Abstract
Background
Prostaglandin D2 (PGD2) is the dominant cyclooxygenase product of mast cells and is an effector of aspirin-induced respiratory reactions in aspirin-exacerbated respiratory disease (AERD).
Objective
We evaluated the role of the innate cytokine thymic stromal lymphopoietin (TSLP) acting on mast cells to generate PGD2 and facilitate tissue eosinophilia and nasal polyposis in AERD.
Methods
Urinary eicosanoids were measured in aspirin-tolerant controls and patients with AERD. Nasal polyp specimens from subjects with AERD and chronic rhinosinusitis were analyzed via qPCR, western blot, and immunohistochemistry. Human cord blood-derived and peripheral blood-derived mast cells were stimulated with TSLP in vitro to assess PGD2 generation.
Results
Urinary levels of a stable PGD2 metabolite (uPGD-M) were 2-fold higher in subjects with AERD relative to controls, and increased further during aspirin-induced reactions. Peak uPGD-M levels during aspirin reactions correlated with reductions in blood eosinophil counts and lung function, and with increases in nasal congestion. Mast cells sorted from nasal polyps expressed PGD2 synthase (hPGDS) mRNA at higher levels than did eosinophils from the same tissue. Whole nasal polyp TSLP mRNA expression correlated strongly with mRNA encoding hPGDS (r = .75), the mast cell-specific marker carboxypeptidase A3 (r = .74), and uPGD-M (r=0.74). The cleaved, active form of TSLP was increased in AERD nasal polyps relative to aspirin-tolerant controls. Recombinant TSLP induced PGD2 generation by cultured human mast cells.
Conclusions
Our study demonstrates that mast cell-derived PGD2 is a major effector of type 2 immune responses driven by TSLP, and suggests that dysregulation of this innate system contributes significantly to the pathophysiology of AERD.
Keywords: Aspirin-exacerbated respiratory disease, Samter's triad, Nasal polyps, Thymic stromal lymphopoietin, Prostaglandin D2, Cysteinyl leukotrienes, Innate immunity, Mast cells, Eosinophils
INTRODUCTION
Eosinophil-rich tissue pathology is an important feature of immune defense against helminths (1), and is also a typical histologic finding in human diseases such as asthma (2), chronic rhinosinusitis (3), and certain gastrointestinal disorders (4). While such pathology can reflect the effector arm of adaptive immune responses involving type 2 T helper (Th2) cells and allergen-specific IgE, a complementary pathway mediated by the innate immune system can drive similar pathology, either alone or as an amplifier of adaptive type 2 responses. This innate type 2 immune pathway is initiated by cytokines such as thymic stromal lymphopoietin (TSLP) (5), interleukin (IL)-33 (6) and IL-25 (7) which derive largely from epithelial and other barrier cells that are disturbed by microbes or toxins. TSLP is an IL-7-like cytokine thought to be important in a number of human diseases including asthma (8), atopic dermatitis (9), and nasal polyposis (10-12), and polymorphic variants of TSLP and TSLPR are risk alleles for asthma and other diseases (13). TSLP induces type 2 cytokine generation by mast cells (14), type 2 innate helper cells (ILC2s) (15, 16) and CD34+ hematopoietic progenitor cells (17), and can activate eosinophils (18) and basophils (19). A monoclonal antibody against TSLP showed promise in a proof-of-concept study in atopic asthmatics (5). To date, however, there has been no direct demonstration of a mechanism by which this system contributes to tissue inflammation and pathology in humans.
Prostaglandin (PG)D2, the dominant cyclooxygenase (COX) pathway product of mast cells, was originally recognized as a bronchoconstrictor (20) and vasodilator (21) when directly administered to human subjects. Subsequently, PGD2 was identified as the preferred ligand for chemoattractant receptor homologue expressed by type 2 cells (CRTH2), a G protein coupled receptor expressed by human type 2 cells, eosinophils, and basophils (22, 23). PGD2 acts at CRTH2 to induce chemotaxis of these cell types in vitro (23), and in vivo in mouse models of allergen-induced pulmonary and cutaneous inflammation (24, 25). Recently, human ILC2s were found to express high levels of CRTH2 (26), and PGD2 was found to potently induce cytokine generation by ILC2s (27). These studies suggest an important role for PGD2, and potentially for mast cells, in the innate type 2 pathway that drives tissue eosinophilia. Whether PGD2 and mast cells are integrated with TSLP in innate type 2 immunity is unknown.
Nasal polyps are outgrowths of inflamed sinonasal mucosa that occur in patients with chronic rhinosinusitis, and often require surgical excision. They are densely infiltrated by eosinophils, contain activated mast cells and relatively large numbers of ILC2s (26, 28, 29), and have increased TSLP expression and activity compared with healthy nasal tissue (10-12). They frequently arise in individuals with no evidence of IgE-dependent allergic sensitization (30). As such, nasal polyps reflect part of the spectrum of tissue pathology induced by the innate type 2 pathway. Nasal polyps are especially aggressive and recurrent in subjects with aspirin-exacerbated respiratory disease (AERD), a distinctive, severe adult-onset respiratory syndrome. AERD is associated with eosinophilic asthma (31) and ongoing activation of mast cells. It is defined by pathognomonic, non-IgE mediated respiratory reactions upon ingestion of aspirin and other drugs that inhibit COX-1. Mast cell activation is a typical feature of these clinical reactions, with systemic release of multiple mediators including tryptase and PGD2 (32). We recently demonstrated that subjects with AERD who generated the highest baseline levels of PGD2, as determined by measurements of a stable metabolite of PGD2 (PGD-M) in the urine, experienced the most severe clinical reactions (33). In the current study, we sought to identify factors that could influence the synthesis of PGD2 in AERD. We tested the hypothesis that TSLP might contribute to PGD2 generation by mast cells, and that PGD2 might, in turn, be an effector of the capacity for TSLP to drive type 2 pathology.
Methods
Patient characterization
Patients between the ages of 18 and 70 years old were recruited at the Brigham and Women's Hospital (Boston, MA) Allergy and Immunology clinic and Asthma Research Center between April 2011 and June 2015 and were classified according to their clinical characteristics. Nonasthmatic controls had no history of asthma or intolerance to aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs). Aspirin-tolerant asthmatic (ATA) controls had physician-diagnosed persistent asthma and had taken aspirin or an NSAID within the previous 6 months without adverse reaction. Patients were suspected of having AERD if they had asthma, nasal polyposis, and a history of respiratory reaction upon ingestion of a COX inhibitor. In all subjects with that compatible clinical history, the diagnosis of AERD was then confirmed with a graded oral challenge to aspirin that resulted in characteristic sinonasal symptoms and/or a decrease in forced expiratory volume in 1 second (FEV1) of at least 15%. Patients were excluded from the study if they smoked cigarettes or were pregnant. All subjects with AERD were treated with the cysteinyl leukotriene (cysLT) 1 receptor blocker montelukast prior to aspirin challenge, and none were on the 5-lipoxygenase (5-LO) inhibitor zileuton prior to the challenge.
For the nasal polyp and nasal tissue studies, patients were recruited and tissue was collected at the time of elective endoscopic sinus surgery from subjects with AERD or from aspirin-tolerant controls with chronic rhinosinusitis (CRS) with and without nasal polyps. Details of the subjects’ characteristics, including age, gender, and asthma severity are included in Table I.
Table I.
Patient characteristics.
| AERD | ATA | Healthy Controls (for urine studies in Fig 1A) | CRS | |
|---|---|---|---|---|
| Number | n = 52 | n = 7 | n = 9 | n = 20 |
| Sex (male:female) | 21:31 | 1:6 | 2:7 | 11:9 |
| Median age (y) [range] | 47 [28-70] | 27 [20-56]* | 35 [25-56]** | 44 [20-67] |
| Asthma (%) | 100% | 100% | 0 | 15% |
| Baseline FEV1 (mean % predicted ±SD) | 89 (± 15) | 84 (± 9) | 96 (± 8) | Unavailable |
| Baseline ACQ7 score (mean ±SD) | 0.60 (± 0.66) | 0.52 (± 0.21) | N/A | Unavailable |
| Receiving daily inhaled corticosteroids (n) | 50/52 | 6/7 | 0/9 | 2/3 |
P-value is 0.023 for ATA ages compared to the AERD patient ages
P-value is 0.036 for HC ages compared to the AERD patient ages.
AERD, Aspirin-exacerbated respiratory disease; ATA, Aspirin tolerant asthmatic; CRS, Chronic rhinosinusitis, FEV1, Forced expiratory volume in 1 second; ACQ7, 7-item Asthma Control Questionnaire.
The institutional human subjects Institutional Review Board approved the study and all subjects provided written informed consent.
Aspirin challenge protocol
Aspirin challenges were performed while patients were not receiving the 5-LO inhibitor zileuton so that cysLT production could be monitored. Patients took their regularly prescribed inhaled corticosteroids with or without long-acting beta agonists the morning of challenge as applicable. All subjects received montelukast (10 mg) the evening prior to aspirin challenge to attenuate the severity of respiratory symptoms during the reaction. All subjects underwent the challenge protocol in one of our outpatient clinics or in the Asthma Research Center at the Brigham and Women's Hospital. Oral aspirin challenges started with 40 mg of aspirin followed by dose increases (81 mg, 162 mg, 325 mg) every 90 minutes. Patients were observed for respiratory symptoms, ocular injection, flushing, rash and abdominal pain. The aspirin dose that caused upper and/or lower respiratory symptoms was recorded as the provocative dose. FEV1 for each patient was recorded at baseline, prior to each dose, and at the time of reaction. Total Nasal Symptom Score (TNSS) questionnaires were recorded by patients at baseline, and again every 30 minutes for three hours after the onset of reaction. Demographic and clinical data were extracted from the medical record at the time of challenge.
Urinary eicosanoid measurements
For AERD subjects who underwent aspirin challenge, urine was collected at baseline (pre-aspirin administration), at the onset of symptoms of their aspirin-induced reaction, and again 90 minutes and 180 minutes after the onset of reaction. Basal urine samples were collected from ATA subjects who had been off NSAIDs for at least one week. Urine samples were stored at −80°C and analyzed via gas chromatography-mass spectrometry at Vanderbilt University. As described previously, concentrations of the major urinary PGD2 metabolite 9α,11β-dihydroxy-15-oxo-2,3,18,19-tetranorprost-5-ene-1,20-dioic acid (PGD-M) were measured and reported as ng/mg creatinine (34).
Peripheral blood leukocyte counts
Blood was collected for complete blood counts (LabCorp, Burlington, NC) from subjects with AERD at baseline (pre-aspirin administration) and again 60 minutes after the onset of their aspirin-induced reaction.
CRTH2 surface expression measurement
Whole peripheral blood was collected in heparinized tubes, kept at room temperature and assayed within one hour of collection. 40 μL of unstimulated blood was incubated for 10 minutes with monoclonal antibodies specific for CD45 (BD Biosciences, San Jose, CA), CCR3 (Biolegend, San Diego, CA), and CRTH2/CD294 (BD Biosciences) or appropriate isotype controls, and was fixed in 1% paraformaldehyde. At least 1,000 CCR3+ cells were recorded for each sample on a BD FACS Canto™ Flow Cytometer and were analyzed with FlowJo Version v.X.0.7. Eosinophils were identified as CCR3+/CD45+ cells with the granulocyte scatter gate and were assayed for the presence of CRTH2.
Polyp procurement and tissue specimen preparation
Nasal tissue was excised at the time of surgery and one tissue segment was immediately preserved in RNAlater® (Qiagen, Valencia, CA) for RNA extraction and the remaining tissue was placed in RPMI with penicillin streptomycin 1 unit/mL for transport to the laboratory. Within 2 hours of surgery the tissue was removed from RPMI and divided into segments. One segment was transferred into CellLytic® MCell Lysis Reagent (Sigma-Aldrich, St. Louis, MO) with 2% protease inhibitor (Roche, Indianapolis, IN) for protein extraction and the tissue was homogenized using a gentleMACS® Dissociator (Miltenyi Biotec, San Diego, CA). The supernatants were stored at −80° C. One se gment was fixed in 4% paraformaldehyde, embedded in Tissue-Tek® O.C.T.™ Compound (Sakura Finetek, Torrance, CA), and kept at −80°C until sectioning. Secti ons of 10 μm thickness were freshly cut, thaw-mounted onto slides, fixed in 4% paraformaldehyde, and stored at 80°C until stained.
For some patients a tissue segment was also placed in media containing 10% fetal bovine serum and chopped with a straight razor blade and then digested with 400 units/mL Type IV collagenase (Worthington Biochemical Corporation, Lakewood, NJ) and 200 μg/mL DNase (Sigma-Aldrich). The resulting suspension was passed through a 70 μm filter to retrieve a single cell suspension for flow cytometric sorting. These cells were stained with monoclonal antibodies against CD45, CD117, CD90, EpCAM, CD31, CCR3 (BD Biosciences) and FcεR1(BioLegend) and were sorted into purified cell populations with a BD FACS Aria™ Fusion Cell-Sorter to collect tissue mast cells (CD45+/CD117hi/ FcεR1+), eosinophils (CD45+/CCR3+ within the granulocyte forward and side scatter gate), fibroblasts (CD45−/EpCAM−/CD31−/CD90+), epithelial cells (CD45−/EpCAM+/CD31−/CD90−) and endothelial cells (CD45−/EpCAM−/CD31+/CD90−) separately. Purified mast cell populations were confirmed by toluidine blue staining and purified eosinophil populations were confirmed with staining comparable to the Wright-Giemsa method (Fisher Scientific, Waltham, MA). Cells were sorted into RNAprotect® (Qiagen) and stored at −20°C until RNA was extracted. Generally, between 3-50 × 103 mast cells were obtained per specimen.
qPCR
RNA was extracted from the whole nasal tissue specimens with Tri Reagent® (Qiagen) and converted to cDNA using the RT2 First Strand Kit (Qiagen). The expression of TSLP, COX-2 (PTGS2), CPA3, hPGDS, 5-LO, and LTC4S transcripts were examined using RT2 SybrGreen qPCR Master Mix (Qiagen).
RNA was extracted from sorted cells with RNeasy Micro Kit® (Qiagen) and converted to cDNA as above. The expression of hPGDS, COX-1, COX-2, and TSLPR transcripts were examined in mast cells and eosinophils, and TSLP expression was measured in epithelial cells, endothelial cells, and fibroblasts as described above. The identities of the sorted cells were further verified by monitoring mRNA encoding eosinophil peroxidase in eosinophils, EpCAM in epithelial cells, PECAM in endothelial cells, and collagen in fibroblasts. Expression levels of transcripts were normalized to the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (all primers from Qiagen).
Western blot analysis
To measure TSLP protein in the nasal polyp tissue, protein-extracted supernatants were used to generate gels and then transferred onto Immun-Blot PVDF Membranes (Bio-Rad, Hercules, CA) and blocked with 5% milk in tris(hydroxymethyl)aminomethane-buffered saline. Blots were incubated with primary rabbit polyclonal anti-TSLP, catalog number ABT330 (Millipore, Danvers, MA), or anti-GAPDH (Cell Signaling Technology, Danvers, MA) antibodies, washed and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Sigma-Aldrich) and visualized by enhanced chemiluminescence (GE HealthCare, Pittsburgh, PA). The molecular weight of TSLP was determined by loading one lane of each gel with 10 μL of SDS-PAGE Molecular Weight Standards, Broad Range (Bio-Rad), and comparing the distance migrated by the protein with the distance migrated by the dye. TSLP protein was quantified by densitometry and expressed relative to GAPDH. To confirm that the staining of TSLP was specific, a blot of 4 nasal polyp samples was probed with a new additional polyclonal anti-TSLP antibody, NB110-55234 (Novus Biologicals, Littleton, CO), which was raised against a known 19 amino acid TLSP peptide. That blot was then stripped of bound antibody and incubated for 1 hour with 1 μL/mL of the neutralizing/blocking peptide NB110-55234PEP (Novus Biologicals), which was used as an immunogen for NB110-55234. The blocked blot was then reprobed with the NB110-55234 antibody to show the disappearance of now blocked protein. To verify that the original antibody used in our studies also recognized the same 10kD and 15kD TSLP bands, the same blot was reincubated with the original anti-TSLP antibody ABT330.
Immunohistochemistry
Frozen sections were prepared from nasal polyps, incubated with mouse anti-human tryptase mAb AA1 (Dako, Carpinteria, CA) or isotype control, and developed with the EnVision+ System-HRP (AEC) for mouse primary Abs (Dako). Sections were counterstained with hematoxylin. For quantification of mast cells, the number of tryptase positive cells in photomicrographs encompassing ~1 mm2 of subepithelial tissue (quantified with Image J software) were counted and expressed per mm2.
Mast cell culture and stimulation
Human cord blood mast cells were derived from CD34+ progenitor cells and cultured for 6 – 8 weeks in RPMI supplemented with stem cell factor (SCF), IL-6, and IL-10 as previously described (35). Mast cell purity was confirmed with toluidine blue staining. 105 cells were incubated for 6 and 24 hours in the presence of TSLP (10 ng/mL) (Peprotech, Rocky Hill, NJ) with SCF at 100 ng/ml. Human peripheral blood mast cells were derived from CD34+ progenitor cells and cultured for 5 weeks as previously described (36) except for the use of StemSpan serum free media (Stem Cell Technologies, Vancouver, Canada) instead of Charcoal-filtered FCS, and with the addition of the retinoic acid receptor antagonist LE540 at 1 μM (Wako Chemicals, Richmond, VA) and human LDL at 10 μg/mL (Stem Cell Technologies), as suggested by Karl Nocka (Pfizer Pharmaceuticals). Mast cell purity was confirmed with toluidine blue staining. 105 cells were incubated for 6 hours in the presence of SCF 10 ng/mL and TSLP 10 ng/mL, IL-33 10 ng/mL (Peprotech), or both TSLP and IL-33 at 10 ng/mL.
The cell supernatants were collected and a PGD2 ELISA (Cayman, Ann Arbor, MI) was performed according to the manufacturer's instructions with the assay performed in duplicate. CysLT and thromboxane (TX)B2 ELISAs (Cayman) were also performed on the cord blood mast cell supernatants according to manufacturer's instructions with the assay performed in duplicate.
Statistical Analysis
The data are presented as the mean plus the standard error of the mean (SEM) unless otherwise stated. Differences in values in normally distributed data were analyzed with the paired or unpaired t-test as appropriate, with significance defined as P < .05, and all tests were 2-tailed. Non-normally distributed data was analyzed with Mann-Whitney with significance defined as P < .05. Linear dependence was measured with Pearson's correlation coefficient.
RESULTS
Study populations and baseline demographics
The AERD and CRS patients were close in age, with the ATA and healthy controls being significantly younger. The baseline FEV1 and Asthma Control Questionnaire (ACQ) scores were similar between the AERD and ATA patients. All patients with AERD and ATA had physician-diagnosed asthma, and 15% of CRS patients had a known diagnosis of asthma (Table I). As the CRS patients were recruited at the time of surgery, not all clinical data was available and some asthma diagnoses may not have been captured. Baseline uPGD-M levels did not correlate significantly with subject age (r = 0.27) or with baseline FEV1 (r = −0.28).
PGD2 production directly mediates effector responses in AERD
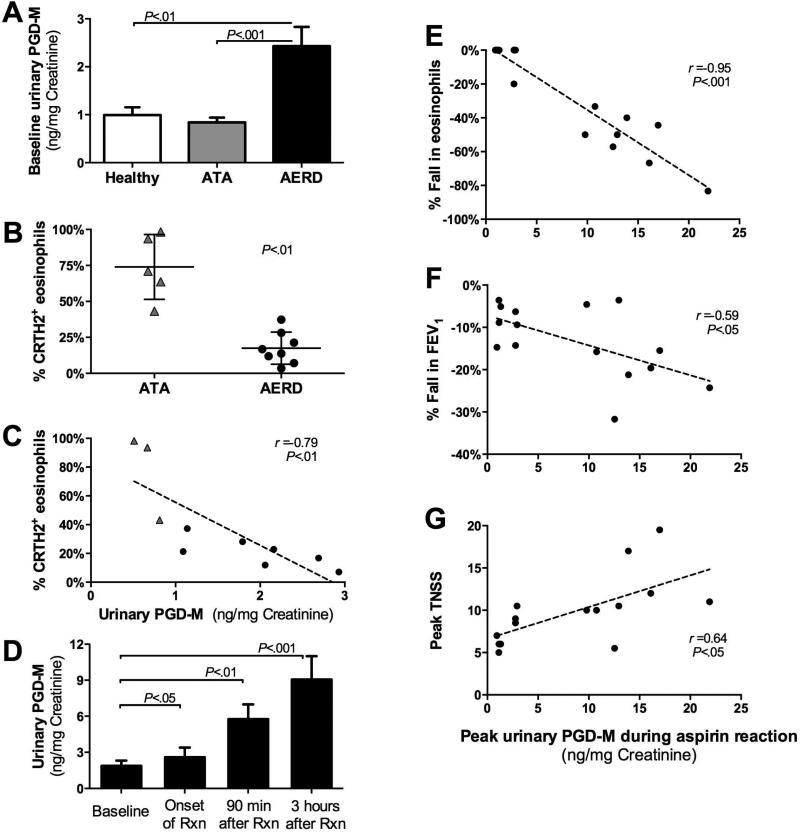
Aspirin challenges in AERD result in respiratory tract mast cell activation by IgE-independent mechanisms (30), presumably reflecting the depletion of homeostatic PGE2. To identify the contributions of PGD2 to the end-organ effects of this activation, we monitored uPGD-M levels and their relationship to clinical and physiologic responses to aspirin challenge. Urine obtained from subjects with AERD before aspirin challenge contained ~2-fold more uPGD-M than did that obtained from ATA and healthy controls (Fig 1, A). All AERD subjects reacted to 162 mg of aspirin or less. There was no significant correlation between provocative aspirin dose and peak uPGD-M level, and there was no relationship between asthma severity (determined by FEV1 and ACQ scores) and uPGD-M level at baseline (data not shown). Cytofluorographic analysis revealed that eosinophils in the blood of subjects with AERD expressed substantially lower levels of surface CRTH2 than did eosinophils in the blood of ATA controls (Fig 1, B). Eosinophil CRTH2 expression inversely correlated with uPGD-M at baseline in subjects with ATA and AERD (r = −0.79; Fig 1, C). Following ingestion of aspirin, all subjects with AERD developed symptoms of sinonasal congestion, sneezing and ocular pruritus, and many also developed chest tightness, wheezing and a decrease in FEV1. During aspirin-induced reactions, uPGD-M increased in a time-dependent manner in all AERD subjects (Fig 1, D). The peak levels of uPGD-M correlated strongly with reductions in blood eosinophil counts (r = −0.95; Fig 1, E), as well as with decreases in FEV1 (r = −0.59; Fig 1, F) and increases in nasal symptom scores (r = 0.64; Fig 1, G).
FIG 1.
PGD2 production mediates effector responses in AERD. A, Baseline uPGD-M levels from healthy controls (n=9), ATA controls (n=7), and subjects with AERD (n=29). B, Surface CRTH2 expression by blood eosinophils from ATA controls and subjects with AERD. C, Correlation of surface CRTH2 expression by blood eosinophils with urinary PGD-M. D, Time-dependent changes in uPGD-M levels during reactions to oral aspirin challenges. E,F,G, Correlations of peak uPGD-M levels with changes in peripheral blood eosinophils (E), FEV1 (F) and TNSS (G). A,D, Data are shown as means +SEM. B, Data are shown as individual points with group mean. C,E,F,G Data are shown as linear dependence measured with Pearson's correlation coefficient.
Mast cells are the primary site of hPGDS expression in nasal polyps
To determine the likely source of PGD2 in the respiratory tissue, we performed cytofluorographic, immunohistochemical, and transcript analyses of nasal polyps that were surgically excised from subjects with AERD and CRS controls.
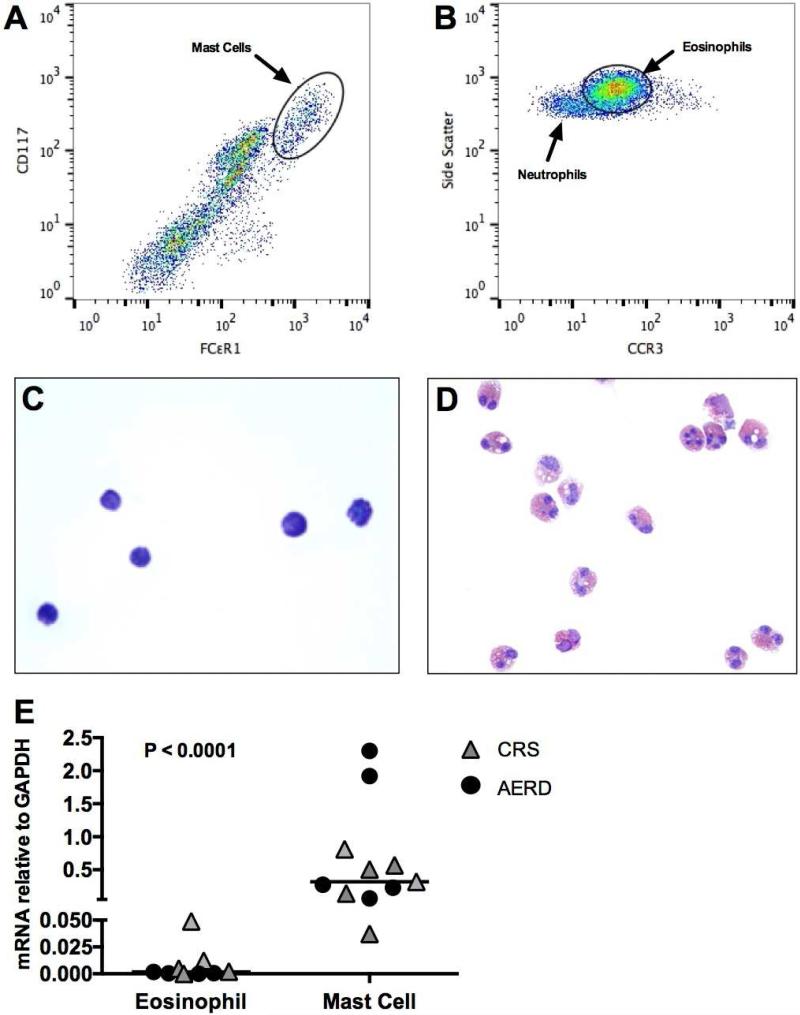
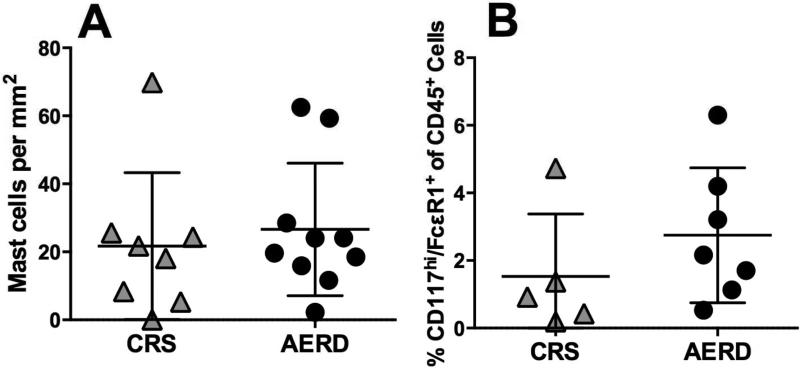
Using flow cytometry, mast cells, defined as CD45+/CD117hi/FcεRI+ within the granulocyte scatter gate, and eosinophils, defined as CD45+/CCR3+ (Fig 2, A and B), were sorted into purified cell populations. Mast cell purity was confirmed with toluidine blue staining (Fig 2, C) and eosinophil purity was confirmed with staining comparable to the Wright-Giemsa method (Fig 2, D). The levels of hPGDS mRNA expressed by the polyp mast cells were 10-3000-fold greater than those expressed by eosinophils obtained from the same samples (P < .0001; Fig 2, E), with similar trends observed in both AERD and CRS samples. COX-2 mRNA expression by mast cells and eosinophils varied between individuals, but the two cell types expressed similar amounts of COX-1 mRNA (see Fig E1) (37), and mast cells expressed significantly higher levels of COX-2 than COX-1. Immunostaining for tryptase revealed no difference in number of mast cells in patients with AERD vs. CRS (Fig 3, A). Flow cytometry of dispersed nasal polyps revealed that mast cells accounted for 0.53-6.3% of all CD45+ cells in the nasal polyps from subjects with AERD, and 0.19-4.7% of all CD45+ cells in the nasal tissue of CRS controls, and also revealed no difference between patients with AERD and CRS (Fig 3, B).
FIG 2.
Expression of PGD2 synthetic enzymes by nasal polyp effector cells. A, B, Cytofluorographic detections of mast cells (A) and eosinophils (B) in enzymatically dispersed nasal polyps. C, Toluidine blue staining of sorted nasal polyp mast cells. D, Staining of sorted nasal polyp eosinophils. E, hPGDS mRNA expression from sorted nasal polyp eosinophils and mast cells. E, Data are shown as individual points with group median as measured by Mann-Whitney test.
FIG 3.
Comparison of nasal tissue mast cell counts in AERD and aspirin-tolerant CRS controls. A, Number of mast cells per mm2 by immunohistochemistry in CRS and AERD. B, Percentage of CD45+ cells that are CD117hi/ FcεR1+ in patients with CRS and AERD. A,B, Data are shown as individual points with group mean.
TSLP expression in nasal polyps correlates with hPGDS and mast cell markers
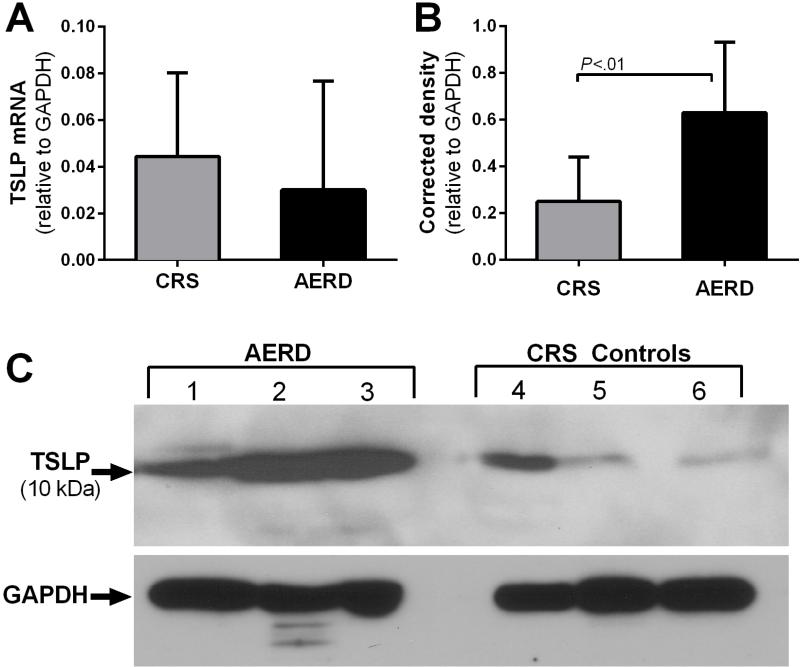
Whole nasal polyps were used to generate RNA and protein for quantitative PCR and western blotting. TSLP mRNA was detected in all nasal polyp samples. Steady-state levels of mRNA encoding TSLP were similar between AERD and CRS subjects (Fig 4, A). There was significantly more proteolytically processed (~10 kDa) TSLP protein in the samples from subjects with AERD than from controls (Fig 4, B and C). The identity of TSLP was confirmed with two separate TSLP antibodies and by blocking with a neutralizing immunogenic peptide (see Fig E2). The presence of the higher molecular weight 15 kDa TSLP band was variable in nasal polyp lysate samples (see Fig E3). Cell sorting of dispersed nasal polyp cells revealed TSLP mRNA derived from EpCAM+ epithelial cells, CD90+ fibroblasts, and PECAM+ endothelial cells (see Fig E4).
FIG 4.
Expression of TSLP in AERD and CRS nasal polyps. A, Whole nasal polyp TSLP mRNA in CRS (n = 7) and AERD (n = 18). B, Densitometric analysis of the cleaved, active form of TSLP from patients with CRS and AERD (n = 11, both groups). C, Total protein levels of TSLP in AERD and CRS measured by Western blotting of three representative samples per group. A,B, Data are shown as mean +SEM.
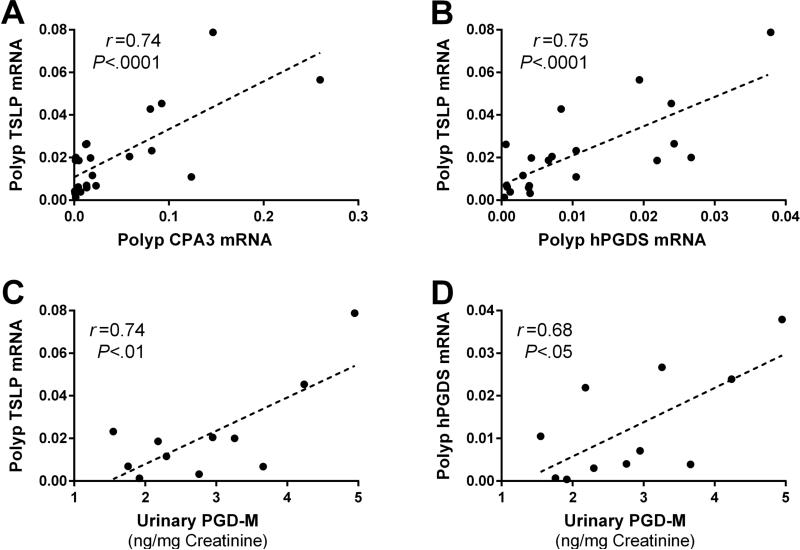
To determine the relationship between TSLP, mast cell phenotype, and PGD2 generation, we compared the levels of TSLP mRNA with mast cell-specific transcripts and PGD2-generating enzymes in the nasal polyps of patients with AERD. The levels of TSLP mRNA correlated strongly with both CPA3 (r = 0.74; P < .0001; Fig 5, A) and with hPGDS (r = 0.75; P < 0.0001; Fig 5, B) transcripts, but not with COX-2, 5-LO, or LTC4 synthase (not shown). Nasal polyp TSLP mRNA correlated significantly with baseline urinary levels of PGD-M (r = 0.74; P < .01; Fig 5, C), but not with those of LTE4 (r = 0.05, not shown). uPGD-M, but not LTE4, also tended to correlate with nasal polyp expression of hPGDS (r = 0.68; P < .05; Fig 5, D) and CPA3 (r = 0.51; P = .08; not shown). Urinary levels of a stable TXA2 metabolite (TX-M) also correlated with TSLP mRNA expression (r = 0.64, not shown). We did not have sufficient numbers of matched urine and polyp samples to determine whether these quantitative relationships were also evident in CRS subjects.
FIG 5.
Relationships between nasal polyp TSLP mRNA expression, mast cell markers, and systemic PGD2 production in AERD. A, Correlation between TSLP and CPA3 mRNA expression. B, Correlation between TSLP and hPGDS mRNA expression. C, Correlation between TSLP expression and baseline uPGD-M. D, Correlation between hPGDS mRNA expression and baseline uPGD-M. A – D, Data are shown as linear dependence measured with Pearson's correlation coefficient.
TSLP stimulates PGD2 production by mast cells in vitro
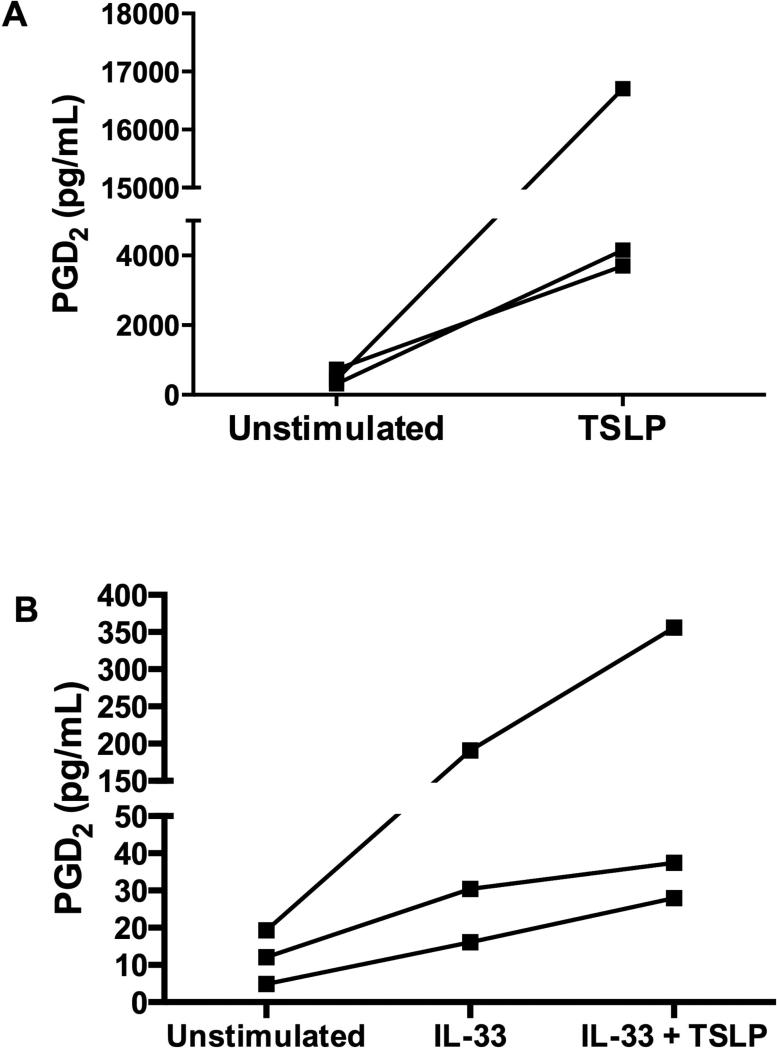
We stimulated human cord blood-derived mast cells with recombinant TSLP to determine whether this cytokine could directly elicit the production of PGD2. Unstimulated mast cells generated small quantities of PGD2. TSLP increased the production of PGD2 at 6 hours in each of three mast cell cultures, although there was significant variability in production of PGD2 between donors, therefore no statistical difference was reached (Fig. 6, A). At 24 hours, the levels of PGD2 production by stimulated mast cells had decreased to ~30% of the levels measured at 6 hours, but was still greater than the unstimulated baseline (not shown). TSLP stimulation also elicited the release of TXA2, as indicated by the detection of the stable metabolite TXB2, but did not induce the formation of cysLTs (see Fig E5).
FIG 6.
In vitro stimulation of mast cells with TSLP. A, TSLP (10 ng/mL) increases human cord blood-derived mast cell generation of PGD2 in vitro at 6 hours. B, TSLP (10 ng/mL) with IL-33 (10 ng/mL) synergistically increases human peripheral blood-derived mast cell generation of PGD2 in vitro at 6 hours compared to IL-33 alone. A, B, Data are displayed for individual experiments with cells from three different donors; differences are not statistically significant.
To test the ability of TSLP to induce PGD2 generation in an alternative human mast cell system, we stimulated human peripheral blood-derived mast cells with recombinant TSLP with and without IL-33. At 6 hours, TSLP alone did not increase peripheral blood-derived mast cell generation of PGD2 above baseline (not shown). However, TSLP and IL-33 together led to greater PGD2 generation over that of IL-33 alone with a 23%, 74%, and 87% increase in PGD2 production in each of the individual donors upon addition of TSLP. This indicates a synergistic effect of IL-33 and TSLP (Fig. 6, B).
DISCUSSION
PGD2 is considered an effector of IgE-dependent type 1 hypersensitivity reactions (38), but its role in innate immunity is largely unexplored. Innate type 2 immune responses, initiated by epithelially-derived cytokines, involve both myeloid (eosinophils, basophils) (23) and lymphoid (ILC2s) (26) effector cells that express CRTH2, suggesting that PGD2 plays a role in this system. AERD in particular, and nasal polyposis in general, frequently occurs in nonatopic individuals (30), yet consistently involves chronic eosinophilic sinonasal pathology, suggesting that innate type 2 immunity contributes to the disease. TSLP is an important component of the innate type 2 immune system that is expressed in nasal polyps and that acts on mast cells in combination with IL-1β and IL-33 to induce their production of type 2 cytokines (14, 39). Given our recent study demonstrating that the severity of clinical reactions to aspirin challenges in AERD relates to the level of PGD2 production (33), we conducted this study to determine the relationship between PGD2 production, mast cell activity, and the potential involvement of TSLP using well-phenotyped patients with and without AERD to reflect a spectrum of PGD2 production.
Aspirin challenges in AERD elicit bronchoconstriction and sinonasal congestion (40), presumably reflecting non-IgE-dependent mast cell activation that is “unbraked” by COX-1 inhibition (41, 42). As blood eosinophil counts decrease during aspirin-induced reactions (43), eosinophil counts simultaneously increase in nasal lavage fluids (44), suggesting that these effector cells are acutely recruited to the site of mast cell activation. This recruitment into the respiratory tissues occurs without changes in the levels of eosinophil-active chemokines. Since eosinophils are CRTH2+, we sought to determine whether PGD2 released during reactions to aspirin might be responsible for their recruitment, along with additional physiologic manifestations of the reaction. During the course of aspirin challenges in a cohort of subjects with AERD, we monitored uPGD-M along with a range of clinical, biochemical and cellular parameters. All subjects were treated with a cysLT1 receptor antagonist to eliminate the potential confounding influences of cysLTs on the lower airway responses to aspirin. The high basal levels of uPGD-M in samples from subjects with AERD (Fig 1, A) were paralleled by significantly lower levels of membrane expression of CRTH2 by their blood eosinophils (Fig 1, B) when compared to ATA controls, suggesting in vivo exposure to high levels of PGD2 leads to CRTH2 receptor downregulation (27, 45). Basal uPGD-M did not correlate with asthma severity. uPGD-M rose during reactions, and peak uPGD-M levels did not correlate with the provocative dose of aspirin, which was 162 mg or below in all AERD subjects. Aspirin selectively inhibits COX-1 at this dosing range. Notably, AERD involves impaired COX-2 expression by nasal polyp cells such as fibroblasts that generate PGE2. As a result, the ratio of PGD2 to PGE2 is substantially higher in polyps from subjects with AERD than CRS controls (41). It seems likely that depletion of residual COX-1-derived PGE2 permits mast cell activation in AERD (46), resulting in PGD2 generation by polyp mast cells, which strongly express COX-2 (see Fig E1). This suggests a mechanism that preserves the capacity of mast cells to generate PGD2 during aspirin challenges. Impaired homeostatic PGE2 generation in the respiratory tissue at baseline could also contribute to the high basal levels of uPGD-M observed in AERD subjects. The correlation between peak uPGD-M levels and the decline in blood eosinophils (Fig 1, E) strongly suggests that PGD2, acting at CRTH2, is a dominant chemotactic stimulus that accounts for effector cell recruitment in this context. Moreover, the relationship between peak uPGD-M during reactions and the decline in FEV1 (Fig 1, F) and the increase in nasal symptoms (Fig 1, G) could well reflect actions of PGD2 at T prostanoid receptors and D prostanoid 1 receptors, which respectively account for the bronchoconstrictive and vasodilatory effects of PGD2 (47, 48). Thus, PGD2 plays a major end-organ effector role in this innate type 2 immune response involving mast cell activation.
We next sought to identify the cellular source(s) of PGD2 in the respiratory tissue and the mechanism(s) responsible for its production. Both mast cells (49) and eosinophils (50) express hPGDS, and both are found in nasal polyps. Quantitative immunohistochemical studies suggest that the number of hPGDS+ cells in nasal polyps correlates with disease severity (51). hPGDS can convert the precursor PGH2 from either COX-1, an exquisitely aspirin sensitive enzyme, or COX-2, which is less aspirin sensitive. Induction of COX-2 expression by mast cells in vitro results in robust, sustained generation of PGD2 (37). We directly monitored the expressions of hPGDS and the COX enzymes in cytofluorographically purified nasal polyp mast cells, comparing them with eosinophils from the same samples (Fig 2, C and D). To our knowledge, this is the first such analysis to be reported. The markedly stronger expression of hPGDS by polyp mast cells compared with eosinophils (Fig 2, E), along with their robust expression of COX-2, (see Fig E1) supports the thesis that mast cells are the primary source of PGD2 in the respiratory tissues. As noted above, COX-2 expression may preserve the ability of mast cells to generate PGD2 when their activation is induced by depletion of COX-1-derived PGE2 in AERD. In turn, the aspirin-resistant generation of PGD2 by mast cells facilitates the baseline pathology, as well as the physiologic responses to aspirin challenge.
Among many cellular targets, TSLP facilitates mast cell development in vivo (52) and mast cell activation in vitro (14). Since TSLP is strongly expressed by nasal polyps (10, 11), we focused on its potential role as a driver of PGD2 generation, both in vivo and in vitro. We found TSLP mRNA and protein in all whole nasal tissue samples, and verified that its transcript was expressed by epithelial cells, fibroblasts, and endothelial cells regardless of disease phenotype (see Fig E4). TSLP undergoes processing by leukocyte-derived proteases, yielding bioactive fragments (12) that are consistent with the 10 kDa size of the molecular species identified by western blotting in our studies (Fig 4C, E2, E3). Remarkably, the levels of TSLP mRNA expression by nasal polyps from AERD patients correlated with polyp CPA3 and hPGDS transcript expression (Fig 5, A and B), as well as with baseline uPGD-M (Fig 5, C) and TX-M (not shown), but not with uLTE4. The correlation between TSLP mRNA, mast cell markers, and PGD2 in vivo suggests a spectrum of AERD pathophysiology that could reflect some combination of inductive effects of TSLP on mast cell markers or the promotion of mast cell proliferation and/or survival by TSLP as suggested by mouse studies (52). Additionally, tissue mast cells promote TSLP expression in a model of allergic rhinitis (53), suggesting that the relationship between mast cells and TSLP in the tissue may be bilateral. The low COX-2 expression at the whole polyp level (due primarily to impaired expression by PGE2-generating structural cells) likely accounts for the lack of relationship between COX-2 and TSLP. Moreover, TSLP induced PGD2 production by human cord blood-derived cultured mast cells (Fig 6, A) and by human peripheral blood-derived cultured mast cells when combined with IL-33 (Fig 6, B) ex vivo, but did not induce cysLT production (see Fig E5). Thus, TSLP may directly and selectively induce PGD2 generation by mast cells in nasal polyps, whereas additional factors may be responsible for inducing cysLT production. Though we have no evidence that TSLP is responsible for the dramatic and acute release of PGD2 during aspirin-induced reactions, we suspect that TSLP is one of the main factors responsible for priming the tissue mast cells for chronic over-production of PGD2.
Our findings strongly link the actions of TSLP to the activation of mast cells and the generation of PGD2 in vivo. PGD2 is a logical candidate effector in AERD due to its persistent high-level production, its capacity to recruit eosinophils and basophils, and its ability to directly induce cytokine production by ILC2s (27) as well as conventional type 2 cells (54). We propose that mast cell-derived PGD2, driven at least in part by TSLP, amplifies and perpetuates the innate, type 2-like axis of inflammation caused by phenotypically altered structural cells and their cytokines. Both anti-TSLP and CRTH2 antagonists are in advanced stages of clinical development for the treatment of asthma. Our findings suggest that both of these modalities may be efficacious in AERD.
Supplementary Material
Key Messages.
TSLP contributes to the generation of PGD2 by mast cells.
PGD2 production correlates with several pathogenic features of AERD.
Anti-TSLP and CRTH2 antagonists are potential treatment modalities to be considered in AERD.
Capsule Summary.
Thymic stromal lymphopoietin (TSLP) contributes to mast cell generation of prostaglandin D2 (PGD2), which in turn facilitates tissue eosinophilia in nasal polyposis and aspirin-exacerbated respiratory disease.
Acknowledgments
This work was supported by NIH grants T32 AI007306-29, AI007306-27, U19 AI095219-01, 5U19AI070412-06, K23HL111113-02 and by generous contributions from the Vinik Family and the Kaye Family.
Abbreviations
- 5-LO
5-lipoxygenase
- ACQ
Asthma Control Questionnaire
- AERD
Aspirin-exacerbated respiratory disease
- ATA
Aspirin tolerant asthmatic
- COX
Cyclooxygenase
- CRS
Chronic rhinosinusitis
- CRTH2
Chemoattractant receptor homologue expressed by Th2 cells
- CysLTs
Cysteinyl leukotrienes
- FEV1
Forced expiratory volume in 1 second
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- hPGDS
Hematopoietic PGD2 synthase
- IL
Interleukin
- ILC2s
Type 2 innate lymphoid cells
- PG
Prostaglandin
- SCF
Stem cell factor
- SEM
Standard error of the mean
- TNSS
Total nasal symptom score
- TSLP
Thymic stromal lymphopoietin
- TSLPR
TSLP receptor
- TX
Thromboxane
- uPGD-M
Urinary PGD metabolite
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference list
- 1.Gurish MF, Humbles A, Tao H, Finkelstein S, Boyce JA, Gerard C, et al. CCR3 is required for tissue eosinophilia and larval cytotoxicity after infection with Trichinella spiralis. Journal of immunology. 2002;168(11):5730–6. doi: 10.4049/jimmunol.168.11.5730. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, et al. Eosinophilic inflammation in asthma. The New England journal of medicine. 1990;323(15):1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Novo CA, Claeys C, Van Cauwenberge P, Bachert C. Expression of eicosanoid receptors subtypes and eosinophilic inflammation: implication on chronic rhinosinusitis. Respiratory research. 2006;7:75. doi: 10.1186/1465-9921-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Straumann A. Eosinophilic esophagitis: emerging therapies and future perspectives. Gastroenterology clinics of North America. 2014;43(2):385–94. doi: 10.1016/j.gtc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Gauvreau GM, O'Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. The New England journal of medicine. 2014;370(22):2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 6.Guo Z, Wu J, Zhao J, Liu F, Chen Y, Bi L, et al. IL-33 promotes airway remodeling and is a marker of asthma disease severity. The Journal of asthma : official journal of the Association for the Care of Asthma. 2014;51(8):863–9. doi: 10.3109/02770903.2014.921196. [DOI] [PubMed] [Google Scholar]
- 7.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. The Journal of allergy and clinical immunology. 2011;128(1):116–24. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. Journal of immunology. 2005;174(12):8183–90. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 9.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature immunology. 2002;3(7):673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Li TL, Zhao F, Xie C, Liu AM, Chen X, et al. Role of thymic stromal lymphopoietin in the pathogenesis of nasal polyposis. The American journal of the medical sciences. 2011;341(1):40–7. doi: 10.1097/MAJ.0b013e3181f20489. [DOI] [PubMed] [Google Scholar]
- 11.Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy, asthma & immunology research. 2011;3(3):186–93. doi: 10.4168/aair.2011.3.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. The Journal of allergy and clinical immunology. 2013;132(3):593–600. e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunological reviews. 2011;242(1):10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. The Journal of experimental medicine. 2007;204(2):253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Science translational medicine. 2013;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vissinga C, Martin T, H.K. J, Comeau MR. TSLP driven expansion and activation of type 2 innate lymphoid cells is augmented by IL-25 and IL-33. Cytokine. 2013;63(3) [Google Scholar]
- 17.Allakhverdi Z, Comeau MR, Smith DE, Toy D, Endam LM, Desrosiers M, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. The Journal of allergy and clinical immunology. 2009;123(2):472–8. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK. IL-3 and TNFalpha increase Thymic Stromal Lymphopoietin Receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clinical and molecular allergy : CMA. 2012;10(1):8. doi: 10.1186/1476-7961-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nature medicine. 2013;19(8):1005–13. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy CC, Robinson C, Tattersfield AE, Holgate ST. The bronchoconstrictor effect of inhaled prostaglandin D2 in normal and asthmatic men. The New England journal of medicine. 1984;311(4):209–13. doi: 10.1056/NEJM198407263110401. [DOI] [PubMed] [Google Scholar]
- 21.Soter NA, Lewis RA, Corey EJ, Austen KF. Local effects of synthetic leukotrienes (LTC4, LTD4, LTE4, and LTB4) in human skin. The Journal of investigative dermatology. 1983;80(2):115–9. doi: 10.1111/1523-1747.ep12531738. [DOI] [PubMed] [Google Scholar]
- 22.Nagata K, Hirai H, Tanaka K, Ogawa K, Aso T, Sugamura K, et al. CRTH2, an orphan receptor of T-helper-2-cells, is expressed on basophils and eosinophils and responds to mast cell-derived factor(s). FEBS letters. 1999;459(2):195–9. doi: 10.1016/s0014-5793(99)01251-x. [DOI] [PubMed] [Google Scholar]
- 23.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. The Journal of experimental medicine. 2001;193(2):255–61. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spik I, Brenuchon C, Angeli V, Staumont D, Fleury S, Capron M, et al. Activation of the prostaglandin D2 receptor DP2/CRTH2 increases allergic inflammation in mouse. Journal of immunology. 2005;174(6):3703–8. doi: 10.4049/jimmunol.174.6.3703. [DOI] [PubMed] [Google Scholar]
- 25.He R, Oyoshi MK, Wang JY, Hodge MR, Jin H, Geha RS. The prostaglandin D(2) receptor CRTH2 is important for allergic skin inflammation after epicutaneous antigen challenge. The Journal of allergy and clinical immunology. 2010;126(4):784–90. doi: 10.1016/j.jaci.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12(11):1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 27.Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. The Journal of allergy and clinical immunology. 2014;133(4):1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. American journal of respiratory and critical care medicine. 2013;188(4):432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw JL, Ashoori F, Fakhri S, Citardi MJ, Luong A. Increased percentage of mast cells within sinonasal mucosa of chronic rhinosinusitis with nasal polyp patients independent of atopy. International forum of allergy & rhinology. 2012;2(3):233–40. doi: 10.1002/alr.21021. [DOI] [PubMed] [Google Scholar]
- 30.Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. American journal of rhinology & allergy. 2014;28(4):287–9. doi: 10.2500/ajra.2014.28.4054. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Haselkorn T, Borish L, Rasouliyan L, Chipps BE, Wenzel SE. Risk factors associated with persistent airflow limitation in severe or difficult-to-treat asthma: insights from the TENOR study. Chest. 2007;132(6):1882–9. doi: 10.1378/chest.07-0713. [DOI] [PubMed] [Google Scholar]
- 32.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. The Journal of allergy and clinical immunology. 2003;111(4):743–9. doi: 10.1067/mai.2003.1387. [DOI] [PubMed] [Google Scholar]
- 33.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. The Journal of allergy and clinical immunology. 2015;135(1):245–52. doi: 10.1016/j.jaci.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrow JD, Guzzo C, Lazarus G, Oates JA, Roberts LJ., 2nd Improved diagnosis of mastocytosis by measurement of the major urinary metabolite of prostaglandin D2. The Journal of investigative dermatology. 1995;104(6):937–40. doi: 10.1111/1523-1747.ep12606209. [DOI] [PubMed] [Google Scholar]
- 35.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. The Journal of experimental medicine. 1999;190(2):267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. Journal of immunology. 2006;177(5):2755–9. doi: 10.4049/jimmunol.177.5.2755. [DOI] [PubMed] [Google Scholar]
- 37.Murakami M, Matsumoto R, Austen KF, Arm JP. Prostaglandin endoperoxide synthase-1 and -2 couple to different transmembrane stimuli to generate prostaglandin D2 in mouse bone marrow-derived mast cells. The Journal of biological chemistry. 1994;269(35):22269–75. [PubMed] [Google Scholar]
- 38.Ono E, Taniguchi M, Mita H, Fukutomi Y, Higashi N, Miyazaki E, et al. Increased production of cysteinyl leukotrienes and prostaglandin D2 during human anaphylaxis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39(1):72–80. doi: 10.1111/j.1365-2222.2008.03104.x. [DOI] [PubMed] [Google Scholar]
- 39.Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. The Journal of allergy and clinical immunology. 2012;130(1):225–32. e4. doi: 10.1016/j.jaci.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Rubin P, Cohn J, et al. Direct evidence for a role of the mast cell in the nasal response to aspirin in aspirin-sensitive asthma. The Journal of allergy and clinical immunology. 1994;94(6 Pt 1):1046–56. doi: 10.1016/0091-6749(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 41.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergology international : official journal of the Japanese Society of Allergology. 2008;57(4):429–36. doi: 10.2332/allergolint.o-08-545. [DOI] [PubMed] [Google Scholar]
- 42.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(42):16987–92. doi: 10.1073/pnas.1313185110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sladek K, Szczeklik A. Cysteinyl leukotrienes overproduction and mast cell activation in aspirin-provoked bronchospasm in asthma. The European respiratory journal. 1993;6(3):391–9. [PubMed] [Google Scholar]
- 44.Kupczyk M, Kurmanowska Z, Kuprys-Lipinska I, Bochenska-Marciniak M, Kuna P. Mediators of inflammation in nasal lavage from aspirin intolerant patients after aspirin challenge. Respiratory medicine. 2010;104(10):1404–9. doi: 10.1016/j.rmed.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 45.Hamada K, Yamada Y, Kamada Y, Ueki S, Yamaguchi K, Oyamada H, et al. Prostaglandin D2 and interleukin-5 reduce CRTH2 surface expression on human eosinophils. Allergology International. 2004;53:179–84. [Google Scholar]
- 46.Machado-Carvalho L, Roca-Ferrer J, Picado C. Prostaglandin E2 receptors in asthma and in chronic rhinosinusitis/nasal polyps with and without aspirin hypersensitivity. Respiratory research. 2014;15:100. doi: 10.1186/s12931-014-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnston SL, Freezer NJ, Ritter W, O'Toole S, Howarth PH. Prostaglandin D2-induced bronchoconstriction is mediated only in part by the thromboxane prostanoid receptor. The European respiratory journal. 1995;8(3):411–5. doi: 10.1183/09031936.95.08030411. [DOI] [PubMed] [Google Scholar]
- 48.Lai E, De Lepeleire I, Crumley TM, Liu F, Wenning LA, Michiels N, et al. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clinical pharmacology and therapeutics. 2007;81(6):849–57. doi: 10.1038/sj.clpt.6100180. [DOI] [PubMed] [Google Scholar]
- 49.Kanaoka Y, Ago H, Inagaki E, Nanayama T, Miyano M, Kikuno R, et al. Cloning and crystal structure of hematopoietic prostaglandin D synthase. Cell. 1997;90(6):1085–95. doi: 10.1016/s0092-8674(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 50.Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. Journal of immunology. 2011;187(12):6518–26. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okano M, Fujiwara T, Yamamoto M, Sugata Y, Matsumoto R, Fukushima K, et al. Role of prostaglandin D2 and E2 terminal synthases in chronic rhinosinusitis. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2006;36(8):1028–38. doi: 10.1111/j.1365-2222.2006.02528.x. [DOI] [PubMed] [Google Scholar]
- 52.Han NR, Oh HA, Nam SY, Moon PD, Kim DW, Kim HM, et al. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. The Journal of investigative dermatology. 2014;134(10):2521–30. doi: 10.1038/jid.2014.198. [DOI] [PubMed] [Google Scholar]
- 53.Miyata M, Hatsushika K, Ando T, Shimokawa N, Ohnuma Y, Katoh R, et al. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. European journal of immunology. 2008;38(6):1487–92. doi: 10.1002/eji.200737809. [DOI] [PubMed] [Google Scholar]
- 54.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. Journal of immunology. 2005;175(10):6531–6. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.