Abstract
Background
Individuals with cystic fibrosis (CF) have reduced pulmonary function and exercise tolerance. Additionally, these individuals may develop abnormal cardiac function. The implications of abnormal cardiac function on exercise tolerance are unclear in CF.
Objective
Study relationships between exercise cardiac hemodynamics and exercise tolerance in CF.
Methods
17 CF and 25 controls participated in cardiopulmonary exercise testing to measure exercise duration and peak workload (PW). Cardiac index (QI) was measured using acetylene rebreathe and oxygen uptake (VO2) breath-by-breath. Forced expiratory volume 1-second (FEV1) was performed at rest.
Results
Peak QI was 6.7±0.5 vs. 9.1±0.3 mL/min/m2, CF vs. controls, respectively (p<0.05). Linear regressions between QI (R2=0.63 and 0.51) and exercise duration or PW were stronger than VO2 (R2=0.35 and 0.37) or FEV1 (R2=0.34 and 0.36) in CF, respectively (p<0.05).
Conclusion
These data are clinically relevant as they suggest attenuated cardiac function in addition to low airway function relate to exercise tolerance in CF.
Keywords: cardiac output, stroke volume, CFTR, cardiopulmonary exercise test, exercise capacity, functional limitation, pulmonary function
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease affecting >30,000 individuals in the United States with an estimated median survival age of 37 and 40 years in females and males, respectively.1, 2 This disease is caused by gene mutations leading to absent or misplaced CF conductance transmembrane regulator (CFTR) that affects chloride (Cl−) transport as well as attenuated inhibition of epithelial Na+ channels (ENaC) at the apical epithelial membrane layer of airway, intestinal, and exocrine cells.1, 3, 4 The most commonly recognized location of abnormal CFTR and ENaC in CF is the pulmonary system that eventually gives way to diminished global airway function.2, 4 This reduction in airway function has been traditionally suggested to be the primary contributor to reduced exercise tolerance in these individuals.5-7
Several studies suggest attenuated exercise tolerance in CF occurs because of central mechanisms related to abnormal airway function that causes an inability to deliver oxygen-rich blood to metabolically active muscle.5, 6, 8-13 For example, it has been observed that dysregulated movement of Cl− and Na+ within lung epithelial tissue leads to airway obstruction and increased work of breathing caused by inadequate ventilation and oxygen reaching the alveoli.8-12 Further exacerbating this condition, it is suggested that of the ventilated alveoli, reduced structural integrity of the alveoli-capillary membrane layer may attenuate free diffusion of oxygen into perfused pulmonary capillaries resulting in ventilation—perfusion mismatch and dead space perfusion.14 Thus, it is through reductions in gas-transfer and ventilatory function that the pathophysiology of low exercise tolerance has been traditionally viewed in CF.
A large body of evidence in CF suggest disease severity is most closely linked to performance on resting pulmonary function tests (e.g., forced vital capacity [FVC] or forced expiratory volume in 1-second [FEV1]2, 4); whereas, to a lesser extent, it is becoming more recognized that low exercise tolerance is also related to prognosis in these individuals.5-7 However, despite the knowledge of both poor airway function and low exercise tolerance in CF, there is no clearly established direct mechanistic link between reduced airway function and low exercise tolerance in this population. Therefore, it remains possible that pathology in addition to airway dysfunction may contribute to reduced exercise tolerance in CF.
A small number of studies have examined the possibility of abnormal cardiac function with respect the implications this could have in individuals with CF. Among those studies, it has been demonstrated that Cl− movement associated with functional CFTR in cardiac myocytes may directly contribute to increased myocardial contractility resulting from stimulation (e.g., via isoproterenol) of linked β-adrenergic receptor and cyclic adenosine monophosphate pathways in mouse models.15, 16 Whereas, others demonstrate from results of histochemical analyses of human myocardial tissue that CFTR represented by transport protein ABCC7 is nearly completely absent in left ventricular tissue of end-stage heart failure patients compared to individuals with non-failing hearts.17 The relevance of those data are noteworthy as it has been observed in human studies of CF that left ventricular strain using echocardiography (i.e., inotropy)18 and cardiac hemodynamic responsiveness to inhalation of the selective β2-agonist albuterol are attenuated in CF.19 However, despite these and several other findings in CF, to date, it is unclear what clinical implication abnormal cardiac function in the setting of known airway dysfunction is in CF.14-24 Comprehensive clinical phenotyping of CF to include an integrative cardiopulmonary understanding of this disease should enhance approaches to develop and deliver targeted novel therapeutic care for these individuals. The consideration of abnormal cardiac function in CF could be important since the lifespan of these individuals is increasing,25 whereas there is an increased cardiovascular and heart disease risk in adults >40 years, which can be predicted by exercise tolerance.26-28
Therefore, because decreased myocardial contractility in CF cannot be predicted by the magnitude of resting pulmonary dysfunction,18, 23 measures more closely reflecting the physiologic response to exercise, such as exercise cardiac hemodynamics, may demonstrate stronger relationships with exercise tolerance compared to resting pulmonary function in CF. The aim of this study was to test the hypothesis that there is a direct relationship between cardiac hemodynamics and exercise tolerance in CF. Changes in total exercise duration and peak exercise workload (PW) (measures of exercise tolerance) are known to be influenced by adjustments in both oxygen uptake (VO2) and cardiac function in cardiovascular patients.29, 30 Greater relative change in VO2 compared to cardiac hemodynamics from rest to peak exercise would further support our hypothesis that low exercise tolerance in individuals with CF is related to an attenuated rise in cardiac hemodynamics relative to the total duration of exercise and PW.
Material and Methods
Participants
Individuals with CF or healthy individuals serving as controls (CTLs) who volunteered for this study were part of a convenience sample (characteristics, Table 1). Participation of individuals with CF mainly came from provider referrals from a nearby clinic that provided care for individuals with CF. Individuals with CF had mild- to- moderate disease, confirmed by a positive Cl− sweat test (≥60.0 millimole/L Cl−) and genotyping of the ΔF508 mutation of CFTR, which is the most common genotype (∼70% of CF population) of the 1000+ possible genotypes associated with this disease.31, 32 Exclusion criteria included: 1) experienced a pulmonary exacerbation within the last two weeks or pulmonary hemorrhage within six months resulting in greater than 50 cc of blood in the sputum, 2) taking any antibiotics for pulmonary exacerbation, or 3) taking any experimental drugs related to CF. Participation from CTLs came from word of mouth and flyers posted around the University campus. Controls were of moderate fitness, suggested by percent of predicted peak VO2 for healthy individuals33. Additional exclusion criteria for CF, while also applying to CTLs included: 1) history of hypertension, cardiac, metabolic, neurologic, orthopaedic, or other diseases affecting the neuromuscular system, 2) history of smoking, or 3) those who were not able to engage in exercise (e.g., known orthopedic limitations or musculoskeletal disorders). The protocol was reviewed and approved by the University Institutional Review Board. All participants provided written informed consent prior to study.
Table 1. Participant characteristics at rest.
| CTL | CF | P | |
|---|---|---|---|
|
|
|||
| N = 25 | N = 17 | 0.22 | |
| Gender, male/female | 15/10 | 13/4 | 0.27 |
| Height, cm | 174 ± 2 | 168 ± 2 | 0.04 |
| Weight, kg | 72 ± 3 | 62 ± 3 | 0.01 |
| BMI, kg/m2 | 24 ± 1 | 22 ± 1 | 0.12 |
| Hemoglobin, g/dL | 14.7 ± 0.3 | 14.7 ± 0.5 | 0.76 |
| Peak VO2, %predicted | 96 ± 5 | 55 ± 6 | <0.01 |
| Pulmonary function test parameters | |||
| FVC, L | 4.8 ± 0.2 | 3.6 ± 0.3 | <0.01 |
| FVC, %predicted | 96 ± 2 | 80 ± 5 | 0.01 |
| FEV1, L | 3.8 ± 0.2 | 2.6 ± 0.2 | <0.01 |
| FEV1, %predicted | 94 ± 3 | 69 ± 6 | <0.01 |
| FEV1/FVC | 0.8 ± 0.0 | 0.7 ± 0.0 | <0.01 |
| FEF25-75, L/sec | 3.8 ± 0.2 | 2.0 ± 0.3 | <0.01 |
| FEF25-75, %predicted | 90 ± 5 | 51 ± 8 | <0.01 |
Data are mean ± SEM or as n. CTL, healthy controls; CF, cystic fibrosis; BMI, body mass index; VO2, oxygen uptake; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; FEF25-75, forced expiratory flow at 25.0 – 75.0% of forced vital capacity. Group comparisons were performed using Wilcoxon rank-sum tests for all variables except n and gender, which were compared by using χ2 tests.
The use of a convenience sample in this study that was not matched on body anthropometry is consistent with previous studies in CF, as these individuals are recognized to demonstrate smaller body stature (e.g., weight, body mass index, body surface area) due to nutritional deficiency associated with this disease.8, 11, 18, 20, 21 Moreover, matching groups on pulmonary function tests generally cannot be accomplished as healthy individuals serving as CTLs would not be expected to demonstrate airway function similar to CF at any age. However, with regard to testing our primary study outcome, the N for each group based on computed effect sizes34 met appropriate power calculations >0.80 and >0.70 for testing relationships between cardiovascular or airway function with exercise tolerance in both CF and CTLs, respectively.
Overall Protocol
The following procedures were performed before and/or during incremental cardiopulmonary exercise testing (CPET) on a stationary upright cycle ergometer (Corival Lode B.V., Netherlands) on a single testing day in an environmentally controlled physiological laboratory.
Upon arrival to the testing lab, participants were fitted with a 12-lead electrocardiogram (Marquette Electronics, Milwaukee, WI) to continuously monitor heart rate (HR) and rhythm, while resting pulmonary function tests according to American Thoracic Society standards were performed.35 Next, participants performed resting measures of cardiac output (Q), VO2, carbon dioxide production (VCO2), respiratory rate (RR), tidal volume (VT), minute ventilation (VE), and end-tidal partial pressure of carbon dioxide (PETCO2).
Cardiopulmonary exercise testing
Peak exercise VO2, total exercise duration, and PW were determined from symptom limited CPET performed according to guidelines of the American Thoracic Society.30, 36 This test consisted of 3 minute stages whereby the increment of workload increase ranged from 15 to 40 watts (mean initial workload was 24±1 vs. 33±1 watts for CF vs. CTLs, respectively; p<0.05) determined using each individual's body surface area (BSA) and type and intensity of the individual's typical pattern of physical activity recorded using an exercise vital sign questionnaire instrument.30, 36, 37 Participants were asked to maintain a pedal rate between 60 and 80 revolutions/minute until volitional fatigue.
During CPET, breath-by-breath ventilation (Medical Graphics CPX/D, St. Paul, MN) was continuously monitored and averaged every three seconds at rest and throughout exercise. For analyses, indices were calculated as averages of 30 second intervals at the end of each exercise stage. Cardiac output was measured within the final 30 seconds of each exercise stage. Pulse oximetry via finger sensor (Nellcor N-600 Pulse Oximeter, Boulder, CO) was used to estimate peripheral oxygen saturation percentage. At rest and near the end of each exercise stage, manual sphygmomanometry was used to assess systolic (SBP) and diastolic (DBP) blood pressures, and rate of perceived exertion (RPE, 6 to 20 Borg scale) was also assessed.
The following were calculated at rest and at peak exercise: mean arterial pressure (MAP=DBP+1/3(SBP−DBP)); arterio-venous oxygen content difference (Ca-vO2=(VO2/QI)/10); stroke volume (SV=Q/HR). Cardiac output and SV were indexed to BSA because abnormal deviations in overall body size are known to influence comparisons of exercise measurements of cardiac hemodynamics (QI and SVI, respectively),38 which is relevant to this study since CF are known to have abnormally low body size.39 Reserve measures for QI, SVI, and VO2 were calculated as the percent (%) change at peak exercise from resting values.
Measurement of cardiac output using inert gas breathing
Inert gas breathing using the CO2 rebreathe method during exercise has been previously validated in CF,21 whereas nitrous oxide rebreathing has been validated in other patients with lung disease.21, 40 These gases have lower blood solubility and, hence, are less sensitive to pulmonary blood flow (i.e., Q) compared to acetylene gas that is used in this study.41
Measurement of Q occurred near the end of each exercise workload using the acetylene rebreathe technique as described by our group in a separate study design.42 Briefly, participants breathed through a pneumotachometer (Hans Rudolph, Kansas City, MO) that was connected to a non-rebreathing automatic pneumatic switching Y-valve (Hans Rudolph, Kansas City, MO), which was integrated to a mass spectrometer (Perkin Elmer MGA-1100, Wesley, MA) for continuous gas sampling analysis. The inspiratory port of the switching Y-valve allowed for rapid switching between breathing room air and the 5.0 L anesthesia rebreathe bag (Hans Rudolph, Kansas City, MO) containing 1.0 to 3.5 L of test gas (0.65% acetylene [C2H2], 9.0% helium [He], 55.0% nitrogen, and 35.0% O2 depending on initial VT of the subject as described previously.42
High blood solubility acetylene disappears in mixed venous blood in a direct relationship with the rate of pulmonary capillary blood flow.42, 43 Therefore, Q can be calculated via the rate of disappearance of acetylene with each breath determined from the slope of the exponential disappearance of acetylene with respect to the insoluble gas He.42, 43
Statistical analyses
Parametric data are presented as mean ± standard error of the mean (SEM). Homogeneity of data variance was assessed using Levene's test. Wilcoxon rank-sum tests were used to compare continuous variables between groups, whereas categorical variables were compared using χ2−tests. Within group differences rest to peak exercise were assessed using paired Student's t-tests, or one-way ANOVA with Bonferroni post-hoc correction for comparisons of reserves.
Ordinary least squares regressions with coefficient of determination (R2) and 95% confidence limits (CL) via univariate modeling were used to explain variance between FVC (rest), FEV1 (rest), VO2 (peak exercise), SVI (peak exercise), or QI (peak exercise) with total exercise duration or PW. Interpretation of R2 from regression models were based on calculated power and effect size standards of Cohen equal to:34 modest = 0.02, moderate = 0.15, and strong ≥ 0.25. Step-wise linear regression model analyses were also used to assess which variables out of FVC (rest), FEV1 (rest), VO2 (peak exercise), HR (peak exercise), SVI (peak exercise), or QI (peak exercise) predicted total exercise duration or PW best. Entrance into the step-wise model required variables to reach an alpha set at 0.15, whereas to remain in the model, variables had to reach an alpha set at <0.05. For all other testing, two-tailed significance was determined using an alpha set at 0.05. Computations were made using SAS statistical software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Participants
All 42 participants in this study completed CPET without adverse events. Shown in Table 1, the sample size for CF compared to CTLs did not differ significantly (N = 17 vs. 25, P = 0.22). Individuals with CF were of similar age compared to CTLs (mean: 23±2 vs. 27±2 years, respectively; P = 0.17). Sex distribution between CF and CTLs did not differ significantly (male/female: 13/4 vs. 15/10, respectively, P = 0.27). Individuals with CF had lower body weight and BSA (CF = 1.7±0.0 vs CTLs = 1.9±0.0 m2, P = 0.01) compared to CTLs. Airway function was significantly lower in CF compared to CTL. Percent achieved of predicted peak VO2 was significantly lower in CF compared to CTLs.33
Resting and CPET cardiovascular and ventilatory responses
Table 2 shows that individuals with CF demonstrated significantly lower HR, SVI, QI, and SaO2 compared to CTLs at rest. In contrast, RPE, VO2 (adjusted for body weight), VO2, VCO2, RR, VE, VT, PETCO2, Ca-vO2, SBP, DBP, and MAP were similar between groups.
Table 2. Between group comparisons for cardiopulmonary exercise test variables at rest and peak exercise.
| Rest | Peak Exercise | |||
|---|---|---|---|---|
| CTL | CF | CTL | CF | |
|
|
||||
| Total exercise duration, seconds | - | - | 960 ± 41 | 729 ± 48* |
| Workload, watts | - | - | 179 ± 11 | 100 ± 9* |
| RPE (6-20, Borg) | 6.0 ± 0.0 | 6.0 ± 0.0 | 17.5 ± 0.2† | 16.8 ± 0.3† |
| Cardiac hemodynamics | ||||
| SVI, mL/m2 | 40 ± 3 | 31 ± 3* | 51 ± 2† | 45 ± 3 |
| QI, L/min/m | 3.1 ± 0.2 | 2.6 ± 0.2* | 9.1 ± 0.3† | 6.7 ± 0.5*† |
| Ventilation or gas exchange | ||||
| VO2, mL/kg/min | 5.9 ± 0.3 | 6.5 ± 0.4 | 33.2 ± 2 .0† | 22.6 ± 2 .2* † |
| VO2, L/min | 0.4 ± 0.0 | 0.4 ± 0.0 | 2.3 ± 0.1† | 1.4 ± 0.1* † |
| VCO2, L/min | 0.3 ± 0.0 | 0.4 ± 0.0 | 26 ± 0.2† | 1.6 ± 0.2 * † |
| RR, breaths/min | 19 ± 2 | 22 ± 1 | 38 ± 2† | 36 ± 2 |
| VE, L/min | 14 ± 1 | 16 ± 1 | 82 ± 4 | 56 ± 3* † |
| VT, L | 0.8 ± 0.05 | 0.8 ± 0.07 | 2.2 ± 1.2 | 1.6 ± 1.2* |
| PETCO2, mm Hg | 30 ± 1 | 30 ± 1 | 35 ± 1 | 36 ± 1 |
| Ca-vO2, mL/100 mL/m2 | 5.8 ± 0.7 | 6.4 ± 1.1 | 15.7 ± 1.0† | 13.3 ±l.8† |
| SaO2, % | 97.8 ± 0.3 | 95.7 ± 0.4* | 96.9 ± 0.3 | 93.8 ± 1.2* |
| Heart rate or blood pressure | ||||
| HR, beats/min | 81 ± 3 | 93 ± 4* | 177 ± 2 † | 145 ± 7* † |
| SBP, mm Hg | 110 ± 2 | 106 ± 2 | 158 ± 4† | 142 ± 6* † |
| DBP, mm Hg | 71 ± 1 | 70 ± 2 | 61 ± 4 | 67 ± 4 |
| MAP, mm Hg | 84 ± 1 | 82 ± 2 | 93 ± 3† | 92 ± 4 |
Data are presented as mean ± SEM. CTL, control (n = 25); CF, cystic fibrosis (n = 17); RPE, rate of perceived exertion; SVI, stroke volume index; QI, cardiac index; VO2, oxygen uptake; VCO2, carbon dioxide output; RR, respiratory rate; VE, minute ventilation; VT, tidal volume; PETCO2, end-tidal partial-pressure carbon dioxide; Ca-vO2, arterial-mixed venous oxygen content difference; SaO2, peripheral oxygen saturation; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure. Between group comparisons were performed using Wilcoxon rank-sum tests for all variables. Within group comparisons were performed using paired Student's t-tests.
p<0.05, CTL vs. CF.
p<0.05, rest vs. peak exercise.
At peak exercise, individuals with CF demonstrated significantly lower PW and total exercise duration compared to CTLs (Table 2). However, RPE in CF was equivalent to CTLs. Additionally, out of all the cardiovascular and ventilatory variables measured at peak exercise, QI, VO2 (adjusted for body weight), VO2, VCO2, VE, VT, and SBP were significantly lower in CF compared to CTLs.
Reserve cardiovascular and ventilatory responses
Illustrated in Figure 1 are SVI, QI, and VO2 reserves (% increase from rest to peak exercise). Between group differences were not significant for SVI (P = 0.16), whereas CTLs had higher QI (P = 0.01) and VO2 (P < 0.001) reserves compared to CF. In both CF and CTLs, there were within group differences between SVI, QI, and VO2 reserves. In particular, there were significant differences between SVI and VO2 (CF, P < 0.001; CTLs, P < 0.001), SVI and QI (CF, P < 0.001; CTLs, P < 0.001), and QI and VO2 (CF, P < 0.001; CTLs, P < 0.001).
Figure 1.
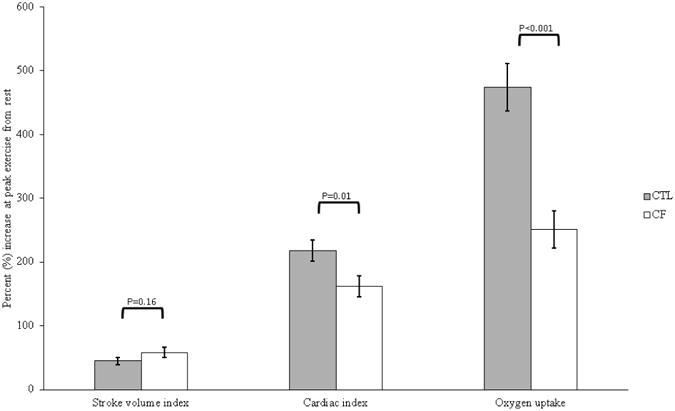
Cardiac hemodynamic and oxygen uptake reserve. Data presented as mean ± standard error of the mean representing stroke volume index (SVI), cardiac index (QI), or oxygen uptake (VO2) reserve calculated as the percent (%) increase at peak exercise from rest. CTL = healthy controls; CF = cystic fibrosis.
Predictors of exercise tolerance
Presented in Figure 2, there were significant relationships for FVC and FEV1 with both total exercise duration and PW in CF, which were stronger in CTLs. Power for each relationship test exceeded 0.80 for both CF and CTLs.
Figure 2.
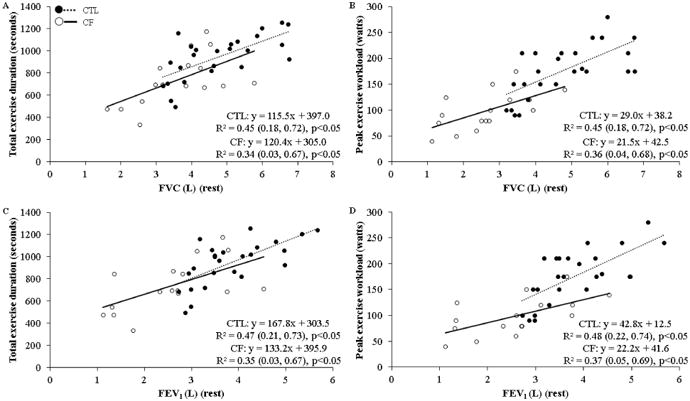
Pulmonary function at rest and exercise tolerance relationships. Data represents results of univariate linear regressions (coefficient of determination [R2] with 95% confidence limits) between total exercise duration or peak exercise workload with either, A and B) forced vital capacity (FVC) (rest), C and D) forced expiratory volume in 1-second (FEV1) (rest), respectively. CTL = healthy controls; CF = cystic fibrosis.
Illustrated in Figure 3, there were strong relationships between total exercise duration or PW and VO2 (peak exercise), SVI (peak exercise), or QI (peak exercise) in CF. In contrast, these same relationships ranged from moderate to strong in CTLs. The power for all relationships in CF were >0.80, and >0.70 in CTLs.
Figure 3.
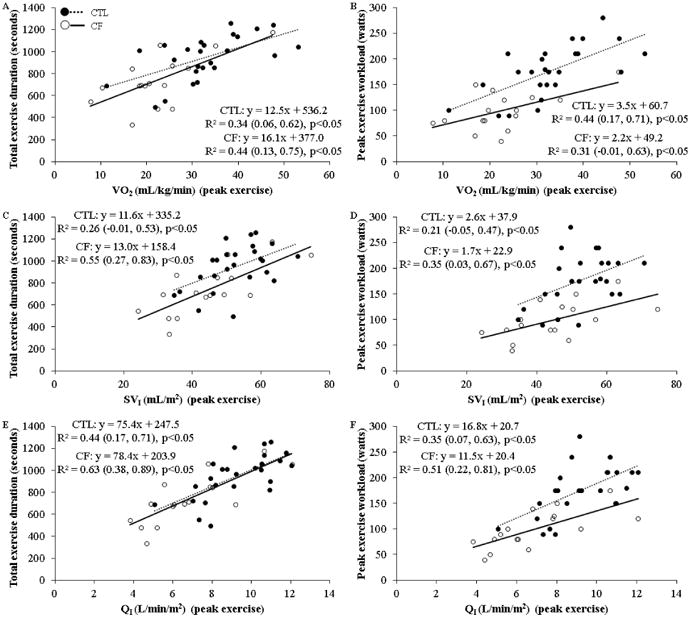
Peak exercise oxygen uptake and cardiac hemodynamic exercise tolerance relationships. Data represents results of univariate linear regressions (coefficient of determination [R2] with 95% confidence limits) between total exercise duration or peak exercise workload with either, A and B) oxygen uptake (VO2) (peak exercise), C and D) stroke volume index (SVI) (peak exercise), or E and F) cardiac index (QI) (peak exercise), respectively. CTL = healthy controls; CF = cystic fibrosis.
All univariate linear regressions (R2 values) in Figures 2 and 3 remained within the 95% CL of one another for both groups. Therefore, upon further testing, step-wise linear regression analyses considering FVC (rest), FEV1 (rest), VO2 (peak exercise), HR (peak exercise), SVI (peak exercise), or QI (peak exercise) for each group was performed (Table 3 and 4 for CF and CTLs, respectively). The step-wise model for total exercise duration for CF resulted in a single step where QI entered and stayed in the final model. In contrast, the exercise duration step-wise model for CTLs included seven steps, which resulted in the greatest amount of the model variance being explained by FVC, SVI, and QI.
Table 3. Cystic fibrosis, step-wise regression models for predicting total exercise duration or peak exercise workload.
| R2 | Semi-partial R2 | Intercept | b | β | P-value | |
|---|---|---|---|---|---|---|
|
|
||||||
| Total exercise duration (sec) | ||||||
| Step 1 (final step-wise model) | ||||||
| QI | 0.63* | - | 204 | 78 | 0.79 | <0.001 |
| Peak exercise workload (watts) | ||||||
| Step 1 | ||||||
| HR | 0.63* | - | -255 | 2.3 | - | <0.001 |
| Step 2 (final step-wise model) | ||||||
| HR | 0.74* | 0.39 | -244 | 2.0 | 0.67 | <0.001 |
| SVI | - | 0.35 | - | 1.0 | 0.36 | 0.01 |
Data presented as step-wise linear regressions. Variables entered the model upon reaching an alpha set at 0.15, whereas variables remained in the final model upon reaching an alpha set at <0.05. Independent variables used for entry into step-wise regressions were FVC, forced vital capacity (rest); FEV1, forced expiratory volume in 1-second (rest); VO2, oxygen uptake (peak exercise); HR, heart rate (peak exercise); QI, cardiac index (peak exercise); or SVI, stroke volume index (peak exercise). Dependent variables were total exercise duration or peak exercise workload. Coefficient of determination (R2) is for the overall model at each step. β, standardized slope. P-value = alpha value for each parameter in regression models.
p<0.05, for the model at each step.
Table 4. Controls, step-wise regression models for predicting total exercise duration or peak exercise workload.
| R2 | Semi-partial R2 | Intercept | b | β | P-value | |
|---|---|---|---|---|---|---|
|
|
||||||
| Total exercise duration (sec) | ||||||
| Step 1 | ||||||
| FEVI | 0.52* | - | 268 | 175 | - | <0.001 |
| Step 2 | ||||||
| FEVI | 0.66* | 0.52 | 12.8 | 128 | - | <0.01 |
| QI | - | 0.14 | - | 47 | - | <0.01 |
| Step 3 | ||||||
| FEVI | 0.72* | 0.52 | 173 | 123 | - | <0.001 |
| SVI | - | 0.06 | - | -15 | - | 0.04 |
| QI | - | 0.14 | - | 118 | - | <0.01 |
| Step 4 | ||||||
| FEVI | 0.76* | 0.52 | 131 | 19 | - | 0.77 |
| SVI | - | 0.06 | - | -14.9 | - | 0.04 |
| QI | - | 0.14 | - | 125 | - | <0.01 |
| FVC | - | 0.04 | - | 76 | - | 0.09 |
| Step 5 (FEVI removed) | ||||||
| SVI | 0.76* | 0.08 | 133 | -14.9 | - | 0.03 |
| QI | - | 0.44 | - | 127 | - | <0.001 |
| FVC | - | 0.23 | - | 87 | - | <0.001 |
| Step 6 | ||||||
| SVI | 0.79* | 0.08 | 2674 | -65 | - | 0.04 |
| QI | - | 0.44 | - | 419 | - | 0.02 |
| FVC | - | 0.23 | - | 88 | - | <0.001 |
| HR | - | 0.03 | - | -14.7 | - | 0.10 |
| Step 7 (final step-wise model) | ||||||
| SVI | 0.76* | 0.08 | 133 | -14.9 | -0.65 | 0.03 |
| QI | - | 0.44 | - | 127 | 1.12 | <0.001 |
| FVC | - | 0.23 | - | 87 | 0.51 | <0.001 |
| Peak exercise workload (watts) | ||||||
| Step 1 | ||||||
| FVC | 0.45* | - | 38 | 29 | - | <0.001 |
| Step 2 | ||||||
| FVC | 0.67* | 0.45 | -15 | 22 | - | <0.001 |
| VO2 | - | 0.22 | - | 2.6 | - | <0.001 |
| Step 3 (final step-wise model) | ||||||
| FVC | 0.73* | 0.24 | -161 | 21 | 0.48 | <0.01 |
| VO2 | - | 0.44 | - | 2.2 | 0.42 | <0.01 |
| HR | - | 0.06 | - | 0.9 | 0.26 | 0.05 |
Data presented as step-wise linear regressions. Variables entered the model upon reaching an alpha set at 0.15, whereas variables remained in the final model upon reaching an alpha set at <0.05. Independent variables used for entry into step-wise regressions were FVC, forced vital capacity (rest); FEV1, forced expiratory volume in 1-second (rest); VO2, oxygen uptake (peak exercise); HR, heart rate (peak exercise); QI, cardiac index (peak exercise); or SVI, stroke volume index (peak exercise). Dependent variables were total exercise duration or peak exercise workload. Coefficient of determination (R2) is for the overall model at each step. β, standardized slope. P-value = alpha value for each parameter in regression models.
p<0.05, for the model at each step.
The step-wise model for PW in CF resulted in two steps resulting in a final model that included SVI and HR as significant predictors. In contrast, the step-wise model for PW in CTLs included three steps, with FVC, VO2, and HR remaining as the strongest predictors of PW.
Discussion
In this study our group demonstrates for the first time that QI and SVI responses at peak exercise directly relate with total exercise duration and PW associated with CPET in individuals with CF. Traditional resting measures of FVC and FEV1, which are commonly used to assess CF prognosis,2, 4 did not show relationships with total exercise duration or PW that exceeded the strength of peak exercise QI and SVI in individuals with CF. Additionally, despite peak VO2 relating to prognosis and commonly used to estimate exercise tolerance in CF,5-7 peak VO2 in this study did not predict either total exercise duration or PW when considered in parallel with cardiac hemodynamics and pulmonary function measures in CF.
The relationships observed between peak exercise QI and SVI with both total exercise duration and PW in this study supports results from previous studies showing that abnormal cardiac function is present at rest in individuals with CF.18-20, 22, 23 However, in extending those observations, this study demonstrates abnormal cardiac function persists during exercise and is strongly related to exercise tolerance in CF. These data provide critical insight concerning the potential importance of evaluating cardiac function during exercise to improve the pathophysiologic understanding of low exercise tolerance in individuals with CF. This is relevant because as the lifespan of individuals with CF increases, with the median age of survival estimated at 37 and 40 years in men and women, respectively,1, 2, 25 it is also important to recognize that the risk for cardiovascular and heart disease increases at ages >40 years, with exercise tolerance serving as a primary factor in predicting disease prognosis.26-28
Cardiac hemodynamics and exercise tolerance in CF
Cystic fibrosis is a genetic disease impairing the normal expression of CFTR while affecting multiple organ system function.1, 3, 9 This disease is traditionally associated with deranged ion regulation within the pulmonary system, leading to a phenotype of perpetual ventilation and gas exchange dysfunction with hallmark signs and symptoms, including low FEV1 and reduced exercise tolerance.1, 4, 8, 10, 14 In this light, although the underlying mechanisms remain unclear, FEV1 has been demonstrated to be a strong correlate of exercise tolerance in CF, and therefore the traditional paradigm for low exercise tolerance in CF has centered on understanding pathophysiology related to airway dysfunction.8, 10, 11 However, in considering only low airway function as an explanation for attenuated exercise tolerance, this may greatly underestimate the overall integrated influence that cardiac and pulmonary systems share during exercise (e.g., ventilation—perfusion matching). Despite showing in this study the presence of pulmonary dysfunction in CF that is consistent with the findings of others,8, 10, 11 these data underscore the need to assess both pulmonary and cardiac function to determine exercise tolerance meaningfully. The present study further demonstrates that peak exercise cardiac performance, but not VO2, is a strong determinant of total exercise duration and PW in individuals with CF. This is also an important finding because it demonstrates that a high or low VO2 in addition to abnormal airway function do not necessarily imply exercise tolerance in CF.
Our observation that peak QI and SVI are lower in CF compared to healthy individuals is consistent with Koelling et al.20 who also shows abnormal cardiac function is present during exercise in individuals with CF. Furthermore, and although studied at rest, Sellers et al.18 demonstrates that left ventricular strain (i.e., inotropy) is reduced in individuals with CF. Whereas Van Iterson et al.19 shows stimulation of β2-adrenergic receptors (i.e., inotropy) via inhalation of the selective β2-agonist albuterol results in attenuated increases in QI, SVI, cardiac power, and stroke work in CF compared to healthy individuals. It is therefore not surprising that while also demonstrating evidence of impaired cardiac function in CF, Ionescu et al.22 suggests there may be a link between cardiac and pulmonary function in CF. Those authors present evidence suggesting that the magnitude of right ventricular dysfunction is associated with disease severity measured by airway function in CF.22 Lastly, in a separate clinical population who are known to demonstrate concurrent cardiac and pulmonary impairment (i.e., heart failure), Solbach et al.17 for the first time observed that expression of protein transporter ABCC7 (representing CFTR) in left ventricular tissue of end-stage heart failure patients is nearly entirely absent compared to expression of ABCC7 in left ventricular tissue of healthy individuals. The connection of those findings in heart failure patients with individuals with CF is unknown, but suggests a potentially critical role for the expression of myocardial CFTR in contributing to normal cardiac function in CF.
Clinical implications
Cystic fibrosis is well-recognized to have debilitating effects on pulmonary function and exercise tolerance.4-7, 9-11 The measurement of airway function at rest to predict prognosis is an extremely useful tool in this population.2, 4 However, although smaller in number, a growing body of evidence suggests cardiac function as it relates to hemodynamically challenging settings such as exercise, may also be indicative of CF disease severity.14, 19-23 The abnormal rise and reduced Q reserve may reflect the severity of integrated cardiopulmonary dysfunction, which is likely to coincide with poor performance on other traditional assessments that are already used in the clinical setting (i.e., pulmonary function tests). The quantification of Q at rest and/or during exercise does not need to take on an invasive and technically challenging approach in the clinical setting. Several studies in patients with pulmonary diseases show Q can be measured using noninvasive techniques that agree with direct measurement standards.21, 40, 44 This study in addition to others show inert gas breathing may be an efficacious option for Q measurement in patients with obstructive lung diseases (e.g., CF and chronic obstructive pulmonary disease), whereas others show that imaging techniques such as echocardiography may also be used to assess several aspects of cardiac function in addition to Q in CF (e.g., myocardial strain).14, 18-23, 40, 44 These and other (e.g., arterial waveform analysis45) technical approaches for the non-invasive measurement of Q can be readily available in the clinical setting without the need for intensive external expertise and training such as is needed for thermodilution or direct Fick measurements of Q. Additionally, implementation of CPET can be performed efficaciously using several approaches that have been well-studied and may be more practical than walking tests for individuals with CF who may demonstrate abnormal gait during walking due to skeletal muscle weakness.30 Thus, because of the new findings in this study, in addition to those previously showing the importance of exercise tolerance and abnormal cardiac function in CF,5-7, 14, 18-23 assessment of cardiac function during CPET may be an important clinical care aspect that should be considered in the therapeutic approach for individuals with CF.
Limitations
Individuals participating in this study were of a convenience sample that did not include a balanced comparison of participants across groups. However, the imbalanced sample size of groups and comparisons performed in this study are consistent with other studies in CF.8, 11, 18, 20, 21 To match body anthropometry and airway function between CF and CTLs is challenging as the phenotype of CF inherently includes abnormally low body size due to nutritional deficiencies associated with this disease, in addition to the well-known airway limitations.39 Therefore, it is uncommon to find healthy individuals to serve as CTLs that could readily match CF on baseline demographics and airway function. Moreover, had groups been matched according to absolute workload for CPET, the CTL group would have been performing cycling at much lower exercise intensities leading to long duration tests not reflective of a true CPET.30 Nevertheless, our assessment of cardiovascular reserve function between groups reflects the relative changes in VO2, SVI, and QI within group and supports the attenuated absolute responses of these variables during CPET in CF. The primary aim of this study was to assess within group relationships between cardiac function and exercise tolerance. Thus, the CF sample size and relationships that were observed met acceptable power and effect sizes for interpretability.34 The calculated post-hoc power exceeded 0.80 at an alpha of 0.05 suggesting the possibility of both Type I and II errors were minimal for these tests of relationships within CF.
Measurement of cardiac hemodynamics did not occur using the invasive direct Fick method, and therefore unknown bias in hemodynamic measurements using the acetylene rebreathe technique, particularly related to potential changes in ventilation, cannot be ruled out. However, inert gas rebreathing is non-invasive and relates with direct Fick measurements of cardiac hemodynamics at rest and during exercise in healthy individuals or in cardiac (e.g., heart failure) or obstructive pulmonary (e.g., CF and chronic obstructive pulmonary disease) patients.21, 40, 43, 44, 46 Further, our group has previously demonstrated that the variance in lung diffusion47 does not influence relationships between acetylene rebreathe measured QI during CPET in CF. Nevertheless, in an alternative method to inert gas breathing, it has been demonstrated in detail that newer methodological approaches associated with echocardiography can be used effectively with high reliability during exercise to estimate cardiac hemodynamics.48-50 However, in the absence of highly trained expertise by both sonographer and reader, this technique may be subject to well-known limitations (e.g., high user-dependence relating to image-acquisition and skill of the echocardiographic reader) that are recognized to introduce measurement bias, particularly during exercise.51-53 Thus, inert gas breathing using the highly blood soluble acetylene-gas41 rebreathe technique may provide a more favorable estimation of cardiac hemodynamics during exercise in CF compared to echocardiography, CO2 rebreathing, or nitrous oxide rebreathing. Finally, it should be note that cardiac function measures in the present study have not been conferred with a comprehensive examination by a cardiologist, and therefore interpretability of our assessment of cardiac function in individuals with CF is hypothesis generating for future studies of invasive measurement of cardiac hemodynamics during exercise in this population.
Conclusions
These data suggest that individuals with CF demonstrate shorter total exercise duration and lower PW compared to CTLs, which may be related to abnormal cardiac function. Although the prognostic potential of abnormal cardiac function during CPET is relevant in CF, there is no consensus recommendation for this assessment in the clinical setting as there has not been an extensive examination in this field concerning the influence of CF on abnormal cardiac function as it relates to exercise tolerance. Nevertheless, this study has shown that in moderate severity CF, attenuated QI and SVI appear equally responsible for the shortened total exercise duration and low PW compared to low FVC and FEV1. Our observations may provide critical insight concerning the nature of the integrative pathophysiology of low exercise tolerance in CF, while adding novel information for an improved understanding of the CF phenotype that extends beyond the pulmonary system. Future studies examining the relationship between low exercise tolerance and low cardiac hemodynamics should look to investigate the role of abnormal integrated cardiopulmonary function relating to ventilation—perfusion mismatch at the central circulation and reduced perfusion and oxygen delivery at the peripheral circulation.
Acknowledgments
This research was supported by the National Institutes of Health (HL108962-03). Thank you to the individuals who participated in this study.
Abbreviations
- BSA
body surface area
- Ca2+
calcium
- Ca-vO2
arterio-venous oxygen content difference
- CF
cystic fibrosis
- CFTR
cystic fibrosis conductance regulator
- Cl−
chloride
- DBP
diastolic blood pressure
- ENaC
epithelial Na+ channel
- FEV1
forced expiratory volume in 1-second
- FVC
forced vital capacity
- HR
heart rate
- MAP
mean arterial pressure
- Na+
sodium; PETCO2; end-tidal carbon dioxide
- PW
peak workload
- Q
cardiac output
- QI
cardiac index
- RPE
rate of perceived exertion
- RR
respiratory rate
- SaO2
oxygen saturation
- SBP
systolic blood pressure
- SD
standard deviation
- SV
stroke volume
- SVI
stroke volume index
- VO2
oxygen uptake
- VCO2
carbon dioxide production
- VE
minute ventilation
- VT
tidal volume
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Courtney M. Wheatley, Email: Wheatley.Courtney@mayo.edu.
Sarah E. Baker, Email: Baker.Sarah@mayo.edu.
Wayne J. Morgan, Email: wmorgan@arc.arizona.edu.
Eric M. Snyder, Email: snyd0180@umn.edu.
References
- 1.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161:233–41. doi: 10.7326/M13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, Stanojevic S. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. The European respiratory journal. 2015;45:670–9. doi: 10.1183/09031936.00119714. [DOI] [PubMed] [Google Scholar]
- 3.Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–8. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 4.Kerem E, Viviani L, Zolin A, MacNeill S, Hatziagorou E, Ellemunter H, Drevinek P, Gulmans V, Krivec U, Olesen H, Group EPRS. Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. The European respiratory journal. 2014;43:125–33. doi: 10.1183/09031936.00166412. [DOI] [PubMed] [Google Scholar]
- 5.Moorcroft AJ, Dodd ME, Webb AK. Exercise testing and prognosis in adult cystic fibrosis. Thorax. 1997;52:291–3. doi: 10.1136/thx.52.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. The New England journal of medicine. 1992;327:1785–8. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Chapron J, Hubert D, Kanaan R, Honoré I, Paillasseur JL, Aubourg F, Dinh-Xuan AT, Dusser D, Fajac I. Prognostic value of six minute walk test in cystic fibrosis adults. Respiratory medicine. 2013;107:1881–1887. doi: 10.1016/j.rmed.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Lands LC, Heigenhauser GJ, Jones NL. Analysis of factors limiting maximal exercise performance in cystic fibrosis. Clinical science. 1992;83:391–7. doi: 10.1042/cs0830391. [DOI] [PubMed] [Google Scholar]
- 9.Leroy S, Perez T, Neviere R, Aguilaniu B, Wallaert B. Determinants of dyspnea and alveolar hypoventilation during exercise in cystic fibrosis: impact of inspiratory muscle endurance. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2011;10:159–65. doi: 10.1016/j.jcf.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Pastre J, Prevotat A, Tardif C, Langlois C, Duhamel A, Wallaert B. Determinants of exercise capacity in cystic fibrosis patients with mild-to-moderate lung disease. Bmc Pulm Med. 2014;14:74. doi: 10.1186/1471-2466-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troosters T, Langer D, Vrijsen B, Segers J, Wouters K, Janssens W, Gosselink R, Decramer M, Dupont L. Skeletal muscle weakness, exercise tolerance and physical activity in adults with cystic fibrosis. The European respiratory journal. 2009;33:99–106. doi: 10.1183/09031936.00091607. [DOI] [PubMed] [Google Scholar]
- 12.Dunnink MA, Doeleman WR, Trappenburg JC, de Vries WR. Respiratory muscle strength in stable adolescent and adult patients with cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2009;8:31–6. doi: 10.1016/j.jcf.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Godfrey S, Mearns M. Pulmonary function and response to exercise in cystic fibrosis. Arch Dis Child. 1971;46:144–51. doi: 10.1136/adc.46.246.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wheatley CM, Foxx-Lupo WT, Cassuto NA, Wong EC, Daines CL, Morgan WJ, Snyder EM. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2011;10:45–53. doi: 10.1016/j.jcf.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Sellers ZM, De Arcangelis V, Xiang Y, Best PM. Cardiomyocytes with disrupted CFTR function require CaMKII and Ca2+-activated Cl− channel activity to maintain contraction rate. The Journal of physiology. 2010;588:2417–2429. doi: 10.1113/jphysiol.2010.188334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sellers ZM, Naren AP, Xiang Y, Best PM. MRP4 and CFTR in the regulation of cAMP and beta-adrenergic contraction in cardiac myocytes. Eur J Pharmacol. 2012;681:80–7. doi: 10.1016/j.ejphar.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solbach TF, Paulus B, Weyand M, Eschenhagen T, Zolk O, Fromm MF. ATP-binding cassette transporters in human heart failure. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:231–43. doi: 10.1007/s00210-008-0279-6. [DOI] [PubMed] [Google Scholar]
- 18.Sellers ZM, McGlocklin L, Brasch A. Strain rate echocardiography uncovers subclinical left ventricular dysfunction in cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2015;14:654–60. doi: 10.1016/j.jcf.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Van Iterson EH, Karpen SR, Baker SE, Wheatley CM, Morgan WJ, Snyder EM. Impaired cardiac and peripheral hemodynamic responses to inhaled beta2-agonist in cystic fibrosis. Respir Res. 2015;16:103. doi: 10.1186/s12931-015-0270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koelling TM, Dec GW, Ginns LC, Semigran MJ. Left ventricular diastolic function in patients with advanced cystic fibrosis. Chest. 2003;123:1488–94. doi: 10.1378/chest.123.5.1488. [DOI] [PubMed] [Google Scholar]
- 21.Lands LC, Heigenhauser GJ, Jones NL. Cardiac output determination during progressive exercise in cystic fibrosis. Chest. 1992;102:1118–23. doi: 10.1378/chest.102.4.1118. [DOI] [PubMed] [Google Scholar]
- 22.Ionescu AA, Ionescu AA, Payne N, Obieta-Fresnedo I, Fraser AG, Shale DJ. Subclinical right ventricular dysfunction in cystic fibrosis. American journal of respiratory and critical care medicine. 2012;163:1212–8. doi: 10.1164/ajrccm.163.5.9908005. [DOI] [PubMed] [Google Scholar]
- 23.Ozcelik N, Shell R, Holtzlander M, Cua C. Decreased right ventricular function in healthy pediatric cystic fibrosis patients versus non-cystic fibrosis patients. Pediatric cardiology. 2013;34:159–164. doi: 10.1007/s00246-012-0407-4. [DOI] [PubMed] [Google Scholar]
- 24.Sellers ZM, Kovacs A, Weinheimer CJ, Best PM. Left ventricular and aortic dysfunction in cystic fibrosis mice. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2013;12:517–24. doi: 10.1016/j.jcf.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. American journal of respiratory and critical care medicine. 2011;183:1463–1471. doi: 10.1164/rccm.201009-1478CI. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D Framingham Heart S. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. The Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 28.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. Jama. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 29.Weber KT, Janicki JS. Cardiopulmonary exercise testing for evaluation of chronic cardiac failure. Am J Cardiol. 1985;55:22A–31A. doi: 10.1016/0002-9149(85)90792-1. [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic S and American College of Chest P. ATS/ACCP Statement on cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2003;167:211–77. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 31.Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Annals of human genetics. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 32.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, LeGrys VA, Massie J, Parad RB, Rock MJ, Campbell PW. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. The American review of respiratory disease. 1984;129:S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. A power primer. Psychological bulletin. 1992;112:155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 35.Society AT. ATS/ERS Task Force: Standardisation of lung function testing. The European respiratory journal. 2005;26:319–338. [Google Scholar]
- 36.Hulzebos H, Werkman M, Van Brussel M, Takken T. Towards an individualized protocol for workload increments in cardiopulmonary exercise testing in children and adolescents with cystic fibrosis. Journal of Cystic Fibrosis. 2012;11:550–554. doi: 10.1016/j.jcf.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Coleman KJ, Ngor E, Reynolds K, Quinn VP, Koebnick C, Young DR, Sternfeld B, Sallis RE. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44:2071–2076. doi: 10.1249/MSS.0b013e3182630ec1. [DOI] [PubMed] [Google Scholar]
- 38.de Simone G, Devereux RB, Daniels SR, Mureddu G, Roman MJ, Kimball TR, Greco R, Witt S, Contaldo F. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation. 1997;95:1837–43. doi: 10.1161/01.cir.95.7.1837. [DOI] [PubMed] [Google Scholar]
- 39.Sinaasappel M, Stern M, Littlewood J, Wolfe S, Steinkamp G, Heijerman HG, Robberecht E, Döring G. Nutrition in patients with cystic fibrosis: a European Consensus. Journal of Cystic Fibrosis. 2002;1:51–75. doi: 10.1016/s1569-1993(02)00032-2. [DOI] [PubMed] [Google Scholar]
- 40.Saur J, Trinkmann F, Doesch C, Scherhag A, Brade J, Schoenberg SO, Borggrefe M, Kaden JJ, Papavassiliu T. The impact of pulmonary disease on noninvasive measurement of cardiac output by the inert gas rebreathing method. Lung. 2010;188:433–40. doi: 10.1007/s00408-010-9257-0. [DOI] [PubMed] [Google Scholar]
- 41.Cander L. Solubility of inert gases in human lung tissue. Journal of applied physiology. 1959;14:538–540. [Google Scholar]
- 42.Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Physiol (1985) 2005;99:1985–91. doi: 10.1152/japplphysiol.00348.2005. [DOI] [PubMed] [Google Scholar]
- 43.Triebwasser JH, Johnson RL, Burpo RP, Campbell JC, Reardon WC, Blomqvist CG. Noninvasive determination of cardiac output by a modified acetylene rebreathing procedure utilizing mass spectrometer measurements. Aviat Space Environ Med. 1977;48:203–9. [PubMed] [Google Scholar]
- 44.Hoeper MM, Maier R, Tongers J, Niedermeyer J, Hohlfeld JM, Hamm M, Fabel H. Determination of cardiac output by the Fick method, thermodilution, and acetylene rebreathing in pulmonary hypertension. American journal of respiratory and critical care medicine. 1999;160:535–41. doi: 10.1164/ajrccm.160.2.9811062. [DOI] [PubMed] [Google Scholar]
- 45.Perel A, Wesselink W, Settels J. The Nexfin Monitor—A Totally Non-Invasive Cardiac Output Monitor Anaesthesia, Pharmacology, Intensive Care and Emergency Medicine APICE. Springer; 2011. pp. 103–108. [Google Scholar]
- 46.Agostoni P, Cattadori G, Apostolo A, Contini M, Palermo P, Marenzi G, Wasserman K. Noninvasive measurement of cardiac output during exercise by inert gas rebreathing technique: a new tool for heart failure evaluation. J Am Coll Cardiol. 2005;46:1779–81. doi: 10.1016/j.jacc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Van Iterson EH, Baker SE, Wheatley CM, Morgan WJ, Snyder EM. The Capacity for Gas Transfer Within Lungs Does Not Influence the Relationship Between Oxygen Uptake and Cardiac Hemodynamics During Exercise in Cystic Fibrosis Patients. Paper presented at: American Thoracic Society; 2015; Denver. [Google Scholar]
- 48.Stöhr EJ, McDonnell B, Thompson J, Stone K, Bull T, Houston R, Cockcroft J, Shave R. Left ventricular mechanics in humans with high aerobic fitness: adaptation independent of structural remodelling, arterial haemodynamics and heart rate. The Journal of physiology. 2012;590:2107–2119. doi: 10.1113/jphysiol.2012.227850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart GM, Yamada A, Kavanagh JJ, Haseler LJ, Chan J, Sabapathy S. Reproducibility of Echocardiograph-Derived Multilevel Left Ventricular Apical Twist Mechanics. Echocardiography. 2015 doi: 10.1111/echo.13020. [DOI] [PubMed] [Google Scholar]
- 50.Balmain B, Stewart G, Yamada A, Chan J, Haseler L, Sabapathy S. The impact of an experimentally induced increase in arterial blood pressure on left ventricular twist mechanics. Experimental physiology. 2016;101:124–134. doi: 10.1113/EP085423. [DOI] [PubMed] [Google Scholar]
- 51.Mutlak D, Aronson D, Lessick J, Reisner SA, Dabbah S, Agmon Y. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? CHEST Journal. 2009;135:115–121. doi: 10.1378/chest.08-0277. [DOI] [PubMed] [Google Scholar]
- 52.Dhutia NM, Zolgharni M, Willson K, Cole G, Nowbar AN, Dawson D, Zielke S, Whelan C, Newton J, Mayet J. Guidance for accurate and consistent tissue Doppler velocity measurement: comparison of echocardiographic methods using a simple vendor-independent method for local validation. European Heart Journal-Cardiovascular Imaging. 2014;15:817–827. doi: 10.1093/ehjci/jeu040. [DOI] [PubMed] [Google Scholar]
- 53.Marwick TH, Nemec JJ, Pashkow FJ, Stewart WJ, Salcedo EE. Accuracy and limitations of exercise echocardiography in a routine clinical setting. J Am Coll Cardiol. 1992;19:74–81. doi: 10.1016/0735-1097(92)90054-q. [DOI] [PubMed] [Google Scholar]


