Abstract
One of the hallmarks of cancer is resistance to programmed cell death, which maintains the survival of cells en route to oncogenic transformation and underlies therapeutic resistance. Recent studies demonstrate that programmed cell death is not confined to caspase-dependent apoptosis, but includes necroptosis, a form of necrotic death governed by Receptor-Interacting Protein 1 (RIP1), RIP3, and Mixed Lineage Kinase Domain-Like (MLKL). Necroptosis serves as a critical cell-killing mechanism in response to severe stress and blocked apoptosis, and can be induced by inflammatory cytokines or chemotherapeutic drugs. Genetic or epigenetic alterations of necroptosis regulators such as RIP3 and cylindromatosis (CYLD), are frequently found in human tumors. Unlike apoptosis, necroptosis elicits a more robust immune response that may function as a defensive mechanism by eliminating tumor-causing mutations and viruses. Furthermore, several classes of anticancer agents currently under clinical development, such as SMAC and BH3 mimetics, can promote necroptosis in addition to apoptosis. A more complete understanding of the interplay among necroptosis, apoptosis, and other cell death modalities is critical for developing new therapeutic strategies to enhance killing of tumor cells.
Keywords: necroptosis, cancer, RIP1, RIP3, MLKL
1. Introduction
Eliminating unwanted or damaged cells through cell death is essential for fundamental biological processes such as animal development, tissue homoeostasis, and stress response. Deregulated cell death contributes to a number of human diseases including cancer and inflammation. During oncogenic transformation, neoplastic cells become resistant to cell death, which allows them to survive and acquire additional oncogenic mutations [1]. Cell death is also one of the most critical anticancer mechanisms triggered by a variety of chemotherapeutic drugs. Thus, targeting cell death pathways is an attractive strategy for developing novel anti-cancer therapies.
Cell death can be classified into several major forms based on morphological features and biochemical characteristics, including apoptosis, necrosis, autophagic death, and mitotic catastrophe [2]. Apoptosis and necrosis represent the two most distinctive and best understood cell death modalities. Apoptosis, known as programmed cell death, is highly regulated, while necrosis was previously thought to be a random and passive process without much regulation. However, this traditional view has been changed by studies in the past two decades that led to the identification of a regulated form of necrosis. It was observed that inhibition of caspases, proteases that are essential for apoptosis, did not suppress but exacerbated cell death with signs of necrosis in certain cell types [3]. Receptor-interacting kinase 1 (RIP1; RIPK1) was shown to be a critical regulator of necrosis in caspase-inhibited cells [4]. Chemical biology studies identified a small-molecule inhibitor of this type of cell death [5], which was subsequently shown to be the inhibitor of RIP1 [6]. Several recent studies demonstrated RIP3 as the downstream mediator of RIP1 [7–9], and Mixed Lineage Kinase Domain-Like (MLKL) as the key player in the execution of cell death [10]. A number of in vivo studies have established the physiological and pathological relevance of necrosis [11]. This type of cell death has now been defined as necroptosis [5], which is regulated by and requires RIP1, RIP3 and MLKL [2]. Accumulating evidence indicates that necroptosis functions as a safeguard mechanism for killing cancer cells that fail to die by apoptosis, suggesting a pivotal role in cancer biology and therapy.
2. Regulation and mechanisms of necroptosis
2.1 Characteristics of necroptosis
Necroptosis has several distinctive characteristics compared to other types of cell death, in particular, apoptosis (Table 1). Necroptotic cells share several morphological features of necrosis, including early loss of plasma membrane integrity, translucent cytosol, and swelling mitochondria (Figure 1) [12]. In contrast, apoptotic cells lack these features, and are characterized by cell shrinkage, plasma membrane blebbing, and condensed and fragmented nuclei and organelles (Figure 1). At the biochemical level, necroptotic cells show marked depletion of cellular ATP and leakage of intracellular contents, in contrast to apoptosis, which is a more energy-consuming process requiring a relatively higher level of cellular ATP. At the molecular level, necroptosis is caspase-independent and signals through RIP1, RIP3 and MLKL, while apoptosis requires caspase activation and is mediated by interplays of the Bcl-2 family proteins or activation of death receptors. Another key feature of necroptosis is that permeabilization of plasma membrane can lead to release of so-called Damage Associated Molecular Patterns (DAMPs), such as high-mobility group box 1 (HMGB1) protein and mitochondrial DNA [13, 14], which can trigger a robust immune response and inflammation [15]. In contrast, corpses of apoptotic cells are engulfed and then dissolved through the process phagocytosis [16], which does not typically incur a strong immune response [17].
Table 1.
Comparison of necroptosis and apoptosis markers
| Necroptosis | Apoptosis |
|---|---|
| Morphological markers | |
| Plasma membrane swelling and rupture | Plasma membrane blebbing |
| Cytoplasm swelling and vacuolization | Cytoplasm fragmentation; apoptotic body formation |
| No nuclear fragmentation | Nuclear condensation and fragmentation |
| Mitochondria and organelle swelling | Organelle fragmentation |
| Biochemical markers | |
| ATP depletion | ATP increase |
| ROS production | ROS production |
| Calcium and sodium influx | Mitochondria outer membrane permeabilization |
| Molecular markers | |
| Signaling by the Bcl-2 family proteins | Signaling by RIP1, RIP3, and MLKL |
| Regulation by death receptors | Regulation by death receptors |
| Inhibition by caspases | Caspase activation |
| Random DNA degradation | DNA digestion by endonucleases |
| Extracellular release of DAMPs, such as HMGB1 and mitochondrial DNA | Cytosolic release of mitochondrial cytochrome c, SMAC and AIF |
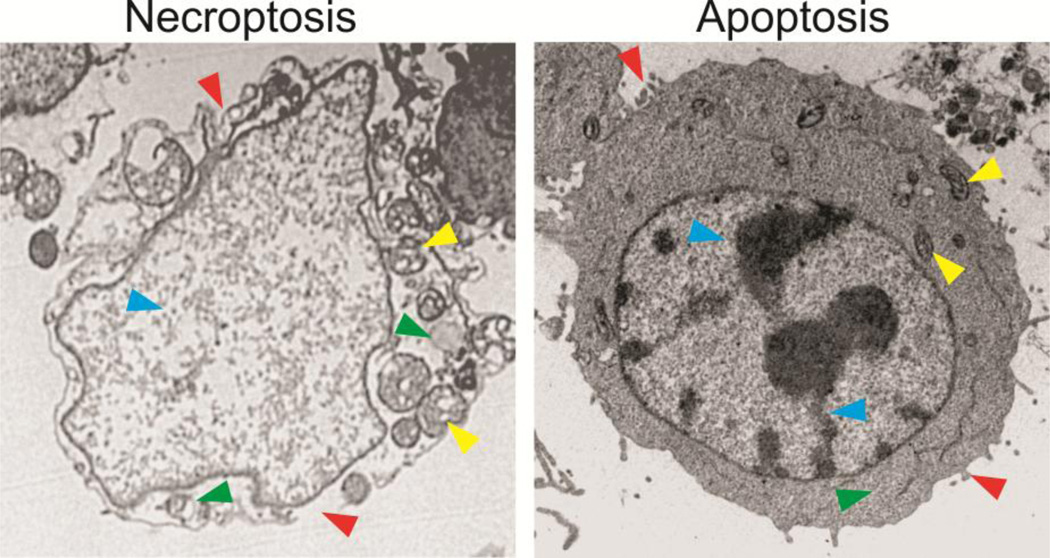
Figure 1. Morphological features of necroptosis and apoptosis in cancer cells.
HT29 colon cancer cells treated with an anticancer drug for 48 hours were analyzed by transmission electron microscopy. The cell undergoing necroptosis shows plasma membrane rupture and permeabilization, compared to the intact plasma membrane with blebbing in the apoptotic cell (red arrowheads). The necroptotic cell exhibits cytoplasm swelling and vacuolization, which are absent in the apoptotic cell (green arrowheads). The necroptotic cell has swelled mitochondria, in contrast to those in the apoptotic cells (yellow arrowheads). The necroptotic cell also lacks condensed and fragmented nuclei seen in the apoptotic cell (blue arrowheads).
Despite these distinctive features, necroptosis is tightly connected with apoptosis and other cell death programs, which presents interesting opportunities as well as challenges to understand the regulation and relative contributions of different cell death forms. Choices of the cell death pathways are dependent upon a variety of factors such as cell type, genetic background, stimuli, and intracellular redox, PH and ion levels. Under most experimental conditions, apoptosis is the default cell death program, and necroptosis serves as a fail-safe mechanism to eliminate stressed cells that fail to undergo apoptosis. However, necroptosis can be predominant under certain circumstances, such as viral infection and exposure to certain natural products [18, 19]. More often, a continuum of necroptosis and apoptosis was observed in cells [20, 21]. Levels of stress and extent of cellular damage also affect choices of cell death programs. Increased stress levels and intensified death signals can switch cell death from apoptosis to necroptosis [22]. The interrelationship between necroptosis and other cell death pathways is complicated and has been a subject of extensive investigations.
2.2 Molecular mechanisms of necroptosis
Necroptosis is often triggered by stimuli that also induce apoptosis via the extrinsic pathway, such as members of the tumor necrosis factor (TNF) family of cytokines including TNF-α, FAS ligand (FASL; CD95), and TNF-related apoptosis-inducing ligand (TRAIL) [23]. Upon ligand stimulation, activated receptors of these stimuli interact with RIP1 through their respective death domains, and recruit cellular inhibitor of apoptosis proteins (cIAPs), such as cIAP1 and cIAP2, to form a plasma membrane associated complex (Figure 2), which leads to activation of pro-survival NF-κB and mitogen-activated protein kinases (MAPKs) [24]. During this process, RIP1 becomes polyubiquitinated by cIAPs and other E3 ubiquitin ligases. Auto-ubiquitination and subsequent degradation of cIAPs, which is stimulated by second mitochondria-derived activator of caspases (SMAC) or small-molecule SMAC mimetics (also known as IAP inhibitors) [25–27], promotes deubiquitination of RIP1 by deubiquitinases, including cylindromatosis (CYLD) and A20 [28, 29]. This results in RIP1dissociation from the plasma membrane and its conversion from a pro-survival into a pro-death protein (Figure 2). RIP1 binding to FAS-Associated Death Domain (FADD) recruits procaspase-8 [26], leading to activation of caspase-8 and induction of apoptosis (Figure 2). The activated caspase-8 inhibits necroptosis by cleaving the core regulators of necroptosis such as RIP1 and RIP3 [30, 31]. If caspase-8 activity is ablated by caspase inhibitors or genetic knockout, the mode of cell death switches to necroptosis. RIP3 and RIP1 bind to each other through their respective homotypic interaction motif (RHIM) domains to form a functional amyloid signaling complex [7–9, 32], leading to auto-phosphorylation of RIP3 at serine 227 and subsequent recruitment of the pseudokinase MLKL [10]. It is important to note that unlike RIP3, which is consistently pro-death, RIP1 is pleotropic, and can both positively and negatively regulate necroptosis and RIP3 activity. For example, RIP1 was shown to suppress spontaneous RIP3 activation in the cytosol [33].
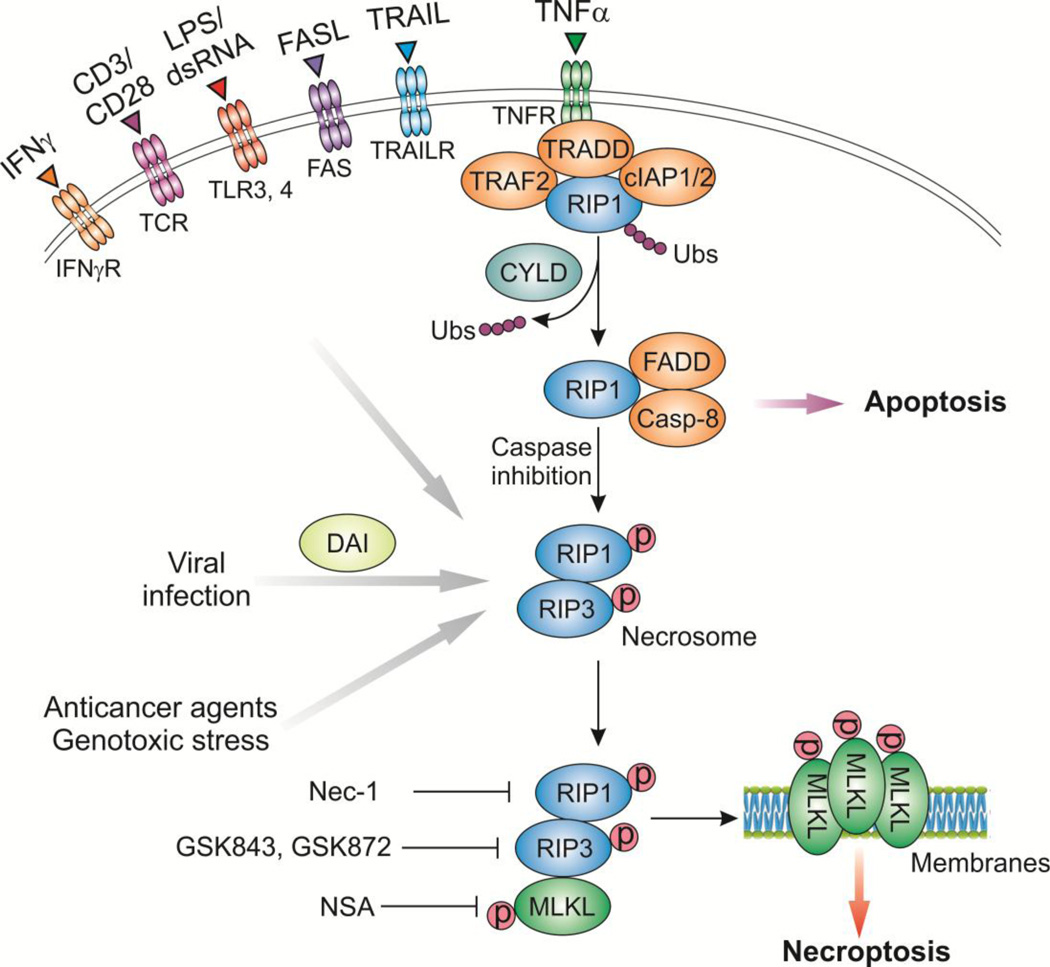
Figure 2. Necroptosis regulators and pathways.
Upon TNF-α stimulation, the activated TNF receptor (TNFR) interacts with RIP1 and recruits cIAP1 and cIAP2 to form a plasma membrane associated complex, resulting in RIP1 polyubiquitination (Ubs). Inhibition of cIAPs (by SMAC or SMAC mimetics) leads to deubiquitination of RIP1 by CYLD and dissociation of RIP1. RIP1 then binds to FADD and procaspase-8 to form a complex, which activates caspase-8 (Casp-8) and leads to apoptosis induction. If caspase-8 activity is inhibited, RIP1 binds to RIP3 to form necrosome and promotes RIP3 auto-phosphorylation and subsequent activation, allowing RIP3 to recruit and phosphorylate MLKL. This results in oligomerization of MLKL, membrane insertion of MLKL oligomers, disruption of plasma and intracellular membrane integrity, and necroptotic death. Other necroptotic stimuli, including FASL, TRAIL, LPS, dsRNA (such as poly (I:C)) and interferon γ (IFNγ), can stimulate their respective receptors to activate RIP1 and/or RIP3 to promote necroptosis. Viral infection directly activates RIP3 through DAI. Anticancer agents and genotoxic stress can also induce RIP1/RIP3-dependent necroptosis. Several inhibitors of necroptosis have been developed, including the RIP1 inhibitor necrostatin-1 (Nec-1), the RIP3 inhibitors GSK843 and GSK872, and the MLKL inhibitor necrosulfonamide (NSA).
In addition to death-receptor ligands, necroptosis can also be triggered by other stimuli, including engagement of Toll-like receptors (TLRs) 3 and 4 by lipopolysaccharides (LPS) and double-stranded RNA (dsRNA) or analogs such as polyinosinic-polycytidylic acid (poly(I:C)), T-cell receptor stimulation, interferons (IFNs), DNA damage and viral infection (Figure 2) [23]. Depending on the stimulus, RIP3 can be activated through RIP1-dependent or -independent mechanisms that often involve a RHIM-RHIM interaction. For example, TLRs can directly activate RIP3 without RIP1 via another RHIM-containing protein known as TRIF [34, 35]. Upon viral infection, DNA-dependent activator of interferon regulatory factors (DAI), a RHIM-containing cellular protein, can activate RIP3 directly, likely through a RHIM-RHIM interaction [36]. After herpes simplex virus 1 (HSV-1) infection, the viral ribonucleotide reductase large subunit (ICP6) interacts with RIP3 through a RHIM-RHIM interaction to trigger necroptosis and host defense, which do not require CYLD and TNFR [37, 38]. In addition, cells with high levels of RIP3 or enforced oligomerization of RIP3 can bypass the requirement of RIP1 to induce necroptosis [33]. Interferons induce RIP1/RIP3-dependent necroptosis through transcriptional activation of interferon responsive genes [39, 40].
Biochemical, chemical biology, and genetic studies have identified MLKL as a major executioner of necroptosis downstream of RIP1/RIP3 [41]. MLKL is normally kept inactive as a monomer in the cytosol by its kinase-like domain [10]. Upon activation, RIP3 binds to MLKL through its kinase domain to promote MLKL phosphorylation at threonine 357 and serine 358 residues [10], which destabilizes the monomeric structure of MLKL and promotes its oligomerization [42, 43]. The oligomerized MLKL, which has affinity for phosphatidylinositol lipids and cardiolipin, translocates from the cytosol to the plasma and intracellular membranes, where it disrupts membrane integrity to promote necroptotic death (Figure 2) [42–44].
Several different mechanisms have been proposed to explain how oligomerized MLKL causes cell death. The complex formed by RIP1, RIP3 and MLKL can promote phosphorylation of the long form of phosphoglycerate mutase family member 5 (pGAM5L), which stimulates auto-phosphorylation of the short form of pGAM5 (pGAM5S) [45]. pGAM5S subsequently binds to the mitochondrial fission factor, dynamin-related protein 1 (Drp1), and activates its GTPase activity to promote mitochondrial fission and cell death [10, 45]. However, the role of the pGAM5-Drp1 axis and mitochondria in necroptosis appears to be context-dependent [46, 47]. It was also suggested that oligomerized MLKL can promote influx of calcium through the calcium channel TRPM7 protein [44], or influx of sodium [48], to promote cell death. Nonetheless, the question still remains as to whether the observed influx of ions in necroptotic cells is a consequence or the cause of cell death [34]. Production of reactive oxygen species (ROS) due to disruption of mitochondrial membrane integrity by MLKL may also contribute to necroptotic death [49]. Therefore, the execution mechanism downstream of MLKL in necroptosis remains to be elucidated.
Genetic studies in mice have demonstrated the roles of RIP1, RIP3, MLKL, and other necroptosis regulators in vivo. Knockout (KO) of RIP1 or RIP3 rescued developmental defects, embryonic lethality, and inflammation caused by KO of caspase-8 or FADD [30, 50–52]. KO of MLKL blocked TNF-induced necroptotic death [41, 53]. A deubiquitinase defective CYLD allele rescued extensive necroptosis and inflammation caused by conditional deletion of FADD in the skin [54]. Consistent with its pleotropic functions, RIP1 sometimes shows pro-survival activity and inhibits both apoptosis and necroptosis. RIP1-KO mice die of systemic inflammation at birth, which is rescued by combined loss of caspase-8 and RIP3 [55–57]. Conditional KO of RIP1 caused lethal intestinal pathology due to FADD/caspase-8-mediated apoptosis in the intestinal epithelial cells [58, 59]. These findings reinforce the notion that necroptosis is actively suppressed by apoptosis or apoptotic regulators to ensure normal development and tissue homeostasis.
2.3 Other forms of regulated necrosis
In addition to necroptosis, several other forms of regulated necrosis have been described. DNA alkylating agents, glutamate toxicity, oxidative stress, hypoxia, and ischemia can trigger necrosis that is dependent on poly(ADP-ribose) polymerase (PARP1), which catalyzes poly(ADP-ribosyl)ation of proteins involved in DNA damage response. PARP1-dependent cell death is mediated by translocation of apoptosis-inducing factor (AIF) from the mitochondrial intermembrane space into the nucleus [60], and activation of calpain proteases [61, 62]. PARP1-mediated necrotic death was due to energy crisis caused by depletion of nicotinamide adenine dinucleotide (NAD), and subsequently that of ATP [63]. Cells using aerobic glycolysis to support their bioenergetics such as cancer cells are more sensitive to this form of death [20].
Another form of regulated necrosis is mediated by the mitochondrial function of the tumor suppressor p53. In response to oxidative stress, p53 accumulates in the mitochondrial matrix and triggers mitochondrial permeability transition pore (PTP) opening and necrosis by interacting with the PTP regulator cyclophilin D [64], leading to ATP depletion and cell death [22]. In this case, the killing activity of p53 is independent of its transcriptional activation function [22]. In addition, several other non-apoptotic cell death modalities, such as pyroptosis and ferroptosis, are also considered to be regulated necrosis [17]. However, it has been difficult to distinguish different forms of necrosis due to lack of distinctive molecular markers. In general, these other forms of necrosis are not considered to be necroptosis due to a lack of evidence for a requirement of RIP1 or RIP3 [63].
2.4 Detection of necroptosis
Due to lack of specific molecular markers of necroptosis, a combination of approaches is often required to distinguish necroptosis from other cell death modalities. For cultured cells, transmission electron microscopy (TEM) is commonly used to provide morphological evidence of necrosis (Figure 1). Extracellular release of HMGB1, loss of cell viability, and depletion of ATP can be used as markers of necroptosis, but do not distinguish necroptosis from other types of necrotic death. More definitive evidence for necroptosis is the requirement of RIP1, RIP3, and/or MLKL, which can be established using approaches such as gene targeting, siRNA/shRNA knockdown, kinase-dead or interacting domain deficient mutants, or small-molecule inhibitors. The RIP1 inhibitor necrostatin-1 (Nec-1) has been widely used, which inhibits both catalytic and allosteric functions of RIP1 by inducting conformational changes [65]. The MLKL kinase inhibitor necrosulfonamide (NSA) [10], and the RIP3 inhibitors GSK843 and GSK872 [35, 66], can also be used for this purpose (Figure 2). Due to the pleiotropic effects of RIP1, RIP3 and MLKL serve as more specific markers of necroptotic death.
Biochemical markers are commonly used to study necroptosis. Activation of RIP1, RIP3, and MLKL in necroptosis can be detected by changes in their phosphorylation status or membrane accumulation using immunoblotting or immunostaining [42, 67]. Necrosome formation can be detected by RIP1/RIP3 and RIP3/MLKL interactions or complex formations using immunoprecipitation or other methods [7]. It is more challenging to study necroptosis in vivo. Increased mRNA and protein expression of RIP1, RIP3 and MLKL is indicative of predisposition to necroptosis [68]. Necroptotic death can be verified by decreased cell death or tissue injury in RIP1, RIP3, or MLKL deficient animals, or the effects of their respective small-molecule inhibitors [68]. For human specimens, necroptotic cell death is suggested by cell loss detected by H&E staining, and positive staining of Tdt-mediated dUTP nick end labeling (TUNEL), but negative staining of apoptosis markers such as active caspase-3. HMGB1 release could be measured by ELISA assays in the blood samples from patients [69].
3. Necroptosis as a safeguard mechanism against cancer
Accumulating evidence suggests that similar to apoptosis, necroptosis also functions as a barrier against tumor development. During tumor progression, a variety of genetic, epigenetic, and expression alterations of key necroptosis regulators are involved, which might allow tumor cells to evade the fate of necroptotic death, and possibly immune surveillance.
3.1 Alterations of necroptosis regulators in cancer
A number of studies reported downregulation or mutations in the core necroptotic machinery, including RIP1, RIP3, MLKL and CYLD, in various types of cancer (Table 2). RIP3 and CYLD were found to be markedly downregulated in chronic lymphocytic leukemia (CLL), which compromises TNF-α-induced necroptosis in CLL cells [70]. The downregulation of CYLD in CLL is due to transcriptional repression by lymphoid enhancer-binding factor 1 (LEF1), a downstream effector of the Wnt/β-catenin pathway [70]. RIP1 and RIP3 expression is significantly decreased in colon, breast, and lung cancer cells and primary tumors compared to corresponding normal tissues, which compromises the response of cancer cells to necroptotic stimuli [71–73]. Silencing of RIP1 and RIP3 in cancer cells is due to promoter hypermethylation [71, 73], or transcriptional suppression by hypoxia [72]. Furthermore, population studies indicate that single nucleotide polymorphisms (SNPs) in the genomic region of RIP3 are associated with increased risk of non-Hodgkin’s lymphoma [74].
Table 2.
Necroptosis regulators and their alterations in cancer
| Proteins | Role in necroptosis |
Function in necroptosis | Alterations in cancer | References |
|---|---|---|---|---|
| RIP1 | Core machinery | Binds to RIP3 to form necrosome | Loss of expression | [8, 72, 119] |
| RIP3 | Core machinery | Binds to RIP1 to form necrosome; phosphorylates MLKL | Loss of expression; promoter methylation | [7–9, 71, 72] |
| MLKL | Core machinery | Phosphorylation by RIP3; oligomerizes and inserts into membranes | Downregulation; mutations | [10, 42, 44, 75, 76] |
| CYLD | Signaling | Deubiquitinates RIP1; triggers ripotosome formation | Frequent mutations; downregulation | [28, 70, 78, 81] |
| cIAP1, 2 | Inhibition | Polyubiquitinate RIP1; block ripotosome formation | Overexpression; amplification | [25–27, 82, 102] |
| Caspase-8 | Inhibition | Cleaves RIP1, RIP3 and CYLD | Mutations | [30, 31, 120] |
| FADD | Inhibition | Inhibits necrosome formation | Rare mutations | [30, 51, 52, 121] |
| pGAM5 | Execution | Activates Drp1 GTPase activity | [45] | |
| Drp1 | Execution | Promotes mitochondrial fission | [45] | |
| Bcl-XL | Inhibition | Binds to pGAM5 | Overexpression | [84, 85, 122] |
Mutations in highly conserved residues located in the C-lobe of the MLKL pseudokinase domain, including F398I and L291P, have been identified in human stomach adenocarcinomas [75]. These loss-of-function mutations abrogate the ability of MLKL to promote necroptosis [41]. Downregulation of MLKL in pancreatic adenocarcinomas was associated with decreased patient survival [76].
CYLD was initially identified as a tumor suppressor gene mutated in Familial Cylindromatosis [77]. Germline and somatic mutations of CYLD have been detected in a variety of tumors [78], and are associated with poor clinical outcomes following chemotherapy [79]. CYLD was also found to be negatively regulated at the transcriptional level by Snail, which promotes tumor progression in malignant melanoma [80]. CYLD mRNA expression was shown to be downregulated in colon and hepatocellular carcinoma cells and tumor samples [81].
Another mechanism leading to compromised necroptosis in cancer cells is overexpression of pro-survival proteins such as IAPs. IAPs, including cIAP1, cIAP2 and XIAP, are often aberrantly expressed in a variety of human tumors due to genomic amplification, elevated mRNA and protein expression, or loss of their endogenous inhibitors such as SMAC [82]. These changes in IAPs have been linked to poor prognosis of cancer patients [82]. Aberrant activation of IAPs was thought to primarily affect apoptosis in cancer cells by inhibiting caspases and promoting the pro-survival NF-κB signaling [83]. It is now clear that cIAP depletion facilitates TNF-α-induced necroptosis [8]. Furthermore, Bcl-XL, a pro-survival Bcl-2 family member often overexpressed in cancer [84], could also suppress necroptosis [85]. To what extent necroptosis is affected by alterations of IAPs, Bcl-XL, and other pro-survival factors remains to be established by ruling out the effects on apoptosis.
3.2 Necroptosis in immune surveillance of cancer
The immune system is believed to function as a primary defense system against cancer by identifying and destroying nascent tumors via a collective action of several immune cell types and effector molecules [86]. In contrast to apoptosis, which seems to be immunologically quiescent, necroptosis is often a more potent inducer of immune response through the release of DAMPs into tissue microenvironment [17]. As a consequence, necroptotic cells can recruit inflammatory cells to survey the extent of tissue damage, remove associated necroptotic debris, and aid tissue remodeling and repair [87]. Necrosis was also used to explain adaptive immunity in seemingly infection-free situations, such as in spontaneous and therapy-induced tumor rejection [88].
Necroptosis has been shown to trigger activation of specific types of immune cells and adaptive immunity against tumor challenge. Necroptotic cells can provide both antigens and inflammatory stimuli for dendritic cells (DCs), which in turn activate CD8+ T cells through a process called antigen cross-priming [89]. CD8+ T cells primed by immunization with necroptotic cells produced multiple effector cytokines, exhibited in vivo cytolytic activity, and protected mice from tumor challenge [90]. Robust CD8+ T cell cross-priming and subsequent tumor protection are dependent on RIP1 signaling and NF-κB-mediated transcription [90]. RIP3 was recently shown to be critical for the function of natural killer T (NKT) cells by regulating cytokine expression via its effects on pGAM5 and Drp1, and RIP3 KO mice had reduced NKT-cell-mediated immune responses to metastatic tumor cells [91]. These findings suggest that necroptosis may elicit antitumor immunity by activating CD8+ T cells or NKT cells.
On the other hand, inflammation, which can be triggered by necroptosis, is considered to be a tumor-promoting mechanism [1]. Inflammation and NF-κB activation are often detected in early-stage tumors, and facilitate tumor progression by supplying bioactive molecules in the microenvironment to promote tumor cell proliferation and survival, as well as tumor angiogenesis, invasion and metastasis [87, 92, 93]. Mutagenic chemicals released by inflammatory cells, such as ROS, can accelerate genetic evolution of tumor cells [87]. The crosstalk between tumor cell necroptosis and inflammation, which remains to be elucidated, is likely to be significant in tumor development and progression.
3.3 Necroptosis as a defense mechanism against viral infection
Viral infection is a significant etiological factor of tumor development. It is estimated that one in five cancer cases worldwide is caused by viral infection [94]. Up to now, 7 tumor-causing viruses have been identified, including human papillomavirus (cervical carcinoma), Epstein-Barr virus (B-cell lymphoproliferative disease and nasopharyngeal carcinoma), Kaposi's sarcoma herpesvirus (Kaposi's Sarcoma and primary effusion lymphomas), hepatitis B and hepatitis C viruses (hepatocellular carcinoma), and Human T-cell leukemia virus-1 (T-cell leukemia) [94]. Killing and removal of infected cells is a key defensive mechanism against viral infection and subsequent cancer development [95]. Viruses have evolved a variety of mechanisms to suppress cell death and prolong survival of host cells to ensure reliable viral replication and dissemination.
Viruses express caspase 8 inhibitors, Bcl-2-like proteins, IAP-like proteins or other factors to inhibit apoptosis of host cells [96]. Emerging evidence suggests that necroptosis may become paramount for host defense when apoptosis is compromised, and suppressing necroptosis is necessary for effective viral dissemination. For example, cells infected with murine cytomegalovirus (MCMV) can be eliminated by necroptosis mediated by the interaction of RIP3 and DAI, an intracellular sensor of viral DNA, via their respective RHIM domains [36]. MCMV encodes a RHIM-containing protein called viral inhibitor of RIP activation (vIRA), which facilitates MCMV infection by disrupting RIP3 activity and necroptosis [36]. Infection of vaccinia virus induces RIP1/RIP3-dependent necroptosis, and mice with RIP3 KO or knock-in of a RIP1 kinase-dead mutant failed to control viral replication in vivo [97], suggesting that RIP1/RIP3-dependent necroptosis contains vaccinia virus infection. Infection with HSV-1 was restricted by RIP3/MLKL-dependent necroptosis via the interaction between RIP3 and ICP6, the viral ribonucleotide reductase large subunit, through a RHIM-RHIM interaction [37, 38]. In this case, TNF signaling was unnecessary for necroptosis induced by HSV-1 infection. However, another study showed that ICP6 inhibits TNF-α-induced and RIP3-dependent necroptosis by disrupting the RIP1/RIP3 interaction [98]. ICP6 also suppresses apoptosis by inhibiting caspase-8 activity [96], suggesting simultaneous targeting of both apoptosis and necroptosis by viral proteins. Furthermore, human cytomegalovirus (HCMV) blocks TNF-induced necroptosis in RIP3-expressing human cells following RIP3 activation and MLKL phosphorylation [99]. Most of the viruses that were found to induce necroptosis are not considered to be oncogenic, except for HCMV [100]. Direct evidence supporting the role of necroptosis against tumor-causing viruses is still lacking, and warrants further investigation. On the other hand, interferon-induced necroptosis of immune cells allows immune evasion and facilitates infection of some pathogens such as Salmonella Typhimurium [39].
4. Necroptosis in anti-cancer therapies
4.1 Induction of necroptosis by anticancer agents
Commonly used chemotherapeutic drugs, in particular DNA damaging agents, have been shown to induce necroptosis in cancer cells (Table 3). This is probably not surprising because DNA-damage-induced apoptosis is defective in most cancer cells due to p53 mutations and genetic and epigenetic alterations in other key apoptotic regulators [101]. Etoposide, a topoisomerase inhibitor drug, was shown to suppress cIAP expression, thereby inducing necroptosis through a RIP1-containing complex called ripoptosome [102]. The antimitotic drug taxol can induce RIP1-dependent necroptosis mediated through FADD double phosphorylation by the mitotic kinases Aurora-A and Plk1 [103]. 5-Fluorouracil (5-FU), etoposide, and camptothecin induced RIP1/MLKL-dependent, but RIP3-independent necroptosis in caspase-3-deficient colorectal cancer cells [104]. Cisplatin caused RIP3-dependent necroptosis in apoptosis-resistant esophageal cancer cells through necrosome formation and autocrine TNF-α signaling [105]. Epigenetic silencing of RIP3 contribute to resistance to 5-FU, cisplatin, camptothecin, and etoposide in colon and breast cancer cells [71].
Table 3.
Necroptosis-inducing anticancer agents
| Anticancer agents | Mechanisms of necroptosis induction | References |
|---|---|---|
| Clinically used drugs | ||
| Cisplatin | RIP3-dependent; necrosome formation; autocrine TNF-α | [71, 105] |
| Etoposide | XIAP, cIAP1 and cIAP2 depletion; ripoptosome activation | [71, 72, 102] |
| 5-FU | RIP1 activation; autocrine TNF-α | [71, 104, 116, 123] |
| Taxol | FADD depletion; double-phosphorylation of FADD by Aurora-A and Plk1; RIP1 activation | [103] |
| Sorafenib | ROS production; RIP1activation | [106, 107] |
| Interferons | TLR3/TLR4 activation; necrosome formation; independent of RIP1 kinase activity | [34, 124] |
| Investigational agents | ||
| TRAIL | TNFR1 signaling; RIP1/RIP3-dependent | [8, 53, 111] |
| Poly(I:C) | Secretion of IFNs; TLR3/TLR4 activation; Necrosome formation | [34, 110] |
| IAP antagonists | IAP depletion; ripoptosome activation | [7–9] |
| ABT-737, obatoclax | Atg5-dependent necrosome formation on autophagosome | [113] |
| Natural products | ||
| Shikonin | ROS production; RIP1/RIP3 activation | [19] |
| Chal-24 | cIAP1 and cIAP2 degradation; ripoptosome activation | [109] |
| Staurosporine | RIP1/RIP3/MLKL-dependent | [108] |
Necroptosis can also be induced by kinase inhibitors, natural products, or immune modulators. Sorafenib, an FDA-approved multi-kinase inhibitor drug, can cause RIP1-dependent necroptosis in prostate cancer cells through induction of autophagy and interaction of RIP1 with p62/sequestosome 1 [106]. A combination of sorafenib with the histone deacetylase inhibitor Givinostat induced RIP1-dependent necroptosis through ROS production and activation of the BH3-only Bcl-2 family protein Bim [107]. Staurosporine, a pan-kinase inhibitor and a widely used apoptosis inducer, was found to promote RIP1/MLKL-dependent necroptosis in U-937 lymphoma cells under caspase-compromised conditions [108]. Chalcone-24, a chalcone derivative, induced RIP1/RIP3-dependent necroptosis through autophagy induction and cIAP degradation [109]. Shikonin, a naturally occurring naphthoquinone, could promote RIP1-dependent cell death in cancer cells [19]. Interestingly, necroptosis induced by these agents is often accompanied by autophagy, which may be responsible for suppression of apoptosis and bias toward necroptosis. It has been suggested that the viral dsRNA analog Poly (I:C) can be used as an adjuvant for cancer immunotherapies. A recent study demonstrated that stimulation of cervical cancer cells with Poly (I:C) caused RIP3-dependent necroptosis, which promotes activation of dendritic cells (DC) to produce IL-12, a cytokine critical for anti-tumor immunity [110]. This finding suggests that the status of RIP3 in cervical cancer cells determines the response to Poly-(I:C)-based immunotherapy, and needs to be assessed prior to therapy [110].
4.2 Manipulating necroptosis for improving anti-cancer therapies
Alterations of RIP1, RIP3, CYLD and MLKL likely explain defective necroptosis in some cancers, suggesting its restoration as a therapeutic strategy. In contrast to a plethora of necroptosis inhibitors, necroptosis activators have yet to be developed. Several strategies might be useful for restoring necroptosis in cancer cells.
Proapoptotic agents, including TRAIL, IAP inhibitors, and Bcl-2 inhibitors, can induce necroptosis in cancer cells when apoptosis is blocked. In preclinical studies, TRAIL, alone or in combination with other anticancer agents, has shown promising activities. It induces apoptosis in a wide range of cancer cells, while lacking significant toxicity to normal cells [22]. However, TRAIL has failed to show a significant benefit in clinical studies [22]. TRAIL induces necroptosis in cancer cells upon caspase inhibition [8, 53]. Changes in extracellular pH can switch TRAIL-induced cell death from apoptosis to RIP1/RIP3-mediated necroptosis in colon and liver cancer cells [111]. Induction of necroptosis may help to improve the anti-cancer activity of TRAIL in tumors with deregulated apoptosis.
cIAPs are commonly overexpressed in cancers and represent attractive therapeutic targets. Well-characterized structures of cIAPs and the binding interface with their endogenous inhibitor SMAC have facilitated pharmacological interventions. A number of small-molecule mimetics of the IAP-binding AVPI motif in SMAC have been identified, some of which have advanced into clinical trials for treating solid tumors and lymphomas [82]. cIAP inhibition seems to confer susceptibility to necroptosis, as exemplified by the use of SMAC mimetics to induce cIAP degradation and RIP1/RIP3-dependent necroptosis in cancer cells when caspase activation is inhibited [8].
Agents mimicking the BH3 domains of the proapoptotic Bcl-2 family members can restore apoptosis and selectively kill cancer cells that are addicted to antiapoptotic Bcl-2-like proteins for survival [112]. Obatoclax (GX15-070), a small-molecule inhibitor of antiapoptotic Bcl-2-family proteins, triggers necroptotic cell death by promoting necrosome formation on autophagosomal membranes [113]. Obatoclax was also found to induce autophagy-dependent necroptosis and overcome resistance to glucocorticoids in childhood acute lymphoblastic leukemia (ALL) [114]. BH3 mimetics, such as ABT-737 and ABT-263, could stimulate secretion of TNF-α [115], a prototypic necroptosis inducer. It is likely that necroptosis contributes to the cell-killing effect of BH3 mimetics, which are often not strong inducers of apoptosis when used as single agents. Therefore, targeting the Bcl-2 family proteins may provide another means for enhancing necroptosis in cancer cells.
As RIP3 is frequently silenced in cancer cells through promoter hypermethylation, demethylating agents such as decitabine (5-aza-2′-deoxycytidine) can be used to restore RIP3 expression and enhance chemotherapy-induced necroptosis [71]. Chemotherapy-induced necroptosis could also be enabled in p53-deficient colon cancer cells through inhibition of GSK3β [116]. Stimulating the expression and dimerization of RIP3 may be useful for enhancing necroptosis in cancer cells. Recovering CYLD function, either by increasing its expression using inhibitors of Snail or Notch-Hes1, or by inhibiting its SUMOylation to enhance deubiquitinase activity, promoted necroptosis of neuroblastoma cells [117]. Furthermore, elucidating the structural basis of the RHIM-RHIM interaction interfaces may help develop small molecules or peptide mimetics for modulating the key signaling event in necroptosis [118].
5. Future directions and conclusions
The research on necroptosis has been rapidly evolving in recent years, while its role in cancer remains to be a nascent area. Several critical issues remain to be solved. First, the interdependent relationship and crosstalk mechanisms of necroptosis and other forms of cell death need to be better elucidated. Dissecting the precise functional role of necroptosis and underlying mechanisms in tumor development and therapy-induced cell killing will provide new opportunities for more specific manipulation of necroptosis. Second, more specific markers of necroptosis need to be identified. Analysis of necroptosis, especially in vivo and in human tissue samples, has been hindered by the lack of molecular markers. An expanding and improved tool box will likely provide more definitive evidence on aberrant regulation of necroptosis in cancer. Third, more physiologically relevant systems are necessary to study the physiological and pathological roles of necroptosis. Up to now, relatively artificial conditions, such as caspase inhibition and KO of a necroptosis suppressor or apoptosis activator, are necessary for the manifestation of necroptosis phenotypes. Last but not least, the connection between necroptosis and the immune system needs to be clarified in the setting of cancer development and therapies. Recent success of cancer immunotherapy highlights a key role of the immune system in preventing tumor progression, likely more important than previously thought. Clarifying how DAMPs and other signaling molecules released during necroptosis elicit specific types of immune response may be critical for understanding the seemingly opposite functions of immune system in cancer immune surveillance and tumor promotion.
In summary, recent studies have defined RIP1/RIP3/MLKL-dependent necroptosis as a new form of cell death responsible for killing stressed cells that are unable to die by apoptosis. Accumulating evidence suggests that necroptosis functions as an alternative barrier against cancer development, and necroptosis signaling can be explored to develop new anti-cancer therapies.
Supplementary Material
Highlights.
Necroptosis is a form of regulated necrosis that kills apoptosis-deficient cells.
Necroptosis is governed by RIP1, RIP3 and MLKL.
Alterations of necroptosis regulators are frequently found in human tumors.
Anticancer agents can promote necroptosis in addition to apoptosis.
Unlike apoptosis, necroptosis elicits a more robust immune response.
Acknowledgments
We apologize for not being able to cite many excellent original articles by our colleagues due to space limitation. We thank our lab members for critical reading. Research in the authors’ labs is supported U.S. National Institute of Health grants (R01CA106348, R01CA172136 and R01CA203028 to L.Z.; U01DK085570 to J.Y.; and P30CA047904 to University of Pittsburgh Cancer Institute).
Abbreviations
- AIF
apoptosis-inducing factor
- ALL
acute lymphoblastic leukemia
- cIAP
cellular inhibitor of apoptosis protein
- CLL
chronic lymphocytic leukemia
- CYLD
cylindromatosis
- DAMPs
Damage Associated Molecular Patterns
- DCs
dendritic cells
- Drp1
dynamin-related protein 1
- dsRNA
double-stranded RNA
- FADD
FAS-associated death domain
- FASL
FAS ligand
- 5-FU
5-fluorouracil
- HCMV
human cytomegalovirus
- HMGB1
high-mobility group box 1
- HSV-1
herpes simplex virus 1
- IFN
interferon
- KO
knockout
- IL-1α
interleukin-1α
- LPS
lipopolysaccharides
- MAPK
mitogen-activated protein kinase
- MCMV
murine cytomegalovirus
- MLKL
mixed lineage kinase domain-like
- NAD
nicotinamide adenine dinucleotide
- Nec-1
necrostatin-1
- NSA
necrosulfonamide
- PARP1
poly(ADP-ribose) polymerase 1
- pGAM5
phosphoglycerate mutase family member 5
- poly(I:C)
polyinosinic-polycytidylic acid
- PTP
permeability transition pore
- RHIM
respective homotypic interaction motif
- RIP
Receptor-Interacting Protein
- ROS
reactive oxygen species
- SMAC
second mitochondria-derived activator of caspases
- SNP
single nucleotide polymorphism
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TLR
Toll-like receptor
- TUNEL
terminal deoxynucleotidyl transferase mediated dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature immunology. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 5.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature chemical biology. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 6.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nature chemical biology. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 11.Belizario J, Vieira-Cordeiro L, Enns S. Necroptotic Cell Death Signaling and Execution Pathway: Lessons from Knockout Mice. Mediators of inflammation. 2015;2015:128076. doi: 10.1155/2015/128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 13.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damageassociated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Danial NN, Korsmeyer SJ. Cell death. Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 17.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 18.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. The Journal of biological chemistry. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 19.Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo J, Hu X. Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Molecular cancer therapeutics. 2007;6:1641–1649. doi: 10.1158/1535-7163.MCT-06-0511. [DOI] [PubMed] [Google Scholar]
- 20.Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes & development. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guchelaar HJ, Vermes I, Koopmans RP, Reutelingsperger CP, Haanen C. Apoptosis- and necrosis-inducing potential of cladribine, cytarabine, cisplatin, and 5-fluorouracil in vitro: a quantitative pharmacodynamic model. Cancer chemotherapy and pharmacology. 1998;42:77–83. doi: 10.1007/s002800050788. [DOI] [PubMed] [Google Scholar]
- 22.Long JS, Ryan KM. New frontiers in promoting tumour cell death: targeting apoptosis, necroptosis and autophagy. Oncogene. 2012;31:5045–5060. doi: 10.1038/onc.2012.7. [DOI] [PubMed] [Google Scholar]
- 23.Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86. doi: 10.1038/cdd.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 27.Geserick P, Hupe M, Moulin M, Wong WW, Feoktistova M, Kellert B, Gollnick H, Silke J, Leverkus M. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J Cell Biol. 2009;187:1037–1054. doi: 10.1083/jcb.200904158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PloS one. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 30.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell death and differentiation. 2014 doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell host & microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong CQ, Xia W, Zhou R, Zheng C, Han J. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell host & microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc Natl Acad Sci U S A. 2014;111:E3206–E3213. doi: 10.1073/pnas.1407068111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Molecular cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, Bertin J, Gough PJ, Savvides S, Martinou JC, Bertrand MJ, Vandenabeele P. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 44.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, Kidd G, Wakefield R, Frase S, Krautwald S, Linkermann A, Green DR. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 2013;5:878–885. doi: 10.1016/j.celrep.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, Dondelinger Y, Roelandt R, Bruggeman I, Goncalves A, Bertrand MJ, Baekelandt V, Takahashi N, Berghe TV, Vandenabeele P. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell death & disease. 2014;5:e1004. doi: 10.1038/cddis.2013.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFalpha-induced necroptotic signaling and cell death. Oncogene. 2015;34:5796–5806. doi: 10.1038/onc.2015.35. [DOI] [PubMed] [Google Scholar]
- 50.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, Yang Z, Wu SQ, Chen L, Han J. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proceedings of the National Academy of Sciences of the United States of America. 2014 doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J. RIPK1 Regulates RIPK3-MLKL-Driven Systemic Inflammation and Emergency Hematopoiesis. Cell. 2014 doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, Sze M, Gilbert B, Kourula S, Goossens V, Lefebvre S, Gunther C, Becker C, Bertin J, Gough PJ, Declercq W, van Loo G, Vandenabeele P. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 60.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 61.Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moubarak RS, Yuste VJ, Artus C, Bouharrour A, Greer PA, Menissier-de Murcia J, Susin SA. Sequential activation of poly(ADP-ribose) polymerase 1, calpains, and Bax is essential in apoptosis-inducing factor-mediated programmed necrosis. Molecular and cellular biology. 2007;27:4844–4862. doi: 10.1128/MCB.02141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 64.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie T, Peng W, Liu Y, Yan C, Maki J, Degterev A, Yuan J, Shi Y. Structural basis of RIP1 inhibition by necrostatins. Structure. 2013;21:493–499. doi: 10.1016/j.str.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 66.Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, Shen YY, Chen Y, Xiong B, Yang CH, Ding J, Miao ZH. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell death & disease. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McQuade T, Cho Y, Chan FK. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. The Biochemical journal. 2013;456:409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jouan-Lanhouet S, Riquet F, Duprez L, Vanden Berghe T, Takahashi N, Vandenabeele P. Necroptosis, in vivo detection in experimental disease models. Seminars in cell & developmental biology. 2014;35:2–13. doi: 10.1016/j.semcdb.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, Rauvala H, Herman G, Tracey KJ, Lee ML, Andersson J, Tokics L, Treutiger CJ. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 70.Liu P, Xu B, Shen W, Zhu H, Wu W, Fu Y, Chen H, Dong H, Zhu Y, Miao K, Xu W, Li J. Dysregulation of TNFalpha-induced necroptotic signaling in chronic lymphocytic leukemia: suppression of CYLD gene by LEF1. Leukemia. 2012;26:1293–1300. doi: 10.1038/leu.2011.357. [DOI] [PubMed] [Google Scholar]
- 71.Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, Levy JM, Pollyea DA, Jordan CT, Yan P, Frankhouser D, Nicolet D, Maharry K, Marcucci G, Choi KS, Cho H, Thorburn A, Kim YS. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707–725. doi: 10.1038/cr.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moriwaki K, Bertin J, Gough PJ, Orlowski GM, Chan FK. Differential roles of RIPK1 and RIPK3 in TNF-induced necroptosis and chemotherapeutic agent-induced cell death. Cell death & disease. 2015;6:e1636. doi: 10.1038/cddis.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukasawa M, Kimura M, Morita S, Matsubara K, Yamanaka S, Endo C, Sakurada A, Sato M, Kondo T, Horii A, Sasaki H, Hatada I. Microarray analysis of promoter methylation in lung cancers. Journal of human genetics. 2006;51:368–374. doi: 10.1007/s10038-005-0355-4. [DOI] [PubMed] [Google Scholar]
- 74.Cerhan JR, Ansell SM, Fredericksen ZS, Kay NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares W, Macon WR, Jelinek D, Witzig TE, Habermann TM, Slager SL. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007;110:4455–4463. doi: 10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR. The Catalogue of Somatic Mutations in Cancer (COSMIC) Current protocols in human genetics / editorial board, Jonathan L. Haines … [et al.] 2008;Chapter 10(Unit 10):11. doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K, Kowalski J, Kooby DA, El-Rayes BF, Staley CA, 3rd, Adsay NV, Curran WJ, Jr, Landry JC, Maithel SK, Yu DS. Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer. 2013;119:3148–3155. doi: 10.1002/cncr.28144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, Jones C, Hansen J, Blair E, Hofmann B, Siebert R, Turner G, Evans DG, Schrander-Stumpel C, Beemer FA, van Den Ouweland A, Halley D, Delpech B, Cleveland MG, Leigh I, Leisti J, Rasmussen S. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- 78.Blake PW, Toro JR. Update of cylindromatosis gene (CYLD) mutations in Brooke-Spiegler syndrome: novel insights into the role of deubiquitination in cell signaling. Hum Mutat. 2009;30:1025–1036. doi: 10.1002/humu.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends in biochemical sciences. 2010;35:392–399. doi: 10.1016/j.tibs.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 80.Massoumi R, Kuphal S, Hellerbrand C, Haas B, Wild P, Spruss T, Pfeifer A, Fassler R, Bosserhoff AK. Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma. J Exp Med. 2009;206:221–232. doi: 10.1084/jem.20082044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hellerbrand C, Bumes E, Bataille F, Dietmaier W, Massoumi R, Bosserhoff AK. Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis. 2007;28:21–27. doi: 10.1093/carcin/bgl081. [DOI] [PubMed] [Google Scholar]
- 82.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov. 2012;11:109–124. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 83.Gyrd-Hansen M, Meier P. IAPs: from caspase inhibitors to modulators of NF-kappaB, inflammation and cancer. Nat Rev Cancer. 2010;10:561–574. doi: 10.1038/nrc2889. [DOI] [PubMed] [Google Scholar]
- 84.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- 85.Irrinki KM, Mallilankaraman K, Thapa RJ, Chandramoorthy HC, Smith FJ, Jog NR, Gandhirajan RK, Kelsen SG, Houser SR, May MJ, Balachandran S, Madesh M. Requirement of FADD, NEMO, and BAX/BAK for aberrant mitochondrial function in tumor necrosis factor alpha-induced necrosis. Mol Cell Biol. 2011;31:3745–3758. doi: 10.1128/MCB.05303-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 89.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C, Green DR, Oberst A, Albert ML. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science. 2015;350:328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kang YJ, Bang BR, Han KH, Hong L, Shim EJ, Ma J, Lerner RA, Otsuka M. Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling. Nat Commun. 2015;6:8371. doi: 10.1038/ncomms9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 93.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaminskyy V, Zhivotovsky B. To kill or be killed: how viruses interact with the cell death machinery. Journal of internal medicine. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 96.Mocarski ES, Upton JW, Kaiser WJ. Viral infection and the evolution of caspase 8-regulated apoptotic and necrotic death pathways. Nature reviews. Immunology. 2012;12:79–88. doi: 10.1038/nri3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, Lee TH, Chan FK, Pasparakis M, Kelliher MA. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. Journal of immunology. 2014;193:1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. Herpes simplex virus suppresses necroptosis in human cells. Cell host & microbe. 2015;17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES. Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem. 2015;290:11635–11648. doi: 10.1074/jbc.M115.646042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009;11:1–9. doi: 10.1593/neo.81178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 102.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Molecular cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 103.Jang MS, Lee SJ, Kang NS, Kim E. Cooperative phosphorylation of FADD by Aur-A and Plk1 in response to taxol triggers both apoptotic and necrotic cell death. Cancer research. 2011;71:7207–7215. doi: 10.1158/0008-5472.CAN-11-0760. [DOI] [PubMed] [Google Scholar]
- 104.Brown MF, Leibowitz BJ, Chen D, He K, Zou F, Sobol RW, Beer-Stolz D, Zhang L, Yu J. Loss of Caspase-3 sensitizes colon cancer cells to genotoxic stress via RIP1-dependent necrosis. Cell death & disease. 2015;6:e1729. doi: 10.1038/cddis.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Y, Lin Z, Zhao N, Zhou L, Liu F, Cichacz Z, Zhang L, Zhan Q, Zhao X. Receptor interactive protein kinase 3 promotes Cisplatin-triggered necrosis in apoptosis-resistant esophageal squamous cell carcinoma cells. PLoS One. 2014;9:e100127. doi: 10.1371/journal.pone.0100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kharaziha P, Chioureas D, Baltatzis G, Fonseca P, Rodriguez P, Gogvadze V, Lennartsson L, Bjorklund AC, Zhivotovsky B, Grander D, Egevad L, Nilsson S, Panaretakis T. Sorafenib-induced defective autophagy promotes cell death by necroptosis. Oncotarget. 2015;6:37066–37082. doi: 10.18632/oncotarget.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Locatelli SL, Cleris L, Stirparo GG, Tartari S, Saba E, Pierdominici M, Malorni W, Carbone A, Anichini A, Carlo-Stella C. BIM upregulation and ROS-dependent necroptosis mediate the antitumor effects of the HDACi Givinostat and Sorafenib in Hodgkin lymphoma cell line xenografts. Leukemia. 2014;28:1861–1871. doi: 10.1038/leu.2014.81. [DOI] [PubMed] [Google Scholar]
- 108.Dunai ZA, Imre G, Barna G, Korcsmaros T, Petak I, Bauer PI, Mihalik R. Staurosporine induces necroptotic cell death under caspase-compromised conditions in U937 cells. PLoS One. 2012;7:e41945. doi: 10.1371/journal.pone.0041945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He W, Wang Q, Srinivasan B, Xu J, Padilla MT, Li Z, Wang X, Liu Y, Gou X, Shen HM, Xing C, Lin Y. A JNK-mediated autophagy pathway that triggers c-IAP degradation and necroptosis for anticancer chemotherapy. Oncogene. 2014;33:3004–3013. doi: 10.1038/onc.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schmidt SV, Seibert S, Walch-Ruckheim B, Vicinus B, Kamionka EM, Pahne-Zeppenfeld J, Solomayer EF, Kim YJ, Bohle RM, Smola S. RIPK3 expression in cervical cancer cells is required for PolyIC-induced necroptosis, IL-1alpha release, and efficient paracrine dendritic cell activation. Oncotarget. 2015;6:8635–8647. doi: 10.18632/oncotarget.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, Martin-Chouly C, Le Moigne-Muller G, Van Herreweghe F, Takahashi N, Sergent O, Lagadic-Gossmann D, Vandenabeele P, Samson M, Dimanche-Boitrel MT. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell death and differentiation. 2012;19:2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L, Ming L, Yu J. BH3 mimetics to improve cancer therapy; mechanisms and examples. Drug Resist Updat. 2007;10:207–217. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Basit F, Cristofanon S, Fulda S. Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ. 2014;21:1183–1184. doi: 10.1038/cdd.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, Schafer BW, Schrappe M, Stanulla M, Bourquin JP. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 116.Grassilli E, Narloch R, Federzoni E, Ianzano L, Pisano F, Giovannoni R, Romano G, Masiero L, Leone BE, Bonin S, Donada M, Stanta G, Helin K, Lavitrano M. Inhibition of GSK3B bypass drug resistance of p53-null colon carcinomas by enabling necroptosis in response to chemotherapy. Clin Cancer Res. 2013;19:3820–3831. doi: 10.1158/1078-0432.CCR-12-3289. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi T, Masoumi KC, Massoumi R. Deubiquitinating activity of CYLD is impaired by SUMOylation in neuroblastoma cells. Oncogene. 2015;34:2251–2260. doi: 10.1038/onc.2014.159. [DOI] [PubMed] [Google Scholar]
- 118.Li J, Yin Q, Wu H. Structural basis of signal transduction in the TNF receptor superfamily. Advances in immunology. 2013;119:135–153. doi: 10.1016/B978-0-12-407707-2.00005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 120.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M. Apoptosis and cancer: mutations within caspase genes. Journal of medical genetics. 2009;46:497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 121.Shin MS, Kim HS, Lee SH, Lee JW, Song YH, Kim YS, Park WS, Kim SY, Lee SN, Park JY, Lee JH, Xiao W, Jo KH, Wang YP, Lee KY, Park YG, Kim SH, Lee JY, Yoo NJ. Alterations of Fas-pathway genes associated with nodal metastasis in non-small cell lung cancer. Oncogene. 2002;21:4129–4136. doi: 10.1038/sj.onc.1205527. [DOI] [PubMed] [Google Scholar]
- 122.Lo SC, Hannink M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem. 2006;281:37893–37903. doi: 10.1074/jbc.M606539200. [DOI] [PubMed] [Google Scholar]
- 123.Oliver Metzig M, Fuchs D, Tagscherer KE, Grone HJ, Schirmacher P, Roth W. Inhibition of caspases primes colon cancer cells for 5-fluorouracil-induced TNF-alpha-dependent necroptosis driven by RIP1 kinase and NF-kappaB. Oncogene. 2015 doi: 10.1038/onc.2015.398. [DOI] [PubMed] [Google Scholar]
- 124.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3109–E3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.