Abstract
L-dopa-induced dyskinesias (LIDs) are a serious complication of L-dopa therapy for Parkinson's disease. Emerging evidence indicates that the nicotinic cholinergic system plays a role in LIDs, although the pathways and mechanisms are poorly understood. Here we used optogenetics to investigate the role of striatal cholinergic interneurons in LIDs. Mice expressing cre-recombinase under the control of the choline acetyltransferase promoter (ChAT-Cre) were lesioned by unilateral injection of 6-hydroxydopamine. AAV5-ChR2-eYFP or AAV5-control-eYFP was injected into the dorsolateral striatum, and optical fibers implanted. After stable virus expression, mice were treated with L-dopa. They were then subjected to various stimulation protocols for 2 h and LIDs rated. Continuous stimulation with a short duration optical pulse (1-5 ms) enhanced LIDs. This effect was blocked by the general muscarinic acetylcholine receptor (mAChR) antagonist atropine indicating it was mAChR-mediated. By contrast, continuous stimulation with a longer duration optical pulse (20 ms to 1 s) reduced LIDs to a similar extent as nicotine treatment (~50%). The general nicotinic acetylcholine receptor (nAChR) antagonist mecamylamine blocked the decline in LIDs with longer optical pulses showing it was nAChR-mediated. None of the stimulation regimens altered LIDs in control-eYFP mice. Lesion-induced motor impairment was not affected by optical stimulation indicating that cholinergic transmission selectively regulates LIDs. Longer pulse stimulation increased the number of c-Fos expressing ChAT neurons, suggesting that changes in this immediate early gene may be involved. These results demonstrate that striatal cholinergic interneurons play a critical role in LIDs and support the idea that nicotine treatment reduces LIDs via nAChR desensitization.
Keywords: ChAT-Cre, ChR2, Dyskinesias, Nicotinic, Optogenetics, Parkinson's disease, striatum
Introduction
A major limitation of L-dopa therapy for Parkinson's disease is the development of LIDs (Huot et al., 2013; Iravani et al., 2012; Meissner et al., 2011; Obeso et al., 2010; Schapira and Jenner, 2011). These involuntary abnormal movements arise in the majority of patients with continued L-dopa use and can be debilitating. The only drug available for the treatment of LIDs is amantadine, which has variable efficacy, response durability and tolerability (Crosby et al., 2003; Luginger et al., 2000; Rodnitzky and Narayanan, 2014; Thomas et al., 2004). Numerous studies over the last three decades show that alterations in the dopaminergic, serotonergic, glutamatergic, GABAergic, noradrenergic, histaminergic and opioid systems are involved in LIDs; however, identification of clinical therapies targeting these systems has proved elusive (Blandini and Armentero, 2012; Cenci, 2007; Gasparini et al., 2013; Huot et al., 2013; Iravani et al., 2012; Lim et al., 2015; Rylander, 2012).
A novel target that has recently attracted attention is the striatal cholinergic system. The rationale is based on several lines of evidence including studies showing a close anatomical overlap between striatal dopaminergic nerve terminals and cholinergic interneurons (Lim et al., 2014; Zhou et al., 2002). Cholinergic interneurons make up 1-3% of striatal neurons and extend large and dense axonal arbors throughout the striatum that allow them to play a pivotal role in the control of striatal function (Bonsi et al., 2011; Zhou et al., 2002). These neurons fire action potentials at a rate of 5-10 Hz in a variable but ongoing manner that includes single spiking and rhythmic bursting patterns (Aosaki et al., 1995; Raz et al., 1996; Wilson et al., 1990). This constant activity ensures a sufficient level of endogenous ACh to tonically activate mAChRs and nAChRs to regulate GABAergic, dopaminergic and glutamate signaling (Quik and Wonnacott, 2011).
Alterations in cholinergic signaling and its functional interplay with the dopaminergic, GABAergic and glutamatergic systems in the striatum are implicated in movement disorders such as LIDs. Accumulating studies show that nAChR agonists and antagonists reduce LIDs in rodent and nonhuman primate parkinsonian animal models, (Bordia et al., 2008; Huang et al., 2011; Quik et al., 2007; Zhang et al., 2014a; Zhang et al., 2014b), with similar findings in a small clinical trial (http://www.neuraltus.com/pages/news.html). Additionally, mAChR antagonists reduce LIDs possibly by altering basal firing rate and dopamine-dependent excitation of cholinergic interneurons via alterations in extracellular signal-regulated kinase (ERK) signaling (Ding et al., 2011). Moreover, neurotoxic ablation of striatal cholinergic interneurons led to a marked reduction in LIDs (Won et al., 2014). However, the effect of selective activation of striatal cholinergic interneurons on LIDs is unknown.
The objective of the present work was to better understand the role of the striatal cholinergic system in the expression of LIDs. To approach this, we used optogenetics that allows for the selective stimulation of striatal cholinergic interneurons in unilateral 6-OHDA-lesioned ChAT-Cre mice expressing ChR2-eYFP. Two optical stimulation paradigms were used, which involved continuous stimulation of cholinergic neurons with short or longer duration optical pulses. Results show that short optical pulses enhanced LIDs while longer pulses reduced their occurrence via an interaction at nAChRs and mAChRs. These data directly demonstrate a role for striatal cholinergic interneurons and suggest that cholinergic agonists and antagonists may be useful for the treatment of LIDs in Parkinson's disease.
Materials and Methods
Mice
Two sets of homozygous male and female ChAT-internal ribosome entry site-cre knockin mice, purchased from Jackson Laboratory (B6.129S6-Chattm1(cre)Lowl), were used for these studies. Adult mice were group-housed in a temperature- and humidity-controlled environment under a 12 h light/dark cycle with free access to food and water. After a 2-3 d acclimation period, they were treated as depicted in the timeline in Fig. 1. Mice were first lesioned by stereotaxic injection of 6-hydroxydopamine (6-OHDA) to produce nigrostriatal dopaminergic damage (Cenci and Lundblad, 2007; Huang et al., 2011). Briefly, mice were anesthetized with isofluorane (3%), and then placed in a Kopf stereotaxic instrument. A burr hole was drilled through the skull and 6-OHDA (6-7 μg free base/1μl) stereotaxically injected into the ascending medial forebrain bundle at the following coordinates relative to Bregma and the dural surface: AP, −1.2mm; ML, −1.2mm; DV, 4.75mm. All procedures were approved by the Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
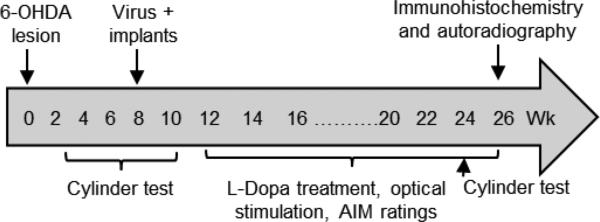
Fig. 1.
Treatment timeline. ChAT-Cre mice were unilaterally lesioned with 6-OHDA into the medial forebrain bundle. They were rated for 6-OHDA-induced motor impairment. Virus expressing ChR2-eYFP or control-eYFP was then injected into the striatum on the lesioned side, and optic fiber placement done. This was followed by L-dopa treatment, optical stimulation and AIM ratings. Mice were rated for motor impairment throughout.
Assessment of motor deficits
6-OHDA-induced motor impairment was assessed after lesioning using the cylinder or forelimb placement test (Fig. 1), a frequently used measure of nigrostriatal damage (Huang et al., 2011; Schallert et al., 2000). In initial experiments, exploratory activity was assessed for a 3 min period in the absence of L-dopa by a blinded rater. In later experiments, mice were rated immediately before and 60 min after L-dopa administration when drug effects are maximal. Wall exploration was expressed in terms of the % use of the impaired contralateral forelimb compared to the total number of forelimb contacts.
Viral expression
Eight wk after 6-OHDA lesioning, mice received a stereotaxic injection of cre-inducible recombinant AAV vector expressing ChR2 (AAV5.EF1a.DIO.hChR2(H134R)-eYFP.WPRE.hGH) or control vector (AAV5.EF1a.DIO.eYFP.WPRE.hGH) into the striatum to induce expression in cholinergic interneurons (Freeze et al., 2013; Kravitz et al., 2010; Nelson et al., 2014; Threlfell et al., 2012; Won et al., 2014). Virus was obtained from the Vector Core at the University of Pennsylvania, with a viral concentration of 1-4 ×1012 particles/ml. One μl virus was injected at the following coordinates: AP +0.8 mm, ML +2.2 mm, DV −2.7 mm. Immediately after viral injection, the mice were implanted with optical fibers (0.2 mm diameter) in the dorsolateral striatum (AP +0.8 mm, ML +2.2 mm, DV −2.5 mm) to allow for in vivo optical stimulation of cholinergic interneurons during behavioral assessment. This striatal area was selected because it plays a major role in LIDs. One wk later the mice were again rated for 6-OHDA-induced motor impairment.
L-dopa treatment and dyskinesia ratings
Four wk after virus administration, mice were injected with L-dopa methyl ester (L-dopa) and benserazide hydrochloride (a DOPA decarboxylase inhibitor given to inhibit the breakdown of L-dopa in the periphery), both purchased from Sigma Aldrich (St. Louis, MO, USA). Two to 3 mg/kg L-dopa were dissolved in saline and injected sc together with a fixed dose of 15 mg/kg benserazide. The mice were injected with L-dopa once daily 5 d per week for 3 wk. L-dopa-induced abnormal involuntary movements (AIMs) were then rated by a blinded rater once weekly. Briefly, the mouse's optical fiber implant was coupled to a laser without stimulation to allow the mice to accommodate to the experimental set up. The mouse was then placed in the cylinder for several min for further acclimation and then subsequently injected with L-dopa. AIMs were scored individually every 5 min for 1 min for a 2-h period after L-dopa injection using a modified mouse dyskinesia scale, in which both frequency and amplitude of different subtypes of AIMs (oral, forelimb, and axial) were rated as previously described (Bordia et al., 2015; Cenci and Lundblad, 2007; Huang et al., 2011; Quik et al., 2013; Quik et al., 2012; Winkler et al., 2002). The original AIMs scale had the drawback that it only took into account the frequency of a dyskinetic movement. Measuring both frequency and amplitude allows for a more complete assessment of AIMs as it offers the advantage that it is sensitive to variations in amplitude during different phases of L-dopa treatment and/or between individual mice (Bordia et al., 2015; Cenci and Lundblad, 2007; Huang et al., 2011; Quik et al., 2013; Quik et al., 2012; Winkler et al., 2002). Each AIM subtype (oral, forelimb, and axial) was scored on a frequency scale from 0 to 4 (0 = no dyskinesia; 1 = occasional dyskinesia displayed for <50% of the observation time; 2 = sustained dyskinesia displayed for >50% of the observation time; 3 = continuous dyskinesia; 4 = continuous dyskinesia not interruptible by external stimuli). Amplitude scores were subdivided as “A”, which indicates oral AIMs without tongue protrusion, forelimb AIMs without the shoulder engaged, and axial AIMs with body twisting <60°, or “B” that indicates oral AIMs with tongue protrusion, forelimb AIMs with the shoulder engaged or axial AIMs with body twisting ≥60°. The stimulation induced changes in AIM scores were consistent in all animals regardless of their amplitude score. Therefore, the overall scores for frequency and amplitude of AIMs used for data analysis were calculated as 1A = 1, 1B = 2, 2A = 2, 2B = 4, 3A = 4, 3B = 6, 4A = 6, 4B = 8 (Bordia et al., 2015; Huang et al., 2011; Quik et al., 2013; Quik et al., 2012). This allows for scores for any one component (axial, oral or forelimb) ranging from 1 to 8, with a maximum possible total score of 24 per time point or 576 for the entire rating period of 2 h since ratings were done every 5 min.
Optical stimulation
The effect of varying optical illumination was determined using stimulation paradigms consistent with those previously described (Freeze et al., 2013; Kravitz et al., 2010; Nelson et al., 2014; Threlfell et al., 2012). Cholinergic neurons were activated using a 473 nm diode laser adjusted such that the power was 1 mW at each fiber tip. Light intensity at 0.2 mm from the tip was calculated to be 15.9 mW/mm2 (corresponding to 73.2 mW/mm2 at the fiber tip) using the model based on direct measurements in mammalian brain tissue for predicted irradiance values developed in the Deisseroth lab (http://web.stanford.edu/group/dlab/cgi-bin/graph/chart.php). Optical stimulation consisted of a single pulse ranging from 1 ms to 1 s in duration delivered every 0.5 s. All experiments were performed using this stimulation frequency, which corresponds to 2 Hz. Pulse durations of 1 to 5 ms are designated as “short pulse stimulation”, while pulse durations ≥ 20 ms are referred to as “longer pulse stimulation”. Stimulation was done for 2 h since L-dopa is effective for such a time period in mice.
For the stimulation studies, the mice were injected with L-dopa 5 days per wk. On the third day of L-dopa treatment (Wednesday), AIMs were rated for 2 h with the mice hooked up to the stimulation set up but in the absence of optical stimulation (No stim). The mice were then optically stimulated and L-dopa-induced AIMs rated on either Thursday or Friday of the same week. It was not possible to stimulate all the mice on one day due to equipment limitations. Any one mouse was only subjected to one stimulation paradigm per wk, with the different paradigms administered on a random basis. The order of stimulation was as follows; wk 3 after L-dopa administration, 1 s; wk 4, 5 ms; wk 5, 100 ms; wk 6, 1 ms; wk 7, 20 ms; wk 8, 5 ms; wk 9, 20 ms; wk 10, 10 ms. The effect of the nAChR mecamylamine and the mAChR antagonist atropine were tested during the last 5 weeks of L-dopa treatment.
Two sets of mice were used for these studies. In the first set, we examined the effect of varying optical pulse stimulation on the expression of LIDs, as described above. Once the optimal stimulation paradigms were determined, we used these mice to test the effect of the nAChR antagonist mecamylamine (1 mg/kg) on LIDs with the drug given 30 min before stimulation. After a 2 wk washout during which maintenance L-dopa treatment was continued, the mice were killed by cervical dislocation and the brains used for ChAT immunofluorescence and autoradiography. Set 1 mice received L-dopa treatment for a total 4.5 months.
The second set of mice was used to evaluate the effect of atropine (1 mg/kg) on optical stimulus-induced alterations in LIDs. These studies were followed by a 2 wk washout period during which maintenance L-dopa treatment was continued. On the day of euthanization, the second set of mice was acutely injected with L-dopa and then immediately stimulated for 1 h with a longer duration optical pulse that reduces LIDs (≥ 20 ms). They were subsequently killed by cervical dislocation and their brain processed for autoradiography, as well as ChAT and c-Fos immunofluorescence. Set 2 mice received L-dopa treatment for a total of 6 months.
Tissue preparation
The brains were quickly removed and bisected at the mid-striatal level. The anterior portion was post-fixed in 4% paraformaldehyde and cryopreserved in 10-30% sucrose for several days. Fixed brains were then sectioned (30 μm) using a cryostat (Leica) for immunofluorescence. The posterior portion of the brain was quick frozen in isopentane on dry ice and stored at −80°C for autoradiography.
Immunofluorescence
Every sixth section spanning the dorsal striatum (A1.4 to A0.2) (total 8 sections per mouse) was used for staining for ChAT or c-Fos. Free floating sections were initially rinsed three times in 0.3% Triton X-100 in PBS, and then exposed to a blocking solution consisting of 3% normal donkey serum in 0.3% Triton X-100 in PBS for 1 h. Sections were then incubated with goat anti-c-Fos (1:600 dilution; Abcam) or goat anti-ChAT (1:200 dilution; Millipore) overnight at 4°C. This was followed by incubation with secondary antibody (donkey anti-goat Alexa-568; dilution 1:200; Invitrogen) for 2 h at RT. Sections were then rinsed in PBS, mounted onto slides with Vectashield mounting medium (Vector Labs), cover slipped and viewed under a Nikon fluorescence microscope (model Eclipse E400).
Images in the dorsolateral striatum were captured at 4x magnifications to view viral expression in striatal neurons and identify the distribution of ChR2-eYFP throughout mouse striatum from A1.4 to A0.2. C-Fos cell quantification in the dorsolateral striatum was performed using images at 10x magnification. Images were first sharpened to enhance nuclear detail using ImageJ software and the threshold adjusted to separate positive stained nuclei from background. Threshold objects of pixel size >60 were counted and expressed as the number of c-Fos positive cells (Nguyen et al., 2015). Cells expressing eYFP and c-Fos+eYFP were counted manually without knowledge of treatment. Three striatal sections at +A1.4 (A1.5-1.3) mm, +A0.8 (A0.9-0.7) mm and +A0.2 (0.3-0.1) mm from bregma were used for cell counting. Mean values were calculated for each animal and considered as an individual observation for statistical analyses.
Dopamine transporter (DAT) autoradiography
Fresh frozen sections (8 μm) were cut at −20°C in a cryostat, thaw-mounted onto poly-L-lysine coated slides, dried, and stored at −80°C. 125I-RTI-121 (specific activity, 2200 Ci/mmol; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) autoradiography was performed as described (Quik et al., 2003). Thawed brain sections were preincubated 2 × 15 min in buffer (50 mM Tris-HCl, pH 7.4, 120 mM NaCl, 5 mM KCl) and then incubated for 2 h with 100 pM 125I-RTI-121 in the same buffer also containing 0.025% BSA and 1 μM fluoxetine. Sections were washed 4 × 15 min in ice cold buffer, 1 × 10 s in ice cold double-distilled H2O, dried and exposed to Kodak MR film (Easterman Kodak Co., Rochester, NY, USA) for several days along with 3H-microscale standards (American Radiolabeled Chemicals, Inc., St. Louis, MO). Nonspecific binding was determined in the presence of the dopamine uptake inhibitor nomifensine (100 μM).
The ImageQuant program from GE Healthcare (Chalfont St. Giles, Buckinghamshire, UK) was used to obtain optical density values from autoradiographic images, which were converted to fmol/mg tissue using standard curves generated from 3H-microscale standards. These were calibrated for 125I autoradiography as described (Artymyshyn et al., 1990). Sample optical density readings were within the linear range of the standards.
Data analyses
Statistical comparisons were done using GraphPad Prism (GraphPad Software Co., San Diego, CA, USA). Since the behavioral data involves integrated or continuous data collected over an extended time frame, that is, the total or area under the time curve (2 h period) of all scores, unpaired or paired t tests or analysis of variance (ANOVA) followed by the indicated multiple comparison test were used. For the molecular studies, t test and ANOVA followed by the indicated multiple comparison test were used. A level of 0.05 was considered significant. Values are the mean ± SEM of the indicated number of mice.
Results
Short optical pulse stimulation of striatal cholinergic interneurons increases L-dopa-induced AIMs
We used transgenic mice expressing cre-recombinase under the control of the choline acetyltransferase promoter (ChAT-Cre) to allow for the selective expression of ChR2-eYFP in cholinergic neurons. All ChAT-Cre mice were first unilaterally lesioned by injection of 6-OHDA into the medial forebrain bundle. Two wk after lesioning ChAT-Cre mice showed a significant decline in forelimb use on the lesioned side compared to the contralateral unlesioned side (Table 1). When the neurodegenerative effects of the toxin were complete several weeks later, the mice were injected with ChR2-eYFP or control-eYFP into the dorsolateral striatum, and fibers implanted to allow for subsequent optical stimulation (Fig. 1). Two wk later, the mice were again rated for motor deficits; forelimb use was reduced on the side affected by the lesion of both ChR2-eYFP and control-eYFP injected mice to a similar extent as for the pre-virus condition (Table 1). Thus, 6-OHDA-induced motor impairment was observed regardless of ChAT-Cre expression, virus injection or optical fiber placement.
Table 1.
Viral expression does not affect 6-OHDA-induced motor deficits in ChAT-Cre mice
| Time | Viral injection | Forelimb use | |
|---|---|---|---|
| 2 wk after lesion | Unlesioned side | No | 76.3 ± 3.3 |
| Lesioned side | No | 23.6 ± 3.3*** | |
| 2 wk after virus and implant | Unlesioned side | Control-eYFP | 77.7 ± 13.0 |
| Lesioned side | Control-eYFP | 22.3 ± 13.0* | |
| Unlesioned side | ChR2-eYFP | 69.1 ± 3.3 | |
| Lesioned side | ChR2-eYFP | 31.0 ± 3.5*** |
ChAT-Cre mice were unilaterally lesioned with 6-OHDA. They were rated for motor impairment by assessing forelimb use 2 wk after lesioning, with a significant decline on the lesioned side compared to the contralateral lesioned side. Virus expressing ChR2-eYFP or Control-eYFP was then injected into the lesioned striatum and optic fiber placement done. Mice were rated for lesion-induced motor deficits 2 wk after virus injection and implant placement. Forelimb use was reduced on the lesioned side in both ChR2-eYFP (n = 11) and Control-eYFP (n = 3) mice, to a similar extent as the pre-virus condition. Values represent the mean ± SEM. Significance of difference from the unlesioned side:
P ≤ 0.05
P ≤ 0.001 using a t-test.
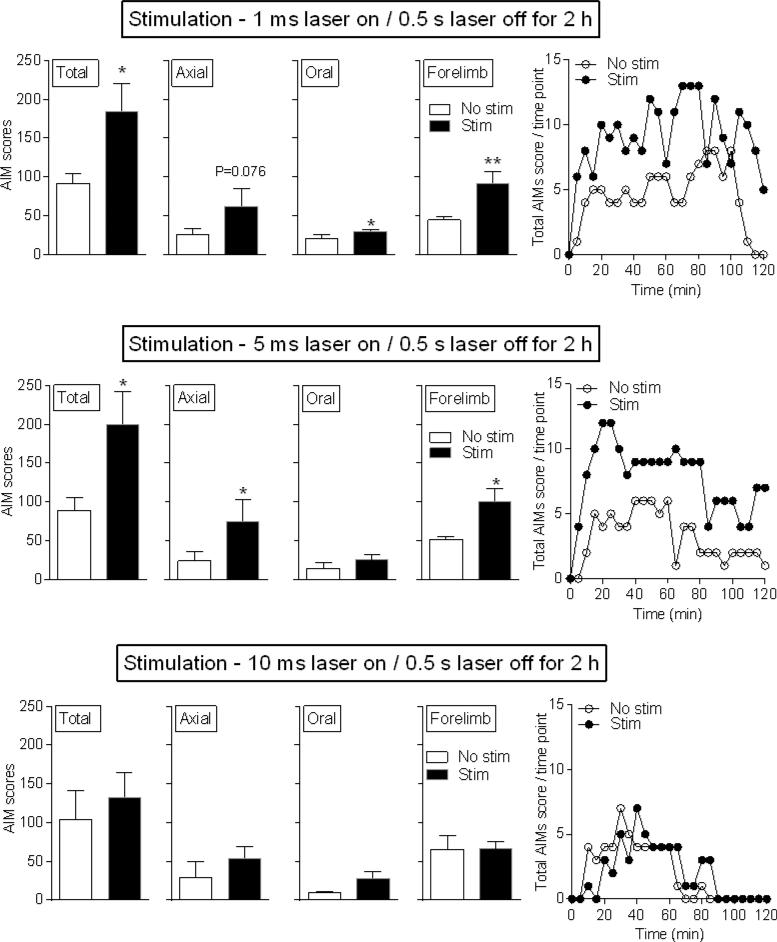
Four wk after viral administration when viral expression is stable (Zhang et al., 2010), the mice were injected with L-dopa (3 mg/kg) plus benserazide (15 mg/kg) (Fig. 1), with AIM scores similar to those previously observed (Bordia et al., 2015; Huang et al., 2011; Quik et al., 2012). The mice were then subjected to short pulse stimulation of 1 or 5 ms laser on followed by 0.5 s laser off (Fig. 2), a paradigm shown to generate single action potentials in vitro (Cachope et al., 2012; Nelson et al., 2014; Threlfell et al., 2012). Ratings were done for a 2 h period because L-dopa treatment elicits AIMs over such a time frame. Short pulse stimulation (1 and 5 ms) significantly increased total L-dopa-induced AIMs due primarily to an increase in axial and forelimb AIMs (Fig. 2). Representative time courses (Fig. 2 right panels) show a stimulation-induced increase in L-dopa-induced AIMs throughout the 2-h stimulation period. A 10 ms laser on / 0.5 s laser off paradigm for a 2 h period did not alter L-dopa-induced AIMs compared to the No stim condition (Fig. 2).
Fig. 2.
Short pulse duration optical stimulation of striatal cholinergic interneurons increases L-dopa-induced AIMs. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with L-dopa. One and 5 ms stimulation resulted in significant increases in total AIMs and various AIM components. By contrast, no change in L-dopa-induced AIMs or AIMs components occurred with 10 ms stimulation. Representative time courses are shown in the right panels. Each bar is the mean ± SEM of 5 mice. Significantly different from No stim, *P ≤ 0.05, **P ≤ 0.01, using a paired t-test.
Lesion-induced motor deficits were not affected by short pulse optical stimulation either on or off L-dopa (Table 2). Although somewhat unexpected, these data are consistent with a recent study showing that optogenetic stimulation of cholinergic neurons did not modulate 6-OHDA-induced motor impairment (Maurice et al., 2015). Thus, short pulse optical stimulation of striatal cholinergic neurons selectively regulates L-dopa-induced AIMs.
Table 2.
Short or longer pulse duration optical stimulation does not affect 6-OHDA-induced motor impairment on or off L-dopa
| Expt | Stimulation | Forelimb use (% total) | |
|---|---|---|---|
| Off L-dopa | On L-dopa | ||
| 1 | None | 21.4 ± 9.5 | 59.4 ± 4.0** |
| 5 ms laser on / 0.5 s laser off for 2h | 29.5 ± 3.6 | 58.3 ± 7.5* | |
| 2 | None | 27.2 ± 7.4 | 59.2 ± 3.7** |
| 20 ms laser on / 0.5 s laser off for 2h | 25.6 ± 6.8 | 61.6 ± 1.8** | |
Unilateral 6-OHDA-lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with L-dopa. Parkinsonism was rated before L-dopa-treatment (Off L-dopa) and 60 min after (On L-dopa) when L-dopa's effects are maximal. The mice received either short (5 ms) or longer (20 ms) pulse duration optical stimulation on wk 8 and 9 of L-dopa treatment, respectively. Values represent the mean ± SEM of 4-5 mice. L-dopa treatment similarly improved forelimb use in the absence and presence of the short (5 ms) (F(2,14) = 28.90) or longer (20 ms) stimulation duration (F(1,16) = 39.19). Significance of difference from Off L-dopa:
P ≤ 0.05
P ≤ 0.01 using two-way ANOVA followed by a Bonferroni post hoc test.
To test if the stimulus-induced increase in AIMs was due to a nonselective effect of light activation, we injected ChAT-Cre mice with control vector (control-eYFP). No increase in L-dopa-induced AIMs was observed with optical stimulation in such mice, with the data shown for the 5 ms laser on / 0.5s laser off condition for 2 h (Table 3). Thus, short pulse optical stimulation of cholinergic interneurons selectively increases AIMs in ChR2-eYFP expressing mice.
Table 3.
Short or longer pulse duration optical stimulation does not affect L-dopa-induced AIMs in mice expressing control-eYFP virus
| Pulse duration | Stimulation paradigm | L-dopa-induced AIMs |
|||
|---|---|---|---|---|---|
| Total | Axial | Oral | Forelimb | ||
| Short | None | 133 ± 62.0 | 33 ± 32.7 | 37 ± 18.6 | 63 ± 28.2 |
| 5 ms laser on / 0.5 s laser off for 2h | 140 ± 55.6 | 33 ± 27.3 | 50 ± 27.4 | 57 ± 24.4 | |
| Longer | None | 110 ± 32.7 | 2.0 ± 2.0 | 76 ± 21.8 | 32 ± 11.6 |
| 20 ms laser on / 0.5 s laser off for 2h | 126 ± 53.2 | 17 ± 16.6 | 58 ± 15.6 | 51 ± 24.6 | |
Unilaterally lesioned ChAT-Cre mice expressing control-eYFP virus in striatum were stimulated using protocols shown to increase and decrease L-dopa-induced AIMs. Values represent the mean ± SEM of 3 mice.
Short optical pulse stimulation of striatal cholinergic interneurons increases L-dopa-induced AIMs in L-dopa-treated non-dyskinetic mice
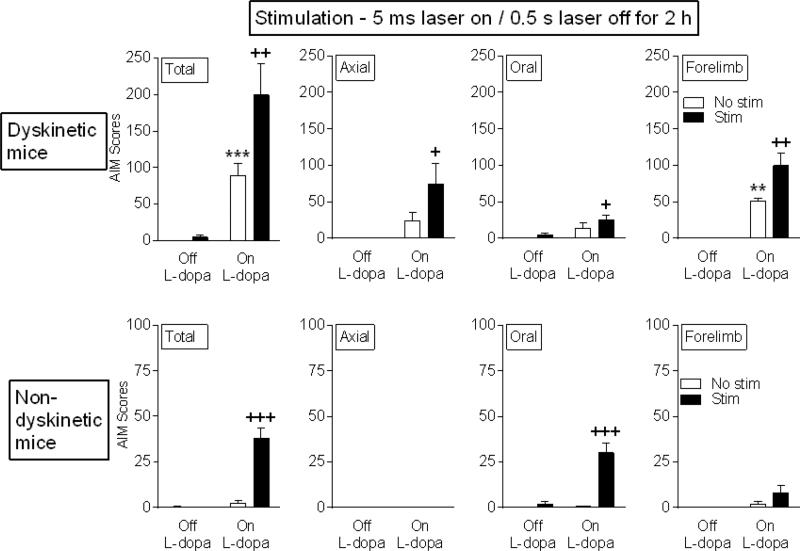
Previous work had shown that L-dopa administration induces AIMs to varying extents, with some animals experiencing little or no dyskinesias despite dopamine precursor treatment (Cenci and Lindgren, 2007; Quik et al., 2012). Such a situation also developed in the current study with a subgroup of 3 ChR2-expressing ChAT-Cre mice exhibiting no AIMs with L-dopa treatment (non-dyskinetic) (Fig. 3). The question arose if optical stimulation could elicit AIMs in non-dyskinetic L-dopa-treated mice. Short pulse optical stimulation (5 ms) did not increase AIMs in dyskinetic or non-dyskinetic mice not receiving L-dopa (Off-L-dopa). However, it significantly increased total AIMs in non-dyskinetic mice after L-dopa injection, suggesting that L-dopa sensitizes striatal systems towards the development of AIMs (Fig. 3 bottom).
Fig. 3.
Short pulse duration optical stimulation of striatal cholinergic interneurons increases L-dopa-induced AIMs in non-dyskinetic L-dopa-treated mice. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with L-dopa, and AIMs measured before (Off L-dopa) and after L-dopa administration (On L-dopa). The majority of mice express AIMs with L-dopa treatment (Dyskinetic), although a subgroup does not (Non-dyskinetic) consistent with previous work. Optical stimulation led to significant increases in total AIMs and in various AIM components in both groups of mice. Each bar is the mean ± SEM of 3-5 mice. Significantly different from Off L-dopa No stim, **P ≤ 0.01, ***P ≤ 0.001; from On L-dopa No stim, +P ≤ 0.05, ++P ≤ 0.01, +++P ≤ 0.001 using a two-way ANOVA followed by a Newman-Keuls post hoc test.
In summary, short pulse optical stimulation (1 and 5 ms) increases L-dopa-induced AIMs in dyskinetic mice and induces their occurrence in L-dopa-treated non-dyskinetic mice. These results support the idea that cholinergic neuron activation induces the expression of L-dopa-induced AIMs.
Longer pulse optical stimulation of striatal cholinergic neurons decreases L-dopa-induced AIMs
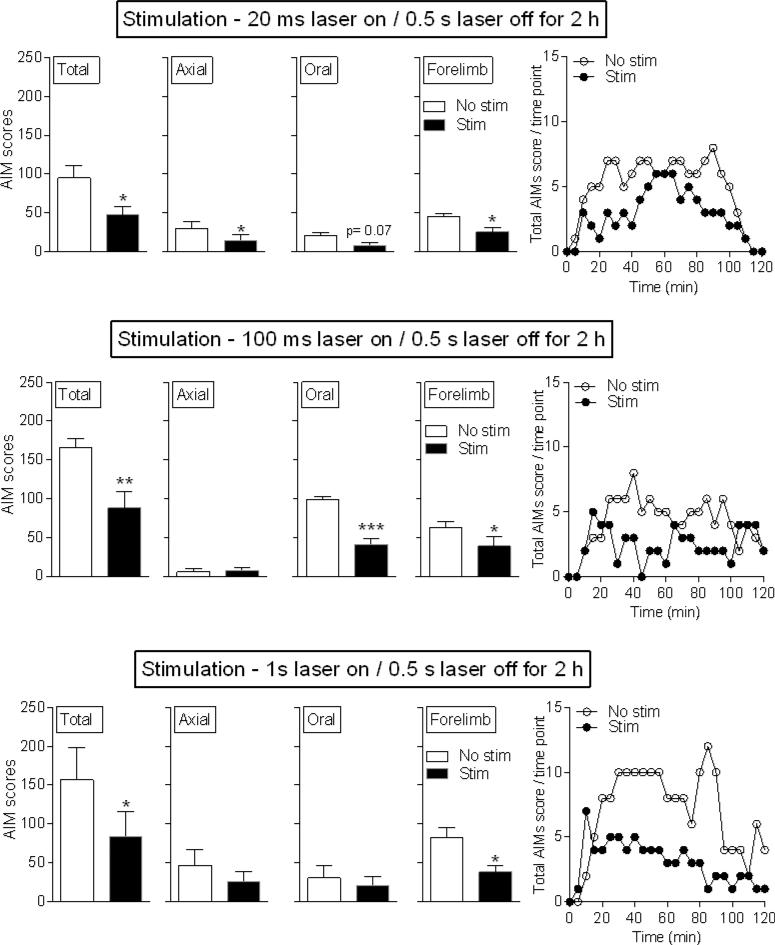
We next tested the effect of longer pulse duration optical stimulation on L-dopa-induced AIMs (Fig. 4). Stimulation ranging from 20 ms to 1 s laser on / 0.5 s laser off decreased AIMs compared to the No stim condition. The decrease appeared to be due primarily to a decline in axial and forelimb AIMs, with a trend for a reduction in oral AIMs. The stimulus-induced decline in AIMs was observed over the 2-h time course of L-dopa action (Fig. 4 right). 6-OHDA-induced motor impairment again was not affected by optical stimulation (Table 2), indicating that cholinergic interneurons selectively regulate motor function linked to L-dopa-induced AIMs.
Fig. 4.
Longer pulse duration optical stimulation of striatal cholinergic interneurons decreases L-dopa-induced AIMs. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with L-dopa. There were significant declines with 20 ms, 100 ms and 1 s stimulation. Representative time courses are shown in the right panels. Each bar is the mean ± SEM of 5 mice. Significantly different from No stim, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, using a paired t-test.
Longer pulse duration stimulation (≥ 20 ms) did not modulate L-dopa-induced AIMs in mice expressing control virus (control-eYFP) (Table 3). Thus, the decrease in L-dopa-induced AIMs was due to activation of cholinergic neurons and not a nonselective effect of light.
In summary, longer pulse stimulation (20 ms to 1 s) selectively reduces L-dopa-induced AIMs (Fig. 4) in contrast to the increase in AIMs observed with short pulse stimulation (1 and 5 ms) (Fig. 2).
The increase in L-dopa-induced AIMs with short pulse optical stimulation is mAChR-mediated
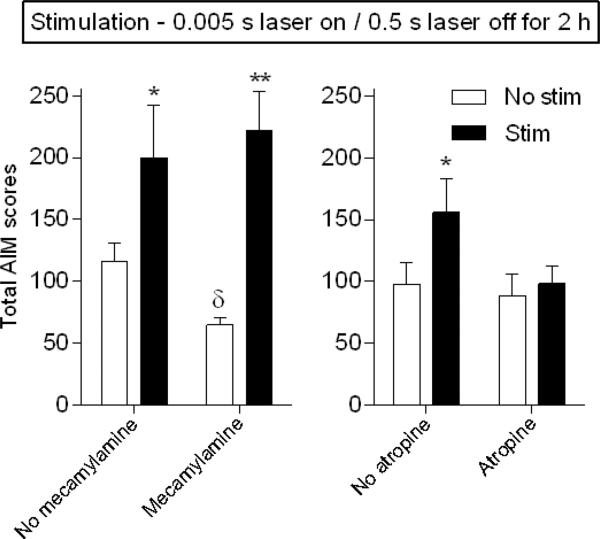
Optical stimulation of cholinergic neurons results in the release of ACh that acts on both nAChR and mAChRs. To evaluate the role of these subtypes on the short pulse (5 ms) stimulation-induced increase in AIMs, we tested the effect of cholinergic receptor blockers. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with the nAChR blocker mecamylamine (Fig. 5 left, 1 mg/kg) 30 min before L-dopa or the mAChR antagonist atropine (Fig. 5 right, 1 mg/kg) 15 min before L-dopa administration. Mecamylamine alone led to a small decline in L-dopa-induced AIMs in the absence of stimulation, as previously shown (Bordia et al., 2010; Bordia et al., 2015), but had no effect on the stimulation-induced increase in AIMs (Fig. 5 left). By contrast, atropine alone had no effect on L-dopa-induced AIMs; however, it blocked the short pulse (5 ms) stimulation-induced increase in AIMs (Fig. 5 right). These data suggest that the short pulse stimulation-induced increase in L-dopa-induced AIMs occurs via mAChRs.
Fig. 5.
The increase in L-dopa-induced AIMs with short pulse duration optical stimulation is mAChR-mediated. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with the nAChR blocker mecamylamine (left panel, 1 mg/kg) 30 min or the mAChR antagonist atropine (right panel, 1 mg/kg) 15 min before L-dopa. Atropine but not mecamylamine treatment blocked the short pulse (5 ms) stimulation-induced increase in AIMs. Each bar is the mean ± SEM of 5-6 mice. Significantly different from own No stim, *P ≤ 0.05, **P ≤ 0.001 using a two-way ANOVA followed by a Newman-Keuls post hoc test; from No mecamylamine No stim, δP ≤ 0.05 using a t-test.
The decline in L-dopa-induced AIMs with longer pulse optical stimulation is both nAChR and mAChR-mediated
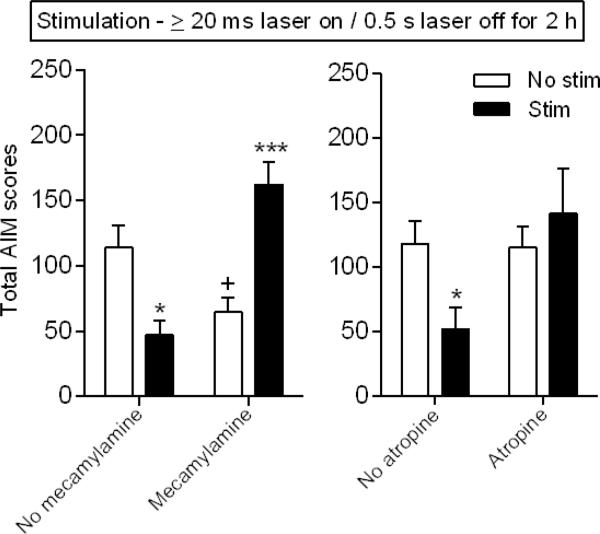
Experiments were also done to determine the role of nAChR and mAChRs in the longer pulse (≥ 20 ms laser on/0.5 s off) stimulation-induced decrease in AIMs (Fig. 6 left panel). Mecamylamine injection alone (1.0 mg/kg) decreased L-dopa-induced AIMs. Optical stimulation alone also decreased L-dopa-induced AIMs. However, no decline in AIMs was observed with combined stimulation and mecamylamine, suggesting that the decrease in AIMs with longer pulse stimulation is nAChR-mediated. The finding that L-dopa-induced AIMs were greater with combined mecamylamine and stimulation than either condition alone was somewhat unexpected and suggested that mAChRs may also be involved. This possibility is supported by the data in Fig. 6 (right panel) which shows that atropine (1.0 mg/kg) prevented the longer pulse stimulation-induced decrease in AIMs.
Fig. 6.
The decline in L-dopa-induced AIMs with longer pulse duration optical stimulation is both nAChR and mAChR-mediated. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP were injected with the nAChR antagonist mecamylamine 30 min (left panel, 1 mg/kg) or the mAChR antagonist atropine (right panel, 1 mg/kg) 15 min before L-dopa. Both mecamylamine and atropine treatment blocked the longer pulse (≥ 20 ms) stimulation-induced decrease in AIMs. Each bar is the mean ± SEM of 5-6 mice. Significantly different from own No stim, *P ≤ 0.05, ***P ≤ 0.001; from no mecamylamine no stim, +P ≤ 0.05 using a two-way ANOVA followed by a Newman-Keuls post hoc test.
Overall these findings suggest that nAChR blockers would be useful for reducing L-dopa-induced dyskinesias. By contrast, mAChR antagonist administration may increase AIM expression.
Selective expression of ChR2-eYFP in striatal cholinergic neurons
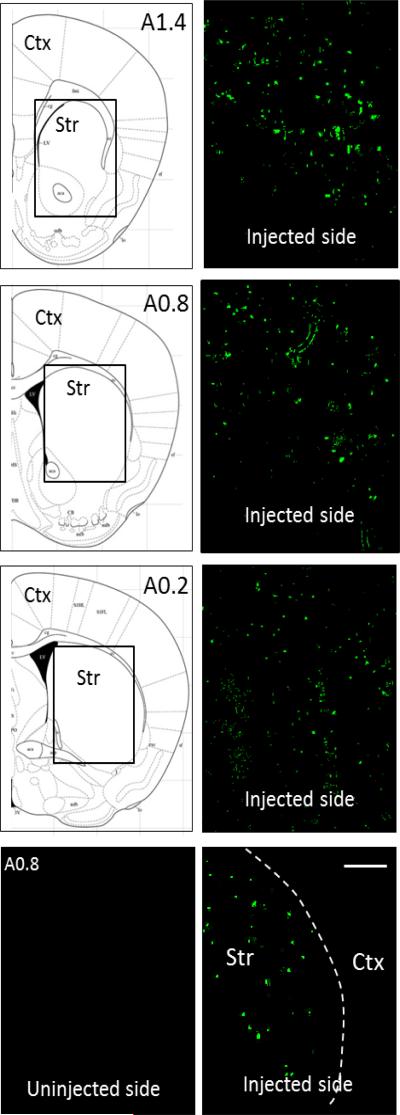
The images in Fig. 7 show expression of ChR2-eYFP throughout the striatum (level A1.4 to A0.2) on the side of the brain injected with virus, with no diffusion into the cortex (Fig. 7 lower right panel). No immunofluorescence was detected on the uninjected side (Fig. 7 lower left panel). Striatal eYFP cell counts showed that variability in striatal viral expression among mice was 16% (n=9).
Fig. 7.
Distribution of ChR2-eYFP throughout mouse striatum. Expression of ChR2-eYFP (green) was present in the striatum from A1.4 to A0.2 on the side of the brain injected with virus. The boxed area in the schematics on the left are depicted in the images on the right. No fluorescence was detected in the striatum on the uninjected side (bottom left panel). There was no diffusion into the cortex (bottom right panel), with the white stippled line depiciting the border between the cortex and striatum. Scale bar=500 μm. Str, striatum; Ctx, cortex.
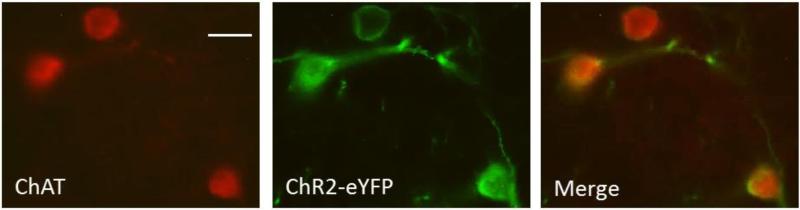
To verify the presence of ChR2-eYFP in striatal cholinergic neurons, fixed striatal slices containing ChR2-eYFP positive neurons were processed for ChAT immunoreactivity (Nelson et al., 2014; Threlfell et al., 2012). The immunofluorescent images show co-expression of ChAT and ChR2-eYFP (Fig. 8), indicating that intrastriatal injection of AAV-ChR2-eYFP led to a selective expression in ChAT neurons in the striatum.
Fig. 8.
Expression of ChR2-eYFP in striatal cholinergic neurons. Images confirming ChR2-eYFP expression in cholinergic neurons as demonstrated using ChAT immunofluorescence. ChAT (red), ChR2-eYFP (green) and the merge. Scale bar = 20 μm.
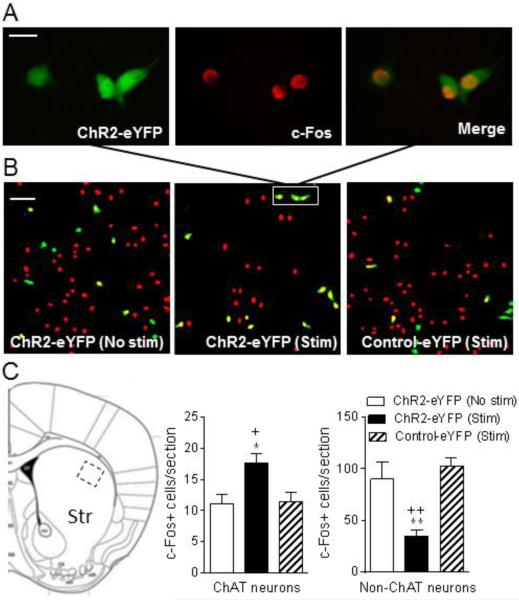
Activation of cholinergic neurons increases c-Fos+ ChAT neurons but decreases c-Fos+ non-ChAT neurons
Increases in the expression of immediate early genes such as c-Fos play an important role in numerous physiological and pathological conditions, including dopamine denervation and dopaminergic drug treatments. For instance, L-dopa treatment induces c-Fos expression in striatum by activation of both D1 and D2 receptors (Ebihara et al., 2011; Paul et al., 1992; Robertson et al., 1989). In the current study, we focused on the dorsolateral striatum because the most pronounced effect is observed in this region (Bishop et al., 2009). Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP (n = 5) or control-eYFP (n = 3) were injected with L-dopa and then immediately stimulated with light for 1 h using the longer pulse optical stimulation paradigm that reduces LIDs (≥ 20 ms). There was also a subset of ChR2-eYFP mice (n = 4) that were injected with L-dopa but not optically stimulated. All mice were killed 1 h after L-dopa administration, and the brains quickly removed and fixed for immunochemistry.
The images in Fig. 9A show that c-Fos activation occurs in ChR2-eYFP expressing ChAT positive neurons. Fig. 9B and C also demonstrate that the decrease in L-dopa-induced AIMs with optical stimulation of cholinergic neurons is associated with a significant increase in L-dopa-induced c-Fos positive striatal cholinergic neurons and decrease in the number of L-dopa-induced c-Fos expressing non-ChAT neurons. Control studies showed that L-dopa-treatment alone increased the number of c-Fos positive cells from 3.3 ± 0.57 in the unlesioned dorsolateral striatum of saline-treated mice to 27 ± 4.9 in the unlesioned dorsolateral striatum of L-dopa-treated mice. This value was further increased in the lesioned dorsolateral striatum of L-dopa-treated mice (101 ± 17). Thus changes in this immediate early gene may play a role in the expression of L-dopa-induced AIMs.
Fig. 9.
Activation of cholinergic neurons increases c-Fos+ ChAT neurons but decreases c-Fos+ non-ChAT neurons. Unilaterally lesioned ChAT-Cre mice expressing ChR2-eYFP or control-eYFP were concurrently injected with L-dopa and optically stimulated for 1 h using longer pulse optical stimulation that reduces LIDs (≥ 20 ms). There was also a subset of ChR2-eYFP mice that were injected with L-dopa but not optically stimulated. They were then killed, the brain quickly removed and fixed for immunofluorescence. A, depicts neurons in the boxed area in B showing cells immunostained with ChR2-eYFP (green, left), c-Fos (red, middle) and the merge (right). B, provides images of eYFP (green), c-Fos (red) and eYFP+c-Fos (yellow) positive neurons of unstimulated ChR2-eYFP mice (No stim, left), ChR2-eYFP mice with stimulation (Stim, middle) and control-eYFP mice with stimulation (Stim, right). Quantitation of the data is provided in C, with the boxed area in the image depicting the dorsolateral striatal area counted. Each bar is the mean ± SEM of n = 4 ChR2-eYFP (No stim), n = 5 ChR2-eYFP (Stim) and n = 3 Control-eYFP (Stim) mice. Significantly different from ChR2-eYFP (No stim), *P ≤ 0.05, **P ≤ 0.01; from Control-eYFP (Stim), +P ≤ 0.05, ++P ≤ 0.01 using a one-way ANOVA followed by a Dunnett's post hoc test. Scale bar = 20 μm and 100 μm in the upper and middle panel, respectively.
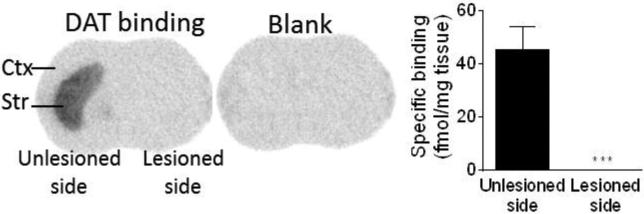
DAT measurements
DAT was evaluated using 125I-RTI-121 autoradiography (Fig. 10). The results show that the current 6-OHDA treatment protocol resulted in a ~99% decline in DAT consistent with previous work (Cenci and Lindgren, 2007; Huang et al., 2011; Quik et al., 2012).
Fig. 10.
6-OHDA treatment decreases striatal DAT in ChAT-Cre mice. 125I-RTI autoradiography demonstrated a 99% decline in the striatal DAT after 6-OHDA administration. Each bar is the mean ± SEM of 9 mice. Significantly different from the unlesioned side, ***P ≤ 0.001 using a t-test.
Discussion
The present results are the first to show that selective in vivo striatal cholinergic interneuron stimulation regulates the expression of LIDs. This was demonstrated by optogenetic activation of striatal cholinergic interneurons in 6-OHDA lesioned dyskinetic mice. Short pulse optical stimulation increased LIDs in parkinsonian ChAT-Cre mice expressing ChR2-eYFP. By contrast, longer pulse optical stimulation, which would result in a greater ACh release, significantly decreased LIDs. Notably, lesion-induced motor impairment was not altered by either short or longer pulse duration optical stimulation. Thus, cholinergic interneurons selectively regulate movement linked to LIDs with no effect on motor function associated with parkinsonism.
Our rationale for the current experiments is based on emerging studies which demonstrate that cholinergic interneurons influence striatal output and are important in the control of voluntary movements and the pathogenesis of various movement disorders (Bonsi et al., 2011; Gonzales and Smith, 2015). Of relevance to the current experiments, recent studies implicate the cholinergic system in LIDs (Ding et al., 2011; Quik et al., 2015; Won et al., 2014). However, the effect of selective activation of cholinergic interneurons to directly determine their role in LIDs has not been investigated. To approach this we optically stimulated cholinergic neurons in stably dyskinetic ChAT-Cre mice expressing ChR2-eYFP virus in the dorsal lateral striatum using various stimulation paradigms.
For our initial studies, we used continuous stimulation with a short optical pulse (1 to 5 ms). Such a stimulation paradigm was used based on previous work by others showing that 1-5 ms optical stimulation evokes single action potentials in cholinergic interneurons (Cachope et al., 2012; Threlfell et al., 2012). Short pulse stimulation increased L-dopa-induced AIMs. This effect was blocked by the general mAChR antagonist atropine, but not the general nAChR antagonist mecamylamine, suggesting it was mediated via mAChRs. Thus short pulse stimulation, which generates a limited amount of ACh release, may briefly activate mAChR, with a consequent increase in LIDs.
Experiments were subsequently done using continuous stimulation with longer optical pulse durations ranging from 20 ms to 1 s. Such paradigms reduced LIDs up to ~50% via an interaction at both nAChRs and mAChRs. Longer laser pulse stimulation of 0.1 s has been shown to induce ChR2-mediated inward currents in cholinergic interneurons that persisted through the entire optical pulse (Cachope et al., 2012). In addition, stimulation durations of 1 s have been shown to induce a burst of action potentials in cholinergic interneurons (Threlfell et al., 2012). Such heightened activity would lead to greater ACh release, and a more prolonged action of ACh at cholinergic receptors. In the case of nAChRs, this would most likely lead to receptor desensitization as nM ACh concentrations desensitize nAChRs on the order of milliseconds (Giniatullin et al., 2005; Paradiso and Steinbach, 2003; Picciotto et al., 2008). Previous microdialysis studies indicate that the amount of striatal ACh released may be on the order of fmol to pmol, which would be sufficient to desensitize nAChRs (Antoniou et al., 1997; Consolo et al., 1987; Kawashima et al., 1991; Mohr et al., 2015). Notably, previous work had shown that chronic agonist treatment decreased LIDs to a similar extent (~50%) as longer pulse optical stimulation. These combined findings provide evidence for the idea that the decrease in LIDs with longer stimulation durations and chronic agonist dosing may arise because of nAChR desensitization (Bordia et al., 2010; Bordia et al., 2015).
The present data show that short pulse stimulation not only increased LIDs in dyskinetic but also in non-dyskinetic L-dopa-treated parkinsonian mice. By contrast, stimulation immediately prior to the daily L-dopa treatment did not elicit LIDs in lesioned mice. Thus, although L-dopa did not induce dyskinesias in all animals, L-dopa-administration appears essential for the development of dyskinesias by priming or sensitizing the system. Previous studies have shown that L-dopa treatment enhances cholinergic interneuron excitability (Ding et al., 2011). Subsequent optical activation of cholinergic interneurons may provide a further stimulus for the expression of LIDs because of activation/inactivation of some common molecular mechanism. For instance, earlier work has demonstrated changes in c-Fos expression with the occurrence of LIDs (Bishop et al., 2009; Ebihara et al., 2011; Paul et al., 1992; Robertson et al., 1989). The current work shows that optical stimulation of cholinergic neurons is also associated with alterations in c-Fos expressing striatal neurons. Combined treatment may modulate levels to a greater extent and initiate LIDs.
Although both short and longer pulse optical stimulation affected LIDs, neither regimen altered the 6-OHDA-induced motor impairment either on or off L-dopa as measured by the forelimb placement test. This lack of effect on motor deficits is in agreement with previous work showing that cholinergic interneuron activation/ablation, nAChR agonists/antagonist treatment and mAChR antagonist treatment did not modulate lesion-induced motor impairment (Ding et al., 2011; Maurice et al., 2015; Quik et al., 2008; Quik et al., 2014). These data directly show that striatal cholinergic interneuron activation selectively regulates LIDs with no effect on motor impairment.
The question arises by what molecular mechanism cholinergic stimulation may regulate LIDs. Previous studies have shown that L-dopa and dopamine agonist treatment induces striatal c-Fos expression in striatum and that inhibition of c-Fos activation attenuates LIDs, providing evidence for a role for c-Fos in these abnormal movements (Bishop et al., 2009; Calon et al., 2000; Ebihara et al., 2011; Hiroi et al., 2002; Paul et al., 1992; Robertson et al., 1989). The current results show that optical stimulation of striatal cholinergic interneurons increased the number of L-dopa-induced c-Fos positive ChAT neurons and decrease the number of L-dopa-induced c-Fos positive non-ChAT neurons. These latter are probably GABAergic MSNs that constitute ~97% of striatal neurons. Since ChAT neurons are important regulators of MSN activity (Threlfell et al., 2012; Tozzi et al., 2011), induction of c-Fos in ChAT neurons may subsequently induce molecular changes in MSNs that contribute to the observed alterations in LIDs. In addition, previous studies indicate that other signaling pathways such as ERK play a role (Ding et al., 2011) possibly by eliciting downstream changes in neuronal plasticity and morphology linked to the development of LIDs (Bido et al., 2015; Cenci and Konradi, 2010; Feyder et al., 2011; Huot et al., 2011; Rangel-Barajas et al., 2011; Santini et al., 2009; Zhang et al., 2013).
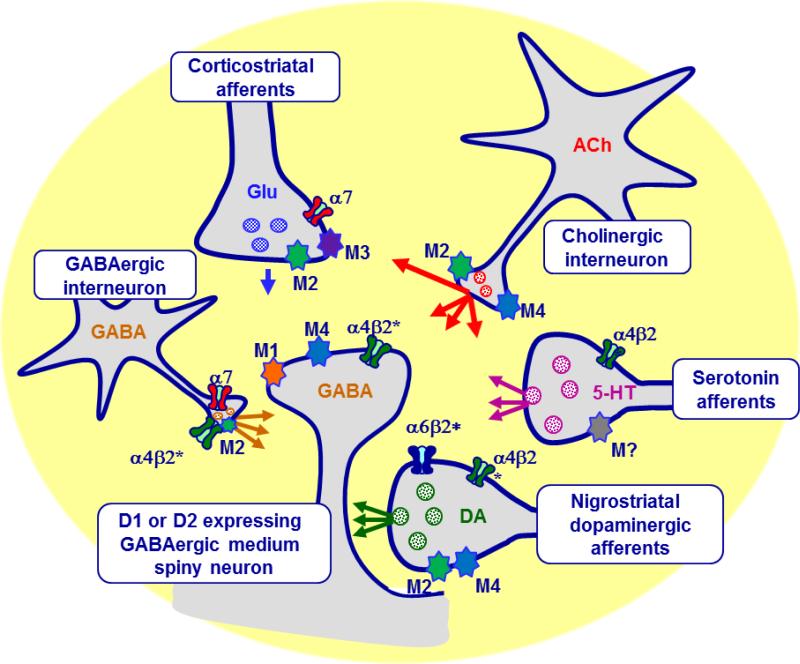
The present results demonstrate that striatal cholinergic signaling significantly influences the expression of LIDs. The cholinergic receptors mediating the effect of ACh on LIDs include both nAChR and mAChR subtypes, as described in Fig. 11 legend (Millar and Gotti, 2009; Quik and Wonnacott, 2011). There are also multiple mAChRs in the striatum with which ACh released from cholinergic interneurons may interact to regulate LIDs (Fig. 11) (Threlfell et al., 2010; Yan and Surmeier, 1996). Tonic cholinergic activity ensures a sufficient level of endogenous ACh to activate nAChR and/or mAChRs and regulate D1- and D2-expressing MSNs (Kaneko et al., 2000; Pakhotin and Bracci, 2007) (Fig. 11). Furthermore, endogenous ACh regulates dopaminergic, serotonergic and glutamatergic signaling as well as fast-spiking interneurons (Gonzales and Smith, 2015; Huot et al., 2013; Koos and Tepper, 2002; Threlfell and Cragg, 2011) (Fig. 11). Thus, multiple striatal systems may be involved in the regulation of LIDs (Blandini and Armentero, 2012; Carta and Bezard, 2011; Duty, 2012; Huot et al., 2011; Huot et al., 2013; Rylander, 2012). In addition, accumulating evidence indicates a role for other brain regions. This includes the cortex, with LIDs correlating with supersensitive excitatory transmission at corticostriatal synapses (Ueno et al., 2014) and high-frequency cortical oscillations (Richter et al., 2013). The cerebellum also plays a role in LIDs possibly via the primary motor cortex (Kishore and Popa, 2014; Kishore et al., 2014). Overall, LIDs appear to be complex in origin with the involvement of numerous brain circuits (Engeln et al., 2015), one of which is the cholinergic system.
Fig. 11.
Schematic representation of the primary striatal neuronal systems. Cholinergic interneurons are the primary source of striatal ACh and play a major role in modifying striatal function by interaction at pre-and post-synaptic nAChRs and mAChRs. ACh regulates the activity of direct and indirect GABAergic MSNs, the main striatal output neurons by acting at α4β2* nAChRs, as well as M1 and/or M4 mAChRs. Additionally, ACh can act at α7 and α4β2* nAChRs, and M2 mAChRs on GABAergic interneurons, as well as α7 nAChRs and M2 and M3 mAChRs located on corticostriatal glutamatergic terminals. Lastly, ACh modulates striatal DA release via an interaction at α6β2*, α4β2*, M2 and/or M4 mAChRs on nigrostriatal dopaminergic and serotonergic terminals, which further regulates the output of direct and indirect pathway MSNs.
Conclusion
L-dopa-induced dyskinesias (LIDs) are a serious complication of L-dopa therapy for Parkinson's disease, for which the pathways and mechanisms are only poorly understood. The present data are the first to show that selective in vivo stimulation of striatal cholinergic interneurons regulates dyskinesias in L-dopa-treated lesioned ChAT-Cre mice, with an increase in LIDs with short pulse stimulation and a decrease with longer duration stimulation. 6-OHDA-induced motor impairment was not affected by any stimulation paradigm indicating that cholinergic transmission primarily regulates dyskinesias. The finding that the longer pulse stimulation-induced decrease in dyskinesias is nAChR-mediated suggests that nAChR drugs may be useful against dyskinesias in Parkinson's disease.
Highlights.
Short pulse stimulation of cholinergic interneurons enhanced dyskinesias.
Longer pulse stimulation of cholinergic interneurons reduced dyskinesias.
Cholinergic neuron stimulation does not affect 6-OHDA-induced motor impairment.
Striatal cholinergic interneurons play a critical role in dyskinesias.
Acknowledgement
This work was supported by National Institutes of Health Grant NS59910. We thank Dr. Alexandra Nelson for excellent discussion and advice.
Abbreviations
- AAV
adeno-associated virus
- ACh
acetylcholine
- AIMs
abnormal involuntary movements
- ANOVA
analysis of variance
- LIDs
L-dopa-induced dyskinesias
- ChAT
choline acetyltransferase
- ChR2
channelrhodopsin 2
- DAT
dopamine transporter
- ERK
extracellular signal regulated kinase1/2
- mAChR
muscarinic acetylcholine receptor
- nAChR
nicotinic acetylcholine receptor
- 6-OHDA
6-hydroxydopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relevant conflict of interest
There are no other conflicts of interest.
References
- Antoniou K, et al. Differential effects of the neuropeptide galanin on striatal acetylcholine release in anaesthetized and awake rats. Br J Pharmacol. 1997;121:1180–6. doi: 10.1038/sj.bjp.0701233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosaki T, et al. Temporal and spatial characteristics of tonically active neurons of the primate's striatum. J Neurophysiol. 1995;73:1234–52. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Artymyshyn R, et al. The use of 3H standards in 125I autoradiography. J Neurosci Methods. 1990;32:185–92. doi: 10.1016/0165-0270(90)90139-7. [DOI] [PubMed] [Google Scholar]
- Bido S, et al. Differential involvement of Ras-GRF1 and Ras-GRF2 in L-DOPA-induced dyskinesia. Ann Clin Transl Neurol. 2015;2:662–78. doi: 10.1002/acn3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, et al. Contribution of the striatum to the effects of 5-HT1A receptor stimulation in LDOPA-treated hemiparkinsonian rats. J Neurosci Res. 2009;87:1645–58. doi: 10.1002/jnr.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Armentero MT. New pharmacological avenues for the treatment of L-DOPA-induced dyskinesias in Parkinson's disease: targeting glutamate and adenosine receptors. Expert Opin Investig Drugs. 2012;21:153–68. doi: 10.1517/13543784.2012.651457. [DOI] [PubMed] [Google Scholar]
- Bonsi P, et al. Centrality of striatal cholinergic transmission in Basal Ganglia function. Front Neuroanat. 2011;5:6. doi: 10.3389/fnana.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, et al. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2008;327:239–47. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- Bordia T, et al. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–38. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, et al. Evidence for a role for alpha6 nAChRs in l-dopa-induced dyskinesias using parkinsonian alpha6 nAChR gain-of-function mice. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, et al. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calon F, et al. Molecular basis of levodopa-induced dyskinesias. Ann Neurol. 2000;47:S70–8. [PubMed] [Google Scholar]
- Carta M, Bezard E. Contribution of pre-synaptic mechanisms to l-DOPA-induced dyskinesia. Neuroscience. 2011;198:245–251. doi: 10.1016/j.neuroscience.2011.07.070. [DOI] [PubMed] [Google Scholar]
- Cenci M, Lindgren H. Advances in understanding l-DOPA-induced dyskinesia. Curr Opin Neurobiol. 2007;17:665–71. doi: 10.1016/j.conb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30:236–43. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Prog Brain Res. 2010;183C:209–233. doi: 10.1016/S0079-6123(10)83011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci. Chapter. 2007;9:1–23. doi: 10.1002/0471142301.ns0925s41. [DOI] [PubMed] [Google Scholar]
- Consolo S, et al. Determination of endogenous acetylcholine release in freely moving rats by transstriatal dialysis coupled to a radioenzymatic assay: effect of drugs. J Neurochem. 1987;48:1459–65. doi: 10.1111/j.1471-4159.1987.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Crosby NJ, et al. Amantadine for dyskinesia in Parkinson's disease. Cochrane Database Syst Rev. 2003:CD003467. doi: 10.1002/14651858.CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, et al. Enhanced striatal cholinergic neuronal activity mediates L-DOPA-induced dyskinesia in parkinsonian mice. Proc Natl Acad Sci U S A. 2011;108:340–345. doi: 10.1073/pnas.1006511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson's disease. CNS Drugs. 2012;26:1017–32. doi: 10.1007/s40263-012-0016-z. [DOI] [PubMed] [Google Scholar]
- Ebihara K, et al. Differential expression of FosB, c-Fos, and Zif268 in forebrain regions after acute or chronic L-DOPA treatment in a rat model of Parkinson's disease. Neurosci Lett. 2011;496:90–4. doi: 10.1016/j.neulet.2011.03.087. [DOI] [PubMed] [Google Scholar]
- Engeln M, et al. Widespread Monoaminergic Dysregulation of Both Motor and Non-Motor Circuits in Parkinsonism and Dyskinesia. Cereb Cortex. 2015;25:2783–92. doi: 10.1093/cercor/bhu076. [DOI] [PubMed] [Google Scholar]
- Feyder M, et al. L-DOPA-Induced Dyskinesia and Abnormal Signaling in Striatal Medium Spiny Neurons: Focus on Dopamine D1 Receptor-Mediated Transmission. Front Behav Neurosci. 2011;5:71. doi: 10.3389/fnbeh.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze BS, et al. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–9. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini F, et al. Metabotropic glutamate receptors for Parkinson's disease therapy. Parkinsons Dis. 2013;2013:196028. doi: 10.1155/2013/196028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, et al. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–8. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gonzales KK, Smith Y. Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann N Y Acad Sci. 2015;1349:1–45. doi: 10.1111/nyas.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, et al. Molecular dissection of dopamine receptor signaling. J Chem Neuroanat. 2002;23:237–42. doi: 10.1016/s0891-0618(02)00010-8. [DOI] [PubMed] [Google Scholar]
- Huang L, et al. Nicotine Reduces L-Dopa-Induced Dyskinesias by Acting at {beta}2 Nicotinic Receptors. J Pharmacol Exp Ther. 2011;338:932–941. doi: 10.1124/jpet.111.182949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot P, et al. Anatomically selective serotonergic type 1A and serotonergic type 2A therapies for Parkinson's disease: an approach to reducing dyskinesia without exacerbating parkinsonism? J Pharmacol Exp Ther. 2011;339:2–8. doi: 10.1124/jpet.111.184093. [DOI] [PubMed] [Google Scholar]
- Huot P, et al. The pharmacology of L-DOPA-induced dyskinesia in Parkinson's disease. Pharmacol Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- Iravani MM, et al. Striatal plasticity in Parkinson's disease and L-DOPA induced dyskinesia. Parkinsonism Relat Disord. 18 Suppl. 2012;1:S123–5. doi: 10.1016/S1353-8020(11)70038-4. [DOI] [PubMed] [Google Scholar]
- Kaneko S, et al. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science. 2000;289:633–7. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- Kawashima K, et al. Determination of acetylcholine release in the striatum of anesthetized rats using in vivo microdialysis and a radioimmunoassay. J Neurochem. 1991;57:882–7. doi: 10.1111/j.1471-4159.1991.tb08233.x. [DOI] [PubMed] [Google Scholar]
- Kishore A, Popa T. Cerebellum in levodopa-induced dyskinesias: the unusual suspect in the motor network. Front Neurol. 2014;5:157. doi: 10.3389/fneur.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore A, et al. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson's disease: clues from dyskinetic patients. Cereb Cortex. 2014;24:2055–67. doi: 10.1093/cercor/bht058. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–35. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–6. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SA, et al. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci. 2014;6:22. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SA, et al. Enhanced histamine H2 excitation of striatal cholinergic interneurons in l-DOPA-induced dyskinesia. Neurobiol Dis. 2015;76C:67–76. doi: 10.1016/j.nbd.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginger E, et al. Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson's disease. Mov Disord. 2000;15:873–8. doi: 10.1002/1531-8257(200009)15:5<873::aid-mds1017>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Maurice N, et al. Striatal Cholinergic Interneurons Control Motor Behavior and Basal Ganglia Function in Experimental Parkinsonism. Cell Rep. 2015;13:657–66. doi: 10.1016/j.celrep.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Meissner WG, et al. Priorities in Parkinson's disease research. Nat Rev Drug Discov. 2011;10:377–93. doi: 10.1038/nrd3430. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–46. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Mohr F, et al. Dysfunctional Presynaptic M2 Receptors in the Presence of Chronically High Acetylcholine Levels: Data from the PRiMA Knockout Mouse. PLoS One. 2015;10:e0141136. doi: 10.1371/journal.pone.0141136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, et al. Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron. 2014;82:63–70. doi: 10.1016/j.neuron.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, et al. Activation of the mouse primary visual cortex by medial prefrontal subregion stimulation is not mediated by cholinergic basalo-cortical projections. Front Syst Neurosci. 2015;9:1. doi: 10.3389/fnsys.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med. 2010;16:653–61. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Pakhotin P, Bracci E. Cholinergic interneurons control the excitatory input to the striatum. J Neurosci. 2007;27:391–400. doi: 10.1523/JNEUROSCI.3709-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso KG, Steinbach JH. Nicotine is highly effective at producing desensitization of rat alpha4beta2 neuronal nicotinic receptors. J Physiol. 2003;553:857–71. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ML, et al. D1-like and D2-like dopamine receptors synergistically activate rotation and cfos expression in the dopamine-depleted striatum in a rat model of Parkinson's disease. J Neurosci. 1992;12:3729–42. doi: 10.1523/JNEUROSCI.12-10-03729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, et al. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. alpha4beta2 nicotinic receptors play a role in the nAChR-mediated decline in ldopa-induced dyskinesias in parkinsonian rats. Neuropharmacology. 2013;71:191–203. doi: 10.1016/j.neuropharm.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Annals of Neurology. 2007;62:588–96. doi: 10.1002/ana.21203. [DOI] [PubMed] [Google Scholar]
- Quik M, et al. Nicotine and Parkinson's disease: implications for therapy. Mov Disord. 2008;23:1641–52. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. Role for alpha6 nicotinic receptors in l-dopa-induced dyskinesias in parkinsonian mice. Neuropharmacology. 2012;63:450–9. doi: 10.1016/j.neuropharm.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–79. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson's Disease. Pharmacol Rev. 2011;63:938–66. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. Alpha7 nicotinic receptors as therapeutic targets for Parkinson's disease. Biochem Pharmacol. 2015 doi: 10.1016/j.bcp.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144:50–9. doi: 10.1016/j.pharmthera.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Barajas C, et al. l-DOPA-induced dyskinesia in hemiparkinsonian rats is associated with up-regulation of adenylyl cyclase type V/VI and increased GABA release in the substantia nigra reticulata. Neurobiol Dis. 2011;41:51–61. doi: 10.1016/j.nbd.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Raz A, et al. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J Neurophysiol. 1996;76:2083–8. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- Richter U, et al. Mechanisms underlying cortical resonant states: implications for levodopa-induced dyskinesia. Rev Neurosci. 2013;24:415–29. doi: 10.1515/revneuro-2013-0018. [DOI] [PubMed] [Google Scholar]
- Robertson GS, et al. L-dopa activates c-fos in the striatum ipsilateral to a 6-hydroxydopamine lesion of the substantia nigra. Eur J Pharmacol. 1989;159:99–100. doi: 10.1016/0014-2999(89)90049-6. [DOI] [PubMed] [Google Scholar]
- Rodnitzky RL, Narayanan NS. Amantadine's role in the treatment of levodopa-induced dyskinesia. Neurology. 2014;82:288–9. doi: 10.1212/WNL.0000000000000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander D. The serotonin system: a potential target for anti-dyskinetic treatments and biomarker discovery. Parkinsonism Relat Disord. 18 Suppl. 2012;1:S126–8. doi: 10.1016/S1353-8020(11)70039-6. [DOI] [PubMed] [Google Scholar]
- Santini E, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–33. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- Schallert T, et al. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov Disord. 2011;26:1049–55. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- Thomas A, et al. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:141–3. [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, et al. Striatal Muscarinic Receptors Promote Activity Dependence of Dopamine Transmission via Distinct Receptor Subtypes on Cholinergic Interneurons in Ventral versus Dorsal Striatum. J Neurosci. 2010;30:3398–408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ. Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci. 2011;5:11. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, et al. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75:58–64. doi: 10.1016/j.neuron.2012.04.038. [DOI] [PubMed] [Google Scholar]
- Tozzi A, et al. The distinct role of medium spiny neurons and cholinergic interneurons in the D(2)/A(2)A receptor interaction in the striatum: implications for Parkinson's disease. J Neurosci. 2011;31:1850–62. doi: 10.1523/JNEUROSCI.4082-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, et al. Morphological and electrophysiological changes in intratelencephalic-type pyramidal neurons in the motor cortex of a rat model of levodopa-induced dyskinesia. Neurobiol Dis. 2014 doi: 10.1016/j.nbd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, et al. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci. 1990;10:508–19. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler C, et al. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–86. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]
- Won L, et al. Striatal Cholinergic Cell Ablation Attenuates L-DOPA Induced Dyskinesia in Parkinsonian Mice. J Neurosci. 2014;34:3090–4. doi: 10.1523/JNEUROSCI.2888-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Surmeier DJ. Muscarinic (m2/m4) receptors reduce N- and P-type Ca2+ currents in rat neostriatal cholinergic interneurons through a fast, membrane-delimited, G-protein pathway. J Neurosci. 1996;16:2592–604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, et al. ABT-089 and ABT-894 reduce levodopa-induced dyskinesias in a monkey model of Parkinson's disease. Mov Disord. 2014a;29:508–17. doi: 10.1002/mds.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, et al. The alpha7 nicotinic receptor agonist ABT-107 decreases L-Dopa-induced dyskinesias in parkinsonian monkeys. J Pharmacol Exp Ther. 2014b;351:25–32. doi: 10.1124/jpet.114.216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–56. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Aberrant restoration of spines and their synapses in L-DOPA-induced dyskinesia: involvement of corticostriatal but not thalamostriatal synapses. J Neurosci. 2013;33:11655–67. doi: 10.1523/JNEUROSCI.0288-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, et al. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]