Abstract
Aim
To conduct an integrative review to examine evidence of pain and associated symptoms in adult (≥ 21 years of age), post-craniotomy, brain tumor patients hospitalized on intensive care units.
Background
Healthcare providers believe craniotomies are less painful than other surgical procedures. Understanding how post-craniotomy pain unfolds over time will help inform patient care and aid in future research and policy development.
Design
Systematic literature search to identify relevant literature. Information abstracted using the Theory of Unpleasant Symptoms’ concepts of influencing factors, symptom clusters and patient performance. Inclusion criteria were indexed, peer-reviewed, full-length, English-language articles. Keywords were ‘traumatic brain injury,’ ‘pain, post-operative,’ ‘brain injuries,’ ‘postoperative pain,’ ‘craniotomy,’ ‘decompressive craniectomy,’ and ‘trephining.’
Data sources
Medline, OVID, PubMed and CINAHL databases from 2000 – 2014.
Review Method
Cooper’s five-stage integrative review method was used to assess and synthesize literature.
Results
The search yielded 115 manuscripts, with 26 meeting inclusion criteria. Most studies were randomized, controlled trials conducted outside of the United States. All tested pharmacological pain interventions. Post-craniotomy brain tumor pain was well-documented and associated with nausea, vomiting and changes in blood pressure and impacted patient length of hospital stay, but there was no consensus for how best to treat such pain.
Conclusion
The Theory of Unpleasant Symptoms provided structure to the search. Post-craniotomy pain is experienced by patients, but associated symptoms and impact on patient performance remain poorly understood. Further research is needed to improve understanding and management of post-craniotomy pain in this population.
Keywords: Brain tumor, craniotomy, pain, Theory of Unpleasant Symptoms, integrative review, literature review, nurses, nursing
INTRODUCTION
Background
Brain tumor is the seventeenth-most diagnosed cancer worldwide, with 256,000 new cases of brain tumor diagnosed in 2012. Men suffer from brain cancer slightly more frequently than women (Bondy et al. 2008, World Cancer Research Fund International 2013, Central Brain Tumor Registry of the United States 2014, Ferlay et al. 2015) and incidence rates are higher in developed countries than in lesser developed countries (Bondy et al. 2008, World Cancer Research Fund International 2013, Central Brain Tumor Registry of the United States 2014). Scientific advances have resulted in improvements in the diagnosis and treatment of brain tumors (Bondy et al. 2008). In fact, one- and five-year survival rates have increased from 7.3% in 1970 to over 18% in 2011 (Informational Services Division of the National Health Services 2010, Cancer Research UK 2014, Ferlay et al. 2015, Queen’s University Belfast 2015).
Approximately 90% of patients with brain tumors undergo craniotomies for excision and removal of the tumor to increase survival (National Cancer Institute 2014). Surgical procedures are generally understood to be painful (McCaffery & Pasero 1999) but less is understood about post-craniotomy pain. Healthcare providers commonly believe that craniotomies are less painful than other types of surgery due to lack of innervation in the brain (Hassouneh et al. 2010, American Brain Tumor Association 2012) and are thus less apt to treat pain. In addition, post-craniotomy pain is often untreated or undertreated due to concerns that it may mask neurological changes in these patients (Talke & Gelb 2005, Durieux & Himmelseher 2007, Lai et al. 2012). Pain is often associated with other symptoms including anxiety and depression (McCaffery & Pasero 1999, Rocha-Filho 2015) and nausea and/or vomiting (Dolin & Cashman 2005). Understanding post-craniotomy pain in brain tumor patients is important because post-operative pain is a common cause of delayed mobilization (Saha et al. 2013), lengthened hospital stay (Chung et al. 1997, Casler et al. 2005, Saha et al. 2013), disability and decreased quality of life (Andrasik et al. 2011, O’Connor & Dworkin 2011). In addition, research has shown that under-treated, generalized post-operative pain is a predictor of the development of persistent pain (Macrae 2001, Dobrogowski et al. 2008, Watt-Watson & McGillion 2011, Wu & Raja 2011, Lamacraft 2012). To date, post-craniotomy pain and the symptoms associated with it is poorly understood. Researchers have called for additional studies to understand influencing factors and associated symptoms of post-craniotomy pain and to determine how to best treat it to prevent negative health outcomes (Talke & Gelb 2005, Roberts 2005, Watson 2011, deOliveira Ribeiro Mdo et al. 2013, Rocha-Filho 2015).
Definitions and Theory
The International Society for the Study of Pain describes pain as a subjective sensory and emotional experience (McCaffery & Pasero 1999, Watt-Watson & McGillion 2011, Gelinas et al. 2013). Pain is a complex symptom comprised of at least four dimensions (intensity, affect, quality and location) (Puntillo et al. 2002, Jensen & Karoly 2011). Physical, psychological, social and cultural factors influence the experience of pain (Melzack 1999, Saha et al. 2013).
The Theory of Unpleasant Symptoms (TOUS), which suggests that symptoms such as pain are multidimensional and interactive, is commonly used to support pain research because it is relevant to practice and can be used as a framework for making decisions related to patient care (Myers 2009, Lenz et al. 2013). The TOUS includes three main concepts: (1) physiological, measureable symptoms experienced by the patient; (2) influencing factors which alter the patient’s experience of the symptom; and (3) patient performance (Lenz et al. 1997, Lenz et al. 2013). Influencing factors are physiological, psychological and situational in nature and can catalyze each other affecting patient performance (Lenz et al. 1997, Lenz et al. 2013). Performance is the impact of the symptom on patient outcomes including functional performance (the ability to physically function) and cognitive performance (the ability to think) (Lenz et al. 1997, Lenz et al. 2013). Researchers using the TOUS have termed groups of associated symptoms as ‘clusters’ (Lenz et al. 1997). This review will also use the term cluster to identify these groups of co-related symptoms.
THE REVIEW
Aim
The aim of this study was to conduct an integrative review using the TOUS as a guiding framework to synthesize and examine what is known about the phenomenon of pain in adult (≥21 years of age), post-craniotomy, brain tumor patients. Specifically, this review sought to answer the following research questions: (1) What is the evidence for post-craniotomy, post-brain tumor pain in adult (≥21 years of age) patients hospitalized on intensive care units?; and (2) What is the evidence for a post-craniotomy symptom cluster associated with pain in adult (≥21 years of age) patients hospitalized on intensive care units?
Design
Cooper’s (2010) integrative review method guided the review. This method of integrative review was chosen because it provides a systematic framework to synthesize the current literature regarding post-craniotomy pain in the brain tumor patient (Whittemore & Knafl 2005, Cooper 2010). Cooper’s method includes five stages: advance formulation of the problem, data collection, data extraction, evaluation, analysis and interpretation (Cooper 2010). The formulation of the problem, the first stage of the method, was informed by a preliminary literature search and the researchers’ clinical experience that suggested a greater understanding of acute post-craniotomy pain was warranted. The authors felt an integrative review was necessary to synthesize the current literature and further the state of the science (Whittemore & Knafl 2005, Cooper 2010).
Search Methods
Data collection, the second stage, consisted of a literature search. Studies were identified for inclusion by purposive searching of electronic databases including Medline, OVID, PubMed and CINAHL. In addition, hand-searching of references and an examination of citations from identified published reviews were conducted. Two experienced reference librarians provided consultation on the search process. Search terms for all databases and searches included traumatic brain injury; pain, postoperative; brain injuries; postoperative pain; craniotomy; decompressive craniectomy; and trephining. Inclusion criteria were as follows: (1) data-based quantitative and qualitative articles focused on post-craniotomy pain in adult brain tumor patients aged 21 or older; (2) published between 1 January 2000 – 12 December 2014; (3) English-language; (4) neurosurgical inpatients; and (5) intensive care unit settings. Abstracts, editorials, dissertations, theses, reviews and articles concerning intraoperative pain control, end-of-life care, or institutional practices were excluded.
Search Outcome
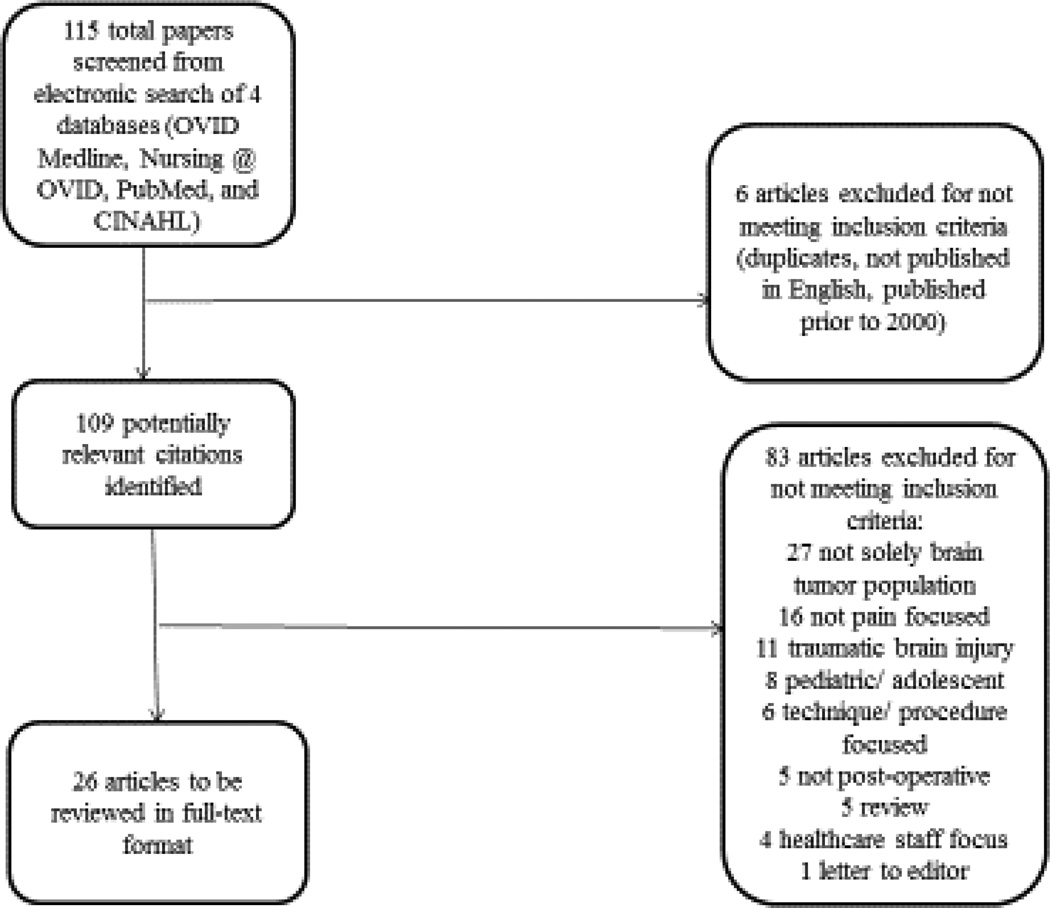
The search strategy generated 115 studies. The studies which were recorded in a Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) diagram. (Figure 1.) A total of 109 potentially relevant studies remained after the initial screening of titles for duplicates, publication in English and publication dates. The remaining abstracts were reviewed for type of study, population, study setting and discussion of pain. After application of the inclusion and exclusion criteria, we eliminated 83 additional articles from review, including five qualitative studies that either did not meet inclusion criteria because they did not focus on pain or the participants were not in-patients. This resulted in a sample of 26 quantitative articles to be reviewed in full-text format (Table 1). Data from eligible studies were abstracted into tables listing general information, level of evidence and concepts defined in the TOUS.
Figure 1.
PRISMA Diagram of Systematic Search
Table 1.
Summarization of Studies
| Author, Year, Country |
Design, Sample Size, Medication | Existence of Pain and Pain Intensity, Rating Scale Used |
|---|---|---|
|
Bala et al.
(2006) India |
Prospective, double-blind RCT; N =
40 Medication: Scalp nerve block (bupivacaine) |
60% experienced moderate-severe pain
in first 12h post-op (control) 25% experienced moderate-severe pain in first 12h post-op (intervention) Rating Scale: NRS; scores 0–22.5 out of 100 |
|
Batoz et al.
(2009) France |
Prospective, single-blinded study; N =
52 Medication: Incisional infiltration (ropivacaine); nalbuphine post-surgery |
VAS scores higher in control
group Persistent pain significantly lower in intervention group at 2 months (56% in control group vs. 8% in intervention group) Rating Scale: VAS; scores 0–35 out of 50 |
|
Biswas and
Bithal (2003) India |
Prospective, double-blind RCT; N =
50 Medication: Incisional infiltration (bupivacaine) vs. fentanyl |
Additional medication needed in 60% of
bupivacaine group and 57% of fentanyl group Rating Scale: VAS; scores 0–4 out of 10 |
|
Ducic et al.
(2012) United States |
Retrospective interview of patients; N
= 7 Medication: None tested |
86% experienced pain greater than
80% on migraine index Rating Scale: VAS; scores 2–10 out of 10 |
|
Ferber et al.
(2000) Poland |
Multi-stage prospective study; N =
35 Medication: Intravenous tramadol |
Pain relief in 50% of patients
receiving one dose; in 88% of patients receiving 2 or 3 doses Rating Scale: VRS; scores 0–4 out of 5 |
|
Girard et al.
(2010) Canada |
Prospective, double-blind RCT; N =
30 Medication: Cervical plexus nerve block (lidocaine and bupivacaine) vs. intravenous morphine bolus |
Similar pain scores between nerve block and
morphine groups Rating Scale: NRS; scores 2–7 out of 10 |
|
Grossman et
al. (2007) Israel |
Open, prospective, double-blind
non-randomized, placebo- controlled study; N = 40 Medication: Incisional infiltration (lidocaine and bupivacaine); metamizol intra-operatively |
13 patients needed additional pain
medication Rating Scale: NRS; scores 0–4 out of 10 |
|
Irefin et al.
(2003) United States |
Prospective study; N =
128 Medication: None tested |
No significant difference in pain scores
between groups Rating Scale: VAS; scores 0–5 out of 10 |
|
Jellish et al.
(2006) United States |
Prospective, double-blind RCT; N =
120 Medication: PCA (morphine or morphine plus ondansetron) |
Up to 76% experienced post-op
pain Administered analgesia was inadequate Rating Scale: VAS; scores 4.5–6.1 out of 10 |
|
Jones et al.
(2009) Australia |
Prospective, double-blind RCT; N =
50 Medication: Intravenous parecoxib; morphine post-operatively |
89% of patients required additional
pain medication (morphine) Pain scores significantly lower in parecoxib group only at 6 hours Rating Scale: VAS; scores 0–35 out of 100 |
|
Law-Koune et
al. (2005) France |
Prospective, double-blind RCT; N =
80 Medication: Incisional infiltration (bupivacaine plus epinephrine) vs. ropivacaine |
Placebo group received more morphine than
bupivacaine or ropivacaine groups (22.2 mg;12.7 mg; 10.5 mg, respectively) Rating Scale: VAS; scores 0–7 out of 10 |
|
Magni et al.
(2005) Italy |
Prospective, randomized, open-label clinical
trial; N = 120 Medication: General anesthesia (sevoflurane- fentanyl vs. propofol-remifentanil) |
10% of ropivacaine group and
6% of sevoflurane group experienced pain at 45 minutes Rating Scale: VAS; scores unclear out of 100 |
|
Magni et al.
(2009) Italy |
Prospective, double-blind RCT; N =
120 Medication: General anesthesia (sevoflurane vs. desflurane) |
22% of sevoflurane group and
17% of desflurane group required additional medication for pain Rating Scale: VAS; scores unclear out of 100 |
|
Morad et al.
(2009) United States |
Prospective RCT (unblinded); N =
64 Medication: as needed intravenous fentanyl vs. PCA (fentanyl) |
Patients in PCA group had significantly lower
pain scores than PRN group (2.53 versus 3.62, respectively) PCA group received significantly more fentanyl Rating Scale: NRS; scores 2–4.7 out of 10 |
|
Nair and
Rajshekhar (2011) India |
Prospective longitudinal study; N =
43 Medication: Oral paracetamol |
5% had moderate pain in first post-op
hour Significant pain reported by 63% of patients during first 48h; severe pain in 12% within first 12h; incidence decreased over first 48h Rating Scale: VAS; not stated out of 10 |
|
Nguyen et al.
(2001) Canada |
RCT; N =
30 Medication: Scalp nerve block (ropivacaine) |
70% of patients in saline group
experienced moderate pain in first 48h post-op Rating Scale: VAS; scores 1.6–4.4 out of 10 |
|
Rahimi et al.
(2006) United States |
Prospective, single-blinded RCT; N =
27 Medication: Oral narcotics vs. oral COX-2 inhibitors |
Pain scores significantly higher in
narcotics-alone group than COX-2 group (p =
0.003) Rating Scale: VAS; scores 2–5.3 out of 10 |
|
Rahimi et al.
(2010) United States |
Prospective, blinded RCT; N =
50 Medication: Oral narcotics vs. tramadol |
Tramadol group had significantly lower pain
scores than narcotics- alone group (p<0.005) Pain scores between groups significantly different (p = 0.001435) Rating Scale: VAS; scores 1–8 (narcotics-along group), 0–7 (tramadol group) out of 10 |
|
Saring-carinkul and Boonsri
(2008) Thailand |
Prospective, double-blind RCT; N =
50 Medication: Incisional infiltration (bupivacaine) |
33% of bupivacaine group pain free at
30 minutes; decreased to 4% at 8 hours 16% of control group pain free at 30 minutes; decreased to 4% at 1 hour Rating Scale: VNS; scores 2.5–3.5 out of 10 |
| Simon et al.
(2011) Hungary |
Prospective RCT; N =
90 Medication: Pre-operative oral diclofenac |
Significant difference in incidence of
pre-operative headache between intervention and control groups (p = 0.0045) 77.7% experienced pain (first post-op day); 69.4% experienced pain (fifth post-op day) Rating Scale: VAS; scores 0–9 out of 10 |
|
Sudheer et al.
(2007) Wales |
Prospective RCT; N =
60 Medication: PCA (morphine vs. tramadol) vs. intramuscular codeine |
4 patients did not require additional
medication in first post- operative hour; 5 had severe pain necessitating withdrawal from study Less pain in morphine and codeine groups; significant residual pain noted in tramadol group Rating Scale: VRS; scores 0–10 out of 10 |
|
Thibault et
al. (2007) Canada |
Retrospective chart review; N =
299 Medication: None tested |
24% experienced mild pain,
51.5% moderate pain, and 24.5% severe pain Overall prevalence of pain = 76% Rating Scale: VRS; scores unclear out of 10 |
|
Ture et al.
(2009) Turkey |
Prospective RCT; N = 80; 75
completed study Medication: Oral gabapentin vs. oral phenytoin |
Pain scores significantly higher in phenytoin
group at 15, 30, and 60 minutes (p < 0.001) Total morphine consumption significantly higher in phenytoin group (p = 0.01) Rating Scale: VAS; scores 0–4 out of 10 |
|
Verchere et
al. (2002) France |
Prospective, blind, RCT; N
=64 Medication: Paracetamol vs. paracetamol plus tramadol vs. paracetamol plus nalbuphine |
Paracetamol-only group stopped quickly due to
inadequate analgesia in 75% of patients Rating Scale: VAS; scores 5–30 out of 100 |
|
Williams,
Pemberton and Leslie (2011) Australia |
Prospective, double-blind RCT; N =
100 Medication: Intravenous parecoxib |
70% of control group and 61%
of parcoxib group needed additional pain medication Rating Scale: VAS; scores 2–5 out of 10 |
|
van der Zwan
et al. (2005) The Netherlands |
Prospective, double-blind RCT; N =
50 Medication: Remifentanil vs. fentanyl |
No significant difference in pain intensity
between groups 13 of remifentanil group (45%) required additional pain medication Rating Scale: VAS; scores 1–4 out of 10 |
RCT: randomized controlled trial; 4NRS: numerical rating scale; VAS: visual analogue scale; VRS: visual rating scale; VNS: visual numeric scale.
Quality Appraisal
In the third stage, two authors completed a quality appraisal on the 26 articles. Using a 3-point scale (yes, no, unclear) described by Gazarian, they rated the studies on nine criteria including aims, design, methods, sample, ethical considerations, results, limitations, implications and sponsorship (2013). The studies were also appraised for bias using the Cochrane Risk of Bias tool. Twenty-one of the studies used a randomized design. Of the five studies that did not use randomization, two were retrospective (Thibault et al. 2007, Ducic et al. 2012) and three were prospective trials (Irefin et al. 2003, Grossman et al. 2007, Nair & Rajshekhar 2011). The team determined that these five studies nonetheless met inclusion criteria and thus all 26 studies are included in the review.
Data Abstraction
The fourth stage includes data analysis and interpretation (Cooper 2010). In this stage, all of the included studies were read in full and relevant data were extracted and tabulated. Table 1 displays the authors’ names; dates and countries of publication; purpose and design; sample, setting and intervention; medication tested; and pain prevalence, incidence and intensity. (Table 1).
Data Synthesis
In the fifth and final stage, the tabulated data were synthesized to address the research questions (Cooper 2010). The authors grouped the data into categories suggested by the TOUS including incidence of pain, influencing factors, cluster and patient performance. (Table 2). Two of the authors (RG & DV) reviewed each study and verified the accuracy of data as presented and over several meetings compiled the results.
Table 2.
Summarization of Studies Using Theory of Unpleasant Symptoms Concepts
| Author, Year | Influencing Factors | Symptom Cluster | Patient Performance |
|---|---|---|---|
| Bala et al. (2006) |
|
--- | --- |
| Batoz et al. (2009) | --- |
|
--- |
| Biswas and Bithal (2003) | --- |
|
--- |
| Ducic et al. (2012) |
|
|
|
| Ferber et al. (2000) | --- | --- |
|
| Girard et al. (2010) | --- |
|
--- |
| Grossman et al. (2007) | --- |
|
--- |
| Irefin et al. (2003) |
|
|
--- |
| Jellish et al. (2006) |
|
|
|
| Jones et al. (2009) | --- |
|
|
| Law-Koune et al. (2005) | --- |
|
|
| Magni et al. (2005) | --- |
|
|
| Magni et al. (2009) | --- |
|
|
| Morad et al. (2009) |
|
|
|
| Nair and Rajshekhar (2011) | --- |
|
|
| Nguyen et al. (2001) |
|
--- | --- |
| Rahimi et al. (2006) |
|
|
|
| Rahimi et al. (2010) | --- | --- |
|
| Saringcarinkul and Boonsri (2008) | --- |
|
|
| Simon et al. (2011) |
|
--- |
|
| Sudheer et al. (2007) |
|
|
--- |
| Thibault et al. (2007) |
|
|
--- |
| Ture et al. (2009) | --- |
|
--- |
| Verchere et al. (2002) | --- |
|
--- |
| Williams, Pemberton, and Leslie (2011) | --- |
|
|
| van der Zwan et al. (2005) |
|
|
--- |
|
Total
Studies Discussing Concept |
11 | 21 | 14 |
NRS: numerical rating scale; VAS: visual analogue scale; VRS: visual rating scale; VNS: visual numeric scale.
RESULTS
Description of the Studies
Of the 26 studies included, all were pharmacological pain management trials (pain medications) and most were randomized, controlled trials (RCTs) (n = 21). The studies included 1892 total patients and were originally designed to test local wound infiltration or medications to control pain (intravenous, intramuscular, oral medications, nerve blocks, general anesthesia) (Table 1). The medications that were tested varied but mostly included bupivacaine, ropivacaine, tramadol, parecoxib, paracetamol and morphine.
The mean ages of the participants in the studies ranged from 45 to 55 and approximately equal numbers of men and women were represented. The comprehensive search identified five qualitative studies; however, these did not meet inclusion criteria (focus not on pain or participants not in-patients) and were excluded from final analysis. The majority of trials took place outside of the United States at non-profit, urban, academic medical institutions. Only one study reported racial characteristics of the sample that consisted mostly of Caucasians (52 versus 12 non-Caucasian) (Morad et al. 2009). Reports included both supratentorial surgeries and infratentorial surgeries with mean lengths of surgery ranging between 200 and 300 minutes.
Main Results
As previously discussed, we used the TOUS as the guiding framework for describing the experiences and cluster associated with post-craniotomy pain in brain tumor patients, which resulted in five categories: (1) evidence of pain; (2) manner of pain assessment; (3) influencing factors; (4) symptom cluster; and (5) patient performance (Tables 1 and 2).
Evidence of Pain
Fifteen studies reported specific percentages of participants experiencing moderate-severe pain. These percentages were as high as 60–96% within the first two days after surgery, despite the use of analgesics. Participants in eight studies required additional pain medications and in one study, inadequate analgesia in 75% of participants necessitated the removal of one study arm (Verchere et al. 2002). In this arm, six of eight patients experienced inadequate analgesia and multiple infusions of additional pain medication were required to reduce pain intensity scores to below 30 (out of 100) (Verchere et al. 2002). An additional study reported the withdrawal of five participants for severe pain in the first post-operative hour (Sudheer et al. 2007).
Manner of Pain Assessment
Measures that were used to assess pain varied but most used one-dimensional assessments of intensity including visual analogue scales (VAS), numerical rating scales (NRS), visual rating scales (VRS), or visual numeric scales (VNS). Study authors did not measure other dimensions of pain such as timing, distress, affect and quality. Twenty-one studies (81%) identified inadequate pain relief.
Influencing factors
Table 2 displays the evidence of post-craniotomy pain, factors that may influence its development, an associated symptom cluster and possible impact on patient performance. Many authors did not report all elements of the TOUS. Eleven of the 26 studies (42%) discussed some physiological, psychological, or situational factors influencing post-craniotomy pain.
Several studies examined physiological influencing factors such as included gender and age but findings were inconsistent. One study found that women tended to experience higher pain levels than men (Morad et al. 2009) while another study found that men were more likely to ask for pain medication than women (Jellish et al. 2006). The impact of age in the development of post-craniotomy pain also was not clear. One study found that older age was associated with less pain (Thibault et al. 2007) while another found increased pain levels in older patients (van der Zwan et al. 2005).
Psychological influencing factors are the patient’s emotional reactions to the disease and can include mood and perceived level of self-sufficiency (Lenz et al. 1997, Lenz et al. 2013). No studies examined psychological factors that may influence the experience of post-craniotomy pain.
Situational factors are found in the social and physical environment and can include surgical positioning, site of surgery and use of anesthetics. Three studies reported less pain among patients with frontal craniotomies (Thibault et al. 2007, Morad et al. 2009 Ducic et al. 2012) and one study found that perioperative nerve blockade decreased the incidence of post-operative pain (Morad et al. 2009). General anesthetics used included sevoflurane and desflurane. The use of sevoflurane resulted in less pain in one study (Magni et al. 2005), while in another, patients receiving sevoflurane required additional medication to control their pain (Magni et al. 2009).
Clusters
Clusters in the TOUS are groups of co-related symptoms that interact, affecting the patient’s symptom experience (Lenz et al. 1997, Lenz et al. 2013). Although the researchers did not explicitly explore ‘symptom clusters,’ 21 (81%) studies discussed symptoms related to pain. Symptoms reported include headache nausea and vomiting, shivering, fatigue, dizziness, respiratory depression, constipation, neurologic changes, increased risk of intracranial bleeding and agitation. The top three most common symptoms described were nausea (15 studies; 58%), vomiting (16 studies; 62%) and changes in blood pressure including, but not limited to, the development of hypertension (9 studies, 35%).
Patient performance
Patient performance is frequently assessed in terms of tangible functional outcomes, such as length of stay, readiness to be discharged and perceived quality of life. Although performance related to post-craniotomy pain was not explicitly examined, almost half of the studies described potential results of post-craniotomy pain (Table 2). However, it was unclear if the impact on patient performance was a direct result of pain, the use of pain medication, or other factors. Other functional performance outcomes reported included increased cost of medication and increased hospital length-of-stay. In two different studies, poorly managed post-craniotomy pain resulted in delayed discharge and altered quality of life (Jellish et al. 2006, Ducic et al. 2012). Four studies described changes in cognitive performance using the proxy measure of level of conscious assessed by the Glasgow Coma Scale (GCS) (Magni et al. 2005, Saringcarinkul & Boonsri 2008, Magni et al. 2009, Williams et al. 2011). Two studies found changes in level of consciousness due to type and amount of analgesic used (Saringcarinkul & Boonsri 2008, Williams et al. 2011) and one identified these changes as being the result of uncontrolled pain (Magni et al. 2005).
DISCUSSION
To our knowledge, this is the first integrative review of data-based studies examining: (1) evidence for post-craniotomy, post-brain tumor pain; and (2) the evidence for a post-craniotomy pain symptom cluster in brain tumor patients. Brain tumors affect many worldwide and pain has been identified as a public health priority. Accordingly, most research on post-craniotomy pain has been conducted in other countries. Research to date has focused solely on pharmacological intervention and fails to explore the multidimensional nature of pain through comprehensive assessment (Leslie & Troedel 2002, Nemergut et al. 2007, Hansen et al. 2011, Guilfoyle et al. 2013). Although pharmacological interventions exist, no one therapeutic medication has been identified as most efficacious (National Pharmaceutical Council 2003, Paolino et al. 2006, Institute of Medicine Committee on Advancing Pain Research 2011, Saha et al. 2013). Our review found that despite the use of 18 different analgesics, moderate to severe pain still occurred among post-craniotomy brain tumor patients and that many patients expressed inadequate pain management resulting in the need for more analgesics. This review provides strong evidence for the existence of post-craniotomy pain and the need for more research to develop evidence-based practice guidelines in this population.
While researchers have begun to study patients’ subjective experiences after craniotomy, such as their fears, expectations and satisfaction (Khu et al. 2010, Milian et al. 2014), these investigations have not yet addressed pain. Patients’ experiences of pain will necessarily be affected by amount of pain control and healthcare provider interaction, but the extent to which these influence post-craniotomy, post-brain tumor patient experience has not yet been made clear. Due to the complicated nature of post-craniotomy pain, further research is warranted to provide evidence-based care.
A full understanding of the post-craniotomy pain experience from the patients’ perspectives would improve assessment of pain, planning of interventions and evaluation of care (Melzack 1999 andrasik et al. 2011, Watt-Watson & McGillion 2011). This review serves as a call to action to describe the context and unfolding of post-craniotomy brain tumor pain from the patient’s perspective and provides evidence to challenge the commonly held belief that post-craniotomy pain is not an important problem (Hassouneh et al, 2010, American Brain Tumor Association 2012).
The intensity of post-craniotomy, post-brain tumor pain is well-documented. Measures such as VASs are capable of reflecting this intensity and change in pain over time (Jensen & Karoly 2011). However, pain intensity is not necessarily correlated with level of patient distress and resulting patient performance (Melzack 1999, Jensen & Karoly 2011, Turk & Melzack 2011, Turk & Robinson 2011, Watt-Watson & McGillion 2011). Consequences such as the development of dysfunction and disability reflect broader dimensions of pain that cannot be assessed by mere measures of intensity and distress (Turk & Melzack 2011, Watt-Watson & McGillion 2011). Current research fails to explore the pain experience beyond intensity and does not address the cluster of associated symptoms that may magnify pain and/or moderate treatment effects.
The limited and conflicting nature of the evidence concerning physiological factors that influence the development of post-craniotomy pain in the brain tumor patient suggests that additional, more comprehensive description is needed. Increased awareness of the experiences of post-craniotomy pain across age groups is needed (Andrasik et al. 2011).
Investigations of the experience of post-craniotomy, post-brain tumor pain by gender could lead to the development of targeted approaches for men and women. Similarly, while incidence of brain tumor is higher in Caucasians than in those of other racial backgrounds (National Cancer Institute 2014), few authors report racial characteristics of the study sample, preventing clear understanding of the manner in which post-craniotomy pain unfolds among different groups.
Psychological factors influencing the development of post-craniotomy, post-brain tumor pain are also thought to be important (McCaffery & Pasero 1999, Melzack 1999 andrasik et al. 2011, Turk & Robinson 2011, Lenz et al. 2013). None of the studies in the review, however, addressed these factor and thus it is not yet clear what role emotions, mood and perceived level of self-sufficiency play in the unfolding and experience of post-craniotomy pain.
Situational factors that affect the unfolding and experience of post-craniotomy pain also need further clarification. Longer surgical time influences length of intensive care unit stays in cardiac patients (Chu et al. 2008) and length of surgery influences the severity of post-operative pain in ambulatory care surgical patients (Chung et al. 1997). In post-craniotomy patients, longer surgeries may increase post-surgical pain due to greater time spent in surgical positions, increased duration of muscle retraction, larger incisions and the potential for more involved surgical procedures (Casler et al. 2005, Ducic et al. 2012). Researchers should therefore investigate the impact of length of surgery on the development of post-craniotomy pain.
More detailed comparisons could also be made if surgical diagnoses were consistently reported. For example, it is known that post-operative headache in occipital surgeries stems from resulting occipital neuralgia (Ducic et al. 2012). Examining the effect of surgical location on development of post-craniotomy headache could lead to better targeted interventions.
The existence of a symptom cluster would call for comprehensive post-craniotomy pain assessment (Melzack 1999 andrasik et al. 2011, Saha et al. 2013). Little is known, however, about the cluster associated with post-craniotomy, post-brain tumor pain. In the current science, effects of pharmaceutical interventions, post-craniotomy pain, other symptoms such as pain and anxiety and patient performance are often confounded. Research that explicates the nature of symptom clusters in this population is needed.
Literature shows that post-operative pain may affect performance by increasing length-of-stay, cost of hospitalization and delaying discharge (Watt-Watson & McGillion 2011, Saha et al. 2013). Some research links post-craniotomy pain to increased length of stay and delayed readiness to be discharged in the traumatic head injury population (Honeybul 2010, Honeybul & Ho 2010). However, only a few studies have examined the impact of post-craniotomy pain on brain tumor patients’ functional and cognitive performance.
In the broader pain literature, untreated acute pain has been correlated with the development of long-term pain due to nervous system plasticity (Melzack 1999, Turk & Robinson 2011, Watt-Watson & McGillion 2011, Ducic et al. 2012). In addition, researchers of general post-surgical pain have shown that inadequate post-operative analgesia has led to the development of persistent pain (Horn & Munafo 1997, McCaffery & Pasero 1999, Watt-Watson & McGillion 2011). Batoz et al. (2009) have shown that improved pain management in post-craniotomy patients during the acute post-operative period decreases the development of persistent pain at two months, but the relationship between post-operative pain management and persistent pain has not been well-studied in post-craniotomy brain tumor patients. Therefore, describing the connection between post-craniotomy pain and patient performance could lead to the development of interventions to prevent or minimize both post-craniotomy pain and its resulting effects.
Over forty years of research have repeatedly illustrated that pain is under-assessed, under-recognized and undertreated. The treatment of post-craniotomy pain is further complicated by a lack of understanding of the manner in which it unfolds over the course of the post-operative period and a reluctance to treat it aggressively for fear of masking neurological changes. The result is an unclear risk-benefit ratio associated with the treatment of post-craniotomy pain in brain tumor patients. Additional research would illuminate the relationship between post-craniotomy pain, influencing factors, associated clusters and patient performance, leading to the development of timely interventions to control pain without increasing risk to patients.
Limitations
This review was limited to examining studies that discussed particular influencing factors, associated clusters and the effect of post-craniotomy, post-brain tumor pain on patient performance. It is possible that studies looking at post-craniotomy pain in a different context were missed. In addition, this review does not represent ongoing or unpublished studies, nor does it include published work that has not undergone the peer review process.
CONCLUSION
Evidence suggests that post-craniotomy, post-brain tumor patients experience significant post-surgical pain but no guidelines have been established to treat this pain. Post-craniotomy pain may influence length of hospital stay, cost of medications, quality of life and development of persistent pain. However, little research has been conducted on the complex nature and experience of post-craniotomy, post-brain tumor pain. Mitigating or preventing post-craniotomy pain in the brain tumor population will likely result in improved patient outcomes. Patient-centered outcomes research should focus on attempting to understand post-craniotomy pain, which will pave the way for the development of timely interventions and standardization of treatment for post-craniotomy pain to improve functional outcomes and quality of life.
Supplementary Material
SUMMARY STATEMENT.
Why is this research or review needed?
Brain tumor patients have long been believed to experience little pain post-craniotomy due to lack of innervation in the brain.
Understanding symptoms correlated with post-craniotomy pain in brain tumor patients will help healthcare providers provide better treatment.
Addressing untreated and undertreated post-craniotomy pain will improve patient-centered outcomes and quality of life.
What are the key findings?
Post-craniotomy patients experience significant levels of pain, but current treatment of post-craniotomy pain lacks evidence-based guidelines.
Post-craniotomy pain in brain tumor patients may be associated with nausea, vomiting and changes in blood pressure and may play a role in healthcare use such as longer hospital stays.
How should the findings be used to influence policy/ practice/ research/ education?
Understanding the manner in which post-craniotomy pain unfolds should inform healthcare providers’ recognition of the symptom.
Recognition of the intensity of post-craniotomy pain and its impact should lead to timely treatment of the symptom and improve patient outcomes.
ACKNOWLEDGEMENT
The authors wish to thank and acknowledge the T32 program leadership of Dr. Susan Rawl.
FUNDING STATEMENT
The work reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number T32NR007066. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by a Jonas Leadership Scholarship from the Jonas Center for Nursing Excellence, as well as the Irene and Nathaniel Aycock Scholarship and the Cheryl A. Bean Scholarship from the Indiana University School of Nursing.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors had no conflicts of interest.
Author Contributions:
- substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
- drafting the article or revising it critically for important intellectual content.
REFERENCES
- American Brain Tumor Association. Surgery. Chicago: American Brain Tumor Association; 2012. [Online]. Available: http://www.abta.org/secure/surgery.pdf. [Google Scholar]
- Andrasik F, Buse D, Lettich A. Assessment of headaches. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. Third. New York: The Guilford Press; 2011. [Google Scholar]
- Bala I, Gupta B, Bhardwaj N, Ghai B, Khosla VK. Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesthesia and Intensive Care. 2006;34(2):224–227. doi: 10.1177/0310057X0603400203. [DOI] [PubMed] [Google Scholar]
- Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. ‘The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumor resection’. Anesthesia Analgesia. 2009;109:240–244. doi: 10.1213/ane.0b013e3181a4928d. [DOI] [PubMed] [Google Scholar]
- Biswas BK, Bithal PK. Preincision 0.25% bupivicaine scalp infiltration and postcraniotomy pain: A randomized double-blind, placebo-controlled study. Journal of Neurosurgical Anesthesiology. 2003;15(3):234–239. doi: 10.1097/00008506-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Bondy M, Scheurer M, Malmer B, Barnholz-Sloan J, Davis F, Il’yasova D, Kruchko C, Mccarthy B, Rajaraman P, Schwartzbaum J, Sadetzki S, Schlehofer B, Tihan T, Wiemels J, Wrensch M, Buffler P. Brain tumor epidemiology: Consense from the Brain Tumor Epidemiology Consortium (BTEC) Cancer. 2008;113(S7):1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Research UK. Brain, other CNS and intracranial tumors survival statistics. St. John Street, London: Cancer Research UK; 2014. [Online]. Available: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/brain/survival/brain-and-central-nervous-system-cancer-survival-statistics#ten 2015]. [Google Scholar]
- Casler J, Doolittle A, Mair E. Enodscopic surgery of the anterior skull base. The Laryngoscope. 2005;115:16–24. doi: 10.1097/01.mlg.0000150681.68355.85. [DOI] [PubMed] [Google Scholar]
- Central Brain Tumor Registry of the United States. 2014 CBTRUS Fact Sheet. Hinsdale, Illinois: Central Brain Tumor Registry of the United States; 2014. [Online]. Available: http://www.cbtrus.org/factsheet/factsheet.html 2015]. [Google Scholar]
- Chu D, Bakaeen F, Wang X, Lemaire S, Coselli J, Huh J. Does the duration of surgery affect outcomes in patients undergoing coronary artery bypass grafting. American Journal of Surgery. 2008;196(5):652–656. doi: 10.1016/j.amjsurg.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Chung F, Ritchie E, SU J. Postoperative pain in ambulatory surgery. Anesthesia Analgesia. 1997;85(4):808–816. doi: 10.1097/00000539-199710000-00017. [DOI] [PubMed] [Google Scholar]
- Cooper H. Research synthesis and meta-analysis: A step-by-step approach. Thousand Oaks: California, Sage Publications, Inc.; 2010. [Google Scholar]
- De Oliveira Ribeiro MDO, Pereira C, Sallum A, Martins-Filho P, Desantana J, Da Silva Nunes M, Hora E. Immediate post-craniotomy headache. Cephalalgia. 2013;33(11):897–905. doi: 10.1177/0333102413479833. [DOI] [PubMed] [Google Scholar]
- Dobrogowski J, Przeklasa-Muszynska A, Wordliczek J. [Persistent post-operative pain] Folia Med Cracov. 2008;49(1–2):27–37. [PubMed] [Google Scholar]
- Dolin S, Cashman J. Tolerability of acute postoperative pain management: Nausea, vomiting, sedation, pruritis and urinary retention. Evidence from published data. British Journal of Anaesthesia. 2005;95(5):584–591. doi: 10.1093/bja/aei227. [DOI] [PubMed] [Google Scholar]
- Ducic I, Jm Felder I, Endara M. Postoperative headache following acoustic neuroma resection: Occipital nerve injuries are associated with a treatable occipital neuralgia. Headache. 2012;52:1136–1145. doi: 10.1111/j.1526-4610.2011.02068.x. [DOI] [PubMed] [Google Scholar]
- Durieux M, Himmelseher S. Pain control after craniotomy: Off balance on the tightrope? Journal of Neurosurgery. 2007;106:207–209. doi: 10.3171/jns.2007.106.2.207. [DOI] [PubMed] [Google Scholar]
- Ferber J, Juniewicz H, Glogowska E, Wronski J, Abraszko R, Mierzwa J. Tramadol for postoperative analgesia in intracranial surgery: Its effect on ICP and CPP. Neurologia i neurochirurgia polska. 2000;34:70–79. [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. Worldwide data. Bedford Square, London: World Cancer Research Fund International; 2015. [Online]. Available: http://www.wcrf.org/int/cancer-facts-figures/worldwide-data 2015]. [Google Scholar]
- Gazarian P. Use of the critical decision method in nursing research: An integrative review. Advances in Nursing Science. 2013;36(2):106–117. doi: 10.1097/ANS.0b013e3182901f8d. [DOI] [PubMed] [Google Scholar]
- Gelinas C, Puntillo KA, Joffe AM, Barr J. A validated approach to evaluating psychometric properties of pain assessment tools for use in nonverbal critically ill adults. Seminars in Respiratory and Critical Care Medicine. 2013;34:153–168. doi: 10.1055/s-0033-1342970. [DOI] [PubMed] [Google Scholar]
- Girard F, Quentin C, Charbonneau S, Ayoub C, Boudreault D, Chouinard P, Ruel M, Moumdjian R. Superficial cervical plexus block for transitional analgesia in infratentorial and occipital craniotomy: a randomized trial. Canadian Journal of Anesthesia. 2010;57(12):1065–1070. doi: 10.1007/s12630-010-9392-3. [DOI] [PubMed] [Google Scholar]
- Grossman R, Ram Z, Perel A, Yusim Y, Zalansky R, Berkenstadt H. Control of postoperative pain after awake craniotomy with local intradermal analgesia and metamizol. Israeli Medical Association Journal. 2007;9:380–382. [PubMed] [Google Scholar]
- Guilfoyle M, Helmy A, Duane D, Hutchinson P. Regional scalp block for postcraniotomy analgesia: A systematic review and meta-analysis. Anesthesia Analgesia. 2013;116(5):1093–1102. doi: 10.1213/ANE.0b013e3182863c22. [DOI] [PubMed] [Google Scholar]
- Hansen MS, Brennum J, Moltke FB, Dahl JB. Pain treatment after craniotomy: Where is the (procedure-specific) evidence? A qualitative systematic review. European Journal of Anaesthesiology. 2011;28(12):821–829. doi: 10.1097/EJA.0b013e32834a0255. [DOI] [PubMed] [Google Scholar]
- Hassouneh B, Centofanti JE, Reddy K. Pain management in post-craniotomy patients: A survey of Canadian neurosurgeons. Canadian Journal of Neurological Science. 2010;38:456–460. doi: 10.1017/s0317167100011872. [DOI] [PubMed] [Google Scholar]
- Honeybul S. Complications of decompressive craniectomy for head injury. Journal of Clinical Neuroscience. 2010;17(4):430–435. doi: 10.1016/j.jocn.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Honeybul S, Ho K. Long-term complications of decompressive carniectomy for head injury. Journal of Neurotrauma. 2010;28(6):929–935. doi: 10.1089/neu.2010.1612. [DOI] [PubMed] [Google Scholar]
- Horn S, Munafo M. Pain: Theory, reserach and intervention. Philadelphia: Open University Press; 1997. [Google Scholar]
- Informational Services Division of the National Health Services. Cancer statistics: Brain and Central Nervous System. Souyth Gyle Crescent, Edinburgh: ISD Scotland; 2010. [Online]. Available: http://www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/Brain-and-Central-Nervous-System/ 2015]. [Google Scholar]
- Institute of Medicine Committee on Advancing Pain Research, C. And Education. Relieving Pain in American: A blueprint for transforming prevention, care, education and research. Washington, D.C: National Academies Press; 2011. [Online]. Available: http://www.ncbi.nlm.nih.gov/books/NBK92516/ 2014]. [PubMed] [Google Scholar]
- Irefin SA, Schubert A, Bloomfield EL, Deboer GE, Mascha EJ. The effect of craniotomy location on postoperative pain and nausea. Journal of Anesthesia. 2003;17:227–231. doi: 10.1007/s00540-003-0182-8. [DOI] [PubMed] [Google Scholar]
- Jellish WS, Leonetti JP, Kristina S, Douglas A, Origitano TC. Morphine/ ondansetron PCA for postoperative pain, nausea and vomiting after skull base surgery. Otolaryngology -- Head and Neck Surgery. 2006;135(2):175–181. doi: 10.1016/j.otohns.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Jensen M, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. Third. New York: The Guilford Press; 2011. [Google Scholar]
- Jones S, Cormack J, Murphy M, Scott D. Parecoxib for analgesia after craniotomy. British Journal of Anaesthesia. 2009;102(1):76–79. doi: 10.1093/bja/aen318. [DOI] [PubMed] [Google Scholar]
- Khu K, Doglietto F, Radovanovic I, Taleb F, Mendelsohn D, Zadeh G, Bernstein M. Patients’ perceptions of awake and outpatient craniotomy for brain tumor: a qualitative study. Journal of Neurosurgery. 2010;112(5):1056–1060. doi: 10.3171/2009.6.JNS09716. [DOI] [PubMed] [Google Scholar]
- Lai LT, Ortiz-Cardona JR, Bendo AA. Perioperative pain management in the neurosurgical patient. Anesthesiology Clinics. 2012;20:347–367. doi: 10.1016/j.anclin.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Lamacraft G. The link between acute postoperative pain and chronic pain syndromes. South African Journal of Anaesthesia and Analgesia. 2012;18(1):45–50. [Google Scholar]
- Law-Koune J-D, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. Scalp infiltration with bupivicaine plus epinephrine or plain ropivicaine reduces postoperative pain after supratentorial craniotomy. Journal of Neurosurgery Anaesthesiology. 2005;17(3):139–143. doi: 10.1097/01.ana.0000171730.41008.da. [DOI] [PubMed] [Google Scholar]
- Lenz E, Gift A, Pugh LC, Milligan RA. Unpleasant symptoms. In: Peterson SJ, Bredow TS, editors. Middle range theories: Application to nursing research. Third. Philadelphia: Wolters Kluwer; 2013. [Google Scholar]
- Lenz E, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. Advances in Nursing Science. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Leslie K, Troedel S. Does anesthesia care affect the outcome following craniotomy? Journal of Clinical Neuroscience. 2002;9(3):231–236. doi: 10.1054/jocn.2001.0934. [DOI] [PubMed] [Google Scholar]
- Macrae W. Chronic pain after surgery. British Journal of Anaesthesia. 2001;87(1):88–98. doi: 10.1093/bja/87.1.88. [DOI] [PubMed] [Google Scholar]
- Magni G, Baisi F, Rosa IL, Imperiale C, Fabbrini V, Pannacchiotti M, Rosa G. No difference in emergence time and early cognitive function between sevoflurane-fentanyl and propofol-remifentanil in patients undergoing craniotomy for supratentorial intracranial surgery. Journal of Neurosurgical Anesthesiology. 2005;17:134–138. doi: 10.1097/01.ana.0000167447.33969.16. [DOI] [PubMed] [Google Scholar]
- Magni G, Rosa IL, Melillo G, Savio A, Rosa G. A comparison between sevoflurane and desflurane anesthesia in patients undergoing craniotomy for supratentorial intracranial surgery. Anesthesia and Analgesia. 2009;109(2):567–571. doi: 10.1213/ane.0b013e3181ac1265. [DOI] [PubMed] [Google Scholar]
- Mccaffery M, Pasero C. Pain: Clinical Manual. Second. Philadelphia: Mosby; 1999. [Google Scholar]
- Melzack R. From the gate to the neuromatrix. Pain. 1999;82:S121–S126. doi: 10.1016/S0304-3959(99)00145-1. [DOI] [PubMed] [Google Scholar]
- Milian M, Tatgiba M, Feigl G. Patient response to awake craniotomy - a summary overview. Acta Neurochirurgica. 2014;156(6):1063–1070. doi: 10.1007/s00701-014-2038-4. [DOI] [PubMed] [Google Scholar]
- Morad AH, Winters BD, Yaster M, Stevens RD, White ED, Thompson RE, Weingart JD, Gottschalk A. Efficacy of intravenous patient-controlled analgesia after supratentorial intracranial surgery: A prospective randomized controlled trial. Journal of Neurosurgery. 2009;111:343–350. doi: 10.3171/2008.11.JNS08797. [DOI] [PubMed] [Google Scholar]
- Myers J. A comparison of the Theory of Unpleasant Symptoms and the conceptual model of chemotherapy-related changes in cognitive function. Oncology Nursing Forum. 2009;36(1):E1–E10. doi: 10.1188/09.ONF.E1-E10. [DOI] [PubMed] [Google Scholar]
- Nair S, Rajshekhar V. Evaluation of pain following supratentorial craniotomy. British Journal of Neurosurgery. 2011;25(1):100–103. doi: 10.3109/02688697.2010.534199. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Adult brain tumors treatment (PDQ) Bethesda: National Institutes of Health; 2014. [Online]. Available: http://www.cancer.gov/cancertopics/pdq/treatment/adultbrain/HealthProfessional/page3. [Google Scholar]
- Council NP, editor. National Pharmaceutical Council. Improving the quality of pain management through measurement and action. Oakbrook Terrace, Illinois: Joint Commission on Accreditation of Healthcare Organizations; 2003. [Google Scholar]
- Nemergut E, Durieux M, Missaghi N, Himmelseher S. Pain management after craniotomy. Best Practice and Research - Clinical Anasethesiology. 2007;21(4):557–573. doi: 10.1016/j.bpa.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Nguyen A, Girard F, Boudreault D, Fugere F, Ruel M, Moumdjian R, Bouthilier A, Caron JL, Bojanowski MW, Girard DC. ‘Scalp nerve blocks decrease the severity of pain after craniotomy’. Neurosurgical Anesthesia. 2001;93:1272–1276. doi: 10.1097/00000539-200111000-00048. [DOI] [PubMed] [Google Scholar]
- O’Connor A, Dworkin R. Assessment of pain and health-related quality of life in chronic pain clinical trials. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. Third. New York: The Guilford Press; 2011. [Google Scholar]
- Paolino A, Ruppert J, Anderson M, Garrison-Lanham J. Guide to the care of the patient with craniotomy post-brain tumor resection. Glenview, Illinois: American Association of Neuroscience Nurses; 2006. [Google Scholar]
- Puntillo K, Wild L, Morris A, Stanik-Hutt J, Thompson C, White C. Practices and predictors of analgesic interventions for adults undergoing painful procedures. American Journal of Critical Care. 2002;11(5):415–431. [PubMed] [Google Scholar]
- Queen’s University Belfast. N. Ireland Cancer Registry. University Road, Belfast: Queen’s University Belfast; 2015. [Online]. Available: http://www.qub.ac.uk/research-centres/nicr/CancerData/OnlineStatistics/Brain/ 2015]. [Google Scholar]
- Rahimi SY, Vender JR, Macomson SD, French A, Smith JR, Alleyne CH., Jr ‘Postoperative pain management after craniotomy: Evaluation and cost analysis’. Neurosurgery. 2006;59(4):852–857. doi: 10.1227/01.NEU.0000232646.35678.D8. [DOI] [PubMed] [Google Scholar]
- Rahimi SY, Alleyne CH, Jr, Vernier E, Witcher MR, Vender JR. ‘Postoperative pain management with tramadol after craniotomy: Evaluation and cost analysis’. Journal of Neurosurgery. 2010;112:268–272. doi: 10.3171/2008.9.17689. [DOI] [PubMed] [Google Scholar]
- Roberts G. Post-craniotomy analgesia: Current practices in British neurosurgical centres - A survey of post-craniotomy analgesic practices. European Journal of Anaesthesiology. 2005;22(5):328–332. doi: 10.1017/s0265021505000554. [DOI] [PubMed] [Google Scholar]
- Rocha-Filho P. Post-craniotomy headache: A clinical view with a focus on the persistent form. Headache. 2015;55(5):733–738. doi: 10.1111/head.12563. [DOI] [PubMed] [Google Scholar]
- Saha P, Chattopadhyay S, Rudra A, Roy S. Pain after craniotomy: A time for reappraisal? Indian Journal of Pain. 2013;27(1):7–11. [Google Scholar]
- Saringcarinkul A, Boonsri S. Effect of scalp infiltration on postoperative pain relief in elective supratentorial craniotomy with 0.5% bupivacaine with adrenaline 1:400,000. Journal of the Medical Association of Thailand. 2008;91(10):1518–1523. [PubMed] [Google Scholar]
- Simon E, Bank J, Gal J, Siro P, Novak L, Fulesdi B, Molinar C. ‘Administration of preemptive analgesia by diclofenac to prevent acute postcraniotomy headache’. Ideggyogyaszati szemle. 2012;65(9–10):302–306. [PubMed] [Google Scholar]
- Sudheer P, Logan S, Terblanche C, Ateleanu B, Hall J. Comparison of the analgesic efficacy and respiratory effects of morphine, tramadol and codeine after craniotomy. Anaesthesia. 2007;62:555–560. doi: 10.1111/j.1365-2044.2007.05038.x. [DOI] [PubMed] [Google Scholar]
- Talke PO, Gelb AW. Editorial: Postcraniotomy pain remains a real headache! European Journal of Anaesthesiology. 2005;22:325–327. doi: 10.1017/s0265021505000542. [DOI] [PubMed] [Google Scholar]
- Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: A retrospective study. Canadian Journal of Anesthesia. 2007;54(7):544–548. doi: 10.1007/BF03022318. [DOI] [PubMed] [Google Scholar]
- Ture H, Sayin M, Karlikaya G, Bingol CA, Aykac B, Ture U. ‘The analgesic effect of gabapentin as a prophylactic anticonvulsant drug on postcranitomy pain: A prospective randomized study’. Neurosurgical Anesthesiology and Neuroscience. 2009;109(5):1625–1631. doi: 10.1213/ane.0b013e3181b0f18b. [DOI] [PubMed] [Google Scholar]
- Turk D, Melzack R. The measurement of pain and the assessment of people experiencing pain. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. Third. New York: The Guilford Press; 2011. [Google Scholar]
- Turk D, Robinson J. Assessment of patients with chronic pain: A comprehensive approach. In: Turk D, Melzack R, editors. Handbook of Pain Assessment. Third. New York: The Guilford Press; 2011. [Google Scholar]
- Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. Journal of Neurosurgical Anesthesiology. 2002;14(2):96–101. doi: 10.1097/00008506-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Watson C. A death knell for codeine for acute pain after craniotomy. Canadian Journal of Neurological Science. 2011;38(3):390–391. doi: 10.1017/s0317167100011744. [DOI] [PubMed] [Google Scholar]
- Watt-Watson J, Mcgillion M. Pain as a symptom outcome. In: Doran D, editor. Nursing Outcomes: The state of the science. Second. Sudbury: Jones and Bartlett Learning; 2011. [Google Scholar]
- Whittemore R, Knafl K. The integrative reivew: updated methodology. Journal of Advanced Nursing. 2005;52(5):546–553. doi: 10.1111/j.1365-2648.2005.03621.x. [DOI] [PubMed] [Google Scholar]
- Williams D, Pemberton E, Leslie K. Effect of intravenous parecoxib on post-craniotomy pain. British Journal of Anaesthesia. 2011;107(3):398–403. doi: 10.1093/bja/aer223. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund International. Comparing more and less developed countries. Bedford Square, London: World Cancer Research Fund International; 2013. [Online]. Available: http://www.wcrf.org/int/cancer-facts-figures/comparing-more-less-developed-countries 2015]. [Google Scholar]
- Wu C, Raja S. Treatment of acute postoperative pain. The Lancet. 2011;377(9784):2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- Van der Zwan T, Baerts W, Perez R, Delange J. Postoperative condition after the use of remifentanil with a small dose of piritramide compared with a fentanyl-based protocol in patients undergoing craniotomy. European Journal of Anaesthesiology. 2005;22(6):438–441. doi: 10.1017/s0265021505000748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.