Abstract
As obligate blood-sucking ectoparasites, to avoid tissue damage, ticks must neutralize the reactive oxygen species (ROS) generated from uptake and digestion of a bloodmeal. Consequently, ticks utilize a battery of antioxidant molecules, including catalase (CAT), an enzyme that converts H2O2 into water and oxygen. Here, we investigated the tick antioxidant machinery by exogenous injection of sublethal doses of H2O2 or paraquat. The relative transcript levels of selected Amblyomma maculatum antioxidant targets in tissues were determined by quantitative reverse transcriptase PCR following treatment. The results showed 2-12 fold increase of target antioxidant gene transcripts signifying the ability of A. maculatum to regulate its antioxidant machinery when exposed to increased ROS levels. Next, RNA interference was used to determine the functional role of CAT in hematophagy, redox homeostasis, and reproductive fitness. CAT gene silencing was confirmed by transcript depletion within tick tissues; however, dsCAT knockdown alone did not interfere with tick hematophagy or phenotype, as confirmed by the resulting differential expression of antioxidant genes, thereby indicating an alternate mechanism for ROS control. Interestingly, dsCAT and the CAT inhibitor, 3-aminotriazole, together reduced tick reproductive fitness via a marked reduction in egg mass and larval eclosion rates, highlighting a role for CAT in tick redox-homeostasis, making it a potential target for tick control.
Introduction
The Gulf Coast tick (Amblyomma maculatum) is an arthropod that has emerged as an increasingly significant public health risk (Paddock and Goddard 2015). Its range is in the American states surrounding the Gulf and in the Eastern Atlantic region, where it is a confirmed vector of the rickettsial pathogen Rickettsia parkeri, which causes a disease similar to Rocky Mountain spotted fever (Paddock et al., 2004). The ability of A. maculatum to offset starvation stress and blood feeding-related stress makes it likely that it possesses a proactive antioxidant system. Complete studies of the tick interactive antioxidant network are presently still lacking, but available literature implicates the extensive arsenal of antioxidant and redox-associated selenoproteins in the ability of the tick tolerate extreme alterations in redox homeostasis associated with long periods of starvation off the host and the acquisition and digestion of blood and bloodmeal-related products on the host (Adamson et al., 2013; Adamson et al., 2014; Budachetri and Karim, 2015).
Higher eukaryotes possess an intricate enzymatic antioxidant system consisting of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), peroxiredoxins, and selenoproteins. These proteins play critical roles in detoxifying elevated levels of reactive oxygen species (ROS), thereby maintaining redox equilibrium. ROS play a significant role in the overall sustainability of an organism, as low levels are integral to the signaling pathways involved in cell proliferation and survival (Holmstöm and Finkel, 2014). But when ROS levels become excessive and imbalanced relative to an organism’s antioxidant response, it can lead to chronic damage to major cellular biomolecules, while extended exposure to a pro-oxidant environment interrupts cellular homeostasis. This critical shift in the cellular redox equilibrium towards a more oxidized environment is defined as “oxidative stress” (Lismont et al., 2015). If an organism is not capable of adapting to environmentally induced oxidative stress through the proper detoxification of ROS, high levels of oxidative stress can result and initiate cell death, with eventual impairment to the fitness of the organism (Jones and Go, 2010; Lismont et al,, 2015).
CAT is a ubiquitous enzyme found in Archaea, prokaryotes and eukaryotes (Kawasaki and Aguirre, 2001; Manduzio et al., 2004; Kashiwagi et al., 1997). Within eukaryotic cells, CAT is localized in the peroxisome where it catalyzes the conversion of reactive hydrogen peroxide (H2O2) to water and molecular oxygen; its expression is tissue specific and highly variable (Zamocky et al., 2008). Production of H2O2 is critical for normal cellular signaling, but its presence can become disruptive in excessive amounts. Excessive H2O2 can damage cells and their surrounding tissues; therefore, proper clearance of it is imperative. CAT has been identified as a contributing factor to the enhancement of longevity in flies and mice (Orr and Sohal, 1994; Schriner et al., 2005; Melov et al., 2000; Sampayo et al., 2003).
Tick blood feeding, which generates toxic levels of ROS, can damage lipids, proteins, and DNA, thus promoting mutation, cellular dysfunction, and cell death. To successfully feed and survive, ticks must prevent the detrimental effects of ROS and utilize any beneficial effects ROS may have; hence, there must be precise regulatory strategies for maintaining proper ROS levels within the tick and possibly at the tick-host interface.
Several studies have shown the importance of native microbiota in regulating several aspects of host physiology including host immunity, obesity, and glucose homeostasis (Kimura et al., 2013). Blood-related oxidative stress has a negative impact on the overall bacterial load in tick tissues (Budachetri and Karim 2015). This study provides strong evidence that ticks have a well-orchestrated, robust antioxidant machinery, which can alleviate the ROS generated in response to endogenous or exogenous stressors. Here, we report that antioxidant levels in ticks increase following exposure to H2O2 and paraquat (PQ). Our data contribute further evidence in support of our hypothesis that induction of elevated ROS in tick tissues triggers differential regulation of antioxidant machinery in these arthropods to alleviate stress.
Results
Bioinformatics Analysis
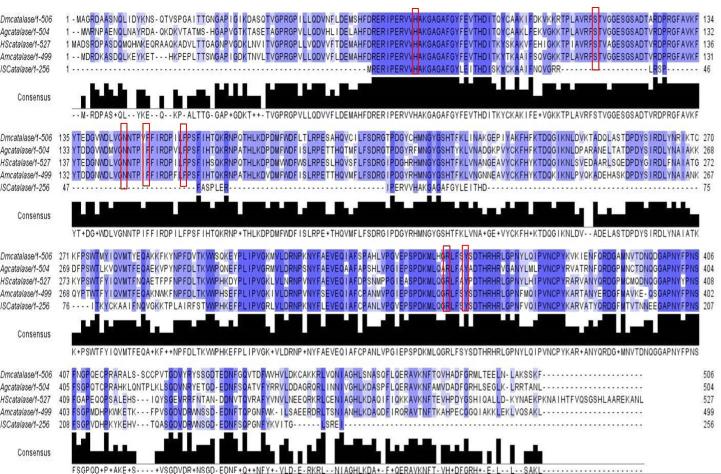
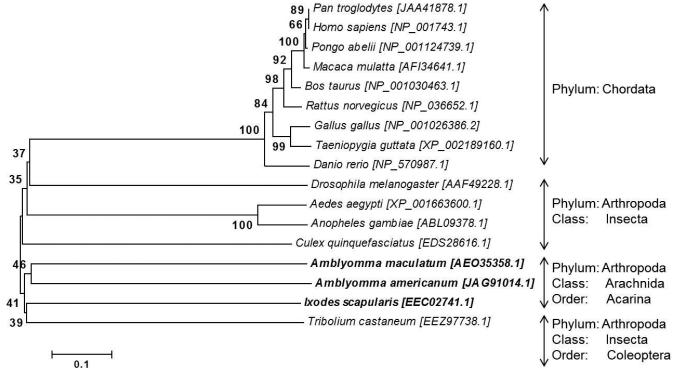
The full-length A. maculatum CAT sequence (AmCAT; GenBank Accession: JO843741.1) was identified in a previous sialotranscriptome project on A. maculatum (Karim et al., 2011). The AmCAT transcript (1500 bp) was translated into its amino acid sequence (499 aa) and compared with the arthropod CAT sequences available in GenBank using ClustalX2 multiple sequence alignments (Larkin et al., 2007). Fig. 1 shows the AmCAT amino acid sequence alignment against Ixodes scapularis, Caenorhabditis elegans, Drosophila melanogaster, Anopheles gambiae, and Homo sapiens. AmCAT shares 68% amino acid sequence identity with CAT from I. scapularis and 68–72% amino acid sequence identity with other arthropods, signifying that the CAT enzyme sequence is conserved among species. Conserved heme-binding residues critical to AmCAT stability and maintenance of a high oxidation state are indicated by red boxes (Fig. 1). Phylogenetic analysis of the AmCAT protein depicts the general expected pattern that recapitulates the species phylogeny for eukaryotic organisms, indicating that it behaves like an orthologous protein. Only a single Cat gene was found on any examined species. A single ancestral AmCAT gene. Fig. 2 shows the pattern of speciation of eukaryotic organisms with AmCAT placed between vertebrates and invertebrates. A single ancestral AmCAT gene, represented by Arthropods and Chordates, has likely diverged into two subgroups, Chordates and arthropods (figure 2). The AmCAT proteins group within the arthropods as expected and were related to Ixodes scapularis, Tribolium castaneum, and Aedes aegypti, Anopheles gambiae, and Culex quinquefasciatus (Figure 2).
Figure 1. Multiple Sequence Alignment of Amblyomma maculatum CAT.
The Clustal-X2 sequence alignment, imported into Jalview2.8, is highlighted blue to indicate the species conservation based on the percentage identity of the following CAT amino acid sequences obtained from GenBank: Amblyomma maculatum CAT (JO843741.1) was aligned with Ixodes scapularis (XM_002400585.1), Caenorhabditis elegans (X82175.1) Drosophila melanogaster (NM_080483.3), Anopheles gambiae (DQ986315.1) and Homo sapiens (NM_001752.3). All seven conserved residues comprising the CAT heme binding domain are indicated by red boxes.
Figure 2. Phylogenetic analysis of CAT from various eukaryotes using the neighbor-joining method.
The eukaryotic organisms used for the phylogenetic analysis are as follows: chimpanzee (Pan troglodytes), human (Homo sapiens), Sumatran orangutan (Pongo abelli), Rhesus macaque (Macaca mullatta), cattle (Bos taurus), brown rat (Rattus norvegicus), chicken (Gallus gallus), zebra finch (Taeniopygia guttata), zebrafish (Danio rerio), fruit fly (Drosophila melanogaster), yellow fever mosquito (Aedes aegypti), malaria mosquito (Anopheles gambiae), southern house mosquito (Culex quinquefasciatus), Gulf Coast tick (Amblyomma maculatum), Lone Star tick (A. americanum), black-legged tick (Ixodes scapularis), and the red flour beetle (Tribolium castaneum). Bootstrap values (1000 replicates) are shown next to the branches. The scale bar represents the rate of amino acid substitutions per position.
Antioxidant response to exogenous oxidative stressors
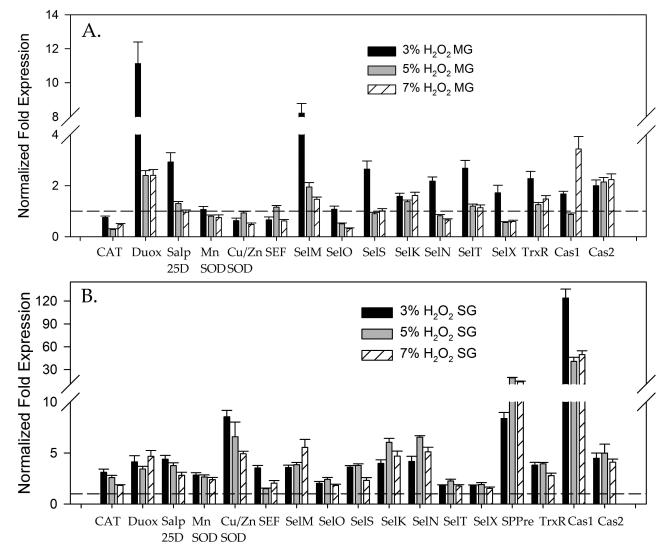
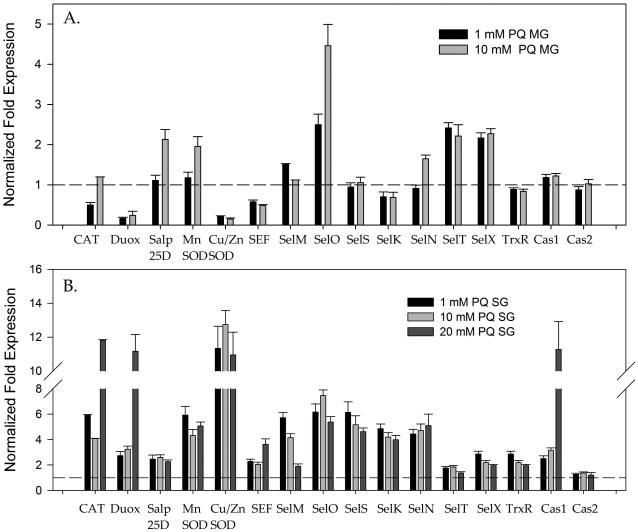
To investigate the antioxidant response and potential alterations in the natural microbiota associated with A. maculatum, incremental doses of the exogenous oxidant, H2O2, were administered to unfed female A. maculatum ticks as an inducer of oxidative stress. Microinjections of either 3%, 5%, or 7% H2O2 were used for assessment, as 9% H2O2 was previously determined to be lethal to the ticks following a 24 h incubation period at room temperature (RT) (data not shown). The transcriptional expression of tick antioxidants was checked following exposure to H2O2 injections in the tick midguts (Fig. 3A) and salivary glands (Fig. 3B). Only Duox (11 fold) or SelM (9 fold) was upregulated in the midguts upon sublethal doses of H2O2 but in the salivary glands almost all the tick antioxidants including the selenoproteins were upregulated ranging from 2-30 fold (Fig. 3B). The sublethal dose of the H2O2 injected did not impact the total oxidative stress level inside the tick tissues (data not shown); this may be a result of robust increases in antioxidants following administration of the injections (Fig. 3B), whereas interestingly, the total bacterial load increased in the tick salivary glands following exposure to H2O2 compared with the phosphate buffered saline (PBS) control (Fig. 5D). As the tick antioxidant response was related to only Duox and SelM in the tick midguts (Fig. 3A), the bacterial load in the tick midguts was depleted after administration of H2O2 injections (Fig. 5C).
Figure 3. Effect of H2O2 injection on modulation of the antioxidant gene network in Amblyomma maculatum tissues.
Tick β-Actin was used as the reference gene for normalization. Transcriptional expression of genes encoding known selenoproteins (CAT, dual oxidase, superoxide dismutase and apoptosis marker genes caspase1, caspase2) in unfed A. maculatum midgut tissues (A) and salivary glands (B) following injection of either 3%, 5% or 7% H2O2 to assess the effects of induced oxidative stress. The gene expression values of all the genes except GSHR are statistically significant (P<0.05) when compared to the control. SG, salivary glands; MG, midgut.
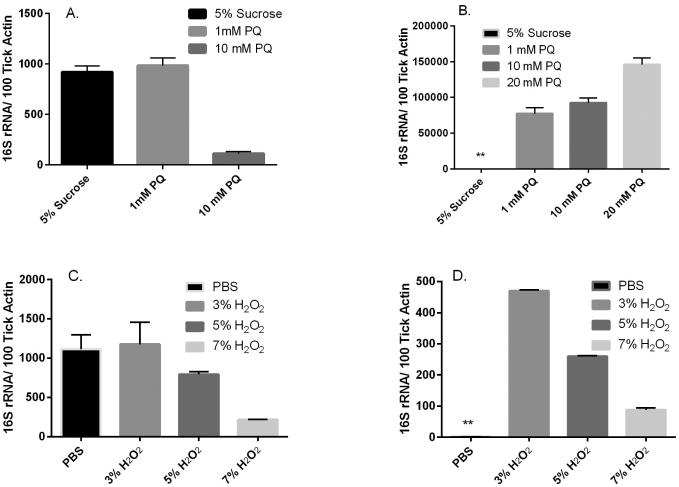
Figure 5. Microbial load estimation within tick tissues following sublethal doses of paraquat (PQ) and H2O2 injection in Amblyomma maculatum.
The effects of sublethal doses of PQ are tissue-specific in the midgut tissues (A) and salivary glands tissues (B). The H2O2 injections affected bacterial colonization in a tissue specific manner in the midgut tissues (C) and salivary glands (D). The bacterial load was estimated based on quantification of overall 16S rRNA gene per 100 ticks and β-Actin by the qRT-PCR method. (**; very low values).
As a secondary means of understanding the interplay between ROS and the robust antioxidant system of A. maculatum, unfed ticks were injected with sublethal doses of 1mM, 10mM, or 20mM PQ and then dissected following a 24 h incubation period. In a previous experiment, 25 mM PQ was found to be lethal to ticks within 6 h post-injection (data not shown).
It was not surprising to see a 2–12 fold up-regulation of antioxidant genes in tick salivary glands exposed to the environmental stressor, PQ, while a 2–5 fold up-regulation of transcript levels was apparent in the gut tissues of the ticks (Fig. 4A,B). Somewhat surprisingly, upregulation of both mitochondrial and cytosolic superoxide dismutases suggests that superoxide was generated within the tick salivary glands, and this process required an increased demand for Mn-SOD and Cu/Zn-SOD to catalyze the superoxide ions into the comparatively less reactive ROS, H2O2. This clearance of H2O2 by the tick antioxidants is represented by the increased expression of CAT (3 to 11-fold) and glutathione peroxidase/Salp25D, both of which are capable of converting hydrogen peroxide into water and molecular oxygen. The antioxidant gene response in the tick midgut tissues upon PQ injection showed that only Salp25D and SelO were upregulated (Fig. 4A). Colonization by bacteria increased in the presence of PQ as observed by quantification of the overall bacterial load in the tick salivary glands; this may have been caused by the aggressive antioxidants (Fig. 5 B). However, in the midgut tissues, as a lesser response to the external stressors, the bacterial load depleted upon receipt of increased PQ concentrations (Fig. 5A).
Figure 4. Effect of paraquat (PQ) injection to modulate the antioxidant gene network in Amblyomma maculatum tissues.
Transcriptional expression of genes encoding known selenoproteins (CAT, dual oxidase, superoxide dismutase and apoptosis marker genes caspase1, caspase2) in unfed A. maculatum midgut tissues (A) and salivary glands (B) following injection of 1 mM or 10 mM paraquat. The gene expression study used tick β-actin as the housekeeping gene. SG, salivary glands; MG, midgut.
Impact of CAT knockdown
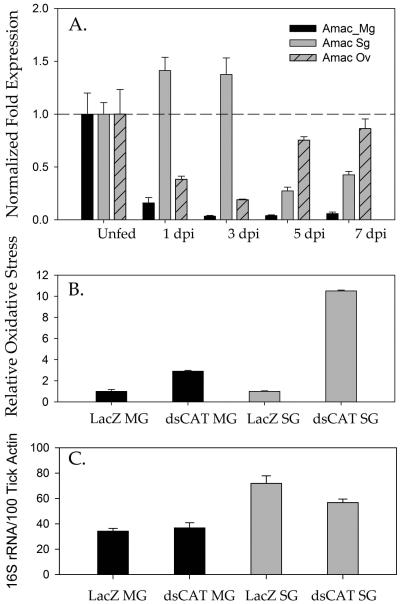
The temporal transcriptional expression of the CAT gene in tick tissues (midguts, salivary glands, and ovaries) was determined across the blood meal period (Fig. 6A). In contrast, the temporal expression of the CAT gene in the tick midgut showed highest expression during the unfed state followed by significant downregulation upon slow-phase blood feeding, a finding that was maintained as the tick transitioned into fast-phase blood feeding through day 7 (Fig. 6A). Intriguingly, transcriptional expression of the CAT gene in tick ovarian tissues increased over the time of the bloodmeal suggesting a potential role for it in egg development and reproductive fitness (Fig. 6A).
Figure 6. Estimation of time-dependent CAT expression in naïve tick tissues upon infestation and the effect on total oxidative stress and bacterial colonization upon CAT silencing.
Temporal transcriptional gene expression of CAT within Amblyomma maculatum midgut (Mg), salivary glands (Sg) and ovarian (Ov) tissues across the blood meal days post infestation (dpi). Relative expression levels across the blood meal were compared with the CAT expression within unfed tissues. B. The total oxidative stress in tick upon silencing of CAT in A. maculatum as determined by protein carbonyl content. C. Total bacterial load estimation upon silencing of CAT in A. maculatum. The bacterial load was estimated by quantification of the 16S rRNA gene per 100 ticks and the β-Actin gene.
To further understand the role of CAT in tick blood feeding and reproductive fitness, CAT was knocked down using RNA interference, and its effect upon the transcriptional gene expression of other tick antioxidant genes, bacterial load, and tick ovipositioning was investigated (Figs. 6, 7). Despite the CAT knockdown, there were no significant changes in tick weight or ovipositioning as determined by egg mass (Fig. 8C). The level of oxidative stress, as quantified by a protein carbonyl content assay, showed a 10-fold increase in the carbonyl content level in the salivary gland tissues indicative of increased oxidative stress as a result of CAT gene knockdown; this suggests a vital role for CAT in preventing protein damage in ticks (Fig. 6B).
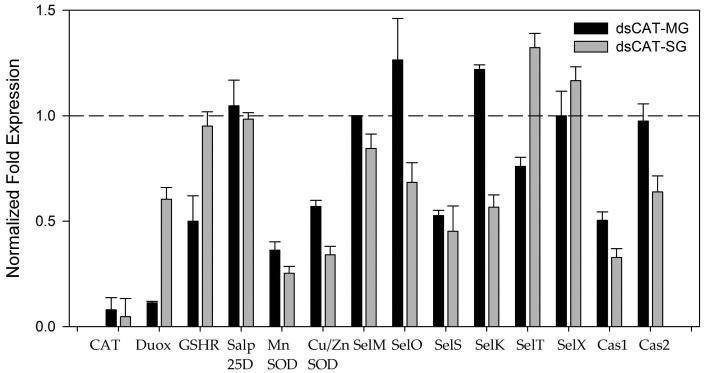
Figure 7. Effect of CAT knockdown (dsCAT) on the antioxidant gene network in Amblyomma maculatum.
Evidence of CAT knockdown upon dsRNA-based silencing of the target gene in tick midguts and salivary glands. Transcriptional expression of tick antioxidant genes including panel of selenoproteins was estimated in the CAT-silenced tick tissues. Transcriptional expression was estimated by qRT-PCR using β-Actin as a reference gene. Transcription of selected target genes in control tick tissues were given a normalized fold expression value of 1, as represented by the dashed line.
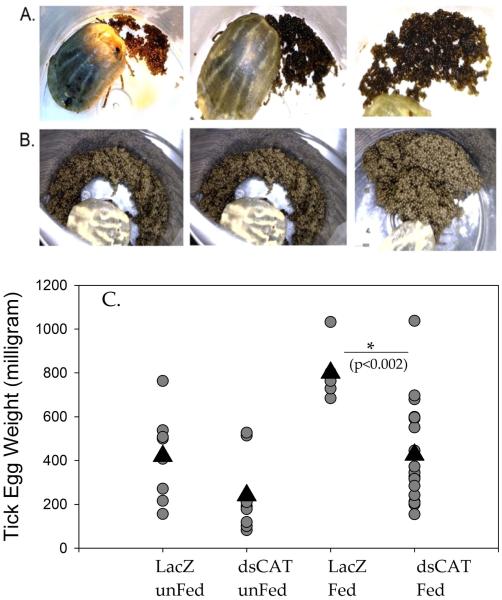
Figure 8. Elucidation of the role played by CAT in ovipositioning in Amblyomma maculatum.
A. Effect on tick ovipositioning when dsRNA-CAT and 3-AT were injected as a cocktail into engorged female ticks. B. Ovipositioning of dsRNA-LacZ (irrelevant control) injected into engorged female ticks. C. Egg weights after dsRNA-CAT silencing (injected at unfed stage) and depletion of mRNA (dsRNA-CAT) and proteins (dsRNA-CAT + AT). Only the cocktail depleting CAT at the mRNA and protein level resulted in significant impairment of ovipositioning based on the egg weights (p=0.002).Triangles indicate the mean egg weight values.
The transcriptional expression of selected tick antioxidant genes was estimated upon depletion of CAT in salivary glands and midgut tissues (Fig. 7). Interestingly, CAT transcript depletion impacted down-regulation of SelS and Duox, both of which are SODs (Fig. 7).
CAT involvement in tick reproductive fitness
Sole depletion of the CAT transcript in unfed ticks did not impact the tick phenotype. However, we noticed a decrease in the weight of an average tick egg (Fig. 8 C). In this experiment, we attempted to deplete both transcript and residual protein activity by using dsRNA and a CAT inhibitor with engorged female ticks. Interestingly, inhibition of transcripts and protein together significantly decreased the total egg weight and eclosion of the larvae (Fig. 8A-B). Control ticks laid eggs from which larvae successfully hatched, while comparatively, the ticks injected with combined dsCAT/3-Amino-1, 2, 4-triazole (3-AT) ovipositioned significantly fewer eggs (p=0.002) .
Discussion
An imbalance between the generation and detoxification of ROS causes oxidative stress, and this has positive or negative repercussions for living organisms. Ticks experience a variety of oxidative stress conditions while attached to the vertebrate host and when free living, and must respond to these conditions before the highly reactive oxidative molecules damage their cellular structures, as both severe and prolonged oxidative stress can trigger cellular apoptosis, necrosis, and death. Therefore, an understanding of the tick antioxidant machinery should provide insight into how ticks avoid tissue damage when experiencing oxidative stress. Such knowledge could be useful for investigating new ways to control tick populations in the absence of effective established methods. Here, we investigated the potential of tick CAT as a target for tick control. Bioinformatics analysis of AmCAT indicate the amino acid sequence homology to other known arthropod and vertebrate genes (Figure 1) and includes likeliness of its subcellular location within the peroxisome lumen through the inclusion of the C-terminal tripeptide, -AKL, fitting the peroxisomal targeting signal 1 (PTS1) tripeptide consensus sequence, S/A/C-K/R/H-L/M (Subramani, 1998; Chen et al., 2012). Moreover, the A. maculatum CAT sequence is close in phylogenetic lineage to Amblyomma americanum, and Ixodes scapularis (Figure 2).
The antioxidant robustness upon the external stressors was observed dominantly in salivary glands, while the midgut tissues did not display a strong antioxidant response in unfed ticks. The relative oxidative stress in tick midgut tissues compared with salivary glands, which has been heavily backed up by antioxidant levels, could be the reason for depletion of native bacterial loads in tick midguts (Fig. 5 A, C) compared to increased microbial loads in the salivary glands (Fig. 5 B, D). In general, PQ is toxic to most vertebrates, but has often been used in biological research as an inducer of oxidative stress because of its ability to induce generation of superoxide ions within eukaryotic cells and tissues (Bus and Gibson, 1984). The injection of PQ increased superoxide levels in salivary glands but depleted Cu/ZnSOD and MnSOD in the tick midgut tissues (Fig. 4). Exogenous PQ has also been shown to induce toxicity in cells by oxidizing NADPH and generating superoxide ions (Bus and Gibson, 1984).
In eukaryotes the CAT enzyme is a tetramer of four polypeptide chains with each monomer composed of more than 500 amino acids that collectively contribute to the formation of four porphyrin heme groups and the binding of a protective NADPH that protects each monomer from being oxidized by its target substrate, H2O2 (Putnam et al., 2000). dsCAT injection in unfed tick and ticks allowed to feed on a host did not impact tick physiology in terms of blood feeding success; this may be caused by a reduced physiological need after attachment of CAT and the dilution effect of the dsRNA that was injected. Increased transcript levels in tick ovarian tissues (Fig. 6A) compared with relatively lower levels in the midguts and salivary glands, suggest that CAT is more important in ovarian tissues. Radulović et al., 2014 showed that time-dependent and tissue-specific expression was up-regulated at the transcript level in the salivary glands during early-phase feeding, suggesting a role for AmCAT in detoxifying the excess H2O2 generated as a result of the high protein synthesis occurring during salivary secretion and establishment of the tick feeding site. The gene expression levels in the tick midguts and salivary glands after CAT silencing suggests lesser compensation (Fig. 7). Surprisingly, although increased oxidative stress upon CAT silencing occurred (Fig. 6B), we did not observe depletion of the microbiome in the silenced tick tissues (Fig. 6C).
Depletion of CAT mRNA alone did not impact tick oviposition; this may have been caused either by dilution of the injected dsRNA-CAT over a prolonged time, or simply due to the presence of active protein synthesized before the degradation of endogenous mRNA. Hence, we designed a set of experiments for engorged A. maculatum that targeted both mRNA and proteins. The dsRNA-CAT and 3-aminotriazole (3-AT) combination injection severely impacted oviposition in A. maculatum (Fig. 8); the exact mechanism for this has yet to be determined, but one study has reported that the enzymatic activity of CAT in removing H2O2 is associated with heme detoxification (Galay et al., 2015). Furthermore, Citelli et al. (2007) reported that injection of the 3-AT CAT inhibitor into engorged Rhiphicephalus microplus females resulted in the inhibition of heme aggregation within hemosomes, as evidenced by heme dispersal throughout the cytoplasm of digestive cells and an alteration in heme detoxification; this finding bears similarities to previous studies where the reproductive output of female An. gambiae CAT knockdown mutants was found to be significantly reduced, indicating that CAT plays a central role in protecting mosquito oocytes and early embryo from ROS damage (DeJong et al., 2007).
Conclusions
Our experiments have shown that A. maculatum possesses tissue-specific and strong antioxidant armaments and that these include a range of selenoproteins that are activated by the external stressors, H2O2 and PQ. The silencing of tick CAT achieved by transcript and protein depletion resulted in a marked impairment of reproductive fitness in A. maculatum, as observed by ovipositioning in the ticks. This study sheds new light on the role played by CAT in the reproductive fitness of ticks.
Experimental Procedures
Ticks
Unfed adult female Gulf Coast ticks (A. maculatum) were obtained from the tick rearing facility within the National Tick Research and Education Resource located at Oklahoma State University, USA. Ticks were maintained according to the methods described previously (Patrick and Hair, 1975). The ticks were maintained in the laboratory in an incubator at 24–26°C, with 90% relative humidity (RH), under a 14 h light and 10 h dark period as described previously (Karim et al., 2002). Ticks blood fed on sheep were handled using Protocol #10042001 approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Southern Mississippi, USA. All animal experiments were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (USA) and with effort made to minimize animal suffering.
Exogenous induction of oxidative stress
For either the H2O2 or PQ experiments, 60 unfed adult female A. maculatum were divided into four groups containing 15 ticks. Three previously determined sub-lethal doses of 3%, 5%, or 7% H2O2, or 1 mM, 10 mM, or 20 mM PQ, plus their respective controls of PBS or 5% sucrose were administered to each of the four experimental groups. A Hamilton syringe was used to inject 1 μL of each of the four substances individually into the tick hemocoel through the posterior dorsal cuticle. Each group of ticks was placed at RT for 24 h following their injections and then dissected to obtain their salivary glands. For each experimental and control group, the salivary glands were pooled and stored for RNA extraction and protein assays (see Tick Tissue Dissection section).
Endogenous induction of oxidative stress through CAT RNA interference and inhibition
Double-stranded RNA (dsRNA), specific for A. maculatum CAT transcripts (AmCAT), was generated using CAT-specific primers (Table 1) to amplify the CAT gene from cDNA previously obtained from partially blood fed salivary glands. The thermocycler settings were 94°C for 2 min, 30 cycles at 94°C for 15 sec, 55°C for 30 sec, 72°C for 50 sec, 72°C for 8 min followed by a 10°C hold. The PCR product, purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA), was used in a secondary PCR reaction using the same primers with the same CAT-specific sequence (Table 1) but with the addition of flanking T7 sequences that allow for the binding of reverse transcriptase and the production of dsRNA. The thermocycler settings for the secondary PCR reaction were 94°C for 90 sec, 30 cycles at 94°C for 15 sec, 61°C for 30 sec, 72°C for 60 sec, 72°C for 8 min followed by a 10°C hold. The secondary PCR product was purified using a QIAquick PCR purification kit (Qiagen) and then sequenced (Eurofins MWG Operon, Louisville, KY) to ensure proper addition of the T7 sequences. After confirming the T7-flanked CAT gene sequence, the secondary PCR product was reverse transcribed into RNA using a T7 Quick High Yield RNA synthesis kit (New England Biolabs, Ipswich, MA) by incubating the PCR product with T7 polymerase overnight at 37°C. The resulting dsRNA was purified by ethanol precipitation and its concentration measured spectrophotometrically using a Nanodrop set to the DNA setting and the product was also visualized by gel electrophoresis using a 2% agarose gel. The CAT dsRNA (dsCAT) was diluted to a working concentration of 1 μg/μl. The same protocol was used to synthesize dsRNA-LacZ (dsLacZ) to be utilized as a control. Forty-five unfed adult female ticks were microinjected with 1 μl of dsCAT or dsLacZ using a 27-gauge needle and then kept overnight within an incubator at 37°C to alleviate needle trauma and promote survival. Surviving ticks were placed in designated and contained cotton cells on a sheep and allowed to blood feed in the presence of A. maculatum males. For sample collection, ten partially-fed experimental and control ticks were removed on days 5 and day 7 post tick infestation, with the remaining ticks allowed to remain attached and blood feed until repletion. The feeding success of the individual ticks was evaluated by recording the attachment duration, repletion weight, and the ability to oviposition (Karim and Adamson, 2012). Partially-fed ticks removed from the dsLacZ or dsCAT groups were dissected to obtain their midguts and salivary gland tissues. For each time point, of the ten ticks, five had their tissues pooled for RNA extraction while the tissues from the other five were pooled for use in enzymatic assays. Twenty females that reached engorgement were divided into two groups. A cocktail of 1 μg/μl dsCAT and 20 mM 3-AT CAT inhibitor was injected in one group of ticks, while the other group was injected with PBS as a control. Replete ticks were separated into individual vials and kept in an incubator (RT, 90% RH) for ovipositioning.
Table 1.
Gene specific primers used in this study.
| Table 1: Gene-specific qRT-PCR primers used | ||||
|---|---|---|---|---|
| Gene | GenBank ID | Forward Primer (5'-3') | Reverse Primer (5'-3') | Size (bp) |
| Actin | JO842238 | TGGCTCCTTCCACCATGAAGATCA | TAGAAGCACTTGCGGTGCACAATG | 169 |
| Catalase | JO843741 | AAAGGACGTCGACATGTTCTGGGA | ACTTGCAGTAGACTGCCTCGTTGT | 173 |
| Duox | Am-41480 | ATGACGCACAGCCTGTATATT | TGTCCAGAGTGAAGACGATTG | 123 |
| GPX (Salp25D) | JO843645 | TGCCGCGCTGTCTTTATTATTGGC | AGTTGCACGGAGAACCTCATCGAA | 102 |
| GSHR | JO844062 | ACCTGACCAAGAGCAACGTTGAGA | ATCGCTTGTGATGCCAAACTCTGC | 170 |
| SEF | KC989559 | TGGCTCCAGAAATGCTGCTCATTG | ACGCCTTTGCGACTCTTCTCCTTA | 157 |
| SelK | JO843326 | AGTTCCAGCAGGTCATCAGTGTCA | TCCAGGAATAGGGCAGTCCATTGT | 132 |
| SelM | JO842653 | ATGATACCTGAATGGCCATCCGCA | TGATCGCGGGTCATCTTCTCCAAA | 171 |
| SelN | KC989560 | TTAGTTTGGACACTGTGGACGGGT | AGGCTTCTCTAACAACGGCACTCA | 150 |
| SelO | KC989561 | AAGCTCGGCCTTGTGAAGAGAGAA | TACAGCACGACAAGAGCTTGGACA | 190 |
| SelS | JO842687 | AGAACAAGTGCACCACAACAGCAG | ATTTCTTGCATCCTTCGACGTGCC | 107 |
| SelT | KC989562 | TCTTTGTGTGTGGAGCCATCGAGA | ACCACACCCGCACGTCATTAAAGT | 81 |
| SelX | JO845128 | ACCACTCTCCTTGGCCATCATTCA | TGCACTTCCCACAGTACACCTTGA | 108 |
| SPPre | JO842653 | ATGGCTACGGTCTACGTGTTGCTT | TGACGAGCATATCAGGTCGGGTTT | 135 |
|
Cu/Zn-SOD
(SOD1) |
JO844140 | GGAACCGAAGACAGCAAGAA | GAGAAGAGGCCGATGACAAA | 143 |
| Mn-SOD (SOD3) | JO843979 | GCATCTACTGGAC AAACCTCTC | GCAGACATCAGGCCTTTGA | 115 |
| TrxR | JO843723 | TGTGACTACACCAACGTGCCTACA | AGTAGCCTGCATCCGTTCCTCTTT | 175 |
| Caspase1 | JO842755 | GAGGAGTCTAGCAGGATGTTTC | ACTGTCATGCTCCGTGTAATC | 127 |
| Caspase2 | JO845022 | GGTGATCGTGATGTCCTGTATG | CGACAGGCCTGAATGAAGAA | 128 |
Tick tissue dissection
Dissections of unfed and partially-fed adult female A. maculatum tissues were performed. The experimental assessments of exogenous oxidative stress via H2O2 or PQ injections required dissection of unfed salivary glands following a 24 h incubation period post-injection. The CAT RNA interference experiment required dissection of partially fed ticks removed from the sheep on day 5 post-infestation. Tick midguts and salivary glands were dissected within four hours of removal of these insects from the sheep. For both the unfed or partially fed ticks, the dissections were performed in ice-cold M-199 buffer (Sigma Aldrich, St. Louis, MO) and tracheal material in the individual tissues was rinsed away with the same ice-cold M-199 buffer, as described by Villarreal et al., 2013. The dissected midguts or salivary glands were pooled based on the experimental sample type described above and then stored in either RNAlater (Ambion, Austin, TX, USA) for later mRNA extraction or in protein storage buffer (0.5 M piperazine N, N0-bis-2-ethane sulfonic acid, 20 mM EGTA, protease inhibitor cocktail; Roche, Indianapolis, IN; 40% glycerol, pH 6.8) followed by storage at −80°C.
Bioinformatics analysis
The full-length A. maculatum CAT (AmCAT) coding sequence (GenBank Accession # JO843741.1) was obtained previously through pyrosequencing of an A. maculatum salivary gland cDNA sequence (Karim et al., 2011). The AmCAT nucleotide sequence was conceptually translated into its amino acid sequence and a ClustalX2 multiple sequence alignment was performed with the protein sequences from I. scapularis (XM_002400585.1), C. elegans (X82175.1), D. melanogaster (NM_080483.3), An. gambiae (DQ986315.1) and H. sapiens (NM_001752.3) obtained through GenBank. The multiple sequence alignment was refined manually and graphically presented using Jalview 2.8 (Larkin et al., 2007; Waterhouse et al., 2009). Phylogenetic relationships were inferred by MEGA6 using the neighbor-joining clustering method (Tamura et al., 2013) with the following protein sequences from eukaryotic organisms obtained from GenBank: chimpanzee (Pan troglodytes) [JAA41878.1], human (H. sapiens) [NP_001743.1], Sumatran orangutan (Pongo abelli) [NP_001124739.1], Rhesus macaque (Macaca mullatta) [AFI34641.1], cattle (Bos taurus) [NP_001030463.1], brown rat (Rattus norvegicus) [NP_036652.1], chicken (Gallus gallus) [NP_001026386.2], zebra finch (Taeniopygia guttata) [XP_002189160.1], zebrafish (Danio rerio) [NP_570987.1], fruit fly (D. melanogaster) [AAF49228.1], yellow fever transmitting mosquito (Aedes aegypti) [XP_001663600.1], malaria transmitting mosquito (An. gambiae) [ABL09378.1], southern house mosquito (Culex quinquefasciatus) [EDS28616.1], Gulf Coast tick (A. maculatum) [AEO35358.1], Lone Star tick (A. americanum) [JAG91014.1] black-legged tick (I. scapularis) [EEC02741.1], and red flour beetle (Tribolium castaneum) [EEZ97738.1].
RNA isolation, cDNA synthesis, and transcriptional expression via qRT-PCR
Total RNA isolation, cDNA synthesis and qRT-PCR were performed as described previously (Browning et al., 2012; Villarreal et al., 2013; Budachetri and Karim, 2015). Briefly, total RNA was extracted from the tick salivary glands or midguts stored in RNAlater using an Illustra RNAspin Mini RNA isolation kit (GE Healthcare, Piscataway, NJ) following the manufacturer’s instructions. The total RNA quantity was determined by Nanodrop analysis. Total RNA (~1 μg) was reverse transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Target gene-specific primers were designed to amplify the cDNA fragments from A. maculatum tissues. All primer sequences used are listed in Table 1. Target mRNA levels were determined using quantitative reverse transcriptase (qRT)-PCR with the CFX96 Real-Time system (Bio-Rad Inc.). cDNA (25 ng), 10 μM of gene-specific primers and 2X iTaq Universal SYBR green supermix (Bio-Rad Inc.) were used in each qRT-PCR reaction mixture and the following thermocycler protocol was used for the reactions: 50°C for 3 min, 95°C for 10 min, 40 cycles at 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. All the samples were run in triplicate along with non-template controls (NTCs) for each set of primers. Actin was used as a reference gene (Browning et al., 2012) to calculate the relative expression of various antioxidant genes. Bio-Rad software was used for data analysis with the 2ΔΔCt method (Bio-Rad CFX manager V3.1, Hercules, CA).
Quantification of oxidative stress
A Protein Carbonyl Content Assay Kit (Sigma Aldrich, USA) was used to quantify the relative oxidative stress levels in the tick salivary glands obtained for the exogenous H2O2 experiment and from the midguts and salivary glands obtained for the CAT RNAi experiment. Tick tissues were extracted for their total protein content as described by Budachetri and Karim, 2015. The protein concentration of the samples was estimated via a Bicinchonic Acid (BCA) Protein Assay Kit (Thermo Scientific, product #23227, Rockford, IL). Salivary gland extract (430 μg/ml) and midgut extract (4210 μg/ml) were used for protein carbonyl content assays for the RNAi samples. The principle of Bear-Lambert’s law was used to calculate incorporation of 2, 4-dinitrophenylhydrazine (DNPH). DNPH reacts with carbonyl groups to form a stable adduct called 2, 4-dinitrophenyl hydrazone, thereby indicating the number of carbonyl groups formed as a result of oxidative stress. 2, 4-dinitrophenyl hydrazone was detected by spectrophotometric analysis at a wavelength of 375 nm. The carbonyl content determined for each experimental sample was normalized to the relative carbonyl content of each experiment’s respective controls for graphical representation and interpretation.
Quantification of total bacterial load
The total bacterial load in the tick tissues was estimated as described previously (Narasimhan et al., 2014; Budachetri et al., 2015). Briefly, 25 ng of tick tissue cDNA, 200 μM of 16S rRNA gene primers and 2X iTaq Universal SYBR green supermix (Bio-Rad Inc., Hercules, CA) were mixed and run on the CFX96 Real-Time system (Bio-Rad Inc.) using the following thermocycler parameters: 94°C for 5 min, 35 cycles at 94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. Standard curves were used for estimating the copy numbers of each gene. The bacterial copy numbers were normalized against A. maculatum actin expression and NTCs were used to ensure the purity of the water used in the reactions.
Statistical analysis
All data are expressed as the mean expression ± the standard error mean (SEM). Statistical significance between the two experimental groups or their respective controls was determined by the Mann–Whitney rank sum test (P-value <0.05). Comparative differences among multiple experimental groups were determined by ANOVA with statistically significant P-values of <0.05 (SigmaPlot ver.11, San Jose, CA, USA). Transcription expression levels were considered by Bio-Rad software (Bio-Rad CFX manager V3.1) to be significantly different between samples for two-fold differences with p-values of <0.05.
Acknowledgements
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (award #AI099919) and the National Institutes of General Medical Sciences (award # P20GM103476) to the MS-INBRE core facility. The funders had no role in the study design, data collection, analysis, decision to publish, or manuscript preparation.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- Adamson S, Browning R, Singh P, Nobles S, Villarreal A, Karim S. Transcriptional activation of antioxidants may compensate for selenoprotein deficiencies in Amblyomma maculatum (Acari: Ixodidae) injected with selK- or selM-dsRNA. Insect Mol Biol. 2014;23:497–510. doi: 10.1111/imb.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson SW, Browning RE, Budachetri K, Ribeiro JMC, Karim S. Knockdown of Selenocysteine-Specific Elongation Factor in Amblyomma maculatum Alters the Pathogen Burden of Rickettsia parkeri with Epigenetic Control by the Sin3 Histone Deacetylase Corepressor Complex. Plos One. 2013;8:e82012. doi: 10.1371/journal.pone.0082012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning R, Adamson S, Karim S. Choice of stable set of reference genes for qRT-PCR analysis in Amblyomma maculatum (Acari: Ixodidae) J Med Entomol. 2012;49:1339–1346. doi: 10.1603/me12123. [DOI] [PubMed] [Google Scholar]
- Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environmental Health Perspectives. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budachetri K, Karim S. An insight into the functional role of thioredoxin reductase, a selenoprotein, in maintaining normal native microbiota in the Gulf Coast tick (Amblyomma maculatum) Insect Molecular Biology. 2015;10:570–581. doi: 10.1111/imb.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citelli M, Lara FA, da Silva Vaz I, Jr, Oliveira PL. Oxidative stress impairs heme detoxification in the midgut of the cattle tick, Rhipicephalus (Boophilus) microplus. Mol Biochem Parasitol. 2007;151:81–88. doi: 10.1016/j.molbiopara.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Chen P, Li J, Liu P, Gao B, Wang Q, Li J. cDNA cloning, characterization and expression analysis of catalase in swimming crab Portunus trituberculatus: cDNA cloning and expression analysis of catalase gene of Portunus trituberculatus. Mol Biol Rep. 2012;39(12):9979–9987. doi: 10.1007/s11033-012-1826-2. doi: 10.1007/s11033-012-1826-2. [DOI] [PubMed] [Google Scholar]
- DeJong RJ, Miller LM, Molina-Cruz A, Gupta L, Kumar S, Barillas-Mury C. Reactive oxygen species detoxification by catalase is a major determinant of fecundity in the mosquito Anopheles gambiae. Proc Natl Acad Sci. 2007;104:2121–2126. doi: 10.1073/pnas.0608407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galay RL, Umemiya-Shirafuji R, Mochizuki M, Fujisaki K, Tanaka T. Iron metabolism in hard ticks (Acari: Ixodidae): The antidote to their toxic diet. Parasitology International. 2015;64:182–189. doi: 10.1016/j.parint.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Holmstöm KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signaling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes. Metab. 2010;12:116–125. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Essenberg RC, Dillwith JW, Tucker JS, Bowman AS, Sauer JR. Identification of SNARE and cell trafficking regulatory proteins in the salivary glands of the lone star tick, Amblyomma americanum (L.) Insect Biochem Mol Biol. 2002;32:1711–1721. doi: 10.1016/s0965-1748(02)00111-x. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One; 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Adamson SW. RNA interference in ticks: a functional genomics tool for the study of physiology. In: Jockusch EL, editor. Small RNAs: Their Diversity, Roles, and Practical Uses. Vol. 42. Storrs; Elsevier: 2012. pp. 119–154. [Google Scholar]
- Kashiwagi A, Kashiwagi K, Takase M, Hanada H, Nakamura M. Comparison of catalase in diploid and haploid Rana rugosa using heat and chemical inactivation techniques. Comp. Biochem. Physiol. Part B. 1997;118:499–503. doi: 10.1016/s0305-0491(97)00216-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki L, Aguirre J. Multiple catalases genes are differentially regulated in Aspergillus nidulans. J. Bacteriol. 2001;183:1434–1440. doi: 10.1128/JB.183.4.1434-1440.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. ClustalW and ClustalX version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lismont C, Nordgen M, Van Veldhoven PP, Fransen M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015;3:35. doi: 10.3389/fcell.2015.00035. doi: 10.3389/fcell.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/ catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- Manduzio H, Monsinjon T, Galap C, Leboulenger F, Rocher B. Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and of glutathione S-transferase. Aquat Toxicol. 2004;7:83–93. doi: 10.1016/j.aquatox.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Rajeevan N, Liu L, et al. Gut Microbiota of the Tick Vector Ixodes scapularis Modulate Colonization of the Lyme Disease Spirochete. Cell Host & Microbe. 2014;15:58–71. doi: 10.1016/j.chom.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Goddard J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae) J Med Entomol. 2015;52:230–252. doi: 10.1093/jme/tju022. [DOI] [PubMed] [Google Scholar]
- Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, et al. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- Radulović Z̆M, Kim TK, Porter LM, Sze S-H, Lewis L, Mulenga A. A 24-48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics. 2014;15:518. doi: 10.1186/1471-2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampayo JN, Olsen A, Lithgow GJ. Oxidative stress in Caenorhabditis elegans: protective effects of superoxide dismutase/catalase mimetics. Aging Cell. 2003;2:319–326. doi: 10.1046/j.1474-9728.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Subramani S. Components involved in peroxisome import, biogenesis, proliferation, turnover, and movement. Physiol Rev. 1998;78:171–188. doi: 10.1152/physrev.1998.78.1.171. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal AM, Adamson SW, Browning RE, Budachetri K, Sajid MS, Karim S. Molecular characterization and functional significance of the Vti family of SNARE proteins in tick salivary glands. Insect Biochem Mol Biol. 2013;43:483–493. doi: 10.1016/j.ibmb.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2 – a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamocky M, Furtmuller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2008;10:1527–1548. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]