Abstract
Seizures occur due to an imbalance between excitation and inhibition, with the balance tipping towards excitation, and glutamate is the predominant excitatory neurotransmitter in the central nervous system of mammals. Since upregulation of expression and/or function of glutamate receptors can contribute to seizures we determined the effects of three antagonists, NBQX, GYKI-52466 and MK 801, of the various ionotropic glutamate receptors, AMPA, NMDA and KA, on acute seizure development in the Theiler’s murine encephalomyelitis virus (TMEV)-induced seizure model. We found that only NBQX had an effect on acute seizure development, resulting in a significantly higher number of mice experiencing seizures, an increase in the number of seizures per mouse, a greater cumulative seizure score per mouse and a significantly higher mortality rate among the mice. Although NBQX has previously been shown to be a potent anticonvulsant in animal seizure models, seizures induced by electrical stimulation, drug administration or as a result of genetic predisposition may differ greatly in terms of mechanism of seizure development from our virus-induced seizure model, which could explain the opposite, proconvulsant effect of NBQX observed in the TMEV-induced seizure model.
Keywords: Theiler’s murine encephalomyelitis virus, Seizures, Viral encephalitis, Picornavirus, Ionotropic glutamate receptors, Glutamate synapses, Excitatory neurotransmitter, Antagonists
Introduction
Seizures occur due to an imbalance between excitation and inhibition with the balance tipping towards excitation (Nadler, 2012). Glutamate is the predominant excitatory neurotransmitter in the central nervous system (CNS) of mammals with fully 60–70% of all synapses being glutamate synapses (Nadler, 2012). Therefore upregulation of expression and/or function of glutamate receptors can contribute to seizures (Dingledine, 2012;Nadler, 2012). There are three subfamilies of ionotropic glutamate receptors, named based on their activation by selective agonists: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate), NMDA (N-methyl-D-aspartate) and KA (kainate) receptors (Dingledine, 2012). AMPA, NMDA and KA receptors are expressed on microglia, astrocytes, oligodendrocytes, and neurons (Gottlieb and Matute, 1997;Hagino et al, 2004;Lees, 2000;Matute, 2006;Murugan et al, 2011;Nadler, 2012;Noda et al, 2000). The region of the brain with the highest density of NMDA and AMPA receptors is the hippocampus, specifically CA1 for AMPA receptors (Rainbow et al, 1984;Wong et al, 1986). KA receptors are found to be most abundant in brain regions where NMDA receptors are least abundant (Nadler, 2012). Nevertheless, hippocampal neurons have been shown to express KA receptors (Paternain et al, 1995). The kinetic properties of KA receptors are intermediate between AMPA and NMDA receptors and there is considerable overlap in agonist action between KA and AMPA receptors (Nadler, 2012). Antagonists to the various ionotropic glutamate receptors, used in this study, include: NBQX [2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline], a highly selective competitive antagonist of AMPA and KA receptors (Sheardown et al, 1990); GYKI-52466 [1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine], a non-competitive antagonist, or negative allosteric modulator, of AMPA receptors (Lees, 2000;Wilding and Huettner, 1995); and MK 801 [(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine], also known as Dizocilpine, a potent non-competitive antagonist of NMDA receptors (Rod and Auer, 1989;Wong et al, 1986). NBQX, GYKI-52466 and MK 801 all readily penetrate into the CNS when administered peripherally (Sheardown et al, 1990;Smith et al, 1991;Vizi et al, 1996;Wong et al, 1986).
Previous studies have shown that NBQX, GYKI-52466 and MK 801 block seizures in various animal seizure models (Barton et al, 2003; Chapman et al, 1991; Loscher and Honack, 1994; Twele et al, 2015). We determined the effects of these antagonists on acute seizure development in the Theiler’s murine encephalomyelitis virus (TMEV)-induced seizure model, an infection-driven animal model for epilepsy [reviewed in (Libbey and Fujinami, 2011)]. In this model, approximately 50% of C57BL/6J mice infected with TMEV experience acute behavioral seizures between days 3 and 10 post-infection (p.i.) (Libbey et al, 2008;Stewart et al, 2010b). The infected mice clear the virus by about day 14 p.i. (Kirkman et al, 2010; Libbey et al, 2011b, 2010), and then approximately 50% of the mice that had acute seizures develop spontaneous seizures (epilepsy) following a latent period (Stewart et al, 2010a). Hippocampal neurons, particularly within the CA1 region, are infected and undergo cell death (Kirkman et al, 2010;Libbey et al, 2008;Stewart et al, 2010b).
We found that, of the three antagonists of ionotropic glutamate receptors tested, only NBQX had an effect on acute seizure development in the TMEV-induced seizure model. Treatment with NBQX resulted in a significantly higher number of mice experiencing seizures, an increase in the number of seizures per mouse, a greater cumulative seizure score per mouse and a significantly higher mortality rate among the mice. Therefore, although NBQX has previously been shown to be a potent anticonvulsant, it has the opposite effect in the TMEV-induced seizure model. It should be noted that animal seizure models in which the seizures are induced by electrical stimulation, drug administration or as a result of genetic predisposition may differ greatly in terms of mechanism of seizure development from our virus-induced seizure model.
Methods
Animal experiments
Four week old, male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal experiments were reviewed and approved by the University of Utah Institutional Animal Care and Use Committee (Protocol #12-09006) and conducted in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council. All animal studies complied with the ARRIVE guidelines. All efforts were made to minimize suffering. Mice were euthanized through an overdose of isoflurane.
TMEV infection
C57BL/6J mice were anesthetized with isoflurane by inhalation and infected intracerebrally (i.c.) with 3 × 105 plaque-forming units (pfu) of the Daniels (DA) strain of TMEV at a final volume of 20 μl per mouse. The site of injection was in the postparietal cortex of the right cerebral hemisphere to a depth of 2 mm [posterior (caudal) and medial of the right eye at approximately bregma −2 mm and interaural +8 mm] (Kirkman et al, 2010). The needle had a William’s collar to limit penetration of the tip to 2 mm. The DA strain of TMEV was propagated as previously described (Tsunoda et al, 1997).
Drug treatment
TMEV-infected mice were treated, via intraperitoneal (i.p.) injection, with MK 801 (1 mg/kg twice daily, Sigma, St. Louis, MO), GYKI-52466 (10 mg/kg twice daily, Sigma) (Nargi-Aizenman et al, 2004) or NBQX (approximately 22.5 mg/kg twice daily, Alomone Labs, Jerusalem, Israel) (Docagne et al, 2007), all in a 25 μl volume, starting on day 2.5 p.i. and stopping on day 10.5 p.i. These doses are tolerated and considered sufficient to block ionotropic glutamate receptor transmission in an efficient and specific manner. Control infected mice were i.p. injected with 25 μl phosphate-buffered saline (PBS).
Seizure scoring
C57BL/6J mice infected with DA were weighed and monitored daily for seizures through day 21 p.i. The monitoring of seizure activity was performed as previously described (Libbey et al, 2011a). Briefly, mice were observed for 2 hours each day immediately following the first injection of antagonist of the day. However, if a mouse experienced a new seizure (i.e. the mouse had not previously been observed having a seizure) immediately following the second injection of antagonist of the day, then the seizure was scored and recorded. Mice were scored for 1 seizure per mouse per day. Seizure activity was graded using the Racine scale: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling (Benkovic et al, 2004;Racine, 1972). Seizure burden was analyzed by assessing both seizure incidence (numbers of mice having seizures, numbers of observed seizures per mouse) and seizure severity [cumulative seizure score per mouse, numbers of mice with the various maximum seizure scores (stages 1–5), numbers of seizures scored as stage 5].
Histology
Mice were euthanized on day 21 p.i. and perfused with PBS, followed by 4% paraformaldehyde solution. Brains were harvested, divided into 5 coronal slabs, embedded in paraffin and cut into 4 μm thick tissue sections. Paraffin sections were stained with Luxol fast blue and analyzed for neuron loss in the hippocampus as previously described (Kirkman et al, 2010). Slides were examined in a blinded fashion using one slide, containing sections from all 5 coronal slabs, per brain (N = 14 to 20 brains per experimental group for antagonist-treated mice and 55 brains per experimental group for PBS-treated mice). The tissue section of only 1 of the 5 coronal slabs per slide contained the hippocampal region of the brain. The extent of neuron loss in the pyramidal cell layer of the hippocampus from CA1 to CA3 was given a graded score as follows: score 0, no damage; score 1, 10–29% cell loss; score 2, 30–59% cell loss; and score 3, >60% cell loss. A score was given for each of the two hippocampi present in a brain and then the scores were summed so the highest possible score for neuron cell loss per brain could be 6 (the highest score, 3, for two regions of the brain).
Viral antigen positive cells were detected on consecutive paraffin sections using TMEV hyperimmune rabbit serum, as previously described (Tsunoda et al, 2001;Zurbriggen and Fujinami, 1989). Astrocytes were detected on paraffin sections using glial fibrillary acidic protein (GFAP) antibody (DAKO Corp., Carpinteria, CA), as previously described (Kirkman et al, 2010). The slides were labeled using the avidin-biotin peroxidase complex technique with 3,3′-diaminobenzidine tetrahydrochloride (Sigma) in 0.01% hydrogen peroxide (Sigma) in PBS. Enumeration of viral antigen positive cells was performed in a blinded fashion with a light microscope using one slide per brain and evaluating the tissue section containing the hippocampal region (N = 15 to 20 brains per experimental group for antagonist-treated mice and 55 brains per experimental group for PBS-treated mice). Viral antigen positive cells were enumerated and summed from each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain. The extent of gliosis was semi-quantified by scoring GFAP+ activated astrocytes in the hippocampus and dentate gyrus in a blinded fashion using one slide per brain (N = 14 to 20 brains per experimental group for antagonist-treated mice and 54 brains per experimental group for PBS-treated mice). Activated astrocytes have larger cell bodies, fatter processes, and stain more intensely for GFAP than quiescent astrocytes. Gliosis was given a graded score as follows: score 0, ≤50 activated astrocytes present; score 0.5, 51–168 activated astrocytes; score 1, 169–285 activated astrocytes; score 1.5, 286–402 activated astrocytes; score 2, 403–519 activated astrocytes; score 2.5, 520–636 activated astrocytes; and score 3, ≥637 activated astrocytes present (Kirkman et al, 2010). A score was given for each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain and then the scores were summed so the highest possible score for gliosis per brain could be 12 (the highest score, 3, for four regions of the brain).
Ricinus communis agglutinin (RCA)-1 lectin histochemistry
Activated microglia and macrophages were identified by biotinylated RCA-1 (Vector Laboratories Inc., Burlingame, CA) as previously described (Suzuki et al, 1988;Tsunoda et al, 1996, 2003, 2007). One slide per brain, with 13 to 20 brains per experimental group for antagonist-treated mice and 55 brains per experimental group for PBS-treated mice, was examined in a blinded fashion. RCA-1+ cells in each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain were enumerated and summed.
Statistical analysis
The program StatView (SAS Institute Inc., Cary, NC) was used for most statistical analyses performed. The Student’s t test was performed for pairwise comparisons (observed seizures per mouse, cumulative seizure score per mouse, weights). Analysis of variance (ANOVA), followed were indicated by the Fisher’s Protected Least Significant Difference (Fisher’s PLSD) post hoc test, was performed to determine group differences for continuous data (RCA-1). The chi-square test was utilized for nominal data (seizures: yes or no; mortality: live or dead; stage 5 seizure: yes or no; seizure score maximums: yes or no). Finally, the unpaired two group Mann-Whitney U test was performed for all nonparametric analyses (neuronal cell loss and gliosis).
Results
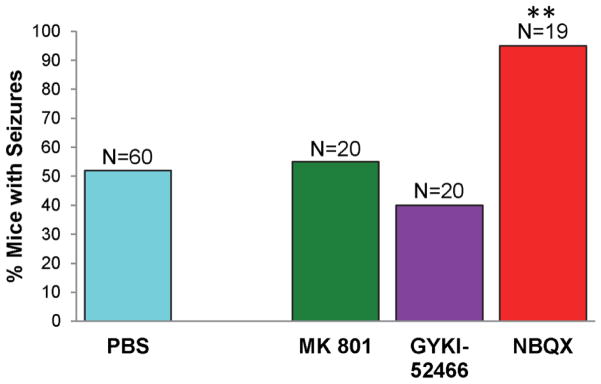
The effect of treatment, with antagonists of ionotropic glutamate receptors, on the development of seizures was examined in the TMEV-induced seizure model. TMEV-infected C57BL/6J mice treated with MK 801, GYKI-52466, NBQX or PBS as control (treatment: day 2.5–10.5 p.i.) were monitored for seizures through day 21 p.i. Mice were observed for 2 hours each day immediately following the first injection of antagonist of the day. The time course of seizure occurrence was not altered by antagonist treatment; antagonist-treated mice experienced seizures between day 3 and 7 p.i. while PBS-treated mice experienced seizures between days 3 and 8 p.i. Treatment with MK 801 or GYKI-52466 did not significantly alter the numbers of mice experiencing seizures compared to PBS treatment (Fig. 1). Treatment with NBQX, however, resulted in a significantly greater number of mice experiencing seizures compared to mice treated with PBS (p<0.001, chi-square) (Fig. 1). Additionally, the mice treated with NBQX experienced 1.9-fold more seizures per mouse (19 mice experienced 44 seizures) than the mice treated with PBS (60 mice experienced 75 seizures) over the course of the study (mice were scored for 1 seizure per mouse per day); this increase was found to be significant (p<0.01, t-test). Likewise, the mice treated with NBQX had a significantly higher (p<0.01, t-test) cumulative seizure score per mouse [10.9 ± 1.4 standard error of the mean (SEM)] compared to the mice treated with PBS (5.8 ± 0.9). Treatment with MK 801 or GYKI-52466 did not significantly alter either of these two seizure burden metrics. However, for mice that were experiencing seizures, only MK 801 demonstrated a significant increase in the numbers of mice that experienced stage 3 (p<0.0001, chi-square) and stage 4 (p<0.001, chi-square) maximum clinical stages, compared to PBS-treated mice (Table 1). There were no significant differences for GYKI-52466 or NBQX for stage 3, 4 or 5 maximum clinical stages or for MK 801 for stage 5 maximum clinical stages (Table 1). Finally, of all of the seizures that were observed, the numbers of seizures that were scored as a stage 5 on the Racine scale (the most severe seizure) were not significantly different between the various antagonist-treated mice (MK 801: 10/19, 52.6%; GYKI-52466:15/21, 71.4%; NBQX: 34/44, 77.3%) and the PBS-treated mice (55/75, 73.3%).
Figure 1.
Percent mice with seizures (Racine scale, stages 3 to 5) in antagonist-treated mice. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466 and NBQX or with phosphate-buffered saline (PBS) as a control (treatment: day 2.5–10.5 post infection). Mice were monitored for seizures through day 21 post infection. Mice were observed for 2 hours each day immediately following the first injection of antagonist of the day. The number of mice in each group is given as N above each column. The percentage of mice with seizures was calculated as follows: (number of mice with seizures/total number of mice infected) × 100. **p<0.001, chi-square test.
Table 1.
Seizures, Racine Scale – Maximum severity of disease.
| Treatment | Number of mice | Number (%) of mice with maximum clinical stages | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| PBS | 31 | 0 | 0 | 2 (6.45) | 2 (6.45) | 27 (87.1) |
| MK 801 | 11 | 0 | 0 | 1 (9.1)‡ | 2 (18.2)** | 8 (72.7) |
| GYKI-52466 | 8 | 0 | 0 | 0 | 1 (12.5) | 7 (87.5) |
| NBQX | 18 | 0 | 0 | 0 | 2 (11.1) | 16 (88.9) |
p<0.001,
p<0.0001, compared to PBS, chi-square test.
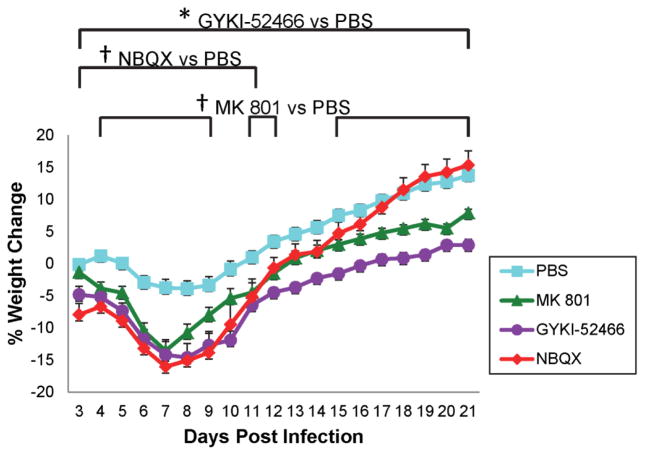
We previously reported (Libbey et al, 2008) that weight loss correlated with mice experiencing seizures. Examination of weight demonstrated that animals treated with GYKI-52466 had significantly lower body weights, compared to the PBS-treated control mice, over the entire course of the study (p<0.01, t-test) (Fig. 2). Animals treated with MK 801 also had lower body weights, compared to PBS treatment, over the entire course of the study and these differences reached significance on days 4–9, 11–12 and 15–21 p.i. (p<0.05, t-test) (Fig. 2). Animals treated with NBQX also had lower body weights, compared to PBS treatment, over most of the study course (excluding days 18–21 p.i.), however, these differences only reached significance for days 3–11 p.i. (p<0.05, t-test) (Fig. 2).
Figure 2.
Weight change in antagonist-treated mice. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466 and NBQX or with PBS as a control (treatment: day 2.5–10.5 post infection). Mice were weighed daily through day 21 post infection. Data represents percent of daily weight in comparison to weight at day −1, given as mean ± standard error of the mean (SEM) for groups of 20 mice (MK 801, GYKI-52466), 19 mice (NBQX) and 60 mice (PBS). †p<0.05, *p<0.01, t-test.
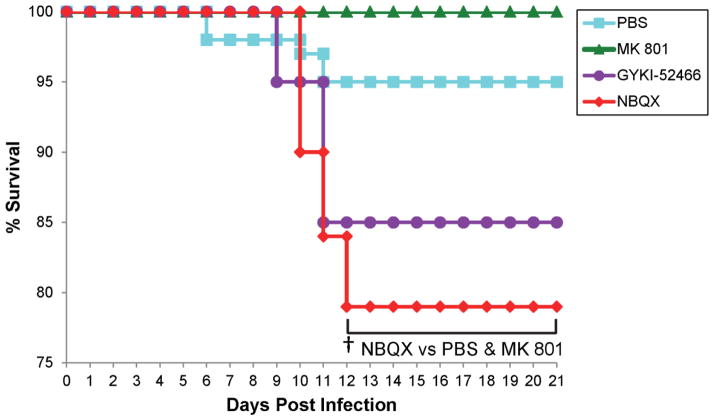
The percentage of mice that were either found dead or were moribund and needed to be euthanized varied with treatment. No mice treated with MK 801 died during the course of the study (Fig. 3). Three mice died over the course of the study in both the PBS-treated group and the GYKI-52466-treated group (Fig. 3). Four mice died in the NBQX-treated group (Fig. 3). Of the mice that died, all but one mouse (PBS group) died between days 9–12 p.i. (Fig. 3). The mortality of the NBQX-treated group was significantly greater than the PBS-treated group and the MK 801-treated group for days 12–21 p.i. (p<0.05, chi-square).
Figure 3.
Mortality in antagonist-treated mice. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466 and NBQX or with PBS as a control (treatment: day 2.5–10.5 post infection). Mice were monitored through day 21 post infection. Data represents percent daily survival in comparison to day 0. The numbers of mice per group at day 0 were 20 mice (MK 801, GYKI-52466), 19 mice (NBQX) and 60 mice (PBS). †p<0.05, chi-square test.
Therefore, of the three antagonists tested, NBQX was the antagonist that had the greatest effect on disease. NBQX-treated mice had significantly greater seizure incidence, both numbers of mice having seizures and numbers of observed seizures per mouse, and significantly greater seizure severity in the cumulative seizure score per mouse metric, but not in the numbers of mice with the various maximum seizure scores or numbers of seizures scored as stage 5 metrics. Additionally, NBQX-treated mice had significantly higher mortality among the mice.
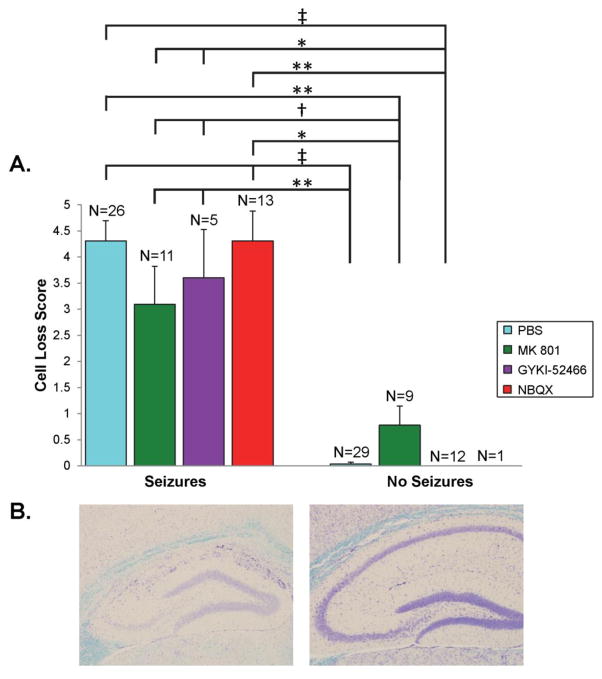
Mice infected with TMEV that experience seizures have a profound loss of CA1 pyramidal neurons (Kirkman et al, 2010;Libbey et al, 2011b). Therefore, the effects of treatment, with antagonists of ionotropic glutamate receptors, on neuron loss in the pyramidal cell layer of the hippocampus from CA1 to CA3 were evaluated, at day 21 p.i., in the TMEV-induced seizure model. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466, NBQX or PBS as control (treatment: day 2.5–10.5 p.i.). Statistical differences in the extent of neuronal cell loss were present between groups of mice that had seizures and those that did not have seizures (Fig. 4A, compare left bars to right bars) with those mice experiencing seizures having greater neuronal cell loss than those mice not experiencing seizures. However, there were no statistical differences between the various treatment groups for those mice that had seizures (left bars) or between the various treatment groups for those mice that did not have seizures (right bars) (Fig. 4A). These results are consistent with previous data demonstrating that mice that experience seizures have more extensive cell loss than mice that do not experience seizures (Kirkman et al, 2010;Libbey et al, 2011b). Figure 4B shows representative images of neuronal cell loss within the pyramidal cell layer of the hippocampus in a NBQX-treated mouse that experienced seizures (left) compared to the one NBQX-treated mouse that did not experience seizures (right). These results also demonstrated that although more mice treated with NBQX experienced seizures, those NBQX-treated mice that experienced seizures did not have demonstrably more neuronal cell loss compared to mice from the other treatment groups that also experienced seizures.
Figure 4.
Neuronal cell loss within the hippocampus of antagonist-treated mice. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466 and NBQX or with PBS as a control (treatment: day 2.5–10.5 post infection). Mice were sacrificed on day 21 post infection. A. Neuronal cell loss was scored as described in the Methods. The number of mice in each group is given as N above each column. Data is given as mean + SEM. †p<0.05, *p<0.01, **p<0.001, ‡p<0.0001, Mann-Whitney U test. B. Representative images are shown for an infected, NBQX-treated mouse that experienced seizures (left) and the one infected, NBQX-treated mouse that did not experience seizures (right).
The effects of treatment, with antagonists of ionotropic glutamate receptors, on viral clearance were evaluated in the TMEV-induced seizure model. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466, NBQX or PBS as control (treatment: day 2.5–10.5 p.i.). Treated and control mice were still able to clear virus infected cells. No appreciable amount of viral antigen positive cells were found in the hippocampus and dentate gyrus of the brains of mice, at day 21 p.i., irrespective of the treatment (data shown as mean ± SEM: MK 801, 0 ± 0; GYKI-52466, 1.06 ± 0.64; NBQX, 0 ± 0; PBS, 0.07 ± 0.07). Therefore, virus does not persist within the brain following antagonist treatment.
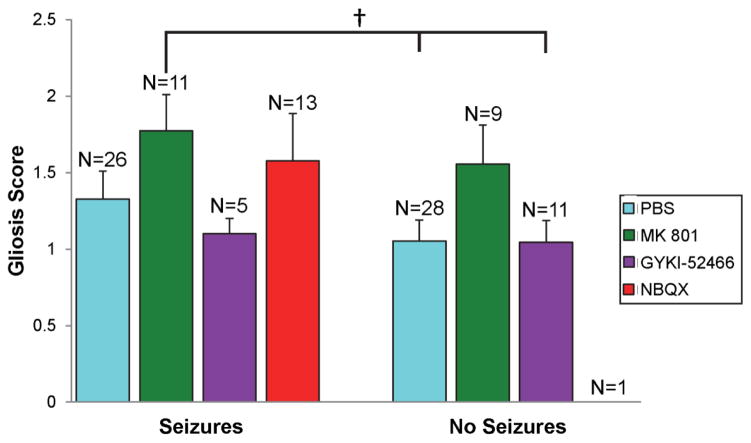
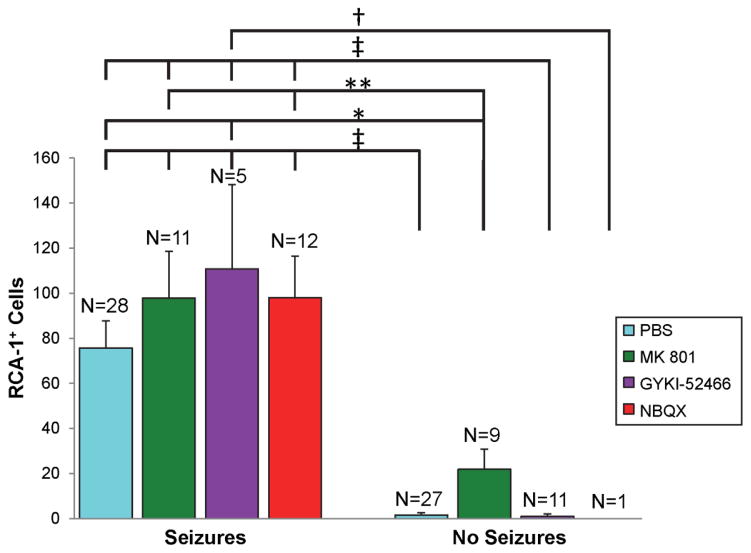
Finally, TMEV-infected mice that experience seizures have more inflammatory changes in the hippocampus (Kirkman et al, 2010;Libbey et al, 2011c). Thus the effects of treatment, with antagonists of ionotropic glutamate receptors, on inflammation in the brain were evaluated in the TMEV-induced seizure model. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466, NBQX or PBS as control (treatment: day 2.5–10.5 p.i.) and gliosis and infiltration of macrophages and/or activation of microglial cells, as markers of inflammation, were evaluated at day 21 p.i. Gliosis was evaluated through staining and scoring of GFAP+-activated astrocytes in the hippocampus and dentate gyrus. For gliosis, the only differences that were significant were between the group of mice that was treated with MK 801 and experienced seizures (significantly more gliosis) and the groups of mice that were treated with PBS and GYKI-52466 and did not experience seizures (p<0.05, Mann-Whitney U) (Fig. 5, compare left bars to right bars). There were no statistical differences between the various treatment groups for those mice that had seizures (compare within left bars only) or between the various treatment groups for those mice that did not have seizures (compare within right bars only) (Fig. 5). Infiltration of macrophages and/or activation of microglial cells were evaluated through staining and counting of RCA-1+ cells in the hippocampus and dentate gyrus. Statistical differences in the numbers of RCA-1+ cells were present between groups of mice that had seizures and those that did not have seizures (Fig. 6, compare left bars to right bars) with those mice experiencing seizures having greater numbers of RCA-1+ cells than those mice not experiencing seizures. In contrast, however, there were no statistical differences in the numbers of RCA-1+ cells between the various treatment groups for those mice that had seizures (left bars) or between the various treatment groups for those mice that did not have seizures (right bars), although, the NBQX group only had one mouse that did not experience seizures (Fig. 6). Interestingly, although more mice treated with NBQX experienced seizures, those NBQX-treated mice that experienced seizures did not have demonstrably more inflammation in the form of gliosis and infiltration of macrophages and/or activation of microglial cells compared to mice from the other treatment groups that also experienced seizures. This suggests that the increase in infected mice having seizures when treated with NBQX is independent of inflammation.
Figure 5.
Gliosis within the hippocampus and dentate gyrus of antagonist-treated mice. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466 and NBQX or with PBS as a control (treatment: day 2.5–10.5 post infection). Mice were sacrificed on day 21 post infection. Gliosis was scored as described in the Methods. The number of mice in each group is given as N above each column. Data is given as mean + SEM. †p<0.05, Mann-Whitney U test.
Figure 6.
Activated microglia/macrophages within the hippocampus and dentate gyrus of antagonist-treated mice. TMEV-infected C57BL/6J mice were treated with MK 801, GYKI-52466 and NBQX or with PBS as a control (treatment: day 2.5–10.5 post infection). Mice were sacrificed on day 21 post infection. RCA-1+ cells were enumerated as described in the Methods. The number of mice in each group is given as N above each column. Data is given as mean + SEM. †p<0.05, *p<0.01, **p<0.001, ‡p<0.0001, ANOVA and Fisher’s PLSD.
Discussion
NBQX competitively binds to the glutamate-binding site on both AMPA and KA receptors (Lees, 1996, 2000). In terms of selectivity, NBQX has been demonstrated to be more potent against AMPA receptors compared to KA receptors (Lees, 2000;Sheardown et al, 1990;Wilding and Huettner, 1996). NBQX treatment was found to protect hippocampal CA1 neurons against ischemic injury (Sheardown et al, 1990), but not against damage caused by status epilepticus (SE) in an adult mouse model (KA injection into the CA1 area of the hippocampus of FVB/N mice) of medically resistant mesial temporal lobe epilepsy (mTLE) (Twele et al, 2015). Protection following ischemia may be through both the direct action of NBQX and its induction of prolonged brain hypothermia (Lees, 2000). In addition, NBQX exerted anti-seizure but not antiepileptogenic effects, when administered after KA-induced SE, in this mouse model of mTLE (Twele et al, 2015). Using the TMEV-induced seizure model, surprisingly we found that NBQX significantly increased the number of mice experiencing seizures compared to control (Fig. 1) and did not protect hippocampal CA1 neurons against damage (Fig. 4). There is precedence for our observation. Berg et al (1993) found that NBQX treatment before and during systemic KA administration caused enhanced toxicity and a lowered seizure threshold. Also, others have found that the antagonistic action of some compounds may be dependent on the local environment, such as the concentration of available agonist (Wahl et al, 1992). Thus, although antagonists at AMPA/KA receptors are usually potent anticonvulsants (Lees, 2000), this is not always the case, and NBQX has the opposite effect in the TMEV-induced seizure model. The mechanism of seizure development in our virus-induced seizure model may differ greatly from that of models where seizures are induced by electrical stimulation, drug administration or as a result of genetic predisposition. These differences may account for the opposite, proconvulsant effect observed in the TMEV-induced seizure model.
Although agonists of AMPA/KA and NMDA receptors are generally considered to be proconvulsants, there is some evidence that agonist activation of some KA receptors can be anticonvulsant. Agonist activation of presynaptic, low affinity KA receptors is thought to reduce glutamate release from presynaptic terminals (Nadler, 2012). It was previously shown that the AMPA/KA receptor agonists, KA and domoate, caused presynaptic depression of excitatory glutamatergic transmission from CA3 projections to the CA1 region in the hippocampus, an effect thought to reduce the transmission of epileptiform activity (Frerking et al, 2001). Moreover, this depressive activity was blocked with CNQX (6-cyano-7-nitroquinoxaline-2,3-dione), another quinoxaline derivative having similar action to NBQX (Catarzi et al, 2007), while GYKI-53655 [1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine], an AMPA receptor antagonist akin to GYKI-52466, failed to have any effect (Frerking et al, 2001). In contrast, others found that NBQX was ineffective at reducing the release of glutamate from synaptosomes when exposed to KA (Chittajallu et al, 1996). Although the reasons for these conflicting results concerning glutamate release are unknown, there are additional studies suggesting a possible role of KA receptor activation in driving inhibitory action in the hippocampus. Work on rat brain slices showed that direct activation of KA receptors (containing the GluR5 subunit) on a subpopulation of inhibitory γ-aminobutyric acid (GABA)ergic interneurons in the CA1 region caused high frequency firing of these neurons resulting in tonic inhibition (dampened excitation) of pyramidal neurons in the hippocampus (Cossart et al, 1998). Furthermore, some models of mTLE display a selective degeneration of these GABAergic interneurons (Cossart et al, 1998;Houser and Esclapez, 1996). Therefore, it is possible that the blockade (antagonism) of some KA receptors could result in a loss of inhibitory tone (disinhibition). Based on these experiments, we hypothesize that KA receptor antagonism with NBQX may reduce protective mechanisms against hyperexcitability, resulting in an increase in the numbers of mice experiencing seizures in the TMEV-induce seizure model. This remains to be tested.
GYKI-52466 is an antagonist that binds to a desensitization site outside of both the glutamate-binding site and the ion channels on AMPA receptors, and this binding decreases the extent and duration of ion channel activation (Lees, 1996, 2000;Vizi et al, 1996). As this mechanism of inhibition is non-competitive, changes in the concentration of glutamate in the synapse do not affect the level of blockade, as happens with competitive antagonists (Lees, 1996;Lees and Leong, 2001;Wilding and Huettner, 1995). Examination of selectivity demonstrated that the concentration of GYKI-52466 that prevents ion channel opening in AMPA receptors has minimal effects at KA receptors, thus distinguishing between these two receptors [reviewed in (Lees, 2000)]. Administration of the AMPA receptor agonist, AMPA, results in hippocampal neuron loss due to both direct toxic effects on neurons and seizure-induced damage, and GYKI-52466 treatment in vivo was found to be minimally effective as a neuroprotective agent against the latter, through its anticonvulsant action, but totally ineffective against the former (Lees and Leong, 2001). We found that GYKI-52466 (AMPA receptor antagonist) had no effect on the numbers of mice experiencing seizures in the TMEV-induced seizure model (Fig. 1) and it did not protect hippocampal CA1 neurons against damage (Fig. 4). This result supports the suggestion that the action of NBQX (AMPA/KA receptor antagonist) is through the KA receptors as opposed to the AMPA receptors in the TMEV-induce seizure model.
MK 801 has been shown to be an antagonist of the NMDA receptor ion channel (Kemp et al, 1987;Wong et al, 1986). MK 801 treatment was found to protect hippocampal neurons against ischemic neuronal necrosis in the rat (Rod and Auer, 1989), and to protect hippocampal CA1 and CA3 neurons against damage in a rat model of mTLE (KA-induced SE), however no antiepileptogenic effects were evident when MK 801 was administered after KA-induced SE (Brandt et al, 2003). We found that MK 801 had no effect on the numbers of mice experiencing seizures in the TMEV-induced seizure model (Fig. 1) and did not protect hippocampal CA1 neurons against damage (Fig. 4).
Mechanisms, other than upregulation of expression and/or function of ionotropic glutamate receptors, potentially responsible for tipping the balance of excitation and inhibition towards excitation and seizures that could occur at the level of the glutamate synapse include an increased number of glutamate synapses, an alteration in the expression and/or function of metabotropic glutamate receptors and increased extracellular glutamate concentration (Nadler, 2012). An increase in the number of glutamate synapses could occur through axonal sprouting of glutamatergic neurons (Bernard, 2012). Mossy fiber sprouting throughout the hippocampus was previously examined, via Timm staining, in the TMEV-induced seizure model and any reorganization at this level was found to be insignificant (Stewart et al, 2010a), however this analysis was performed at 4 to 8 months p.i. Mossy fiber sprouting has not yet been examined during the acute infection period. Enhanced activation of Group I metabotropic glutamate receptors and reduced regulation of glutamate release by Group II and Group III metabotropic glutamate receptors can potentially contribute to the development of seizures (Nadler, 2012). Increased extracellular glutamate concentration could occur due to enhanced glutamate biosynthesis via glutaminase, enhanced glutamate release via synaptic vesicles and vesicular glutamate transporters, impaired metabolic glutamate conversion to glutamine via glutamine synthetase and impaired glutamate clearance from the extracellular space via excitatory amino acid transporters (Nadler, 2012). We are fully aware of these alternate mechanisms and are actively exploring the role that each may play in the development of seizures in the TMEV-induced seizure model. Examination of miniature excitatory postsynaptic currents in the CA3 pyramidal cells of the hippocampus during acute TMEV infection demonstrated significant increases in the amplitude and frequency indicative of changes at the synapse both presynaptically and postsynaptically (Smeal et al, 2012). Therefore, it is quite likely that a combination of mechanisms occurring in concert and involving all aspects of the synapse ultimately results in the development of acute seizures.
The TMEV-induced seizure model is similar to mTLE, a common form of refractory (drug-resistant) epilepsy (Kirkman et al, 2010). It is known that epilepsy-associated alterations in the distribution, density and composition of receptors and voltage-gated ion channels can influence the efficacy of anti-seizure drugs (ASDs) (Schmidt and Loscher, 2005). Therefore, it is possible that changes in the hippocampus specific to mTLE, and/or the TMEV-induced seizure model, could result in the reduced or altered effects of ASDs, and further investigation of possible mechanisms is needed.
Highlights.
Upregulation of glutamate receptors can contribute to seizures
NBQX is a highly selective competitive antagonist of AMPA/KA glutamate receptors
NBQX had an effect on acute seizure development in the virus-induced seizure model
NBQX treatment resulted in a greater seizure burden, both incidence and severity
NBQX, a potent anticonvulsant, has the opposite effect on virus-induced seizures
Acknowledgments
We would like to thank Jordan T. Sim, BA, Mitchell A. Wilson, Kelley M. Ingram and Samantha P. Duzy, for excellent technical assistance, Ana Bea DePaula-Silva, PhD, for many helpful discussions, and Daniel J. Harper for the outstanding preparation of the manuscript.
This work was supported by NIH 5R01NS065714. The funding source had no involvement in the study design, in collection, analysis and interpretation of data, in writing the article or in the decision to submit the article for publication.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate
- ANOVA
Analysis of variance
- ASDs
anti-seizure drugs
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CNS
central nervous system
- DA
Daniels
- GABA
γ-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- GYKI-52466
1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine
- GYKI-53655
1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine
- i.c
intracerebrally
- i.p
intraperitoneal
- KA
kainate
- MK 801
(5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- mTLE
mesial temporal lobe epilepsy
- NBQX
2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline
- NMDA
N-methyl-D-aspartate
- PBS
phosphate-buffered saline
- pfu
plaque-forming units
- p.i
post-infection
- PLSD
Protected Least Significant Difference
- RCA
Ricinus communis agglutinin
- SE
status epilepticus
- SEM
standard error of the mean
- TMEV
Theiler’s murine encephalomyelitis virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barton ME, Peters SC, Shannon HE. Comparison of the effect of glutamate receptor modulators in the 6 Hz and maximal electroshock seizure models. Epilepsy Res. 2003;56:17–26. doi: 10.1016/j.eplepsyres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Benkovic SA, O’Callaghan JP, Miller DB. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 2004;1024:59–76. doi: 10.1016/j.brainres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Berg M, Bruhn T, Johansen FF, Diemer NH. Kainic acid-induced seizures and brain damage in the rat: different effects of NMDA- and AMPA-receptor antagonists. Pharmacol Toxicol. 1993;73:262–268. doi: 10.1111/j.1600-0773.1993.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Bernard C. Alterations in synaptic function in epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] National Center for Biotechnology; Bethesda: 2012. [PubMed] [Google Scholar]
- Brandt C, Potschka H, Loscher W, Ebert U. N-methyl-D-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience. 2003;118:727–740. doi: 10.1016/s0306-4522(03)00027-7. [DOI] [PubMed] [Google Scholar]
- Catarzi D, Colotta V, Varano F. Competitive AMPA receptor antagonists. Med Res Rev. 2007;27:239–278. doi: 10.1002/med.20084. [DOI] [PubMed] [Google Scholar]
- Chapman AG, Smith SE, Meldrum BS. The anticonvulsant effect of the non-NMDA antagonists, NBQX and GYKI 52466, in mice. Epilepsy Res. 1991;9:92–96. doi: 10.1016/0920-1211(91)90018-b. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Dingledine R. Glutamatergic Mechanisms Related to Epilepsy: Ionotropic Receptors. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] National Center for Biotechnology Information; Bethesda: 2012. [PubMed] [Google Scholar]
- Docagne F, Muneton V, Clemente D, Ali C, Loria F, Correa F, Hernangomez M, Mestre L, Vivien D, Guaza C. Excitotoxicity in a chronic model of multiple sclerosis: Neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Kainate receptors depress excitatory synaptic transmission at CA3→CA1 synapses in the hippocampus via a direct presynaptic action. J Neurosci. 2001;21:2958–2966. doi: 10.1523/JNEUROSCI.21-09-02958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Hagino Y, Kariura Y, Manago Y, Amano T, Wang B, Sekiguchi M, Nishikawa K, Aoki S, Wada K, Noda M. Heterogeneity and potentiation of AMPA type of glutamate receptors in rat cultured microglia. Glia. 2004;47:68–77. doi: 10.1002/glia.20034. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res. 1996;26:207–218. doi: 10.1016/s0920-1211(96)00054-x. [DOI] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Wong EH. Non-competitive antagonists of excitatory amino acid receptors. Trends Neurosci. 1987;10:294–298. [Google Scholar]
- Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51:454–464. doi: 10.1111/j.1528-1167.2009.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees GJ. Therapeutic Potential of AMPA Receptor Ligands in Neurological Disorders. CNS Drugs. 1996;5:51–74. [Google Scholar]
- Lees GJ. Pharmacology of AMPA/kainate receptor ligands and their therapeutic potential in neurological and psychiatric disorders. Drugs. 2000;59:33–78. doi: 10.2165/00003495-200059010-00004. [DOI] [PubMed] [Google Scholar]
- Lees GJ, Leong W. In vivo, the direct and seizure-induced neuronal cytotoxicity of kainate and AMPA is modified by the non-competitive antagonist, GYKI 52466. Brain Res. 2001;890:66–77. doi: 10.1016/s0006-8993(00)03080-8. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Fujinami RS. Neurotropic viral infections leading to epilepsy: focus on Theiler’s murine encephalomyelitis virus. Future Virol. 2011;6:1339–1350. doi: 10.2217/fvl.11.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Interleukin-6, produced by resident cells of the central nervous system and infiltrating cells, contributes to the development of seizures following viral infection. J Virol. 2011a;85:6913–6922. doi: 10.1128/JVI.00458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Lack of correlation of central nervous system inflammation and neuropathology with the development of seizures following acute virus infection. J Virol. 2011b;85:8149–8157. doi: 10.1128/JVI.00730-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Once initiated, viral encephalitis-induced seizures are consistent no matter the treatment or lack of interleukin-6. J NeuroVirol. 2011c;17:496–499. doi: 10.1007/s13365-011-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, Fujinami RS. Seizures following picornavirus infection. Epilepsia. 2008;49:1066–1074. doi: 10.1111/j.1528-1167.2008.01535.x. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Kirkman NJ, Wilcox KS, White HS, Fujinami RS. Role for complement in the development of seizures following acute viral infection. J Virol. 2010;84:6452–6460. doi: 10.1128/JVI.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Honack D. Effects of the non-NMDA antagonists NBQX and the 2,3-benzodiazepine GYKI 52466 on different seizure types in mice: comparison with diazepam and interactions with flumazenil. Br J Pharmacol. 1994;113:1349–1357. doi: 10.1111/j.1476-5381.1994.tb17146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C. Oligodendrocyte NMDA receptors: a novel therapeutic target. Trends Mol Med. 2006;12:289–292. doi: 10.1016/j.molmed.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Murugan M, Sivakumar V, Lu J, Ling EA, Kaur C. Expression of N-methyl D-aspartate receptor subunits in amoeboid microglia mediates production of nitric oxide via NF-kappaB signaling pathway and oligodendrocyte cell death in hypoxic postnatal rats. Glia. 2011;59:521–539. doi: 10.1002/glia.21121. [DOI] [PubMed] [Google Scholar]
- Nadler JV. Plasticity of Glutamate Synaptic Mechanisms. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] National Center for Biotechnology Information; Bethesda: 2012. [PubMed] [Google Scholar]
- Nargi-Aizenman JL, Havert MB, Zhang M, Irani DN, Rothstein JD, Griffin DE. Glutamate receptor antagonists protect from virus-induced neural degeneration. Ann Neurol. 2004;55:541–549. doi: 10.1002/ana.20033. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Wieczorek CM, Halpain S. Quantitative autoradiography of binding sites for [3H]AMPA, a structural analogue of glutamic acid. Brain Res. 1984;309:173–177. doi: 10.1016/0006-8993(84)91025-4. [DOI] [PubMed] [Google Scholar]
- Rod MR, Auer RN. Pre- and post-ischemic administration of dizocilpine (MK- 801) reduces cerebral necrosis in the rat. Can J Neurol Sci. 1989;16:340–344. doi: 10.1017/s031716710002919x. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Loscher W. Drug resistance in epilepsy: putative neurobiologic and clinical mechanisms. Epilepsia. 2005;46:858–877. doi: 10.1111/j.1528-1167.2005.54904.x. [DOI] [PubMed] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honore T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Smeal RM, Stewart KA, Iacob E, Fujinami RS, White HS, Wilcox KS. The activity within the CA3 excitatory network during Theiler’s virus encephalitis is distinct from that observed during chronic epilepsy. J NeuroVirol. 2012;18:30–44. doi: 10.1007/s13365-012-0082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Durmuller N, Meldrum BS. The non-N-methyl-D-aspartate receptor antagonists, GYKI 52466 and NBQX are anticonvulsant in two animal models of reflex epilepsy. Eur J Pharmacol. 1991;201:179–183. doi: 10.1016/0014-2999(91)90342-n. [DOI] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler’s virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010a;69:1210–1219. doi: 10.1097/NEN.0b013e3181ffc420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart KA, Wilcox KS, Fujinami RS, White HS. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia. 2010b;51:1418–1428. doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Franz H, Yamamoto T, Iwasaki Y, Konno H. Identification of the normal microglial population in human and rodent nervous tissue using lectin-histochemistry. Neuropathol Appl Neurobiol. 1988;14:221–227. doi: 10.1111/j.1365-2990.1988.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler’s murine encephalomyelitis virus. Acta Neuropathol (Berl) 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Kuang LQ, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am J Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, McCright IJ, Kuang LQ, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler’s murine encephalomyelitis virus variant. J Neuropathol Exp Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am J Pathol. 2007;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS. Prolonged gray matter disease without demyelination caused by Theiler’s murine encephalomyelitis virus with a mutation in VP2 puff B. J Virol. 2001;75:7494–7505. doi: 10.1128/JVI.75.16.7494-7505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twele F, Bankstahl M, Klein S, Romermann K, Loscher W. The AMPA receptor antagonist NBQX exerts anti-seizure but not antiepileptogenic effects in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Neuropharmacology. 2015;95:234–242. doi: 10.1016/j.neuropharm.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Mike A, Tarnawa I. 2,3-Benzodiazepines (GYKI 52466 and Analogs): Negative Allosteric Modulators of AMPA Receptors. CNS Drug Reviews. 1996;2:91–126. [Google Scholar]
- Wahl P, Nielsen B, Krogsgaard-Larsen P, Hansen JJ, Schousboe A, Miledi R. Stereoselective effects of AMOA on non-NMDA receptors expressed in Xenopus oocytes. J Neurosci Res. 1992;33:392–397. doi: 10.1002/jnr.490330305. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Differential antagonism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Mol Pharmacol. 1995;47:582–587. [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Antagonist pharmacology of kainate- and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring receptors. Mol Pharmacol. 1996;49:540–546. [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci U S A. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurbriggen A, Fujinami RS. A neutralization-resistant Theiler’s virus variant produces an altered disease pattern in the mouse central nervous system. J Virol. 1989;63:1505–1513. doi: 10.1128/jvi.63.4.1505-1513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]