Abstract
Introduction
A broad range of subjective and objective assessments have been used to assess balance confidence and balance control in persons with Parkinson's disease (PD). However, little is known about the relationship between self-perceived balance confidence and actual balance control in PD. The purpose of this investigation was to determine the relationship between self-perceived balance confidence and objectively measured static/dynamic balance control abilities.
Methods
Forty-four individuals with PD participated in the study. Patients were stratified into 2 groups based on the modified Hoehn and Yahr (H&Y) disability score: early stage, H&Y≤2.0 and moderate stage, H&Y ≥2.5. All participants completed the activities-specific balance confidence (ABC) scale and performed standing balance and gait initiation tasks to assess static and dynamic balance control. The center of pressure (COP) sway (CE95%Sway) during static balance and the peak distance between the projections of the COP and the center of mass (COM) in the transverse plane (COPCOM) during gait initiation were calculated. Pearson correlation analyses were conducted relating the ABC score and CE95%Sway and COPCOM.
Results
For early stage PD, there was a moderate correlation between ABC score and CE95 %Sway (r=-0.56, R2=0.32, p=0.002), while no significant correlation was found between ABC score and COPCOM (r=-0.24, R2=0.06, p=0.227). For moderate stage PD, there was a moderate correlation between ABC score and COPCOM (r=0.49, R2=0.24, p=0.044), while no correlation was found between ABC score and CE95%Sway (r=-0.19, R2=0.04, p=0.478).
Conclusion
Individuals with different disease severities showed different relationships between balance confidence and actual static/dynamic balance control.
Keywords: Balance confidence, Balance control, Parkinson's disease
Introduction
Parkinson's disease (PD) is one of the most common neurodegenerative diseases affecting older adults. One of the cardinal motor symptoms and disabling features of PD is postural instability. Postural instability may present early in the disease but worsens and becomes more prevalent as disease progresses [1]. Postural instability reduces independence and quality of life and increases the incidence of falls during activities of daily living [2].
Postural instability in PD results from several underlying neuromechanical impairments. For example, automatic postural reactions (APRs) and anticipatory postural adjustments (APAs) that play a role in posture and movement coordination are both impaired in PD [3, 4]. Further, increased co-contraction and subsequent joint stiffness also play a role in postural responses. Taken together, these contribute to greater postural sway during quiet standing [5, 6], reduced ability to respond to external perturbations [3, 7], and impaired performance of self-initiated behaviors that require dynamic postural stability such as gait initiation, standing from sitting, and turning [8, 9, 10].
Not surprisingly, postural instability in PD leads not only to impaired balance control in daily activities, but also to a decrease in balance confidence, or increase in fear of falling [11]. Decreased balance confidence in PD diminishes participation in daily activities, independence from others, and the quality of life [12, 13]. Indeed, it has been recommended that assessment of balance performance should include a subjective measure of psychological aspects such as fear of falling or balance confidence [14]. However, we still lack adequate knowledge about the interrelation of whether balance confidence of persons with PD actually reflect their balance capabilities.
This study examines the relationship between self-perceived balance confidence (i.e. the Activities-specific balance confidence, ABC) and objective measures of postural stability in individuals with PD during static balance and dynamic balance conditions. More specifically, we framed the relationship by investigating balance confidence and balance control in two groups of PD patients stratified based on Parkinson's disability. We hypothesized that the relationship between ABC scale and objective balance measures would be altered by level of parkinsonian disability.
Methods
Participants
Forty-four patients diagnosed with idiopathic PD by a fellowship trained movement disorders neurologist participated in the study. All participants were evaluated using the Unified Parkinson's Disease Rating Scale (UPDRS) and the Modified Hoehn and Yahr (H&Y) scale by a Movement Disorders clinician blinded to the study purpose. None of the patients exhibited any dyskinesia, dystonia, or other signs of involuntary movement. All participants were able to ambulate independently. Participants were then stratified into 2 groups based on the H&Y disability score, which primarily favors balance in determining disability [8]. The 2 groups were: early stage, H&Y≤2.0 (n=27; age, 64.6±8.9(y); mass, 76.2±11.2kg; height, 170.8±8.7cm; UPDRS motor, 19±6; H&Y, 1.8±0.3) indicating unilateral or bilateral disease without impairment in balance; moderate stage, H&Y ≥2.5 (n=17; age, 63.9±8.1(y); mass, 80.0±14.1kg; height, 166.9±9.24cm; UPDRS motor, 25±6; H&Y, 2.6±0.2) indicating mild to moderate disease with some postural instability but are physically independent. All participants were tested in their best medicated state with stable doses of antiparkinsonian medication. All participants signed a written informed consent form approved by the University's Institutional Review Board.
Procedures
Self-perceived balance confidence
The activities-specific balance confidence (ABC) scale is commonly used as a measure of a patient's perceived ability to perform activities without losing balance [15]. The ABC scale is a clinically validated measure in the (a) prediction of falling, and (b) assessment of balance control in daily activities among patients with PD [16, 17]. Participants were asked to rate their self-rated balance confidence level (their ability to not lose their balance, 0-100%) when performing 16 activities of daily living. The 16 daily activity items is scored ranging from 0% (no confident at all) to 100% (completely confident). The overall score was the average score of the 16 items. The minimum and maximum total score are 0 and 100 respectively, and lower scores indicate “lower” balance confidence.
Objective balance measures
Static balance (quiet standing) and dynamic balance (gait initiation) trials were performed along on a 12-m walkway surrounded by an eight-camera optical motion capture system (120 Hz; Vicon Nexus, Lake Forrest, CA). Ground reaction forces (GRFs) and moments under the feet were collected at 360Hz using a force plate (Bertec Corporation, Columbus, OH) mounted level to the laboratory floor. GRFs and moments were used to calculate the instantaneous center of pressure (COP) in the anterior-posterior and medio-lateral direction. Kinematic data were time-synchronized to the kinetic data. Participants wore tight fitted clothing and were barefoot. Thirty five passive reflective markers were attached over bony landmarks. Then, the whole body center of mass (COM) location was computed as the weighted sum of each body segment's COM from a 15 segment biomechanical model based on the VICON Plug-in-Gait model.
During the quiet standing task, participants stood with their feet comfortably on one force plate with the inside edge of their feet spaced 10 cm apart. Participants stood “as still as possible” for three 2 minute trials with their arms comfortably at their side. A 95% confidence ellipse sway area (CE95%Sway) was calculated around the COP motion along both the anterior-posterior and medio- lateral axes [18] (Figure 1A). Traditionally, a smaller value of CE95%Sway indicates greater static balance or postural stability.
Figure 1.
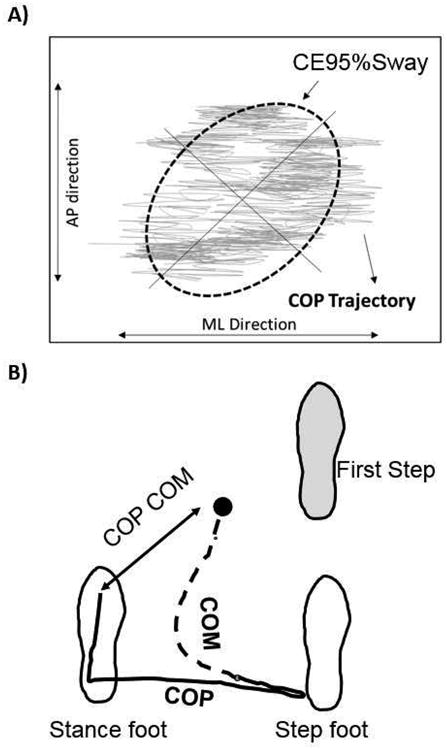
A) CE95%Sway based on raw COP trajectory during static standing tasks, B) Representative record of an overhead view of the path of the center of pressure (COP) and the center of Mass (COM) during forward-oriented gait initiation when stepping with the right foot. The arrow represents the calculated distance between the COP phase and the COM (COPCOM).
For gait initiation (GI) trials, participants began each experimental trial by standing with both feet comfortably spaced on the force-plate. Upon a verbal cue of “ready”, the participants were asked to take a short pause (2–3 s) and then initiate walking at their own comfortable pace, and continue walking the length of the walkway. Foot positioning was marked and held constant for all 5 trials. The distance between the vertical projections of the COM in the transverse plane and the COP was calculated using a customized Matlab program and is referred to as the COPCOM [8, 19, 20] (Figure 1B). Based on previous studies, we calculated the peak separation of the COM and COP during the single support phase of the first step as this time has been shown to be sensitive to disease severity and fall risk, and interpreted that greater COPCOM distances better dynamic balance control [8, 21]
Statistical analysis
Independent t-Tests were conducted to compare all clinical variables and dependent balance measures between early stage and moderate stage PD. Further, Pearson correlation analyses were conducted on the ABC scale score and two experimental balance measures: CE95%Sway and COPCOM. These correlations were first examined with the entire sample and then separately for each group. An a priori alpha level of 0.05 was set for all statistical tests. All statistical analyses were performed using SPSS for Windows 21.0 (Chicago, Illinois).
Results
All participants successfully completed the experimental trials without freezing or loss of balance. Clinical scores of H&Y and UPDRS are significantly different between early and moderate stage PD (H&Y, p=.04; UPDRS, p=.03). Further, ABC score of self-perceived balance confidence was significantly higher in early stage than moderate stage (p=0.047). The mean ABC score of the early stage group was 88 which is 9% greater than the score of the moderate stage group. A significant difference was also detected in dynamic balance measure of COPCOM (p=0.01) between groups, while no significant difference was detected in static balance measure of CE95%Sway (p=0.57) (Table 1).
Table 1.
Mean (± standard deviation) values of balance confidence (ABC score) and balance control characteristics (static balance, CE95%Sway; dynamic balance, COPCOM) in each group.
| Variables\Group | Early (H&Y ≤ 2) | Moderate (H&Y ≥ 2.5) |
|---|---|---|
| ABC score | 87.7 ± 12.7 | 79.2 ±14.8 * |
| CE95%Sway (cm2) | 3.91 ± 1.89 | 4.24 ± 1.92 |
| COPCOM (cm) | 18.4 ± 3.3 | 14.9 ± 3.2 * |
a significant main effect of Group was observed (p ≤ 0.05).
Abbreviations: ABC, The activities-specific balance confidence (ABC) scale; CE95%Sway, 95% confidence ellipse COP sway area during static standing; COPCOM, Maximum distance between COP and COM during gait initiation.
In all participants, balance confidence (ABC scale) was negatively correlated with static balance control (CE95%Sway) (r=-0.42, R2=0.18, p=0.006) (Figure 2), indicating that PD patients with greater balance confidence exhibited better capabilities in static balance, while no statistically significant correlation was found with dynamic balance control (COPCOM) (r=0.19, R2=0.03, p=0.221). However, the relationship between balance confidence and actual balance performance was altered when examining the groups separately (Figure 3). For early stage PD, ABC score correlated with CE95 %Sway (r = -0.56, R2=0.32p=0.002), indicating that early PD patients with greater balance confidence exhibited better capabilities in static balance, whereas no significant correlation was found between ABC score and COPCOM (r=-0.24, R2=0.06, p=0.227). For moderate stage PD, ABC score correlated positively with COPCOM (r=0.49, R2=0.24, p=0.044), indicating that moderate PD patients with greater balance confidence exhibited better capabilities in dynamic balance whereas ABC score and CE95%Sway were not significantly related (r=-0.19, R2=0.04, p=0.478).
Figure 2.
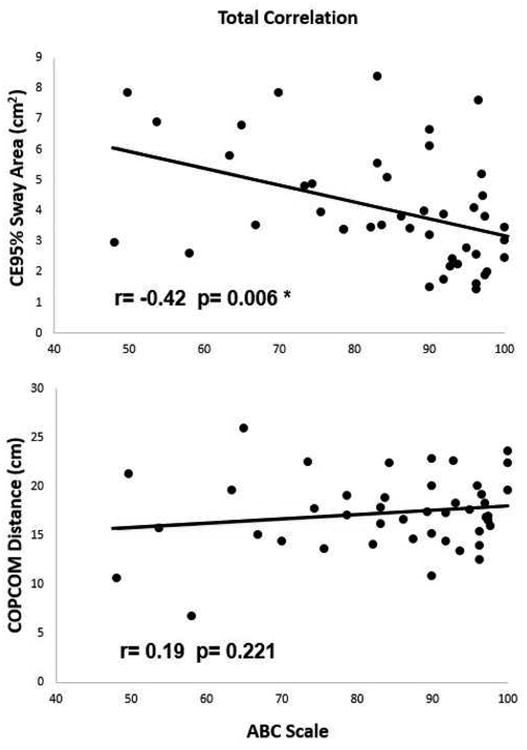
Correlation between balance confidence and static (CE95%Sway)/dynamic balance (COPCOM) in all participants. * p < 0.05
Figure 3.
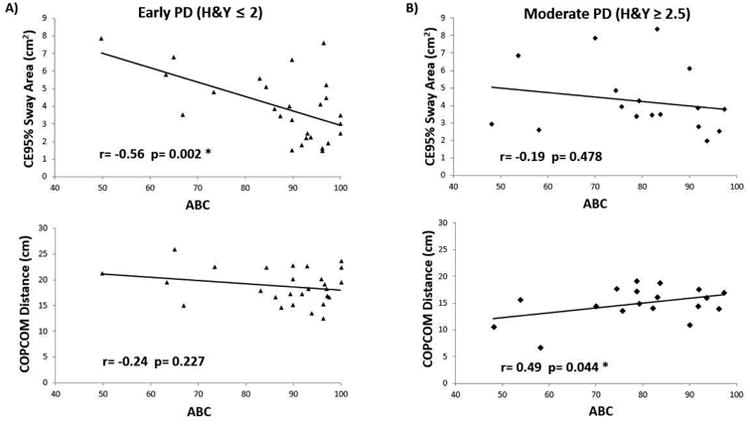
A) Correlation between balance confidence and dynamic/static balance in participants of Early PD (H&Y≤2), B) Correlation between balance confidence and static/dynamic balance in participants of moderate PD (H&Y≥2.5). * p < 0.05
Discussion
This study investigated the relationship between balance confidence and static and dynamic balance controls in individuals at two disease severities of PD (early stage, H&Y≤2.0; moderate stage, H&Y ≥2.5). In order to better determine whether patients' balance confidence actually represents how disabled their balance control really is, a self-perceived balance confidence assessment (ABC scale) and two objective balance measures (Static balance, CE95%Sway; Dynamic balance, COPCOM) were evaluated. Individuals at early stage PD showed higher balance confidence than those at moderate stage PD. The relationships between balance confidence and balance control capabilities were different between early vs. moderate stage PD. Specifically, balance confidence correlated negatively with static balance control in early PD, suggesting that those who report the highest balance confidence also produce the least amount of postural sway. In more disabled participants, there was a statistically significant moderate correlation between balance confidence and dynamic balance control with those with the more balance confidence also producing greater separation of the center of pressure and center of mass during gait initiation tasks.
In early stage PD, there was a strong negative correlation between balance confidence and static balance control, as measured by CE95%Sway. In our study, the balance confidence of participants in early stage PD had a mean ABC score of 87.7, which was similar to that reported previously for healthy old adults [22] and for individuals with PD without a history of falls [23]. This indicates that individuals in early stage PD in our sample were confident in their static balance control and had low fear of falling. It appears that during non-challenging balance tasks this subset of patients' perception of their balance abilities (magnitude of sway) are in agreement with their balance performance. However, during times when the COM is positioned outside of the base of support, like gait initiation, the perception of balance abilities did not match the tolerance (magnitude) of separation. Previously, Chang and Krebs [24] reported that peak COP-COM distances less than 16-18cm differentiated stable from fall-prone older adults. In our early PD cohort, those with COPCOM separations in this range still reported 87.7% confidence in balance control. Indeed, disrupted representation of ability/misjudgments is a possible perceptual component of increased fall risk in persons with PD. Given the known deficits in basal ganglia function that influence processing and integration of sensory input to control posture, individuals with PD may have difficulty perceiving and directing movements. In particular, persons with PD exhibit impairments in assessing proper movement distances [25] and disrupted representation of their external space and the environment [26, 27]. Thus, PD patients may place themselves in situations where there balance abilities do not meet the demands of the task.
Although not statistically significant, the moderate PD group exhibited an 8% larger confidence ellipse during static balance trials and a 20% reduction in the separation of the COPCOM compared to those in the early stage PD. Balance confidence was significantly lower in the moderate stage group (79.2% vs 87.7%). This finding aligns with Johnson et al. [23] who reported ABC scores of 72% in persons with PD who had a history of one or more falls in the previous two years compared to 86.8% in non-fallers. In these more advance patients, dynamic balance, as measured by COPCOM, was significantly and positively correlated with balance confidence. However, static balance performance did not correlate with balance confidence. This finding suggests that with disease progression, perception of balance abilities may be driven more by performance during dynamically challenging tasks than static tasks. Johnson et al. [23] have reported that dynamic balance performance more strongly discriminates PD fallers from non-fallers and healthy controls. Similarly, the extant literature suggest that reductions in the peak COPCOM separation delineates steady versus unstable elders and, as mentioned previously, individuals producing peak separations <16cm are at increased risk of falls [24]. The moderate PD group exhibited a reduced ability to tolerate separation of the COP and COM (14.9cm COPCOM). Collectively, the findings suggest that the perception of balance ability may be aligned with actual dynamic balance control in those with moderate PD.
To our knowledge, this study is the first investigation to explore the underlying relationship between static and dynamic balance control capabilities and balance confidence among individuals with different severities of PD (e.g. early vs. moderate). Previously, Adkin [11] observed a significant correlation between COP sway area and the ABC score by using regression analyses. More recently, Johnson et al. [23] reported a significant moderate correlation (r=-.46) between ABC scores and static balance (CE95%Sway) measured when participants stood with their feet 5cm apart. However, ABC scores were less related to reaction time measures (r=-.29) on dynamic lean to target tasks. These findings underscore that the use of different posturography methods and differences in the stage of disease and severity of impairments may influence our understanding of the relationship between perceived balance abilities and actual static and dynamic performance. Nonetheless, Mak and Pang [28] suggested that balance confidence is a significant risk factor for falls in individuals with PD, highlighting the importance of assessing self-perceived balance confidence level as well as assessing physical performance in estimating fall risk. Our findings confirm the importance of a global assessment of postural control that includes static and dynamic assessment. Several applications using wearable sensors (e.g. portable accelerometer and gyroscope) can be conveniently employed to objectively and sensitively [29] measure postural stability in a clinic environment. Furthermore, it will be important for clinicians to assess balance confidence and to discuss the discrepancies between patients' perception of their balance (confidence) and the objective balance performance. Rehabilitation paradigms could also incorporate ratings of balance confidence during performance of rehabilitation activities to help patients become more aware of the relationships between their feelings and their performance.
Our study has certain limitations. This investigation excluded patients with dyskinesia and dystonia, or other sings of involuntary movement. Thus, it might be difficult to generalize our findings to all PD patients. Indeed, recent findings suggest that dyskinesia at the time of testing has a significant impact on measures of balance performance in persons with PD [30]. Furthermore, this investigation employed only one objective measure for each task, which might not be enough to fully capture balance control capability in individuals with PD. However, static computerized posturography and evaluation of the COPCOM during gait initiation have been shown to be valuable objective and reliable techniques to assess balance control, are able to contribute to differential diagnosis of balance disorders, and are sensitive to treatment effects. In order to determine the relationship between balance confidence and control in individuals with PD, a future investigation should employ diverse assessments including both subjective (e.g. psychological aspects) and objective measures (e.g. movement analysis). In addition, there are other identified clinical factors such as falling experience that can possibly affect balance confidence and performance. We did not measure falls in this population. Johnson et al. [23] reported that those with a history of falls have significantly lower balance confidence compared to non-fallers. We cannot rule out that individuals who are classified as same disease severity level may have different balance confidence based on falling experience. Last, future studies should investigate vestibular, visual, and somatosensory contributions to the observed relationships to aid in the identification of potential mechanisms involved in the observed phenomena.
In conclusion, despite the limitations, the current study documents that mild versus moderate PD patients show different relationships between balance confidence and static and dynamic balance control. The findings of this study will be helpful for both clinicians and researchers to better understand and interpret the assessment of PD patient's balance performance and the underlying psychological mechanisms that may enhance or exacerbate objective balance control. In particular, patients should be made aware of the mismatch between perceived balance confidence and actual balance abilities.
Highlights.
Balance confidence and balance control capabilities in Parkinson's disease (PD) were evaluated.
There were significant correlation between balance confidence and static balance in patients at early disease severity in PD.
Unlike early PD, there were significant correlation between balance confidence and dynamic balance in patients at advanced disease severity in PD.
The relationships between balance confidence and postural control in PD were altered with respect to disease severity.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Bloem BR, van Vugt JP, Beckley DJ. Postural instability and falls in Parkinson's disease. Adv Neurol. 2001;87:209–223. [PubMed] [Google Scholar]
- 3.Carpenter MG, Allum JHJ, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massion J. Postural control systems in developmental perspective. Neurosci Biobehav Rev. 1998;22:465–472. doi: 10.1016/s0149-7634(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 5.Błaszczyk JW, Orawiec R, Duda-Kłodowska D, Opala G. Assessment of postural instability in patients with Parkinson's disease. Exp Brain Res. 2007;183:107–114. doi: 10.1007/s00221-007-1024-y. [DOI] [PubMed] [Google Scholar]
- 6.Horak FB, Nutt JG, Nashner LM. Postural inflexibility in parkinsonian subjects. J Neurol Sci. 1992;111:46–58. doi: 10.1016/0022-510x(92)90111-w. [DOI] [PubMed] [Google Scholar]
- 7.Oude Nijhuis LB, Allum JHJ, Nanhoe-Mahabier W, Bloem BR. Influence of perturbation velocity on balance control in Parkinson's disease. PloS One. 2014;9:e86650. doi: 10.1371/journal.pone.0086650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hass CJ, Waddell DE, Fleming RP, Juncos JL, Gregor RJ. Gait initiation and dynamic balance control in Parkinson's disease. Arch Phys Med Rehabil. 2005;86:2172–2176. doi: 10.1016/j.apmr.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Mak MKY, Hui-Chan CWY. The speed of sit-to-stand can be modulated in Parkinson's disease. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2005;116:780–789. doi: 10.1016/j.clinph.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Morris ME, Huxham F, McGinley J, Dodd K, Iansek R. The biomechanics and motor control of gait in Parkinson disease. Clin Biomech Bristol Avon. 2001;16:459–470. doi: 10.1016/s0268-0033(01)00035-3. [DOI] [PubMed] [Google Scholar]
- 11.Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord Off J Mov Disord Soc. 2003;18:496–502. doi: 10.1002/mds.10396. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SL, Williams CS, Gill TM. Characteristics associated with fear of falling and activity restriction in community-living older persons. J Am Geriatr Soc. 2002;50:516–520. doi: 10.1046/j.1532-5415.2002.50119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. On the nature of fear of falling in Parkinson's disease. Behav Neurol. 2011;24:219–228. doi: 10.3233/BEN-2011-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant MS, Rintala DH, Hou JG, Protas EJ. Influence of fear of falling on gait and balance in Parkinson's disease. Disabil Rehabil. 2014;36:744–748. doi: 10.3109/09638288.2013.814722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JV, Horak FB, Van Tran K, Nutt JG. An alternative clinical postural stability test for patients with Parkinson's disease. J Neurol. 2006;253:1404–1413. doi: 10.1007/s00415-006-0224-x. [DOI] [PubMed] [Google Scholar]
- 17.Schepens S, Goldberg A, Wallace M. The short version of the Activities-specific Balance Confidence (ABC) scale: its validity, reliability, and relationship to balance impairment and falls in older adults. Arch Gerontol Geriatr. 2010;51:9–12. doi: 10.1016/j.archger.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng. 1996;43:956–966. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 19.Corriveau H, Hébert R, Prince F, Raîche M. Intrasession reliability of the ‘center of pressure minus center of mass’ variable of postural control in the healthy elderly. Arch Phys Med Rehabil. 2000;81:45–48. doi: 10.1016/s0003-9993(00)90220-x. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, Shinberg M, Kuchibhatla M, Ray L, Carollo JJ, Schenkman ML. Gait initiation in community-dwelling adults with Parkinson disease: comparison with older and younger adults without the disease. Phys Ther. 2002;82:566–577. [PubMed] [Google Scholar]
- 21.Polcyn AF, Lipsitz LA, Kerrigan DC, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Arch Phys Med Rehabil. 1998;79:1582–1589. doi: 10.1016/s0003-9993(98)90425-7. [DOI] [PubMed] [Google Scholar]
- 22.Donoghue OA, Cronin H, Savva GM, O'Regan C, Kenny RA. Effects of fear of falling and activity restriction on normal and dual task walking in community dwelling older adults. Gait Posture. 2013;38:120–124. doi: 10.1016/j.gaitpost.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Johnson L, James I, Rodrigues J, Stell R, Thickbroom G, Mastaglia F. Clinical and posturographic correlates of falling in Parkinson's disease. Mov Disord Off J Mov Disord Soc. 2013;28:1250–1256. doi: 10.1002/mds.25449. [DOI] [PubMed] [Google Scholar]
- 24.Chang H, Krebs DE. Dynamic balance control in elders: gait initiation assessment as a screening tool. Arch Phys Med Rehabil. 1999;80:490–494. doi: 10.1016/s0003-9993(99)90187-9. [DOI] [PubMed] [Google Scholar]
- 25.Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson's disease. Vision Res. 2005;45:1285–1296. doi: 10.1016/j.visres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee AC, Harris JP, Atkinson EA, Fowler MS. Disruption of estimation of body-scaled aperture width in Hemiparkinson's disease. Neuropsychologia. 2001;39:1097–1104. doi: 10.1016/s0028-3932(01)00032-x. [DOI] [PubMed] [Google Scholar]
- 27.Ryckewaert G, Luyat M, Rambour M, Tard C, Noël M, Defebvre L, Delval A. Self-perceived and actual ability in the functional reach test in patients with Parkinson's disease. Neurosci Lett. 2015;589:181–184. doi: 10.1016/j.neulet.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 28.Mak MKY, Pang MYC. Balance confidence and functional mobility are independently associated with falls in people with Parkinson's disease. J Neurol. 2009;256:742–749. doi: 10.1007/s00415-009-5007-8. [DOI] [PubMed] [Google Scholar]
- 29.Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balnce deficits. Eur J Phys Rehabil Med. 2010;46:239–248. [PMC free article] [PubMed] [Google Scholar]
- 30.Curtze C, Nutt JG, Carlson-Kuhta P, Mancini M, Horak FB. Levodopa Is a Double-Edged Sword for Balance and Gait in People With Parkinson's Disease. Mov Disord. 2015;30:1361–1370. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]


