Abstract
Background
There has been increasing interest in primary surgical treatment of patients with early T classification (T1–T2) oropharyngeal squamous cell carcinoma (OPSCC), with the stated goal of de-escalating or avoiding adjuvant treatment. We sought to determine the degree to which this interest has translated into changes in practice patterns, and the rates of adverse post-operative pathologic features.
Methods
Patients with T1–T2 OPSCC in the National Cancer Database (NCDB) treated from 2004–2013 were categorized as receiving primary surgical or primary radiation-based treatment. Trends in treatment selection and factors related to selection of primary surgery were examined. The rates of adverse pathologic features including positive margins, extracapsular spread (ECS), and advanced T and N stage following surgery were analyzed.
Results
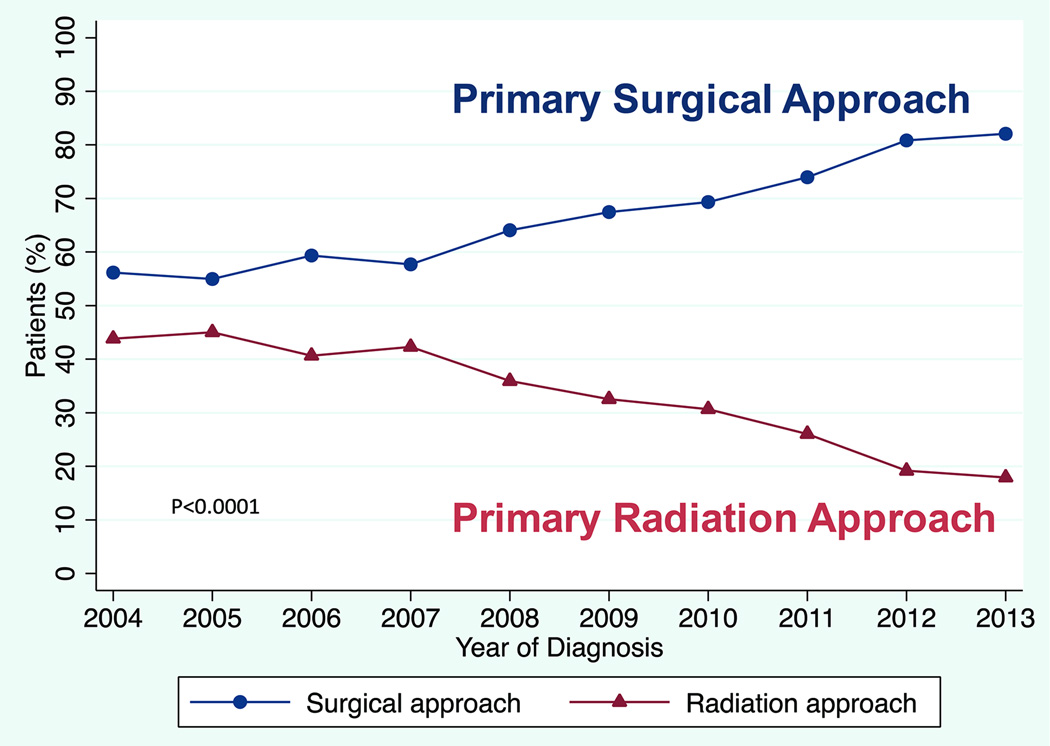
Of 8,768 patients with T1–T2 OPSCC, 68% received primary surgical treatment, increasing from 56% in 2004 to 82% in 2013 (p<0.0001). The highest versus lowest volume hospitals treated 78% versus 59% of patients with primary surgery (O.R. 2.23 C.I. 1.55–3.22, p<0.0001). Higher nodal stage predicted lower rates of primary surgery, but the majority of patients with clinical N2/N3 disease underwent primary surgery. Among surgical patients, positive margins were present in 24% and ECS in 25%. Positive margins decreased over time (p<0.0001) and were seen less often at high volume centers (p<0.0001). Among candidates for single modality therapy (clinical T1–T2/N0–N1), 33% had positive margins and/or ECS, and 47% had at least one adverse feature (T3–T4, N2–N3, positive margins, and/or ECS).
Conclusion
Primary surgical treatment for early T-stage OPSCC has become more widespread.
Keywords: HPV-related oropharyngeal squamous cell carcinoma (OPSCC), trans-oral robotic surgery (TORS), positive margins, extracapsular spread (ECS), treatment selection
INTRODUCTION
The incidence of oropharyngeal squamous cell carcinoma (OPSCC) in the United States (US) is rising, and is projected to double by 2030, to more than 15,000 diagnoses per year.1 In addition to the classic risk factors of chronic tobacco and alcohol exposure, the human papillomavirus (HPV) has emerged as the leading causative agent for OPSCC in the US and other countries.2, 3 Compared to patients with tobacco-associated OPSCC, patients with HPV-associated OPSCC are, on average, younger at diagnosis, have fewer comorbid illnesses, and have a superior prognosis.4–6 At presentation, HPV-associated OPSCC tumors commonly present with low T classification (T1–T2), but advanced stage (Stage III-IV), owing to nodal metastases.7, 8
Uncertainty persists about the most appropriate treatment strategies for patients with OPSCC. Prior to the 1990s, primary surgery and primary radiotherapy were the most commonly employed strategies. In the late 1990s, organ preservation trials demonstrated survival benefits of chemoradiotherapy (CRT) over radiotherapy (RT) alone,9 leading to increased use of CRT in the early 2000s.10, 11 However, there has recently been renewed enthusiasm for primary surgical approaches for the treatment of OPSCC12–14. This has been driven by technologic advances such as transoral robotic surgery (TORS) that enhance the feasibility of surgery for low T classification OPSCC and offer the potential of avoiding or de-escalating adjuvant treatments. TORS was approved by the Food and Drug Administration (FDA) in 2009.
In order to achieve the goal of minimizing the extent of postoperative therapy for patients with early T-stage OPSCC, it is important to select patients appropriately for one of two current standard initial treatment strategies: primary surgery or primary radiation with or without chemotherapy. Specifically, surgical patients should ideally be those in whom the likelihood of adverse pathologic risk factors is low. According to National Comprehensive Cancer Center (NCCN) guidelines, the adverse risk factors that prompt escalation of adjuvant therapy include N2–N3 nodal status, advanced pathologic T classification, positive margins, the presence of lymph nodes with extracapular spread (ECS), and tumor perineural invasion or lymphovascular invasion.15 Conversely if patients have none of these adverse features they may be treated with surgery alone.
While recent work has examined variables associated with patients treated with TORS since FDA approval16, there is limited knowledge of national trends in the primary treatment strategy for patients with early T-stage OPSCC before and during the TORS era, particularly the factors associated with a decision to triage patients to primary surgical therapy, and the success of physicians in selecting patients for surgery in whom adverse pathologic features are not present. The goal of this study was to use a large, high quality US cancer registry to examine these questions at a national level.
MATERIALS AND METHODS
Data Source
The data source for this study was the National Cancer Database (NCDB), a joint program of the Commission on Cancer (CoC) and the American College of Surgeons (ACS) that collects hospital-based registry data on over 80% of U.S. oral cavity and pharynx cases.17 The source files were used in accordance with the NCDB Participant User Files (PUF) data use agreement. This study was given IRB waiver by Memorial Sloan Kettering Cancer Center.
Study Cohort
We identified all patients with clinically staged T1 and T2 OPSCC diagnosed between 2004–2013 who were ≥ 18 years old. We included ICDO codes for the “oropharynx” (ICDO C019, C090, C091, C098, C099, C100, C101, C102, C103, C104, C108, C109, C142). We included only patients with histologically proven squamous cell carcinoma tissue examined by microscope rather than cytology alone; the tissue could be examined from biopsy or surgical pathology specimens. We excluded patients who had received part or all of their treatment outside of the NCDB reporting facility in order to be certain whether or not the various possible primary treatments were in fact delivered, and in what order. Patients were also excluded if treatment information was missing or for whom the sequence of treatments was not clear, and whose staging information was inconsistent with treatment information or could not be assessed (Figure 1). The total cohort with complete data was 8,768 patients.
Figure 1.
Flow diagram of patient inclusion/exclusion
Outcomes
The main outcome was the choice of primary treatment modality. Patients were categorized as receiving “primary radiation” or “primary surgical” treatment. The primary treatment modality was determined to be radiation if they did not receive primary surgery but had undergone radiation. The primary modality was surgery if they received surgery before any radiation or chemotherapy. A “local tumor excision” such as excisional biopsy was not considered primary surgery. For tonsil and other oropharynx all categories of “pharyngectomy” were used; 91% were “Limited/partial pharyngectomy” which includes tonsillectomy, 4% were “Pharyngectomy NOS,” 2% were “total pharyngectomy,” 1% were “radical pharyngectomy,” and the rest were other subclassifications of pharyngectomy. Base of tongue tumors (ICD C019) are listed with other tongue tumors; primary surgical patients were those who underwent at least “glossectomy” and not “local tumor excision.”
A secondary outcome was the presence of adverse pathologic features among those who underwent primary surgical treatment. For this analysis, we included only those patients undergoing primary surgery who had complete information on margin status and ECS (Figure 1).
Tumor characteristics
Clinical T and N classification were used for the analysis of predictors of primary treatment modality since patients who did not have surgery did not have pathologic information. For the secondary analysis of pathologic factors among those undergoing primary surgery, T and N classification were each described according to both clinical and pathologic staging. ECS is defined in the NCDB as either negative, microscopic, macroscopic, or unknown; for this analysis ECS was determined to be positive if micro or macroscopically positive. Margins were defined as positive if there was microscopic or macroscopic “residual tumor.” Close margins are not recorded in the NCDB and would be considered negative by coding rules.
Covariates
Patient sociodemographic factors included age, sex, race, insurance status (grouped as private, uninsured, or government insurance including Medicare and Medicaid), and comorbidities (Charlson-Deyo Comorbidity index18). Hospital volume was defined as the total new OPSCC cases seen over all years from 2004–2013, with hospitals divided into quartiles by volume for analysis. Hospital type was defined as either “academic,” which included Academic/Research programs as well as National Cancer Institute (NCI)-designated Comprehensive Cancer Centers, or “community,” which included Community Cancer Programs, Comprehensive Community Cancer Programs, Integrated Network Cancer Programs, and other specified types of programs.
Analysis
We used descriptive and chi-squared statistics to compare tumor, patient sociodemographic, and hospital factors among patients who underwent primary surgery versus primary radiation treatment. The Cochran-Armitage trend test was used to determine whether the proportion of patients treated with a primary surgical approach (versus primary radiation approach) changed over time. For multivariable analysis we used hierarchical generalized linear models with a logit link to account for clustering of patients within hospitals while evaluating the influence of tumor, patient sociodemographic, and hospital factors on the binary choice of primary treatment modality. The large sample size allowed us to include all variables of interest in the multivariable model and no model selection was necessary. For the secondary analysis of pathologic factors among those undergoing primary surgery, we used descriptive statistics as well as the Cochran-Armitage trend test to examine how these pathologic features changed over time. As a sensitivity analysis for one of these pathologic features (margin positivity), we also performed logistic regression of year on margin positivity to ensure that the trend over time was in a downward direction. P-values <0.05 were considered significant. Analyses were conducted using Stata Statistical Software (Release 12.1; Stata Inc., College Station, TX).
RESULTS
Characteristics of the cohort
We identified 8,768 patients who presented with T1 and T2 OPSCC between 2004–2013 (Table 1). The majority were male (79%), age 50–64 (55%), and were white (92%). The majority had private insurance (68%) and zero comorbidities (82%). Slightly less than half (49%) were treated at community cancer programs. While 26% were treated at higher volume hospitals that saw >50 patients with OPSCC, another 34% were treated at low volume hospitals where only 10 or fewer patients with OPSCC were treated between 2004–2013. Regarding tumor factors, 47% of patients presented with clinically classified T1 tumors, and 53% with T2 tumors, while 26% had clinically or radiographically classified N0 disease, 23% had N1 disease, 47% had N2 disease, and 3% had N3 disease at presentation.
Table 1.
Primary Treatment Modality in Patients with T1–T2 OPSCC
| Characteristic | Overall | Primary Surgical Treatment (vs. Primary XRT) |
Multivariable Analysis of Primary Surgical Treatment |
||
|---|---|---|---|---|---|
|
No. (Column %) |
No. (Row % compared to Primary XRT, not shown) |
p-value |
Adjusted Odds Ratio (95% C.I.) |
p-value | |
| Patients | 8768 | 5967 (68.1%) | |||
| Age at Diagnosis | p=0.265 | ||||
| <50 | 1732 (19.8%) | 1210 (69.9%) | 1 [Reference] | ||
| 50–64 | 4843 (55.2%) | 3287 (67.9%) | 0.78 (0.68 – 0.89) | p<0.0001 | |
| 65–79 | 1940 (22.1%) | 1297 (66.9%) | 0.68 (0.55 – 0.83) | p<0.0001 | |
| ≥80 | 253 (2.9%) | 173 (68.4%) | 0.70 (0.49 – 0.99) | p=0.046 | |
| Sex | p=0.001 | ||||
| Male | 6911 (78.8%) | 4644 (67.2%) | 0.93 (0.81 – 1.06) | p=0.251 | |
| Female | 1857 (21.2%) | 1323 (71.2%) | 1 [Reference] | ||
| Race | p=0.688 | ||||
| White | 8032 (91.6%) | 5463 (68%) | 1 [Reference] | ||
| Black | 492 (5.6%) | 332 (67.5%) | 0.85 (0.67 – 1.06) | p=0.152 | |
| Other | 244 (2.8%) | 172 (70.5%) | 1.10 (0.80 – 1.51) | p=0.556 | |
| Clinical N Stage* | p<0.0001 | ||||
| N0 | 2304 (26.3%) | 1856 (80.6%) | 1 [Reference] | ||
| N1 | 2030 (23.2%) | 1357 (66.8%) | 0.44 (0.37 – 0.51) | p<0.0001 | |
| N2 | 4151 (47.3%) | 2602 (62.7%) | 0.33 (0.28 – 0.38) | p<0.0001 | |
| N3 | 283 (3.2%) | 152 (53.7%) | 0.26 (0.20 – 0.35) | p<0.0001 | |
| Clinical T-Stage* | p=0.245 | ||||
| T1 | 4155 (47.4%) | 2853 (68.7%) | 1 [Reference] | ||
| T2 | 4613 (52.6%) | 3114 (67.5%) | 0.93 (0.84 – 1.03) | p=0.142 | |
| Insurance Status | p=0.007 | ||||
| Private | 5942 (67.8%) | 4091 (68.8%) | 1.19 (1.01 – 1.39) | p=0.036 | |
| Uninsured | 358 (4.1%) | 220 (61.5%) | 0.93 (0.70 – 1.25) | p=0.639 | |
| Government** | 2468 (28.1%) | 1656 (67.1%) | 1 [Reference] | ||
| Charlson-Deyo Comorbidity Count | p=0.029 | ||||
| 0 | 7194 (82%) | 4854 (67.5%) | 1 [Reference] | ||
| 1 | 1279 (14.6%) | 911 (71.2%) | 1.13 (0.97 – 1.30) | p=0.122 | |
| ≥2 | 295 (3.4%) | 202 (68.5%) | 0.95 (0.71 – 1.26) | p=0.705 | |
| Year Diagnosed | p<0.0001 | ||||
| 2004 | 568 (6.5%) | 319 (56.2%) | 1 [Reference] | ||
| 2005 | 644 (7.3%) | 354 (55%) | 0.98 (0.76 – 1.27) | p=0.913 | |
| 2006 | 674 (7.7%) | 400 (59.3%) | 1.09 (0.84 – 1.41) | p=0.484 | |
| 2007 | 747 (8.5%) | 431 (57.7%) | 1.14 (0.89 – 1.46) | p=0.294 | |
| 2008 | 1052 (12%) | 674 (64.1%) | 1.50 (1.18 – 1.89) | p=0.001 | |
| 2009 | 1174 (13.4%) | 792 (67.5%) | 1.78 (1.41 – 2.25) | p<0.0001 | |
| 2010 | 939 (10.7%) | 651 (69.3%) | 1.86 (1.46 – 2.39) | p<0.0001 | |
| 2011 | 979 (11.2%) | 724 (74%) | 2.28 (1.78 – 2.92) | p<0.0001 | |
| 2012 | 970 (11.1%) | 784 (80.8%) | 3.37 (2.60 – 4.37) | p<0.0001 | |
| 2013 | 1021 (11.6%) | 838 (82.1%) | 3.82 (2.95 – 4.96) | p<0.0001 | |
| Facility Type | p<0.0001 | ||||
| Academic/ NCI CCC | 4496 (51.3%) | 3306 (73.5%) | 1 [Reference] | ||
| Community | 4272 (48.7%) | 2661 (62.3%) | 0.91 (0.75 – 1.10) | p=0.334 | |
| Hospital Volume | p<0.0001 | ||||
| 1–10 pts | 3018 (34.4%) | 1791 (59.3%) | 1 [Reference] | ||
| 11–20 pts | 1576 (18%) | 1057 (67.1%) | 1.41 (1.13 – 1.75) | p=0.002 | |
| 21–50 pts | 1883 (21.5%) | 1336 (71%) | 1.52 (1.17 – 1.97) | p=0.002 | |
| >50 pts | 2291 (26.1%) | 1783 (77.8%) | 2.23 (1.55 – 3.22) | p<0.0001 | |
Abbreviations: OPSCC, oropharyngeal squamous cell carcinoma; XRT, external radiation therapy, NCI CCC, national cancer institute comprehensive cancer center; N, nodal; T, tumor.
Bold p-values are <0.05
Both nodal and T stage defined as clinical staging, since this was the only information available before selection of primary treatment modality.
Medicare and Medicaid
Primary treatment selection
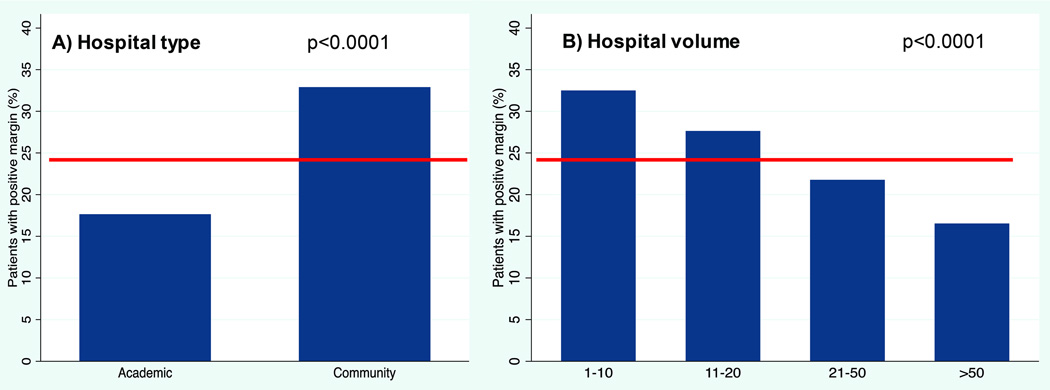
Overall 68% (5967) of patients underwent primary surgical treatment. From 2004–2013 the use of the primary surgical approach increased over time from 56% of patients in 2004 to 82% in 2013 (p< 0.0001; Figure 2). Patients selected for primary surgical treatment tended to have lower nodal disease status at presentation (81% of N0, 67% of N1, 63% of N2, and 54% of N3 selected for surgery, p<0.0001, Table 1 Column 2). Patients undergoing primary surgery tended to have private insurance compared to no insurance or government insurance (69% vs 62% and 67% respectively, p=0.007). Patients treated at academic hospitals received a primary surgical approach more often than those treated at community hospitals (74% vs. 62%, p<0.0001), and patients treated at the highest volume hospitals received a primary surgical approach more often than those treated at the lowest (78% vs. 59%, p<0.0001; Table 1 Column 2).
Figure 2.
Primary treatment approach for patients with T1–T2 OPSCC. N=8,768 including 2,801 (31.9%) receiving primary radiation approach and 5,967 (68.1%) receiving primary surgical approach. There were 568 total patients in 2004, increasing to 1,021 total patients in 2013. P<0.0001 for difference in primary treatment approach over time.
Factors associated with primary surgery
On multivariable analysis, in addition to the year of diagnosis, several tumor and non-tumor factors were associated with the use of a primary surgical approach (Table 1 Column 3). Clinical N0 stage was associated with a higher likelihood of a primary surgical approach (N1 vs N0: O.R. 0.44, C.I. 0.37–0.51, p<0.0001; N2 vs N0: O.R. 0.33, C.I. 0.28–0.38, p< 0.0001; N3 vs N0: O.R. 0.26, C.I. 0.20–0.35, p<0.0001). Younger patients were more likely to undergo a primary surgical approach (age 50–64 vs. <50: O.R. 0.78 C.I. 0.68–0.89, p<0.0001; age 65–79 vs. <50: O.R. 0.68 C.I. 0.55–0.83, p<0.0001). Higher volume hospitals were significantly more likely to use a primary surgical approach compared to the lowest volume hospitals (>50 patients vs 1–10 pts: O.R. 2.23, C.I. 1.55–3.22, p<0.0001). In the multivariable analysis hospital type was not associated with primary treatment approach.
Pathologic factors in patients receiving a primary surgical approach
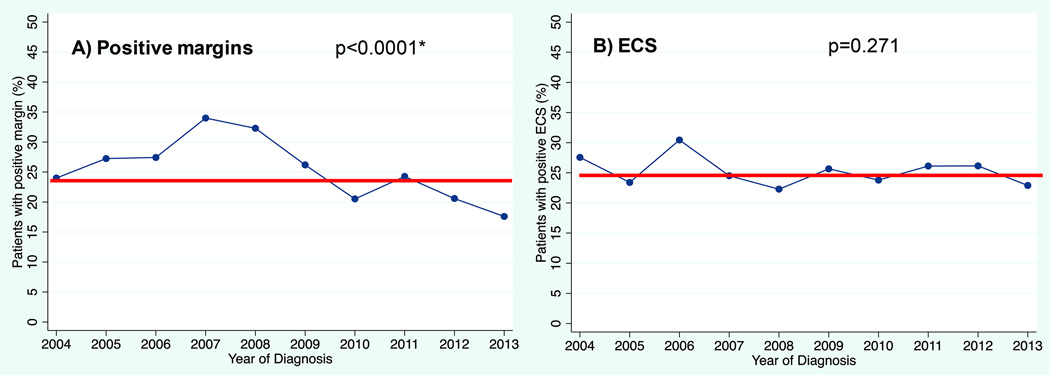
Of the 5,967 primary surgical patients, 4,833 had complete pathologic information regarding T stage, N stage, margins, and ECS. In this group, 24% had positive margins, 25% had ECS, and 43% had either positive margins and/or ECS. There was a significant decrease over time in positive margin rate (p<0.0001, Figure 3a). Margin positivity was highest in 2007 at 34% and dropped to 18% in 2013. Community and low volume hospitals were more likely to have positive margins on final pathology (community 33% positive margin vs. academic 17%, p<0.0001; lowest volume 33% vs. highest volume 17%, p<0.0001, Figure 4). There was no change in rates of ECS over time (p=0.271, Figure 3b). Higher pathologic nodal classification was associated with increased rates of ECS (17% of N1, 38% of N2, 51% of N3; p<0.0001, data not shown).
Figure 3. Change in pathologic factors over time.
A. Positive margin (24% overall) B. ECS (25% overall) P-values are via Cochran-Armitage test. *For margin status, p-value was the same when using logistic regression to ensure the trend was in a downward direction.
Figure 4. Margin positivity by hospital characteristics.
A. Positive margins by hospital type. B. Positive margins by hospital volume. The line represents overall positive margin percentage. P-values are via chi-square statistic.
Overall, 56% of patients undergoing a primary surgical approach had pathologic N2 or N3 nodal classification. Pathologic N-upstaging occurred in 24% of cN0, 39% of cN1 and 3% of cN2a/N2b patients (Table 2a). Pathologic T-upstaging occurred in 17% of cT1 and 11% of cT2 patients. (Table 2b).
Table 2.
| a: Clinical Nodal Stage Compared to Pathologic Stage Among Primary Surgical | ||||||
|---|---|---|---|---|---|---|
| Pathologic N Stage, N (Row %) | ||||||
| Patients | 4833 | N0 1230 (25.5) |
N1 921 (19.1) |
N2a/N2b 2380 (49.2) |
N2c/N3 302(6.3) |
|
| Clinical N Stage, N (Column %) | ||||||
| N0 | 1427 (29.5) | 1085 (76.0) | 172 (12.1) | 158 (11.1) | 12 (0.8) | →24% upstaged |
| N1 | 1143 (23.7) | 77 (6.74) | 625 (54.7) | 415 (36.3) | 26 (2.3) | →39% upstaged |
| N2a/N2b | 2000 (41.4) | 54 (2.7) | 115 (5.8) | 1763 (88.2) | 68 (3.4) | →3% upstaged |
| N2c/N3 | 263(5.4) | 14(5.32) | 9(3.4) | 44 (16.7) | 196(74.5) | |
| b: Clinical Tumor Stage Compared to Pathologic Stage Among Primary Surgical Patients | ||||||
|---|---|---|---|---|---|---|
| Pathologic T Stage, N (Row %) | ||||||
| Patients | 4833 | T1 2287 (47.3) |
T2 2227 (46.1) |
T3 208 (4.3) |
T4 111 (2.3) |
|
| Clinical T Stage, N (Column %) | ||||||
| T1 | 2345 (48.5) | 1948 (83.1) | 358 (15.3) | 24(1.0) | 15(0.6) | →17% upstaged |
| T2 | 2488 (51.5) | 339 (13.6) | 1869 (75.1) | 184 (7.4) | 96(3.9) | →11% upstaged |
Abbreviation: N, nodal.
Clinical stage row totals do not match Table 1 Column 2 because of exclusion of surgical patients with incomplete pathologic information
Abbreviation: T, tumor.
Adverse pathologic factors in candidates for single modality therapy
Among primary surgical patients with complete pathologic information, 2,570 (53%) were clinically T1–T2/N0–N1 and therefore candidates for single modality therapy with surgery. Of these, 723 (28%) were pathologically upstaged out of single modality therapy, to T3–T4 and/or N2–N3. In addition 842 (33%) had positive margins and/or ECS. In total, 1,200 (47%) of patients originally T1–T2/N0–N1 had at least one adverse feature (T3–T4, N2–N3, positive margins, or ECS).
DISCUSSION
The incidence of OPSCC is rapidly increasing in the United States, due to HPV-associated disease. Many of these patients have low T classification (T1–T2) tumors amenable to primary surgical treatment. The potential advantage of this approach is the possibility of avoiding or de-escalating post-operative therapies. To date there has been limited information on trends in the primary treatment for patients with early T-stage OPSCC, or rates of adverse pathologic features in surgically treated patients.
We observed a significant increase in the use of primary surgery and a decrease in primary radiation approach to treating patients with T1–T2 OPSCC between 2004–2013. Although lower clinical nodal stage was associated with a primary surgical approach, primary surgery was nonetheless chosen for nearly two thirds of patients with clinically N2/N3 disease. Non-tumor factors including receiving treatment at a high volume hospital were also associated with selection of a primary surgical approach.
Among the subgroup of patients who had primary surgery, one in four patients had a positive margin. This rate improved over time and there was a lower rate at high volume and academic institutions. One in four primary surgical patients also had ECS. Nearly half (47%) of patients treated with primary surgery who by clinical staging might have been candidates for surgical treatment alone (T1–T2/N0–N1) had at least one adverse feature requiring adjuvant radiation or chemoradiation.
The findings herein have to be considered in the context of important limitations. First, there were a significant number of exclusions due to incomplete staging information, a basic oncologic reporting requirement that should in theory be better represented. The strength of the NCDB for inquiry into practice patterns in head and neck oncology might be examined in separate dedicated analyses given this large amount of missing data. Second, in determining the cohort who had primary surgical therapy, we included patients coded as having a “tonsillectomy” who were listed as a subcategory of “pharyngectomy NOS.” While we cannot be certain that such patients received an oncologic resection, we did exclude from our primary surgical cohort any patient who had a “local tumor excision” or “excisional biopsy,” which would help exclude the simple tonsillectomies done explicitly for biopsy purposes. We also note that all patients in this study classified as having primary surgical therapy had both pathologic T and pathologic N classification recorded, implying primary tumor resection and neck dissection; pathologic N classification especially would not likely be seen in patients having primary radiation therapy. The issue of classification of tonsillectomy as primary surgery or not is important since simple tonsillectomy confers no benefit to these patients and may lead to overtreatment if there are resulting positive margins. Third, HPV status was only routinely recorded in the NCDB after 2010 and so was not accounted for in this analysis. This is important because the relevance of ECS and its relationship to N-upstaging may be different between HPV-related and non-HPV-related disease. Nonetheless, decisions about primary treatment selection over much of the study were probably made without information about HPV status since it was not routinely tested in clinical practice during many of these years. In addition, the NCBD does not record information on pre- or post-operative radiologic work-up and re-operations for positive margin status, factors that may influence treatment decision-making. Finally, information on pathologic features of perineural invasion and lymphovascular invasion are not available in the NCDB, so there may be underestimation of the rates of adverse features among surgically treated patients.
The steepest rise in primary surgical therapy has been observed toward the end of the study period, correlating with 2009 FDA approval of TORS as well as increasing interest in other methods of transoral endoscopic head and neck surgery including transoral laser microsurgery (TLM).19 Hospital volume was also predictive of a primary surgical approach. High volume hospitals were the innovators and early adopters of transoral approaches resulting in a surgical bias. This was probably necessary, since the diffusion of any technology requires innovators who push it forward.20
An important goal in primary treatment selection, especially for HPV-related OPSCC, is to avoid the increased toxicity associated with multimodal adjuvant therapy that would obviate the desired goal (de-escalation) of surgical therapy.4 In NCCN guidelines over the study period, indications for post-operative adjuvant therapy (radiation alone or chemoradiation) include adverse features of advanced pathologic T stage (T3/T4), advanced N stage (N2/N3), positive margins, ECS, perineural invasion, and lymphovascular invasion.15 It should be noted that some data suggest that ECS may not be as important in HPV-related OPSCC,21 and a future prospective trial may negate this as a recommendation for adjuvant chemoradiation.22 Nevertheless during the years of this study and as of this publication, positive margins and ECS are considered indications for adjuvant chemoradiation (triple modality therapy) as a Category 1 recommendation based on RTOG and EORTC data showing benefit in these patients.23–25 In some patients, primary surgical treatment results in none of these features, and the patient is therefore able to avoid any adjuvant treatment with radiation or chemoradiation. To the degree that any of these pathologic factors might be anticipated before treatment selection, there may be opportunities to improve the treatment selection process in an attempt to avoid escalated modalities of adjuvant therapy.
Positive margin status is a factor that should be anticipated before choosing primary surgery. Although there is some debate about the meaning of close or positive margins in HPV-related OPSCC, most surgeons nonetheless strive to minimize this adverse factor.26 Reported positive margin rates in the literature for TORS are as high as 33%.27 The decrease in positive margin status observed over time may be related to improvement in patient selection combined with advancement in surgical skill as experience increased. Consistent with this observation is that positive margins were more commonly observed in low volume hospitals. While continued overall progress in technique might be expected, a ceiling effect of technical improvement may exist where a rate of positive margins persists.
ECS is another pathologic risk factor that should be better anticipated before treatment selection. In this cohort, one in four patients undergoing primary surgery had ECS. One recent study found that greater than half of HPV-related regional metastases were found to have ECS and that CT was not a reliable method for determining the presence of ECS in patients with HPV-related OPSCC.24 In the future, ECS may be considered less important for HPV-associated disease.21, 22 Until that time, better ways to predict ECS will lead to fewer patients selected for primary surgical treatment who end up having escalated post-operative therapy.
More than two out of three patients with clinically advanced nodal disease underwent surgery. These patients are more likely to receive escalated adjuvant therapy secondary to advanced nodal disease and the increased probability of having ECS. Importantly however, primary surgical patients have pathologically staged nodal information, whereas in primary radiation patients, only clinical staging is used to determine need for adjuvant therapy. Pathologic staging may sometimes reveal a lack of nodal metastases seen on pre-operative imaging, leading to less unnecessary adjuvant treatment in this group than if they were treated with primary radiation based on imaging staging alone. Conversely, pathologic staging may lead to important upstaging, identifying patients who might otherwise be undertreated with adjuvant therapies. Specifically, patients with advanced nodal disease (e.g. HPV negative oropharyngeal cancer) may have improved outcomes with primary surgery followed by chemoradiation, but one prospective trial attempting to answer this question was recently closed due to insufficient accrual.28 Alternatively, functional outcomes may be superior in patients with advanced nodal stage undergoing primary surgery followed by a lower dose of radiation as compared to standard concurrent chemoradiation without compromising oncologic outcomes, a question which is currently being examined in an ongoing trial29. Until such data has matured, and with higher rates of HPV positive disease, the high volume of primary surgery in this cohort theoretically requiring escalated adjuvant therapy should be examined.
Patients with cT1–T2 and cN0–N1 tumors are potential candidates for single modality therapy with surgery alone. In this cohort, more than one in four such patients were T or N-upstaged enough to require adjuvant therapy, one in three had positive margins and/or ECS theoretically requiring adjuvant chemoradiation, while slightly more than half had no adverse features requiring adjuvant therapy. While a full clinical assessment of the submucosal extent of tumors involving the base of tongue and tonsil may be difficult, T-upstaging in general would suggest radiographic evaluation under-represented the extent of tumor at the time of pretreatment evaluation. N-upstaging also suggests nodal disease that was more advanced than seen on pretreatment radiologic imaging. Improvements in pretreatment radiologic staging could help better select patients for primary surgery who do not need adjuvant therapy.
Although there is great enthusiasm for the primary surgical approach to treating T1–T2 OPSCC because of the possibility of limiting treatment related morbidity, we have demonstrated elements of suboptimal treatment selection that may ultimately be leading to escalation of adjuvant therapy. The findings shed light on areas where improvement is needed in treatment selection and treatment provision.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflict of interest: None (all authors)
Author Contributions:
Jennifer R. Cracchiolo: Conceptualization, methodology, writing – original draft, writing – review and editing, visualization, and project administration. Shrujal S. Baxi: Conceptualization, methodology, formal analysis, and writing – review and editing. Luc G. Morris: Conceptualization, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, and supervision. Ian Ganly: Writing – original draft, writing – review and editing, and visualization. Snehal G. Patel: Conceptualization and writing – review and editing. Marc A. Cohen: Conceptualization, methodology, formal analysis, investigation, resources, writing – original draft, writing – review and editing, visualization, supervision, and project administration. Benjamin R. Roman: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision, project administration, and funding acquisition.
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedaghat AR, Zhang Z, Begum S, et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119(8):1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 6.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 7.Huang SH, Perez-Ordonez B, Liu FF, et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg D, Begum S, Westra WH, et al. Cystic lymph node metastasis in patients with head and neck cancer: An HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 9.Calais G, Alfonsi M, Bardet E, et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J Natl Cancer Inst. 1999;91(24):2081–2086. doi: 10.1093/jnci/91.24.2081. [DOI] [PubMed] [Google Scholar]
- 10.Chen AY, Zhu J, Fedewa S. Temporal trends in oropharyngeal cancer treatment and survival: 1998–2009. Laryngoscope. 2014;124(1):131–138. doi: 10.1002/lary.24296. [DOI] [PubMed] [Google Scholar]
- 11.Liederbach E, Lewis CM, Yao K, et al. A Contemporary Analysis of Surgical Trends in the Treatment of Squamous Cell Carcinoma of the Oropharynx from 1998 to 2012: A Report from the National Cancer Database. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4560-x. [DOI] [PubMed] [Google Scholar]
- 12.Lorincz BB, Jowett N, Knecht R. Decision management in transoral robotic surgery: Indications, individual patient selection, and role in the multidisciplinary treatment for head and neck cancer from a European perspective. Head Neck. 2015 doi: 10.1002/hed.24059. [DOI] [PubMed] [Google Scholar]
- 13.Mydlarz WK, Chan JY, Richmon JD. The role of surgery for HPV-associated head and neck cancer. Oral Oncol. 2015;51(4):305–313. doi: 10.1016/j.oraloncology.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen AY. A shifting paradigm for patients with head and neck cancer: transoral robotic surgery (TORS) Oncology (Williston Park) 2010;24(11):1030–1032. [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. Head and Neck Cancers NCCN Guidelines Version 1.2015. http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. [Google Scholar]
- 16.Chen MM, Roman SA, Kraus DH, Sosa JA, Judson BL. Transoral Robotic Surgery: A Population-Level Analysis. Otolaryngol Head Neck Surg. 2014;150(6):968–975. doi: 10.1177/0194599814525747. [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Holsinger FC. New Approaches: Robotics and Endoscopic Head and Neck Surgery. In: Harrison LB, Sessions RB, Kies M, editors. Head and Neck Cancer: A Multidisciplinary Approach. 4th 2013. [Google Scholar]
- 20.Rogers EM. Diffusion of innovations. 5th. New York, NY: Free Press; 2003. [Google Scholar]
- 21.Sinha P, Lewis JS, Jr, Piccirillo JF, Kallogjeri D, Haughey BH. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118(14):3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 22.Haughey BH. Post Operative Adjuvant Therapy De-intensification Trial for Human Papillomavirus-related, p16+ Oropharynx Cancer (ADEPT) [accessed 8-25-15, 2015]; Available from URL: ClinicalTrials.gov: NCT01687413.
- 23.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 25.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 26.Weinstein GS, O'Malley BW, Jr, Rinaldo A, Silver CE, Werner JA, Ferlito A. Understanding contraindications for transoral robotic surgery (TORS) for oropharyngeal cancer. Eur Arch Otorhinolaryngol. 2015;272(7):1551–1552. doi: 10.1007/s00405-014-3331-9. [DOI] [PubMed] [Google Scholar]
- 27.Genden EM, Kotz T, Tong CC, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121(8):1668–1674. doi: 10.1002/lary.21845. [DOI] [PubMed] [Google Scholar]
- 28.Radiation Therapy Oncology Group. RTOG 1221 Protocol Information. [accessed May 26th, 2015]; Available from URL: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1221. [Google Scholar]
- 29.Ferris RECOG. Transoral Surgery Followed By Low-Dose or Standard-Dose Radiation Therapy With or Without Chemotherapy in Treating Patients With HPV Positive Stage III-IVA Oropharyngeal Cancer: NCT01898494. [accessed January 12th, 2016]; Available from URL: https://clinicaltrials.gov/ct2/show/NCT01898494.