Abstract
Combination of dietary/herbal spice curcumin (Cur) and COX inhibitors has been tested for improving therapeutic efficacy in pancreatic cancer (PC). The objective of this study was to identify agent with low toxicity and COX-independent mechanism to induce PC cell growth inhibition when used along with Cur. Anti-cancer NSAID, Tolfenamic acid (TA) and Cur combination was evaluated using PC cell lines. L3.6pl and MIA PaCa-2 cells were treated with Cur (5–25 μM) or TA (25–100 μM) or combination of Cur (7.5 μM) and TA (50 μM). Cell viability was measured at 24–72 h post-treatment using CellTiter-Glo kit. While both agents showed a steady/consistent effect, Cur+TA caused higher growth inhibition. Anti-proliferative effect was compared with COX inhibitors, Ibuprofen and Celebrex. Cardiotoxicity was assessed using cordiomyocytes (H9C2). The expression of Sp proteins, survivin, and apoptotic markers (Western blot), caspase 3/7 (caspase-Glo kit), Annexin-V staining (flow cytometry), reactive oxygen species (ROS) and cell cycle phase distribution (flow cytometry) were measured. Cells were treated with TNF-α and NF-kB translocation from cytoplasm to nucleus was evaluated (immunofluorescence). When compared to individual agents, combination of Cur+TA caused significant increase in apoptotic markers, ROS levels and augmented NF-kB translocation to nucleus. TA caused cell cycle arrest in G0/G1 and the combination treatment showed mostly DNA synthesis phase arrest. These results suggest that combination of Cur+TA is less toxic and effectively enhance the therapeutic efficacy in PC cells via COX-independent mechanisms.
Keywords: Pancreatic Cancer, Tolfenamic acid, Curcumin, Sp1, Combination treatment
1. INTRODUCTION
Herbal products have been extensively studied for their medical benefits for a long time [1–3]. Curcumin (Cur) is a natural phenolic compound, and an active ingredient found in the dietary spice Turmeric (Curcuma longa L.). Cur [1, 7-bis-(4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione] has a wide spectrum of biological actions against inflammation, ischemia, cancer, and aging. Extensive research over the last 50 years has indicated that Cur can prevent and treat cancer [4, 5]. Anti-carcinogenic effects of Cur have been observed in several malignancies including pancreatic cancer (PC) [6], [7–10]. PC is an aggressive disease with poor prognosis and survival often depending on mutational status of certain signaling molecules [11]. Phase I clinical trials indicated that Cur can be safely administered at very high doses (6 g/day) [12]. However, low bioavailability was noticed when administered orally. Phase II trial also supported the biologic activity of Cur in PC patient showing a marked tumor regression [13].
Certain strategies such as drug delivery systems, synthetic analogs have been tested to overcome the bioavailability issues [14–19]. Combination of Cur with other agents was also investigated in some cancers[20]. Cur also showed radiosensitization response in cervical carcinoma cells[21]. These studies suggest that Cur could be effective when used in a combination therapy. Combination of Cur and gemcitabine (Gemzar) was tested in a clinical trial conducted at MD Anderson Cancer Center. Another clinical trial has been approved for testing the combination of Cur, Gemzar and a non-steroidal anti-inflammatory drug (NSAID), Celebrex for treating metastatic PC. While the effect of Cur in combination with the above candidates is relatively well studied, it is also important to see other potential contributing targets especially COX-independent mechanisms for improving the anti-cancer activity of Cur. In this study, we have tested a combination involving an inhibitor of Specificity protein (Sp) transcription factors along with Cur.
The Sp-family of transcription factors regulate variety of genes involved in critical processes ranging from cell cycle, proliferation, cell differentiation, apoptosis and associated with a number of human cancers [22–26]. Sp1 is a negative prognostic factor for survival in some cancer patients [27, 28]. It is postulated that Sp (Sp1, Sp3 and Sp4) transcription factors bind to GC-rich promoter sites regulate key sets of genes responsible for cancer cell proliferation and survival [26]. Previous laboratory studies from our group and others demonstrated the significance of targeting Sp proteins for the treatment of various cancers [29–32]. After screening several small molecules (NSAIDs) representing different structural classes to target Sp proteins in pre-clinical models for PC, tolfenamic acid (TA) was introduced as an effective anti-cancer agent[32]. TA decreased PC cell growth and inhibited metastasis in orthotopic mouse model via inducing the degradation of Sp1, Sp3, and Sp4 [32].
In current study, we investigated the effect of co-treatment of Cur and TA on PC cell growth. The individual and combined treatment using the optimized doses for each agent was tested using L3.6pl and MIA PaCa-2 cells. The anti-proliferative effect of other NSAIDs, Ibuprofen (Ibu) and Celebrex (Cel) were compared with the effect of TA. Cell viability results were corroborated with the effect on expression of Sp1, survivin and the markers associated with apoptosis (apoptotic cell population, cleavage of PARP and the activity of caspases 3/7). Since the cell growth inhibition was massive with the combination treatment, the cell cycle phase distribution and was also determined following the individual and combined treatment of Cur and TA. Furthermore, the activation of reactive oxygen species (ROS) was measured using flow cytometry and we assessed the effect on translocation of NF-kB from cytoplasm to nucleus via immunofluorescence.
2. MATERIAL AND METHODS
2.1 Cell Lines and Chemicals
Human pancreatic cancer cells (L3.6pl, MIA PaCa-2) and cardiomyocyte cells (H9C2) were used in this study. L3.6pl cells were obtained from the University of Texas MD Anderson Cancer Center, Houston, TX. MIA PaCa-2 and H9C2 cells were purchased from ATCC (Manassas, VA). Cells were grown in DMEM media supplemented with fetal bovine serum and 1% antibiotic (Pen/Strep) and maintained at 37°C with 5% CO2.
Antibodies were purchased from Santa Cruz Biotechnology, Santa Cruz, CA (Sp1, Sp3); R&D Systems, Minneapolis, MN (survivin); Cell Signaling Technology, Danvers, MA (c-PARP). Tolfenamic acid, Ibuprofen, Celebrex, dimethyl sulfoxide (DMSO), protease inhibitor, and β-actin were purchased from Sigma Chemical Co. (St. Louis, MO). Kits were purchased from BD Biosciences, San Jose, CA (Annexin-V/7-AAD); Pierce, Rockford, IL (BCA protein assay) and Promega, Madison, WI (CellTiter-Glo and Caspase-Glo 3/7). Reagents were obtained from Mediatech, Manassas, VA (PBS, DMEM); Invitrogen, Carlsbad, CA (cell lysis buffer) and Pierce, Rockford, IL (SuperSignal West Dura).
2.2 Cell Viability
Cell viability of PC cells and cardiomyocytes (H9C2) was measured with CellTiter-Glo kit. PC cells were plated in 96-well plates (Lonza, Basel, Switzerland) and treated with DMSO (Control) or increasing (25–100 μM) concentrations of NSAIDs (TA, Ibuprofen and Celebrex) or Cur (5–25 μM). At indicated time post-treatment (24/48/72 h) assay reagent (1:1 ratio) was added. After 15 min incubation in dark, luminescence was measured using FLUOstar Optima (BMG Labtech) or Synergy HT (BioTek) plate reader. Data (triplicate samples) were normalized to control cells and presented as percent viable cells.
2.3 Combination index
To determine whether simultaneous treatment with TA and Cur results in synergistic effect, the combination index (CI) was calculated using the method of Chou-Talalay [33]. PC cells were incubated with various concentrations of TA and Cur (at constant ratio of 50:7.5) for 48h. Cell viability was measured using CellTiter-Glo reagent and CI values were calculated for the drug interactions using the CalcuSyn software (version 2.0. Biosoft, Cambridge, UK). CI value of less than 1.0 was considered to indicate synergism.
2.4 Apoptosis (Annexin-V staining) using Flow Cytometry
L3.6pl and MIA PaCa-2 cells were treated with vehicle (DMSO) or TA (50 μM) or Cur (7.5 μM) or both (TA+Cur). Apoptotic cells were measured using the annexin V apoptosis detection kit (Annexin V-PE/7-AAD). Briefly, cells were collected at 48 h post-treatment and incubated with annexin V-PE and 7-AAD in 1X binding buffer for 15 min. After the incubation, cells were processed to determine the percentage of pre-apoptotic, apoptotic and necrotic cells via flow cytometry (BD FACSCalibur flow cytometer). Data were collected and analyzed by FlowJo software (Tree Star, Inc., Ashland, OR).
2.5 Western Immunoblot
L3.6 pl and MIA PaCa-2 cells were treated with DMSO or 50 μM of TA or 7.5 μM of Cur or both (TA+Cur) and cells were harvested at 48 h post-treatment. Protein extracts were prepared and the expression of proteins of interest was determined via Western blot analysis as described previously[32].
2.6 Caspase activation assay
The effect of TA or Cur or TA+Cur on effector caspases activity was evaluated using Caspase-Glo 3/7 kit. PC cells were seeded and treated as described above. After 48h, the assay reagent (1:1 ratio) was added to each well. Plates were mixed and incubated in dark for ~60 min. Luminescence was measured using the FLUOstar Optima (BMG Labtech) plate reader. All samples were measured in triplicate and the data were shown as mean±SEM.
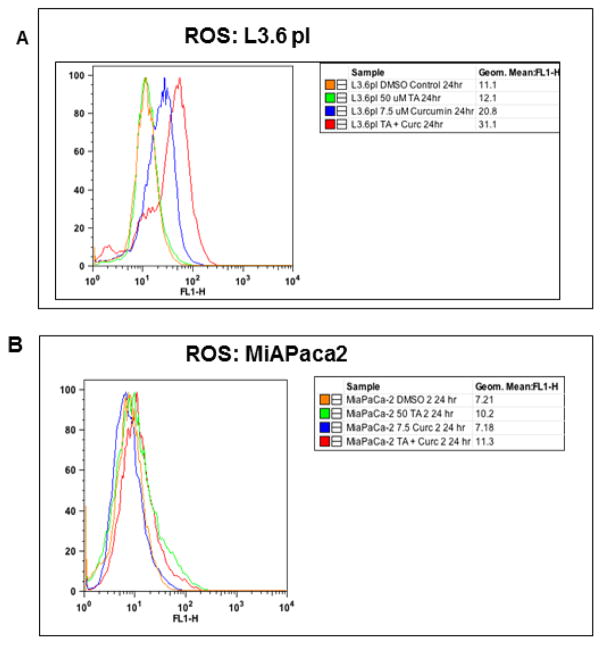
2.7 Estimation of Reactive Oxygen Species
The levels of ROS were measured with an oxidative stress indicator, CM-H2DCFDA using flow cytometry. Briefly, PC cells were treated with TA or Cur or TA+Cur or H2O2 (positive control). After 24h cells were harvested and incubated with 5 μM of CM-H2DCFDA for 30 min at 37°C in dark. Fluorescence was quantified and the geometric mean fluorescence was used as average fluorescence value for each population.
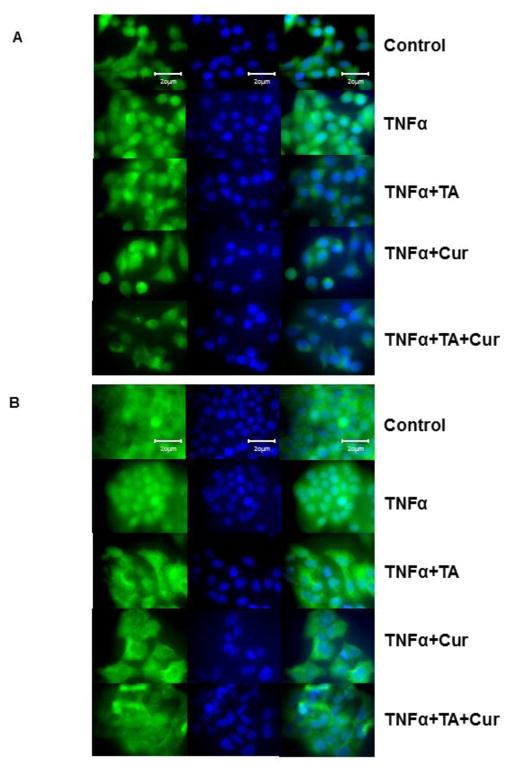
2.8 NF-kB Translocation
PC cells were seeded on cover slips and grown in 6-well plates. Cells were treated with TNFα (15 ng/ml) to induce the translocation of NF-κB in to the nuclei. Simultaneously, cell lines were treated with TA (50 μM) or Cur (7.5 μM) or TA+ Cur. After 24h treatment, PC cells were fixed treating with cold methanol (10 min), washed with PBS and then blocked with goat serum for 1 hr. Then cells were incubated with (1:200) NF-κB antibody for 2 h, washed with 0.1% PBS-Tween and incubated with goat anti-rabbit antibody (1:400) conjugated with Alexa Fluor 488 for 2 h. Cells were mounted on glass slides using Vectashield Mounting Medium with DAPI. Images were acquired using Olympus AX70 upright fluorescence microscope.
2.9 Statistical analysis
Data were analyzed using one way ANOVA. Results were expressed as mean±SEM. Significance between treated and untreated cells were evaluated and p value <0.05 was considered as significant.
3. RESULTS
3.1 TA and Cur combination showed higher efficacy on cell growth inhibition
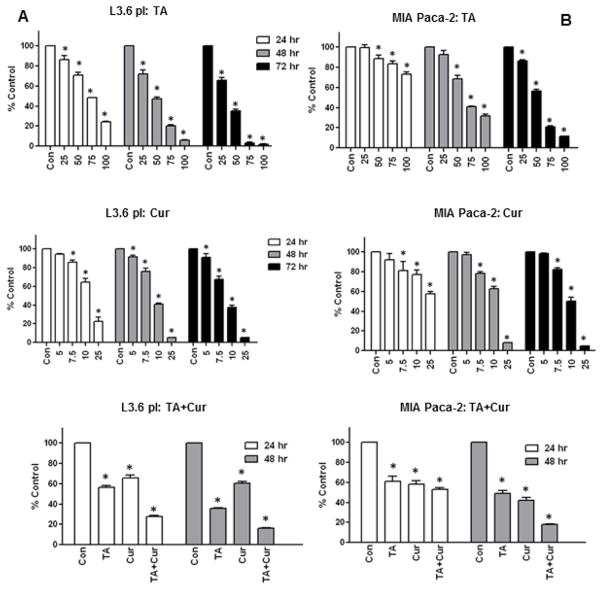
The effect of Cur+TA combination was investigated using two PC cell lines. L3.6pl and MIA PaCa-2 cells were treated with vehicle or TA (25/50/75/100 μM) or Cur (5/7.5/10/20 μM) and the cell viability was assayed for 24, 48, and 72 h. TA or Cur treatment inhibited both cell lines showing a dose- and time-dependent response (Figure 1). TA-induced cell growth inhibition was 30–50% with 50 μM dose and 60–80% with 75 μM dose at 48h post-treatment. Similarly, Cur (7.5 μM) inhibition was 24% and 22% respectively in L3.6pl and MIA PaCa-2 cells at 48h. The detailed information on percent inhibition at 24–72h post-treatment is given in Supplementary data (Tabl1&2; Figure S1&S2. The IC50 values (in μM) of TA were 68.2 (48h) and 51.8 (72h) for MIA PaCa-2 and 42.3 (48h), 33.8 (72h) for L3.6 pl cells. The IC50 values of Cur were lower than TA, MIA PaCa-2: 11.62 (48h), 10.03 (72h); L3.6pl: 9.4 (48h), 8.9 (72h). PC cell growth is significantly inhibited when tested with TA+Cur combination. This combination caused more than double in PC cell growth inhibition at 48 h post-treatment (Figure 1).
Figure 1. Anti-proliferative effect of TA and Cur on PC growth.
(A) L3.6 pl and (B) MIA PaCa-2 cells were treated with DMSO (Control) or TA (25–100 μM) or Cur (5–25 μM) and cell viability was measured after 24, 48 and 72 h using CellTiter-Glo kit. The combination effect of Cur (7.5 μM) and TA (50 μM) was determined at 24 and 48 h post-treatment. Bars represent % viable cells (mean±SEM) normalized to control. All treatments were done in triplicate and the bars marked with ‘*’ are significantly different from corresponding controls (p<0.05).
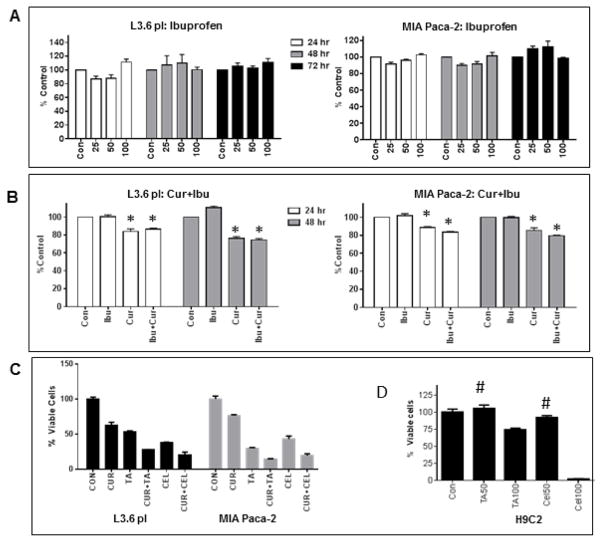
In order to evaluate specificity of TA, the anti-proliferative effect of two other NSAIDs Ibuprofen and Celeberex were also tested. Interestingly, Ibuprofen did not show anti-proliferative effect on PC cells up to 100 μM and the combination with Cur was also not beneficial (Figure 2A&B). The cell growth inhibition caused by Celebrex (individual and combination with Cur) was comparable to TA showing dose/time-dependent response. Since, NSAIDs are known to cause cardiotoxicity; both TA and Celebrex were tested for cytotoxicity using cardiomyocytes (H9C2 cells). TA caused no toxicity at 50 μM but inhibited about 20% cell growth at 100 μM; however Celebrex inhibited >25% and 90% growth respectively at 50 and 100 μM doses.
Figure 2. Effect of Ibu and Cel on PC cell proliferation.
(A) L3.6 pl and MIA PaCa-2 cells were treated with vehicle (Control) or Ibu (25, 50 and 100 μM) and cell viability was determined at 24 and 48 h post-treatment. (B) L3.6 pl and MIA PaCa-2 (D) cells were treated with vehicle (Control) or Ibu (50 μM) or Cur (7.5 μM) or both and cell viability was determined at 24 and 48 h. Bars (% viability compared to control) represents mean±SEM of triplicate determination. ‘*’ indicates significantly different from corresponding controls (p<0.05). (C) L3.6 pl and MIA PaCa-2 cells were treated with vehicle (Control), 50 μM of TA or Cel, 7.5 μM of Cur, TA+Cur, Cel+Cur. (D) H9C2 cells were treated with vehicle, 50 μM of TA or Cel, or 100 μM of TA or Cel. Cell viability was measured at 48 h post-treatment. Data shown indicates % viable cells over control. Bars represent mean±SEM (n=3). In C and D, except the bars marked with ‘#’, all values are significantly different from corresponding controls (p<0.05).
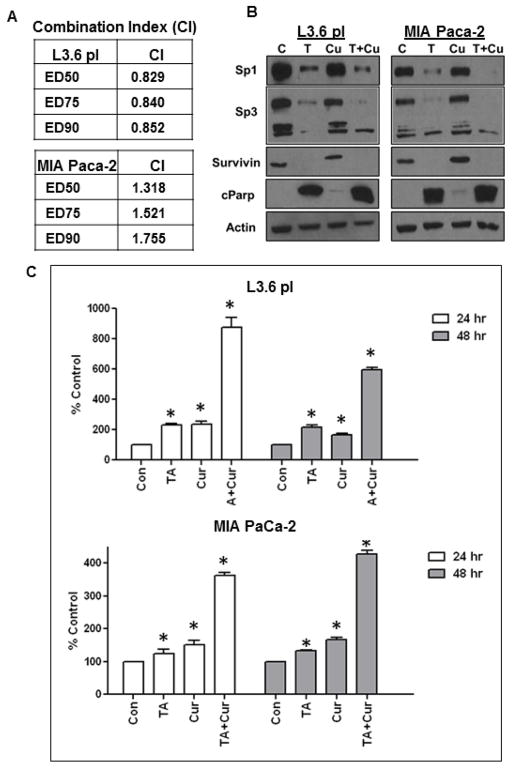
To determine the potential mechanism of TA-Cur interaction, combination index (CI) values were calculated using the Chou-Talalay median effects analysis for drug interactions [33]. The CI values were calculated using CalcuSyn software (Biosoft, Cambridge, UK). A CI value of less than 1.0 indicates synergism in the interaction. As seen in Figure 3A, the CI values were < 1.0 for L3.6 pl and > 1.0 for MIA PaCa-2, indicating that the combination of TA and Cur exhibited synergistic inhibitory effects on viability of L3.6 pl but not MIA PaCa-2 cells.
Figure 3. TA and Cur combination inhibits the expression of Sp proteins and survivin and up-regulates apoptotic markers c-PARP expression and caspase 3/7 activity.
(A) Combination Index: PC cells seeded in 96-well plates were treated with different concentrations of TA and Cur (at TA:Cur ratio of 50:7.5) for 48h. Cell growth was determined at 48h by CellTiter-Glo reagent. Cell viability data were used to calculate the Combination Index (CI) according to the method of Chou and Talalay using CalcuSyn software. CI values were calculated at different effect levels, ED50, ED75, and ED90 (B) PC cells were treated with TA (50 μM) or Cur (7.5 μM) or both for 48 h and protein extracts were prepared. The expression of Sp1, Sp3, survivin, c-PARP and β-Actin (loading control) was determined by Western blot analysis. (C) L3.6 pl and MIA PaCa-2 cells were treated with vehicle (Control) or TA (50 μM) or Cur (7.5 μM) or both for 48 h. Caspase 3/7 levels were measured using caspase-Glo 3/7 kit. Bars represent (mean±SEM) % increase compared to control (n=3). Bars marked with an asterisk are significantly different from corresponding control (p<0.05).
3.2 Combination of Cur+TA inhibits Sp1, Sp3 and survivin expression
PC cells were treated with TA (50 μM), Cur (7.5 μM) and both for 48h. Protein extracts were prepared, the expression of Sp1, Sp3, survivin, and β-actin (loading control) was determined via Western blot analysis. TA decreased the expression of Sp1 and Sp3 which was accompanied, by a similar decrease of survivin expression in both cell lines (Figure 3B); however Cur did not show any effect. Notably, Cur+TA treatment resulted in an increase in the inhibition of Sp proteins. The changes observed in Sp proteins (expression) are consistent with PC cell growth inhibition.
3.3 Cur and TA Combination induces apoptosis
The role of effector caspases is well established in apoptotic pathways. Cur and TA caused a dose-dependent response and induced caspase-3/7 activity. While TA or Cur caused about 2-fold increase, the combination made 4-fold increase in both cell lines (Figure 3C). In order to characterize molecular markers associated with TA-induced apoptosis, the expression of cleaved PARP was evaluated at 48h post-treatment. The expression of pro-apoptotic marker, c-PARP was determined by Western blot analysis. While, TA up-regulated the cleavage of PARP; the effect of Cur+TA was more prominent (Figure 3B).
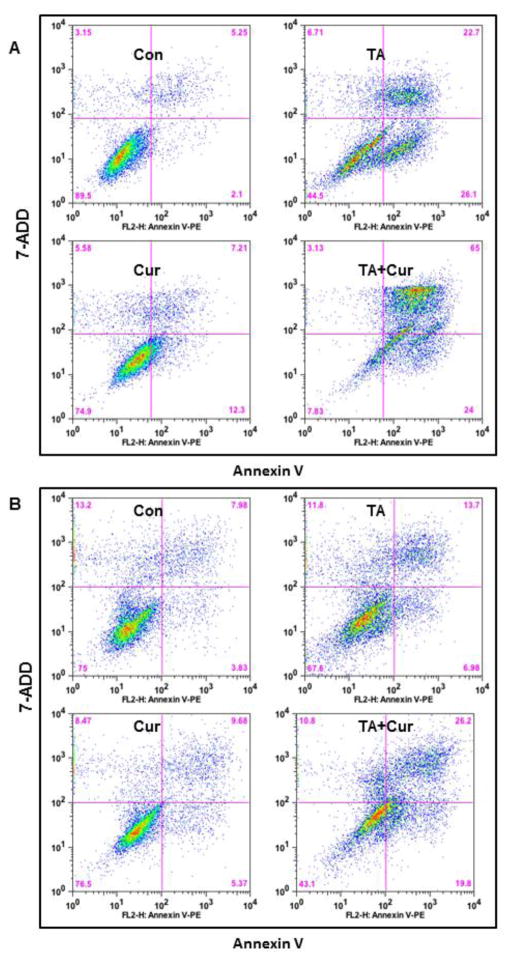
The effect of Cur+ TA on PC cell apoptosis was further determined using flow cytometry. As evinced by Annexin-V staining (Figure 4), TA (50 μM) or Cur (7.5 μM) or combination treatment caused a significant increase in apoptosis. The percentage of apoptotic cells in L3.6pl were increased with each treatment but this increase was more effective with Cur+TA co-treatment (Con: 5.25%; TA: 22.7%; Cur: 7.21%; TA+Cur: 65%) and MIA PaCa-2 (Con: 7.98%; TA: 13.7%; Cur: 9.58; TA+Cur: 26.2%).
Figure 4. TA and Cur treatment increases apoptotic cell population in PC cells.
(A) L3.6 pl and (B) MIA PaCa-2 cells were treated with DMSO (control) or TA (50 μM) or Cur (7.5 μM) or TA+Cur for 48 h. The apoptotic cell population was evaluated with Annexin-V staining using flow cytometry and the data were analyzed with FlowJo software.
3.4 Cur and TA increases ROS levels
The effect of individual and combination effect of Cur and TA on ROS was determined in PC cells. At 24h post-treatment, the effect of TA, Cur and TA+Cur for inducing ROS was measured. In L3.6 pl cells, Cur and Cur+TA resulted in 2 and 3-fold increase respectively in ROS levels; however the effect on MIA PaCa-2 cells was marginal (Figure 5). These results demonstrate that Cur alone or in combination with TA induces ROS in L3.6pl cells.
Figure 5. Effect of TA and Cur on ROS levels in PC cells.
(A) L3.6 pl and (B) MIA PaCa-2 cells were cultured in the presence of TA (50 μM) or Cur (7.5 μM) or combination of TA+ Cur. After 24 h, ROS levels were determined with CM-H2DCFDA kit (Invitrogen) using flow cytometry. Results were processed using FlowJo software.
3.5 Cur and TA suppresses NF-kB translocation
The effect of TA and /or Cur treatments on NF-kB translocation into nucleus was tested. Cells were treated with TNFα to induce translocation of NF-kB. The translocation of NF-kB from cytoplasm to nuclei was evaluated via immunofluorescence. As seen in Figure 6A (MIA PaCa-2) and 6B (L3.6pl), the intensity of NF-kB staining was clearly lowered in nucleus when compared to cytoplasm in all treated groups in both cell lines. The inhibition of NF-kB translocation in TA+Cur treated cells was higher when compared to the cells treated with single (TA or Cur) agent.
Figure 6. Effect of TA and Cur on NF-kB translocation in PC cells.
(A) MIA PaCa-2 and (B) L3.6 pl cells were cultured in the presence of vehicle (Control) or TNFα (15 ng/ml) and treated with TA (50 μM) or Cur (7.5 μM) or TA+Cur. Cells were examined to assess the translocation of NF-kB (into nucleus) by immunofluorescence. Images (60X) were captured using an upright fluorescence microscope (Olympus AX70). Representative images are shown in the figure. Con: Control; TA: Tolfenamic acid (50 μM); Cur: Curcumin (7.5 μM); TA+CUR: Tolfenamic acid and Curcumin.
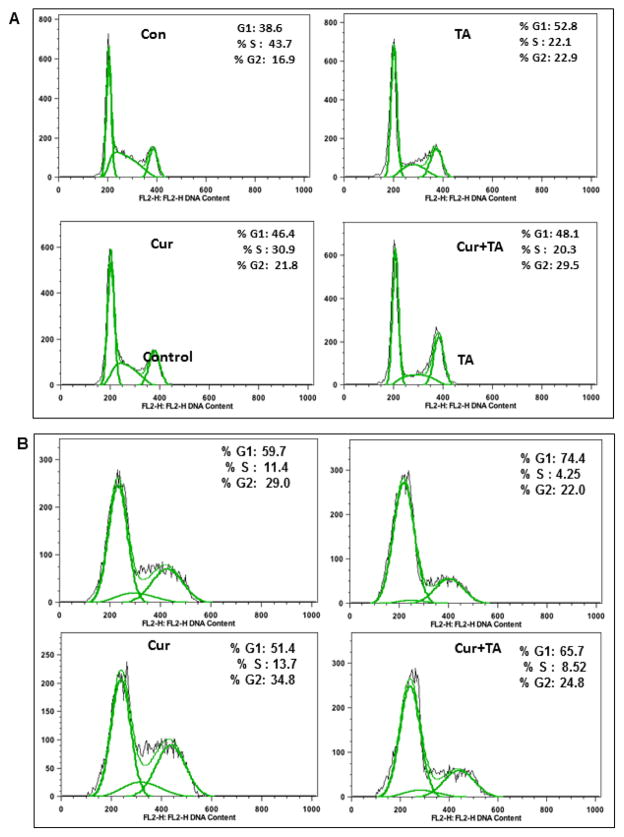
3.6 Combination of Cur+TA modulates cell cycle phase distribution
The combination of TA and Cur caused increased cell growth inhibition (Figure 1). Since an induction of apoptosis alone may not explain such response, the effect on cell cycle arrest was also evaluated using flow cytometry (Figures 7). In L3.6 cells, TA caused early phase (G1) arrest (Con: 38.6%; TA: 52.8%), Cur accrued more cells in G2 phase (Con: 16.9%; Cur: 21.8%), while the combination of TA and Cur showed some increase of cells in both G1 and G2 phases but also consistently low percentage in ‘S’ phase (Figure 7A). In MIA PaC-2 cells TA caused a G1 arrest (Con: 59.7%; TA: 74.4%) while Cur treatment resulted in slight accumulation of cells in G2 (Con: 29.0%; Cur: 34.8%). The combination treatment revealed marginal response on G1 and ‘S’ phases of cell cycle (Figure 7B). These results indicate that cell cycle arrest could be partially responsible for the cell growth inhibition.
Figure 7. Effect of TA and Cur on cell cycle in PC cells.
Human PC cells (A) L3.6 pl and (B) MIA PaCa-2 were cultured in the presence of DMSO (Control), TA (50 μM), Cur (7.5 μM) or TA+Cur for 48 h. Cell cycle phase distribution was measured by flow cytometry. Representative histograms (with peaks) are shown in the figure.
4. DISCUSSION
PC is the fourth most common causes of cancer-related deaths and the National Cancer Institute (NCI) estimates that 43,140 new cases and 36,800 deaths will occur due to pancreatic cancer (PC) in one year in the United States alone [34]. The prognosis of this malignancy is very poor, and the 5 year survival rate is less than 5%. While the major contributing molecular changes in PC include mutation and inactivation of several oncogenes including tumor suppressor genes, the aggressive nature of this malignancy is co-related with perturbations in growth factors and their receptors that affect the downstream signal transduction pathways involved in the control of growth and differentiation [35, 36]. There is an increasing interest in identifying novel agents that acts against specific targets that are involved in driving the growth and survival of cancer cells. Transcription factors (Sp proteins, NF-kB) could be such potential targets for the intervention in PC therapy.
In this study we investigated a strategy using a combination of small molecule and Sp inhibitor (TA) and a natural product with therapeutic properties (Cur) for inducing treatment efficacy in PC cells (Figure 1). In order to verify if such efficacy is specific to this particular combination, we have tested other combinations using two NSAIDs, Ibuprofen and Celebrex (Figure 2). Ibuprofen is a common NSAID largely used for the treatment of mild pain and fever and is known to have a relatively good side effects profile. Ibuprofen did not show anti-proliferative effect in PC cells. Celebrex has been tested in clinical trials for treating some cancers (https://clinicaltrials.gov). In this investigation, results indicated that Celebrex caused PC cell growth inhibition similar to TA either in dose curves or in combination treatment (Figure 2) but showed significantly high cytotoxicity when compared to TA in H9C2 cardiomyocyte cells (approximately 4-fold more at 100 μM dose) suggesting that TA may cause lower cardiac side effects than Celebrex. These results further justify the reason for testing TA in this combination treatment.
Combination treatments involving phytochemicals have shown beneficial response for inhibiting cancer cell growth[37]. Several mechanisms have been described for the apoptosis in gastrointestinal tissues[38]. Apoptosis may occur through extrinsic and intrinsic pathways but both of these pathways converge on effector caspases 3/7 [39]. In this investigation, the results showed an increase in the expression of c-PARP (Figure 3B), apoptotic cell population (Figure 4) and the activation of effector caspases 3/7 (Figure 3C). Furthermore, the combination of Cur+TA also upregulated ROS in L3.6 pl cells (Figure 5). It is evident from Annexin-V staining that L3.6 pl cells are undergoing apoptosis at a much higher rate than MIA PaCa-2 cells. High levels of ROS could lead to cell death by activating apoptotic pathways. These results are in agreement with the combination index analysis, which demonstrates that the effect of TA+Cur combination on cell viability was synergistic only in L3.6 pl cells (Figure 3A).
The anti-proliferative effect of TA and Cur+TA combination was accompanied by a significant decrease in the expression of Sp1, Sp3, and survivin proteins (Figure 3B). Sp proteins regulate genes that are critical in the cellular processes (e.g., apoptosis and cell cycle arrest) associated with cancer [40, 41]. TA targets Sp1 and Sp3 transcription factors, which mediate the expression of survivin [42]. Survivin is an inhibitor of apoptosis protein and mediates apoptosis, cell division, and induces mitogenesis [39, 43, 44]. The inhibition of survivin could have partially contributed to the activation of caspases3/7 (Figure 3C).
Inducing ROS to high levels has been proposed as a therapeutic strategy in cancer treatment, however ROS also play role in cancer initiation. Earlier studies revealed that Cur and TA have the ability to increase ROS in cancer cells [45, 46]. Our results demonstrate that the combination of TA and Cur resulted in increased ROS levels compared to single drug treatment (Figure 5). The induction of ROS is much higher in L3.6pl, while MiaPaCa-2 cells showed a marginal increase. In fact, the percentage of apoptotic cells was much higher in L3.6pl cells when compared to MIA PaCa-2 cells following the combination treatment (Figure 4). ROS can cause DNA damage and subsequent cell death; therefore it could be responsible for higher cell death in L3.6pl cells. It is known that ROS can impact tumor initiation but at high levels it inhibits cancer cells and tumor growth. Both Cur and TA have been shown to increase ROS levels in L3.6pl cells. Both Cur [45] and TA [46] are known to generate ROS and cause subsequent DNA damage via modulating transcription factors [46] and/or microRNAs (miR-27a and miR-20a) [45]. Since both agents can induce ROS, the combination can further potentiate such response in susceptible cells. The current results demonstrate that an increase in ROS due to combination of Cur and TA plays an important role for inducing anti-proliferative effect in L3.6pl cells.
Transcription factor NF-κB regulates genes associated with inflammation, angiogenesis, apoptosis, cell growth, and invasion [47]. In this study results showed that the combination of TA+Cur inhibits the translocation of NF-kB into the nucleus (Figure 6). It is known that Cur and TA modulate NF-κB signaling in some cancer models [48, 49]. These results suggest that TA might be contributing to induce the effect of Cur for inhibiting NF-kB translocation and the co-treatment with both the agents is effective at least partially due to modulation of NF-kB associated signaling. Our results also showed that TA caused cell cycle arrest in both L3.6 pl and MIA PaCa-2 cells. Flow cytometry analysis (Figure 7) revealed that TA treatment results in accumulation of cells in G0/G1 phase in both cell lines. The combination treatment of TA+Cur in MIA PaCa-2 cells accumulated cells in G0/G1 and also affected the DNA synthesis phase (S). In L3.6 pl cells, each agent showed high response showing the effect at multiple phases (low DNA synthesis and cell arrest in G2/M). Since the combination is affecting multiple phases in cell cycle it may also contributing towards PC cell growth inhibition.
In summary, this study shows that food spice Cur and small molecule TA combination inhibits PC cell growth by inducing apoptosis and causing cell cycle arrest. The anti-proliferative effect of TA and TA+Cur combination is accompanied by a significant decrease in the expression of Sp1, Sp3 and survivin, induction of ROS and inhibition of NF-kB translocation. Among the two cell lines, L3.6 pl cells showed higher sensitivity with single agent or with combination treatment. The combination of TA+Cur caused higher growth inhibition in L3.6 pl cells than MIA PaCa-2 cells. This response is accompanied with modulation of multiple processes including apoptosis and cell cycle. The induction of apoptotic markers (c-PARP, caspase 3/7) and ROS as well as cell cycle arrest were more obvious in L3.6pl cells when compared to MIA PaCa-2. Association of ROS and NF-kB in modulating signaling cascades and inducing undesired effects of chemotherapy in pancreatic cancer was previously reported [50, 51], The combination of Cur+TA affects both ROS and NF-kB for inducing higher therapeutic efficacy in PC cells. Overall, these results suggest that suppression of Sp proteins and NF-kB signaling is primarily involved in the beneficial effect of this combination. Even though, research on Cur and TA reported anti-cancer activity in multiple cancers, it is the first study to test the combination of these two agents for potentiating anti-proliferative effect in PC cells. This therapeutic strategy provides promising activity and better tolerance than standard cytotoxic therapies currently being used in pancreatic cancer. Cur [48] and TA [52] have been tested (individually) for their chemopreventive properties and both of these agents showed a positive response in animal models. Cur exhibited cancer prevention in a clinical study. A phase IIa clinical trial showed that Cur was effective in reducing the formation of number of aberrant crypt foci revealing the evidence for the prevention of colorectal neoplasia [53]. Therefore, apart from therapeutic applications, this combination may be tested in future to focus on its chemopreventive properties.
Supplementary Material
Acknowledgments
This work was partially supported by a grant from the Shirley E. Noland Foundation (awarded to RB), Institute for Cancer Research and Pre-clinical Services, UNTHSC (RB & JS). JKV is supported by a grant (1P20 MD006882) from the National Institute on Minority Health and Health Disparities, NIH.
Footnotes
CONFLICT OF INTEREST
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langmead L, Rampton DS. Review article: herbal treatment in gastrointestinal and liver disease--benefits and dangers. Aliment Pharmacol Ther. 2001;15:1239–52. doi: 10.1046/j.1365-2036.2001.01053.x. [DOI] [PubMed] [Google Scholar]
- 2.Tunali-Akbay T, Sener G, Salvarli H, Sehirli O, Yarat A. Protective effects of Ginkgo biloba extract against mercury(II)-induced cardiovascular oxidative damage in rats. Phytother Res. 2007;21:26–31. doi: 10.1002/ptr.2007. [DOI] [PubMed] [Google Scholar]
- 3.Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol. 2006;25:79–84. doi: 10.1191/0960327106ht589oa. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annual review of nutrition. 2010;30:173. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambrechts S, Decloedt J, Neven P. Breast cancer prevention: lifestyle changes and chemoprevention. Acta Clin Belg. 2011;66:283–92. doi: 10.2143/ACB.66.4.2062570. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal DK, Mishra PK. Curcumin and its analogues: Potential anticancer agents. Medicinal Research Reviews. 2010;30:818–60. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. biochemical pharmacology. 2006;71:1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Shukla PK, Khanna VK, Ali MM, Khan MY, Srimal RC. Anti-ischemic effect of curcumin in rat brain. Neurochemical research. 2008;33:1036–43. doi: 10.1007/s11064-007-9547-y. [DOI] [PubMed] [Google Scholar]
- 9.Ströfer M, Jelkmann W, Depping R. Curcumin Decreases Survival of Hep3B Liver and MCF-7 Breast Cancer Cells: The Role of HIF (Original Article) Strahlentherapie und Onkologie. 2011;187:393–400. doi: 10.1007/s00066-011-2248-0. [DOI] [PubMed] [Google Scholar]
- 10.Pericleous M, Rossi RE, Mandair D, Whyand T, Caplin ME. Nutrition and pancreatic cancer. Anticancer Res. 2014;34:9–21. [PubMed] [Google Scholar]
- 11.Rachakonda PS, Bauer AS, Xie H, Campa D, Rizzato C, Canzian F, et al. Somatic mutations in exocrine pancreatic tumors: association with patient survival. PLoS One. 2013;8:e60870. doi: 10.1371/journal.pone.0060870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, Abrial C, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 13.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 14.Kurita T, Makino Y. Novel curcumin oral delivery systems. Anticancer Res. 2013;33:2807–21. [PubMed] [Google Scholar]
- 15.Ranjan AP, Mukerjee A, Helson L, Gupta R, Vishwanatha JK. Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: inhibition of tumor growth and angiogenesis. Anticancer Res. 2013;33:3603–9. [PubMed] [Google Scholar]
- 16.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–75. [PubMed] [Google Scholar]
- 17.Seeta Rama Raju G, Pavitra E, Nagaraju GP, Ramesh K, El-Rayes BF, Yu JS. Imaging and curcumin delivery in pancreatic cancer cell lines using PEGylated alpha-Gd2(MoO4)3 mesoporous particles. Dalton Trans. 2014;43:3330–8. doi: 10.1039/c3dt52692e. [DOI] [PubMed] [Google Scholar]
- 18.Nagaraju GP, Zhu S, Ko JE, Ashritha N, Kandimalla R, Snyder JP, et al. Antiangiogenic effects of a novel synthetic curcumin analogue in pancreatic cancer. Cancer Lett. 2015;357:557–65. doi: 10.1016/j.canlet.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Nagaraju GP, Zhu S, Wen J, Farris AB, Adsay VN, Diaz R, et al. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett. 2013;341:195–203. doi: 10.1016/j.canlet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Cheah YH, Nordin FJ, Sarip R, Tee TT, Azimahtol H, Sirat HM, et al. Combined xanthorrhizol-curcumin exhibits synergistic growth inhibitory activity via apoptosis induction in human breast cancer cells MDA-MB-231. Cancer Cell Int. 2009:9. doi: 10.1186/1475-2867-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Molecular pharmacology. 2008;73:1491–501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. Journal of cellular physiology. 2001;188:143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 23.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Molecular and cellular endocrinology. 2002;195:27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Le X, Abbruzzese JL, Peng Z, Qian CN, Tang H, et al. Constitutive Sp1 activity is essential for differential constitutive expression of vascular endothelial growth factor in human pancreatic adenocarcinoma. Cancer research. 2001;61:4143–54. [PubMed] [Google Scholar]
- 25.Torgeman A, Mor-Vaknin N, Aboud M. Sp1 is involved in a protein kinase C-independent activation of human T cell leukemia virus type I long terminal repeat by 12-O-tetradecanoylphorbol-13-acetate. Virology. 1999;254:279–87. doi: 10.1006/viro.1998.9556. [DOI] [PubMed] [Google Scholar]
- 26.Abdelrahim M, Smith R, 3rd, Burghardt R, Safe S. Role of Sp proteins in regulation of vascular endothelial growth factor expression and proliferation of pancreatic cancer cells. Cancer research. 2004;64:6740–9. doi: 10.1158/0008-5472.CAN-04-0713. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Wei D, Huang S, Peng Z, Le X, Wu TT, et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:6371–80. [PubMed] [Google Scholar]
- 28.Yao JC, Wang L, Wei D, Gong W, Hassan M, Wu TT, et al. Association between expression of transcription factor Sp1 and increased vascular endothelial growth factor expression, advanced stage, and poor survival in patients with resected gastric cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:4109–17. doi: 10.1158/1078-0432.CCR-03-0628. [DOI] [PubMed] [Google Scholar]
- 29.Basha R, Ingersoll SB, Sankpal UT, Ahmad S, Baker CH, Edwards JR, et al. Tolfenamic acid inhibits ovarian cancer cell growth and decreases the expression of c-Met and survivin through suppressing specificity protein transcription factors. Gynecol Oncol. 2011;122:163–70. doi: 10.1016/j.ygyno.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Eslin D, Lee C, Sankpal UT, Maliakal P, Sutphin RM, Abraham L, et al. Anticancer activity of tolfenamic acid in medulloblastoma: a preclinical study. Tumour Biol. 2013;34:2781–9. doi: 10.1007/s13277-013-0836-6. [DOI] [PubMed] [Google Scholar]
- 31.Sankpal UT, Abdelrahim M, Connelly SF, Lee CM, Madero-Visbal R, Colon J, et al. Small molecule tolfenamic acid inhibits PC-3 cell proliferation and invasion in vitro, and tumor growth in orthotopic mouse model for prostate cancer. Prostate. 2012;72:1648–58. doi: 10.1002/pros.22518. [DOI] [PubMed] [Google Scholar]
- 32.Abdelrahim M, Baker CH, Abbruzzese JL, Safe S. Tolfenamic acid and pancreatic cancer growth, angiogenesis, and Sp protein degradation. J Natl Cancer Inst. 2006;98:855–68. doi: 10.1093/jnci/djj232. [DOI] [PubMed] [Google Scholar]
- 33.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–81. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 34.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 35.Kern SE. Advances from genetic clues in pancreatic cancer. Current opinion in oncology. 1998;10:74–80. doi: 10.1097/00001622-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Korc M. Role of growth factors in pancreatic cancer. Surgical oncology clinics of North America. 1998;7:25–41. [PubMed] [Google Scholar]
- 37.Nessa MU, Beale P, Chan C, Yu JQ, Huq F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer Res. 2012;32:4843–50. [PubMed] [Google Scholar]
- 38.Timmons J, Chang ET, Wang JY, Rao JN. Polyamines and Gut Mucosal Homeostasis. J Gastrointest Dig Syst. 2012:2. [PMC free article] [PubMed] [Google Scholar]
- 39.Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239–52. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 40.Deniaud E, Baguet J, Mathieu AL, Pages G, Marvel J, Leverrier Y. Overexpression of Sp1 transcription factor induces apoptosis. Oncogene. 2006;25:7096–105. doi: 10.1038/sj.onc.1209696. [DOI] [PubMed] [Google Scholar]
- 41.Chae JI, Jeon YJ, Shim JH. Downregulation of Sp1 is involved in honokiol-induced cell cycle arrest and apoptosis in human malignant pleural mesothelioma cells. Oncol Rep. 2013;29:2318–24. doi: 10.3892/or.2013.2353. [DOI] [PubMed] [Google Scholar]
- 42.Xu R, Zhang P, Huang J, Ge S, Lu J, Qian G. Sp1 and Sp3 regulate basal transcription of the survivin gene. Biochem Biophys Res Commun. 2007;356:286–92. doi: 10.1016/j.bbrc.2007.02.140. [DOI] [PubMed] [Google Scholar]
- 43.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–15. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis. 2007;28:1133–9. doi: 10.1093/carcin/bgm047. [DOI] [PubMed] [Google Scholar]
- 45.Gandhy SU, Kim K, Larsen L, Rosengren RJ, Safe S. Curcumin and synthetic analogs induce reactive oxygen species and decreases specificity protein (Sp) transcription factors by targeting microRNAs. BMC Cancer. 2012;12:564. doi: 10.1186/1471-2407-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeong JB, Choi J, Baek SJ, Lee SH. Reactive oxygen species mediate tolfenamic acid-induced apoptosis in human colorectal cancer cells. Arch Biochem Biophys. 2013;537:168–75. doi: 10.1016/j.abb.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–82. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 48.Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005;223:181–90. doi: 10.1016/j.canlet.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 49.Shao HJ, Lou Z, Jeong JB, Kim KJ, Lee J, Lee SH. Tolfenamic Acid Suppresses Inflammatory Stimuli-Mediated Activation of NF-kappaB Signaling. Biomol Ther (Seoul) 2015;23:39–44. doi: 10.4062/biomolther.2014.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh AP, Arora S, Bhardwaj A, Srivastava SK, Kadakia MP, Wang B, et al. CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor kappaB: implications for bidirectional tumor-stromal interactions. J Biol Chem. 2012;287:39115–24. doi: 10.1074/jbc.M112.409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arora S, Bhardwaj A, Singh S, Srivastava SK, McClellan S, Nirodi CS, et al. An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor kappaB- and hypoxia-inducible factor 1alpha-mediated up-regulation of CXCR4. J Biol Chem. 2013;288:21197–207. doi: 10.1074/jbc.M113.484576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maliakal P, Abdelrahim M, Sankpal UT, Maliakal C, Baker CH, Safe S, et al. Chemopreventive effects of tolfenamic acid against esophageal tumorigenesis in rats. Invest New Drugs. 2012;30:853–61. doi: 10.1007/s10637-010-9622-0. [DOI] [PubMed] [Google Scholar]
- 53.Carroll RE, Benya RV, Turgeon DK, Vareed S, Neuman M, Rodriguez L, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354–64. doi: 10.1158/1940-6207.CAPR-10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.