeneic bone marrow transplantation: a phase I/IIa clinical trial. Biol Blood Marrow Transplant. 2014;20:58–65. doi: 10.1016/j.bbmt.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 103.Barr ML, Meiser BM, Eisen HJ, et al. Photopheresis for the prevention of rejection in cardiac transplantation. Photopheresis Transplantation Study Group. N Engl J Med. 1998;339:1744–1751. doi: 10.1056/NEJM199812103392404. [DOI] [PubMed] [Google Scholar]
- 104.Jardine MJ, Bhandari S, Wyburn KR, et al. Photopheresis therapy for problematic renal allograft rejection. J Clin Apher. 2009;24:161–169. doi: 10.1002/jca.20199. [DOI] [PubMed] [Google Scholar]
- 105.Benden C, Speich R, Hofbauer GF, et al. Extracorporeal photopheresis after lung transplantation: a 10-year single-center experience. Transplantation. 2008;86:1625–1627. doi: 10.1097/TP.0b013e31818bc024. [DOI] [PubMed] [Google Scholar]
- 106.Urbani L, Mazzoni A, Colombatto P, et al. Potential applications of extracorporeal photopheresis in liver transplantation. Transplant Proc. 2008;40:1175–1178. doi: 10.1016/j.transproceed.2008.03.071. [DOI] [PubMed] [Google Scholar]
- 107.Greinix HT, Volc-Platzer B, Kalhs P, et al. Extracorporeal photochemotherapy in the treatment of severe steroid-refractory acute graft-versus-host disease: a pilot study. Blood. 2000;96:2426–2431. [PubMed] [Google Scholar]
- 108.Flowers ME, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 109.Lamioni A, Parisi F, Isacchi G, et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–850. doi: 10.1097/01.tp.0000157278.02848.c7. [DOI] [PubMed] [Google Scholar]
- 110.Di Renzo M, Sbano P, De Aloe G, et al. Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients. Clin Exp Immunol. 2008;151:407–413. doi: 10.1111/j.1365-2249.2007.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maeda A, Schwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 112.Maeda A, Schwarz A, Bullinger A, et al. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181:5956–5962. doi: 10.4049/jimmunol.181.9.5956. [DOI] [PubMed] [Google Scholar]
- 113.George JF, Gooden CW, Guo L, et al. Role for CD4(+)CD25(+) T cells in inhibition of graft rejection by extracorporeal photopheresis. J Heart Lung Transplant. 2008;27:616–622. doi: 10.1016/j.healun.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 114.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schmitt S, Johnson TS, Karakhanova S, et al. Extracorporeal photophoresis augments function of CD4+CD25+FoxP3+ regulatory T cells by triggering adenosine production. Transplantation. 2009;88:411–416. doi: 10.1097/TP.0b013e3181aed927. [DOI] [PubMed] [Google Scholar]
- 116.Suchin KR, Cassin M, Washko R, et al. Extracorporeal photochemotherapy does not suppress T- or B-cell responses to novel or recall antigens. J Am Acad Dermatol. 1999;41:980–986. doi: 10.1016/s0190-9622(99)70257-4. [DOI] [PubMed] [Google Scholar]
- 117.French LE, Alcindor T, Shapiro M, et al. Identification of amplified clonal T cell populations in the blood of patients with chronic graft-versus-host disease: positive correlation with response to photopheresis. Bone Marrow Transplant. 2002;30:509–515. doi: 10.1038/sj.bmt.1703705. [DOI] [PubMed] [Google Scholar]
- 118.Budde H, Kolb S, Salinas Tejedor L, et al. Modified extracorporeal photopheresis with cells from a healthy donor for acute graft-versus-host disease in a mouse model. PLoS One. 2014;9:e105896. doi: 10.1371/journal.pone.0105896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hannani D. Extracorporeal Photopheresis: Tolerogenic or Immunogenic Cell Death? Beyond Current Dogma. Front Immunol. 2015;6:349. doi: 10.3389/fimmu.2015.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vallion R, Bonnefoy F, Daoui A, et al. Transforming growth factor-beta released by apoptotic white blood cells during red blood cell storage promotes transfusion-induced alloimmunomodulation. Transfusion. 2015;55:1721–1735. doi: 10.1111/trf.13031. [DOI] [PubMed] [Google Scholar]
- 121.Quezada SA, Fuller B, Jarvinen LZ, et al. Mechanisms of donor-specific transfusion tolerance: preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102:1920–1926. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- 122.Divito SJ, Wang Z, Shufesky WJ, et al. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694–2705. doi: 10.1182/blood-2009-10-251058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang Z, Divito SJ, Shufesky WJ, et al. Dendritic cell therapies in transplantation revisited: deletion of recipient DCs deters the effect of therapeutic DCs. Am J Transplant. 2012;12:1398–1408. doi: 10.1111/j.1600-6143.2012.04060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perruche S, Zhang P, Liu Y, et al. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]


