SUMMARY
Microglia maintain homeostasis in the brain, but whether aberrant microglial activation can cause neurodegeneration remains controversial. Here, we use transcriptome profiling to demonstrate that deficiency in frontotemporal dementia (FTD) gene progranulin (Grn) leads to an age-dependent, progressive up-regulation of lysosomal and innate immunity genes, increased complement production, and enhanced synaptic pruning in microglia. During aging, Grn−/− mice show profound microglia infiltration and preferential elimination of inhibitory synapses in the ventral thalamus, which lead to hyperexcitability in the thalamocortical circuits and obsessive-compulsive disorder (OCD)-like grooming behaviors. Remarkably, deleting C1qa gene significantly reduces synaptic pruning by Grn−/− microglia, and mitigates neurodegeneration, behavioral phenotypes and premature mortality in Grn−/− mice. Together, our results uncover a previously unrecognized role of progranulin in suppressing aberrant microglia activation during aging. These results represent an important conceptual advance that complement activation and microglia-mediated synaptic pruning are major drivers, rather than consequences, of neurodegeneration caused by progranulin deficiency.
INTRODUCTION
Microglia are innate immune cells that repair injury and maintain homeostasis in the central nervous system (CNS)(Ransohoff and Perry, 2009). Previous studies indicate that microglia employ a diverse repertoire of proteins in the innate immune system to regulate synapse formation and maintenance. For example, in early postnatal life, microglia use the classical complement pathway to regulate synapse development in the lateral geniculate nucleus (Schafer et al., 2012; Stevens et al., 2007). During the aging process, however, progressive accumulation of complement C1qa in the dentate gyrus of hippocampus promotes cognitive decline and memory impairments (Stephan et al., 2013). In contrast, loss of complement C3 protein protects against age-dependent declines in synaptic and dendritic spine density in the CA3 region of the hippocampus, and rescues attenuation of long-term potentiation (LTP)(Shi et al., 2015). In addition, microglia also use fractalkine receptor CX3CR1 to regulate the growth and maintenance of dendritic spines on hippocampal neurons, which in turn serve as the structural basis of synapse formation (Paolicelli et al., 2011). Finally, genetic ablation of microglia in the adult brain further reveals the essential role of microglia in the maintenance of synaptic functions and motor learning (Parkhurst et al., 2013). Given these results, it has been proposed that excessive microglial activation may contribute to the pathogenesis of neurodegenerative diseases (Aguzzi et al., 2013). However, whether microglial activation directly contributes to neurodegeneration remains unclear.
In this study, we investigate the role of aberrant microglial activation as the primary pathogenic factor for frontotemporal dementia (FTD), the second most common dementia affecting patients younger than 65 years of age (Rascovsky et al., 2011; Ratnavalli et al., 2002). We focus on the autosomal dominant mutations in the human progranulin (GRN) gene, which cause a drastic reduction in progranulin (PGRN) levels, contributing to the pathogenesis of one the most common forms of familial frontotemporal lobar degeneration (FTLD)(Baker et al., 2006; Cruts et al., 2006; Finch et al., 2009; Ghidoni et al., 2008; Sleegers et al., 2009). Several studies indicate that PGRN is a key regulator of inflammation and that PGRN deficiency causes an aberrant increase in phagocytosis and pro-inflammatory cytokine production in microglia and macrophages (Kao et al., 2011; Martens et al., 2012; Yin et al., 2010). Furthermore, when exposed to the neurotoxin MPTP, both global Grn knockout (Grn−/−) and microglia-specific Grn knockout (Cd11b-Cre;Grnfl/fl) mutant mice show a much more robust increase in microglial activation and neuronal loss (Martens et al., 2012), supporting the idea that PGRN is required to suppress excessive microglial activation. Finally, rare homozygous GRN mutations in humans have been shown to cause neuronal ceroid lipofuscinosis (NCL), which shares similar neuropathological features with FTLD patients with GRN mutations (Gotzl et al., 2014; Smith et al., 2012). The connection between FTLD caused by GRN mutations and NCL is intriguing because neuroinflammation is a prominent and consistent feature in animal models of NCL and lysosomal storage diseases (Castaneda et al., 2008; Cotman et al., 2013). Together, these data suggest that PGRN deficiency may lead to a spectrum of neurodegenerative conditions in a dose-dependent manner.
Despite evidence supporting the role of PGRN in microglia function, it remains unclear how PGRN deficiency causes microglia activation and how PGRN-deficient microglia contribute to neurodegeneration in the aging brain. It is equally unclear whether blocking microglia activation in chronic PGRN deficiency could mitigate neurodegeneration. Here, we show that loss of PGRN causes an age-dependent up-regulation of lysosomal and innate immunity genes in microglia, which increases complement production and synaptic pruning activity by microglia to preferentially eliminate inhibitory synapses in the ventral thalamus. These defects lead to hyperexcitability in the thalamocortical circuits and obsessive-compulsive disorder (OCD)-like grooming behaviors. Remarkably, blocking complement activation significantly reduces synaptic pruning by Grn−/− microglia, and mitigates neurodegeneration, behavioral phenotypes and premature mortality in Grn−/− mice. These results uncover a previously unrecognized role of PGRN in suppressing microglia activation, and support an important conceptual advance that complement activation and microglia activation are major drivers, rather than consequences, of neurodegeneration caused by PGRN deficiency.
RESULTS
Transcriptional Profiling Reveals Age-dependent Lysosomal and Innate Immunity Defects in Grn−/− Microglia
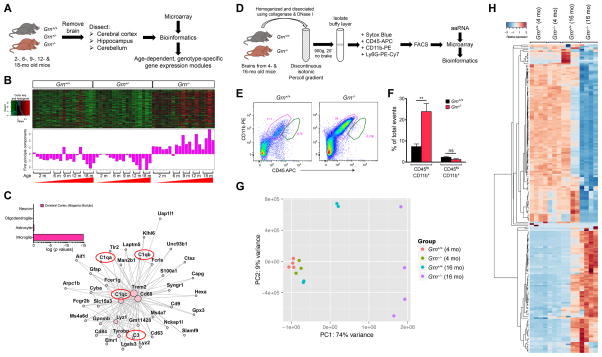
To investigate how PGRN deficiency contributes to neurodegeneration during aging, we analyzed the transcriptomes of the cerebral cortex, hippocampus and cerebellum in 2, 6, 9, 12 and 18 month old Grn+/+, Grn+/− and Grn−/− mice. We used weighted correlation network analysis (WGCNA) to identify highly correlated gene modules that were either up- or down-regulated in an age-dependent and genotype-specific manner (Figure 1A)(Langfelder and Horvath, 2008; Zhang and Horvath, 2005). Principal component analyses showed no difference in the transcriptomes between Grn+/+ and Grn+/− brain regions, but revealed an age-dependent up-regulation of one particular gene module in the cerebral cortex (magenta module)(Figure 1B), hippocampus (purple module) and cerebellum (pink module) of Grn−/− mice (Figure S1A). DAVID gene ontology (GO) analyses of these modules revealed two major categories, the lytic vacuole genes in the lysosomal pathway and genes related to innate immune responses (Table S1). Compared to an independent cell type enrichment dataset (Zhang et al., 2014), genes in the magenta module (cerebral cortex) showed exclusive association with the microglial lineage (p = 6.52 × 10−14)(Figure 1C, inset). System-level analyses of the top 40 genes from magenta, purple and pink modules revealed extensive topographical overlap (Figure 1C, Figure S1B–C), supporting functional interactions among these genes. At the center of this interconnected network were complement genes (C1qa, C1qb, C1qc and C3), CD68 and Trem2, which have been implicated in innate immunity, lysosomal function and microglial activation (Wang et al., 2015), respectively. These results are consistent with the cell-type enrichment transcriptome data, which shows that Grn mRNA is >50-fold enriched in microglia (>881.4 FPKM) (Figure S1D)(Zhang et al., 2014), and support that microglia contribute to the age-dependent transcriptional up-regulation of lysosomal and innate immunity genes in the aging Grn−/− brain.
Figure 1. Transcriptome profiling in Grn+/+, Grn+/− and Grn−/− mice reveal age-dependent up-regulation of lysosomal and innate immunity genes in microglia.
(A) Diagram showing the procedures to characterize the transcriptomes of specific brain regions in Grn+/+, Grn+/− and Grn−/− mice during aging. (B) Weighted correlation network analysis (WGCNA) identifies highly correlated gene modules that are age-dependently up-regulated in the cerebral cortex of Grn−/− mice. (C) The top 40 genes from the magenta (cerebral cortex) module are highly enriched with microglial genes (inset), and there is extensive topographical overlap among their expression patterns, especially for complements C1qa, C1qb, C1qc and C3, Cd68 and Trem2. (D) Diagram showing the procedures to isolate microglia from 4 and 16 month old Grn+/+ and Grn−/− mice using discontinuous isotonic Percoll gradient, FACS, preparation of amplified antisense RNA (aaRNA), microarray and bioinformatics analyses. (E) Dissociated cells from 16 month old Grn+/+ and Grn−/− mice are incubated with CD11b PE [clone M1/70](Invitrogen), CD45 APC [clone Ly5](eBioscience), and anti-Ly6G PE-Cy7 [clone 1A8](BD Biosciences), and sorted by Beckman-Coulter MoFlo XDPs flow cytometer. (F) Microglia are defined as CD45lo;CD11b+, whereas macrophages are defined as CD45hi;CD11b+ population in FACS. Data are presented as % of total events. Student’s t test, n = 3 for Grn+/+ and Grn−/− mice. ** indicates p < 0.01, ns, not significant. (G) Principle component analysis of the top 500 most variable transcripts from the FACS-sorted microglia from 4 and 16 month old Grn+/+ and Grn−/− mice. (H) Hierarchical clustering analyses of the top 200 transcripts from FACS-sorted microglia from 4 and 16 month old Grn+/+ and Grn−/− mice. See also Figure S1 and Table S1.
To validate the brain region-specific transcriptome profiling results, we isolated microglia from 4 and 16 month old Grn+/+ and Grn−/− mice using Percoll gradients and fluorescence-activated cell sorting (FACS) with microglia/macrophage/neutrophil markers, CD11b PE, CD45 APC and anti-Ly6G PE-Cy7 (Figure 1D). This approach revealed a 4-fold increase in the relative abundance of microglia (defined as CD45lo;CD11b+, pink gate in Figure 1E–F) in 16 month old Grn−/− mice, but no detectable increase in macrophage number (defined as CD45hi;CD11b+;Ly6G-, green gate in Figure 1E). We then analyzed the transcriptional profiles of FACS-sorted 4 and 16 month old Grn+/+ and Grn−/− microglia using principle component analysis of the top 500 most variable transcripts, and showed that the 4 month old Grn+/+ and Grn−/− microglia replicates clustered closely, suggesting that loss of PGRN had only modest effects on the transcriptional profiles of microglia at this age (Figure 1G). In contrast, 16 month old Grn−/− microglia showed much more profound alterations in transcriptional profiles than 16 month old Grn+/+ microglia (Figure 1G). Hierarchical clustering analyses of the top 200 transcripts confirmed that the transcriptional profiles of Grn+/+ and Grn−/− microglia were similar at 4 month old, but became drastically different at 16 month old (Figure 1H). Together, these results support that PGRN is required to suppress aberrant microglial activation during aging.
Characterizations of PGRN in the Endolysosomal Pathway in Microglia
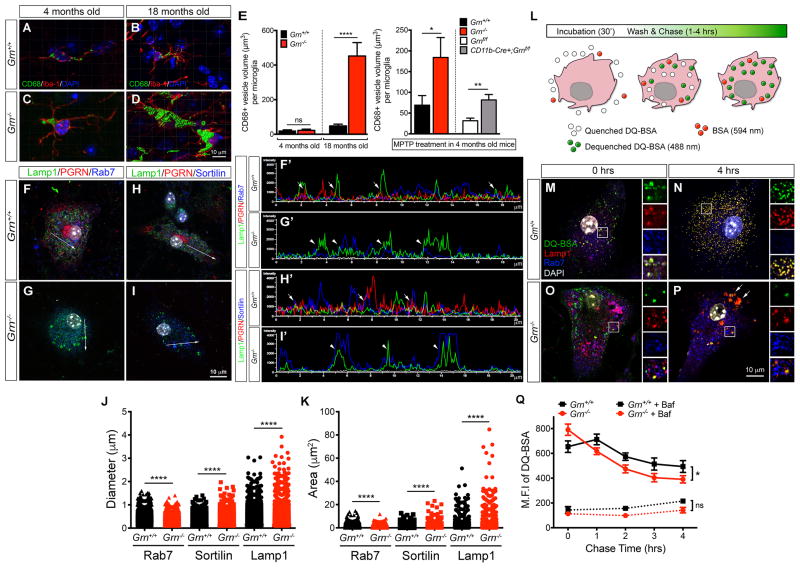
To characterize how microglia contribute to the up-regulation of lysosomal and innate immune response genes in Grn−/− brain, we analyzed lysosomal morphology in the microglia of 4 and 18 month old Grn+/+ and Grn−/− brains. Using CD68 as a marker for lysosomes and Iba-1 for microglia, we found that Grn−/− microglia showed a marked increase in lysosomal size at 18 month old (Figure 2A–E). Although microglia in 4 month old Grn+/+ and Grn−/− brains showed no difference in the lysosomal size, treatment with neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced a significant increase in the size of CD68+ lysosomes in Grn−/− microglia (Figure 2E). Similar MPTP-induced increase in lysosomal size was also detected in the microglia of Cd11b-Cre;Grnf/f mutants, suggesting that the lysosomal defects in Grn−/− microglia may be tightly coupled to their activation state.
Figure 2. Lysosomal defects and increased complement production by Grn−/− microglia.
(A–D) Confocal images of Iba-1+ microglia from the ventral thalamus of 4 and 18 month old Grn+/+ and Grn−/− brains show CD68+ lysosomes in the cytoplasm. Images were captured from Grn+/+ and Grn−/− brains (n = 3 per age), and processed for 3D reconstruction of the lysosomes using Imaris software. (E) Quantification of CD68+ lysosome volume in the ventral thalamus of 4 and 18 month old Grn+/+ and Grn−/− mouse brains (left panel) and in 4 month old Grn+/+ and Grn−/− mouse brains after MPTP treatment (right panel). Volumes are expressed as μm3 per microglia. Student’s t test, n = 3 per group. * indicates p < 0.05, ** p < 0.01, and **** p < 0.001. (F–I) Confocal images of primary microglia cultured from neonatal Grn+/+ and Grn−/− mouse brains. Microglia are labeled with antibodies for Lamp1/PGRN/Rab7 or Lamp1/PGRN/Sortilin. Arrows indicate regions in each microglia where fluorescent signal intensity plots are obtained using Nikon NIS-Elements. (F′-I′) Fluorescent signal intensity plots of Lamp1+ (green), PGRN+ (red), Rab7+ (blue) and Sortilin+ (blue) vesicles in Grn+/+ and Grn−/− microglia. Arrows in F′ and H′ indicate partial overlap of Lamp1+;PGRN+ or PGRN+;Sortilin+ signals, respectively. (J–K) Quantification of the size of Lamp1+, Sortilin+ and Rab7+ vesicles in Grn+/+ and Grn−/− microglia. Student’s t test, **** indicates p < 0.001. (L) Diagram showing DQ-BSA assays in cultured microglia. Open circles are quenched DQ-BSA, green circles are dequenched DQ-BSA and red circles are BSA-conjugated with 568nm fluorophore. (M–P) Representative images of Grn+/+ and Grn−/− microglia after 30′ incubation with DQ-BSA (0 hrs) or 4 hrs after washing (4 hrs). Scale bar in P is 10 μm. Insets to the right of each panel represent enlarged images in the boxed area in M-P. Arrows in P indicate dequenched DQ-BSA signals in Lamp1+ lysosomes. (Q) Quantification of the maximal fluorescence intensity (M.F.I.) of dequenched DQ-BSA in Grn+/+ (n = 5) and Grn−/− microglia (n = 7). Bafilomycin inhibits lysosomal acidification and protein degradation in Grn+/+ and Grn−/− microglia. Two-way ANOVA, * indicates p < 0.05. See also Figure S2.
To further characterize the lysosomal defects in Grn−/− microglia, we examined the subcellular localization of PGRN and the effects of PGRN deletion on lysosome morphology. Using confocal microscopy and fluorescent signal intensity plots of PGRN and markers for early endosomes (Rab5), late endosomes (Rab7), trans-Golgi network (Sortilin), and early lysosomes (Lamp1), we showed that PGRN did not co-localize with Rab5+ vesicles in Grn+/+ microglia (data not shown), but was detected in Golgi apparatus and in vesicles that were immediately adjacent to and partially overlapped with Rab7+, Sortilin+ and Lamp1+ vesicles (Figure 2F, F′, H, H′, arrows and Figure S2). In contrast, Grn−/− microglia showed many enlarged Lamp1+ vesicles that co-expressed Rab7 or Sortilin (Figure 2G, G′, I, I′, arrowheads and Figure S2).
Quantification using NIH ImageJ further revealed that Rab7+ vesicles were modestly reduced in size in Grn−/− microglia, whereas the size of Lamp1+ or Sortilin+ vesicles was significantly increased (Figure 2J–K and Figure S2). Given the phagocytic activity of microglia, we asked whether loss of PGRN might alter endolysosomal functions. To this end, we incubated Grn+/+ and Grn−/− microglia with BSA conjugated with fluorophore that emitted signal at 594 nm for 30′, and found no difference in Grn+/+ and Grn−/− microglia to endocytose BSA (data not shown). We then incubated Grn+/+ and Grn−/− microglia with a green BODIPY dye conjugated to BSA (DQ-BSA)(10 μg/ml), which upon proteolysis in acidic lysosomal compartments becomes dequenched and releases bright fluorescent signals (Figure 2L)(Vazquez and Colombo, 2009). After incubation with DQ-BSA for 30′, the intracellular trafficking of dequenched DQ-BSA signals in microglia were monitored using confocal microscopy and flow cytometry at 0, 1, 2, 3 and 4 hours. At the end of 30′ incubation, Grn−/− microglia contained ~20% more dequenched DQ-BSA than Grn+/+ microglia, most were located in Lamp1+ and Rab7+ vesicles (Figure 2M, O, Q). Interestingly, 4 hours after washing, Grn+/+ microglia still retained >75% of the dequenched DQ-BSA signals within the Lamp1+ vesicles (Figure 2N, Q). In contrast, only 49.3% of dequenched DQ-BSA signals remained in Lamp1+ vesicles in Grn−/− microglia (Figure 2P, Q). These results indicate that Grn−/− microglia are more efficient in processing materials via the endolysosomal pathway. To further demonstrate that the lysosomal functions in Grn−/− microglia are indeed intact, we used bafilomycin A1 (5 nM), a vacuolar type H+-ATPase inhibitor, to block acidification and protein degradation in lysosomes (Yoshimori et al., 1991). This treatment completely blocked DQ-BSA signals in Grn+/+ and Grn−/− microglia, with no detectable differences (Figure 2Q).
Complement Protein C1qa Promotes Synaptic Pruning by Grn−/− Microglia
To investigate the functional consequences of the lysosomal defects in Grn−/− microglia, we asked whether this phenotype promotes activation of innate immunity and thereby promoting neurodegeneration. Among the innate immune response genes up-regulated in Grn−/− brain, we focused on the complement system because complement-mediated synaptic pruning is a key mechanism in modulating neural circuit functions (Schafer et al., 2012; Stephan et al., 2013; Stevens et al., 2007). Furthermore, aberrant lysosome-mediated cleavage of complement protein C3 induces proinflammatory cytokine production in T lymphocytes and contributes to the pathogenesis of autoimmune disease (Liszewski et al., 2013). To characterize the role of complements in PGRN deficiency, we performed QRT-PCR using mRNA from Grn+/+ and Grn−/− primary cortical neurons and neonatal microglia, and showed that C1qa and C3 mRNA levels were much more abundant in Grn−/− microglia and further up-regulated upon treatment with lipopolysaccharide (LPS)(Figure S3A–B). FACS analyses using antibodies specific for C1qa, C3 and C3b confirmed more abundant complements in primary microglia from neonatal Grn−/− mouse brain in regular culture conditions and upon LPS treatment (Figure S3C–D). Finally, confocal microscopy showed that Grn−/− microglia acutely isolated from neonatal Grn−/− mouse brains expressed abundant C3 proteins that co-localized with lysosomal marker Lamp1 and secretory marker Grasp55 (Figure S3E–H), supporting that C3 proteins are processed in the lysosomes and released via the secretory pathway. In addition, microglia in 12 month old Grn−/− brains contained abundant C1qa in the cytoplasm, whereas microglia in Grn+/+ brains had very low C1qa expression (Figure S3I–N).
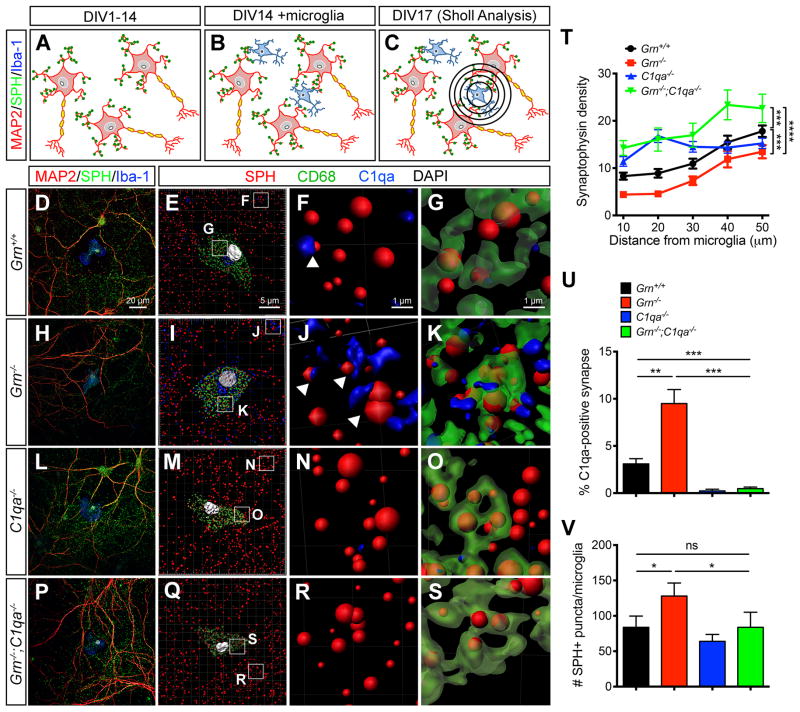
Given the marked increases in C1qa and C3 in Grn−/− microglia, we reasoned that increased complement production and up-regulation of lysosomal genes might allow Grn−/− microglia to be more efficient in pruning synapses. To test this, we designed a co-culture system in which wild type cortical neurons were plated at low density to allow uniform synapse development for 14 days in vitro (DIV14)(Figure 3A). Concurrently, microglia were cultured from Grn+/+ and Grn−/− neonatal brains, and added to cortical neurons at 1:3 ratio at DIV14. The co-cultures continued for 3 more days before they were collected for immunostaining and analysis (Figure 3B–C). To show the effect of microglia in synaptic pruning, we used a modified Sholl analysis to quantify the amount synaptophysin+ synapses around the cell body of microglia (Figure 3C). We also used the Imaris software to perform 3D reconstruction of confocal images to quantify the number of C1qa-tagged synapses and the number of synapses within the lysosomes of microglia.
Figure 3. Increased synaptic pruning activity in Grn−/− microglia requires C1qa.
(AC) Diagrams showing microglia-neuron co-cultures and Sholl analyses to quantify synapses around microglia. (D–S) Confocal images showing the presence of synapses (SPH+) around Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− microglia (Iba1+)(D, H, L, P). Imaris 3D image reconstruction of the microglia-neuron co-cultures at a lower magnification (E, I, M, Q). Higher magnification shows the presence of C1qa immediately adjacent synapses (F, J, N, R) and C1qa-tagged synapses inside CD68+ lysosomes in microglia (G, K, O, S). Scale bar 20 μm in D, 5 μm in E, and 1 μm in F and G. (T) Synaptic density around Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− microglia. *** p < 0.005, **** p < 0.001, two-way ANOVA, n = 4 for all genotypes. (U–V) Quantification of the percentage of C1qa-tagged synapses outside microglia (U), and the number of SPH+ synapse inside microglia (V). * p < 0.05, ** p < 0.01, *** p < 0.005, Student’s t test, n = 4 per genotype. See also Figure S3.
In Grn+/+ microglia-neuron co-cultures, the synaptic density adjacent to the cell body of microglia was low, with progressive increase in areas distant from microglia (Figure 3D, T [black line]). About 3% of synapses in Grn+/+ microglia-neuron co-cultures were immediately adjacent to C1qa-positive signals (Figure 3E–F), suggesting that these synapses were “tagged” by C1qa for removal. Indeed, several synapses were identified in CD68+ lysosomes within the cytoplasm of Grn+/+ microglia (Figure 3G). In contrast, the synaptic density surrounding Grn−/− microglia was significantly lower than that around Grn+/+ microglia (Figure 3H, T [red line]). Furthermore, there were more C1qa-positive synapses both outside Grn−/− microglia and within the CD68+ lysosomes inside Grn−/− microglia (Figure 3I–K, T–V).
In C1qa−/− microglia-neuron co-cultures, the synaptic density around microglia was much higher, especially within a 30 μm radius (Figure 3L–M, T [blue line]). No C1qa was detected near or within the synapse, again supporting the idea that C1qa was made by microglia, not by neurons (Figure 3N, U). The number of synapse within CD68+ lysosomes in C1qa−/− microglia was also significantly lower than that in Grn−/− microglia (Figure 3O, V), indicating that loss of C1qa reduced the synaptic pruning activity of microglia. To determine if loss of C1qa could mitigate synaptic pruning by Grn−/− microglia, we performed microglia-neuron co-cultures using microglia from Grn−/−;C1qa−/− neonatal mice and showed that the synaptic density around Grn−/−;C1qa−/− microglia was indeed much higher compared to that in Grn−/− and Grn+/+ microglia-neuron co-cultures (Figure 3P, T [green line]). Similar to the results from C1qa−/− microglia, no C1qa was detected near or at the synapse in Grn−/−;C1qa−/− microglia-neuron co-cultures, and the number of synapses within the lysosomes of Grn−/−;C1qa−/− microglia was also reduced (Figure 3R–V). Together, these results support that the increase of C1qa in Grn−/− microglia promotes, whereas loss of C1qa in Grn−/−;C1qa−/− microglia reduces synaptic pruning activity.
Age-dependent Complement Activation at the Grn−/− Thalamic Synapses
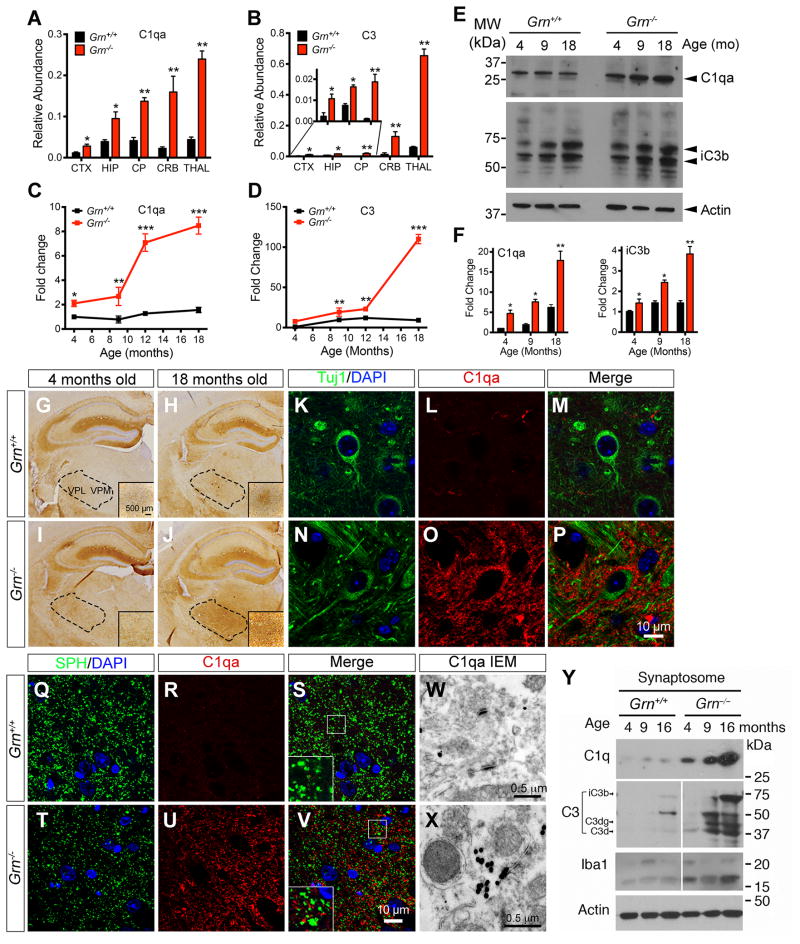
Having observed the increased synaptic pruning activity in Grn−/− microglia, we asked whether specific regions in Grn−/− mouse brains are more severely affected by the increases in complements and microglia, and how this might contribute to neurodegeneration. To test this, we performed QRT-PCR using mRNA from cerebral cortex, hippocampus, caudate/putamen, cerebellum and thalamus from 12 month old Grn+/+ and Grn−/− brains, and showed that C1qa and C3 mRNA were most abundant in the thalamus in Grn−/− brains, with an age-dependent increase (Figure 4A–D). In addition, western blots showed that both C1qa and C3 cleavage product iC3b were age-dependently up-regulated in the thalamus of Grn−/− mice (Figure 4E–F).
Figure 4. Age-dependent C1qa accumulation at the synapses of the ventral thalamus in Grn−/− mice.
(A–B) QRT-PCR detects the relative abundance of C1qa and C3 mRNA in cerebral cortex (CTX), hippocampus (HIP), caudate-putamen (CP), cerebellum (CRB) and thalamus (THAL) of 12 month old Grn+/+ and Grn−/− mice. (C–D) QRT-PCR shows the progressive increase of C1qa and C3 mRNA in the thalamus of 4, 9, 12 and 18 month-old Grn−/− mice. (E–F) Western blots showing the relative abundance of C1qa and C3 proteins in the thalamus of 4, 9 and 18 month-old Grn+/+ and Grn−/− mice. * p < 0.05, ** p < 0.01, *** p < 0.005, Student’s t test. Error bars indicate s.e.m. n = 3 per age for Grn+/+ and Grn−/− mice. (G–J) Immunostains in 4 and 18-month old Grn+/+ and Grn−/− mouse brain detect C1qa signals in the VPM and VPL thalamic nuclei (dotted areas). Scale bar in G is 500 μm. (K–P) Confocal images using neuronal marker TuJ1 and C1qa antibodies in the ventral thalamus of 12 month-old Grn+/+ and Grn−/− mice. Scale bar in P is 10 μm. (Q–V) Colocalization of synaptophysin and C1qa in the ventral thalamus of 12 month-old Grn+/+ and Grn−/− mice. Scale bar in V is 10 μm. (W–X) Immunogold EM detects C1qa deposits in synapses in the ventral thalamus of 12 month-old Grn+/+ and Grn−/− mice. Scale bars are 0.5 μm. (Y) Western blots using synaptosomes from 4, 9 and 16 month-old Grn+/+ and Grn−/− brains detect C1qa and cleaved C3 proteins.
Given the role of complements in synaptic pruning, we asked whether C1qa and C3 were deposited near or at the synapses in vivo. To address this, we performed immunostains and showed very low C1qa signal in the ventral thalamus of Grn+/+ mice at 4 and 18 month old (Figure 4G–H). In contrast, C1qa staining intensity showed a modest increase around the ventral thalamic nuclei of Grn−/− mice at 4 month old, and a much more prominent increase at 18 month old (Figure 4I–J). Whereas no detectable C1qa was found in the cell body of Grn+/+ or Grn−/− thalamic neurons, abundant C1qa was found in the neuropil surrounding the cell bodies and processes of Grn−/− neurons (Figure 4K–P).
To test if C1qa was deposited near or at synapses in the ventral thalamus of Grn−/− brain, we performed double labeling using antibodies for C1qa and the presynaptic marker synaptophysin (SPH). Consistent with our prediction, most C1qa signals were in close proximity to SPH+ puncta in the thalamus of Grn−/− mice. No detectable C1qa signals were found near the synapses in Grn+/+ brain (Figure 4Q–V). In addition, immunogold electron microscopy (IEM) showed that C1qa was immediately adjacent to synapses in Grn−/− thalamus, but not in Grn+/+ thalamus (Figure 4W–X). Finally, to provide biochemical evidence for complement deposition at the synapses of Grn−/− mice, we used discontinuous sucrose gradient to isolate synaptosomes from 4, 9 and 16 month old Grn+/+ and Grn−/− mouse brains (Carlin et al., 1980), and showed an age-dependent increase in C1qa and multiple C3 cleavage products in the synaptosomes from Grn−/− brain (Figure 4Y).
Removing C1qa in Grn−/−;C1qa−/− Mice Protects Synapse Loss, Restores Thalamic Microcircuit Function, Mitigates OCD-like Behaviors and Improves Survival
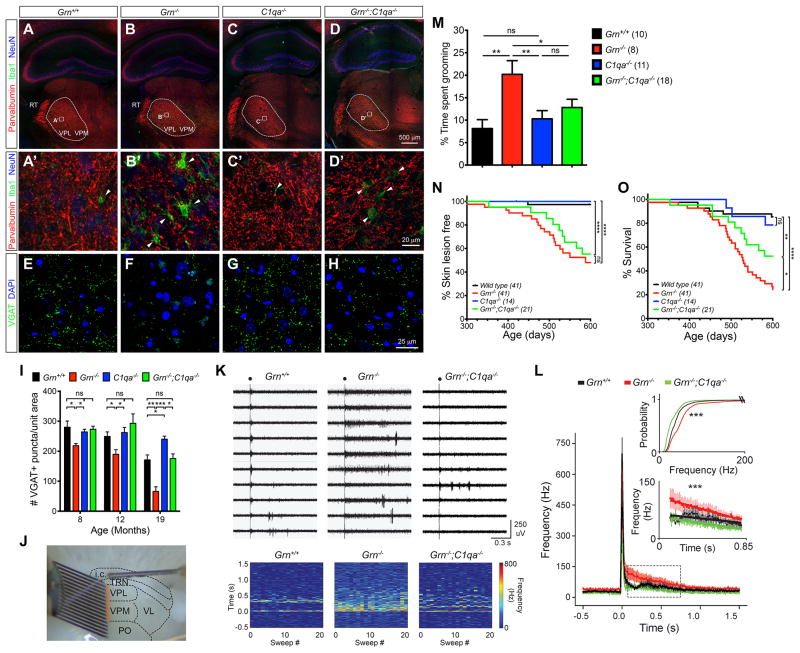
To determine whether synapse loss is a major phenotype in Grn−/− mouse brain and whether C1qa removal might protect synapse loss, we established an aging cohort of Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mice, and examined microgliosis and synaptic density in the ventral thalamus from 2 to 19 month old. During aging, the number of microglia in the ventral thalamus of Grn+/+ and C1qa−/− mice only showed a very modest increase, with inconspicuous cytoplasm and thin, delicate processes, consistent with resting, quiescent microglia morphology (Figure 5A–B, E–F, I). In contrast, Grn−/− mice showed an age-dependent increase in microglia in the ventral thalamus, and the majority of Grn−/− microglia exhibited abundant cytoplasm with short, prominent processes consistent with reactive microglia (Figure 5C–D, 5I). Interestingly, compared to Grn−/− mice, Grn−/−;C1qa−/− mice showed a consistent and significant reduction in the number of microglia in the ventral thalamus at 8, 12 and 19 month old (p = 0.0006, two-way ANOVA)(Figure 5G–I). Furthermore, Grn−/−;C1qa−/− microglia showed mixed morphology similar to Grn+/+ or Grn−/− microglia (Figure 5H). Using SPH as a marker for synaptic density, we found no detectable loss of synapse in the ventral thalamus of Grn+/+ and C1qa−/− mice during aging (Figure 5J, L, N). In contrast, Grn−/− mice showed significant reductions in SPH density in the ventral thalamus at 4, 7, 12 and 19 month old, whereas SPH density in Grn−/−;C1qa−/− mice was almost completely preserved (Figure 5K, M–N). These results are consistent with data from microglia-neuron co-cultures, and support the essential role of C1qa in mediating synaptic pruning by Grn−/− microglia in vivo.
Figure 5. Reduced microglia number and preservation of synaptic density in the ventral thalamus of Grn−/−;C1qa−/− mutant mice.
(A–H) Immunostains for Iba-1 in coronal sections of 12 month-old Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mouse brains at the level of anterior hippocampus. The square dotted boxes in panels A, C, E and G highlight the ventral thalamus, where the higher magnification images are obtained. Scale bar 500 μm in A and 50 μm in B. (I) Iba-1+ microglial density in the ventral thalamus of Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mouse brains at 2, 4, 7, 12 and 19 month-old. *** p < 0.005, **** p < 0.001, two-way ANOVA, n = 4 per genotype per age. (J–M) Confocal images of synaptophysin in 12 month-old Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− brains. Scale bar 10 μm in J. (N) Synaptophysin density in Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− brains at 2, 4, 7, 12 and 19 month-old. * p < 0.05, ** p < 0.01, *** p < 0.005, ns, not significant. Student’s t test, n = 4 per genotype per age.
Given the progressive loss of SPH in the ventral thalamus of Grn−/− mice, we asked whether synaptic pruning preferentially affects excitatory or inhibitory synapses. Within the ventral thalamus, neurons in the ventral posterolateral (VPL) and ventral posteromedial (VPM) nuclei project to layer IV of somatosensory cortex and receive excitatory inputs from neurons in layer VI (Figure S4A–B). The sole source of inhibition to this reciprocally excitatory circuit comes from parvalbumin-positive (Parv+) inhibitory neurons in the thalamic reticular nuclei (TRN), which co-express vesicular GABA transporter VGAT (Figure 6A–A′, Figure S4C–J). To investigate whether C1qa protein deposits in the ventral thalamus of Grn−/− mice can perturb the balance of excitatory and inhibitory synaptic inputs, we used confocal images to show that C1qa proteins extensively surrounded both VGLUT2+ excitatory and VGAT+ inhibitory synapses in the ventral thalamus of Grn−/− mice (Figure S5A–D). Although C1qa deposits showed no preference for excitatory or inhibitory synapses, the number of Parv+ synapses in the VPM and VPL of 12 month old Grn−/− mice was significantly reduced (Figure 6B–B′). Many Parv+ synapses were in close proximity to Iba1+ microglial processes, suggesting that Grn−/− microglia were actively pruning these synapses. Similar to these data, the number of VGAT+ synapses also showed significant reduction in the ventral thalamus of Grn−/− mice from 8 to 19 month old (Figure 6E–F, I). In contrast, the number of VGAT+ synapses in Grn−/−;C1qa−/− mice showed complete preservation (Figure 6G–I). Remarkably, unlike the VGAT+ synaptic phenotype, the number of VGLUT2+ excitatory synapses showed no reduction in Grn−/−, C1qa−/− or Grn−/−;C1qa−/− mice (Figure S5E–I).
Figure 6. Removing C1qa in Grn−/−;C1qa−/− mice protects synaptic pruning, restores thalamic microcircuit function, mitigate OCD-like behaviors and improves survival.
(A–D) Confocal images of parvalbumin (Parv) show the projection of Parv+ neurons in the reticular nucleus (TRN) to the ventroposterior medial (VPM) and ventroposterior lateral (VPL) nuclei in the ventral thalamus of 12 month old Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mice. Dashed lines highlight VPM and VPL nuclei, and squares regions higher magnification in A′-D′. Scale bar is 500 μm in D, and 20 μm in D′. (E–H) Confocal images of VGAT+ synapses in the ventral thalamus in 19 month old Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mice. Scale bar is 25 μm in H. (I) Quantification of VGAT+ synaptic density in the ventral thalamus of 8, 12 and 19 month old Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mice. Student’s t test, * indicates p < 0.05, ** p < 0.01 and ns, not significant. (J) Image of a thalamic slice showing the stimulating electrode in the internal capsule and the 16-channel linear array silicon probe that records multiunit firing in VPM and VPL. (K) Representative multiunit recordings (orange box in J) from the ventral thalamus of Grn+/+, Grn−/− and Grn−/−;C1qa−/− mice. Black circle indicates stimulation artifact. Bottom, rate meters showing consistent evoked spike rate across sweeps of stimulations (X-axis) relative to time (Y-axis). (L) Peri-stimulus time histogram of the population data from 6 Grn+/+, 7 Grn−/− and 4 Grn−/−;C1qa−/− mice. Inset bottom: enlargement of the black dashed box in (L) showing the slope of the response is significantly different among genotypes (***, p < 0.0001, F = 10.5554). Inset top: plot of the relative probability of eliciting AP firing frequencies among Grn+/+, Grn−/−, and Grn−/−;C1qa−/− mice analyzed by the Kolmogorav-Smirnov Test (Grn+/+ vs. Grn−/−, *** p < 0.0001, D = 0.5783; Grn+/+ vs. Grn−/−;C1qa−/−, *** p < 0.0001, D = 0.4980; Grn−/− vs. Grn−/−;C1qa−/−, *** p < 0.0001, D = 0.7751). Error bars, s.e.m., (M) Grooming activities in Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mice is expressed as percentage of total time. * p < 0.05, ** p < 0.01, ns, not significant, Student’s t test. (N–O) Kaplan-Meier curve for skin lesion onset and survival in Grn+/+, Grn−/−, C1qa−/− and Grn−/−;C1qa−/− mice. * p < 0.05, **** p < 0.001, ns, not significant, Long-rank (Mantel-Cox) test. See also Figures S4, S5 and S6, and Supplemental Movie S1.
To characterize how loss of inhibitory synapses in the ventral thalamus of Grn−/− mice might alter circuit function, we performed multiunit array extracellular recording in VPM and VPL in freshly prepared brain slices from 12 month old Grn+/+ and Grn−/− mice (Figure 6J). This approach preserves an intact intra-thalamic circuit and has been instrumental in determining the thalamic function in rodents (Paz et al., 2011; Paz et al., 2013). In the ventral thalamus of Grn+/+ mice, electrical stimulation of the internal capsule generated occasional bursts of action potentials (AP)(Figure 6K). In contrast, the same stimulation in the ventral thalamus of Grn−/− brain slices evoked a long-lasting and sustained tonic firing, with significant increase in AP firing frequency and the relative probability of eliciting AP (p < 0.0001)(Figure 6L). Remarkably, loss of C1qa in Grn−/−;C1qa−/− mice completely reversed the hyperexcitability phenotype observed in the Grn−/− mice and restored the evoked firing pattern in the thalamus to a pattern similar to Grn+/+ mice (Figure 6K–L).
Dysfunction in the thalamus and striatum has been implicated in obsessive compulsive disorder (OCD), a key clinical feature in FTD (Fitzgerald et al., 2011; Rascovsky et al., 2011). Consistent with hyperexcitability in the ventral thalamus, Grn−/− mice exhibited increased grooming activity, consisting of bouts of high frequency repetitive movement that covered the snout, face, ear and back (Supplemental Video 1). These excessive grooming behaviors began at 8 month old and persisted at 12 month old (Figure 6M and Figure S6A). Due to excessive grooming, >60% of Grn−/− mice developed severe skin ulcerations. In addition, ~10% of Grn−/− mice also developed motor dysfunction, including unsteady gait and imbalance (Supplemental Video 1). These two phenotypes contributed to the early mortality in Grn−/− mice (median survival for Grn−/− mice was 529 days, compared to 735 days in Grn+/+ mice, p < 0.0001, log rank [Mantel-Cox] test)(Figure 6N–O). In contrast, Grn−/−;C1qa−/− mice showed significantly reduced grooming activity, more modest skin lesions, and improved survival (Figure 6M–O and Figure S6B–D). These results support that blocking complement activation via C1qa gene deletion can mitigate the neurodegenerative phenotypes in Grn−/− mice.
Complement Activation As Biomarkers for FTLD With GRN Mutations
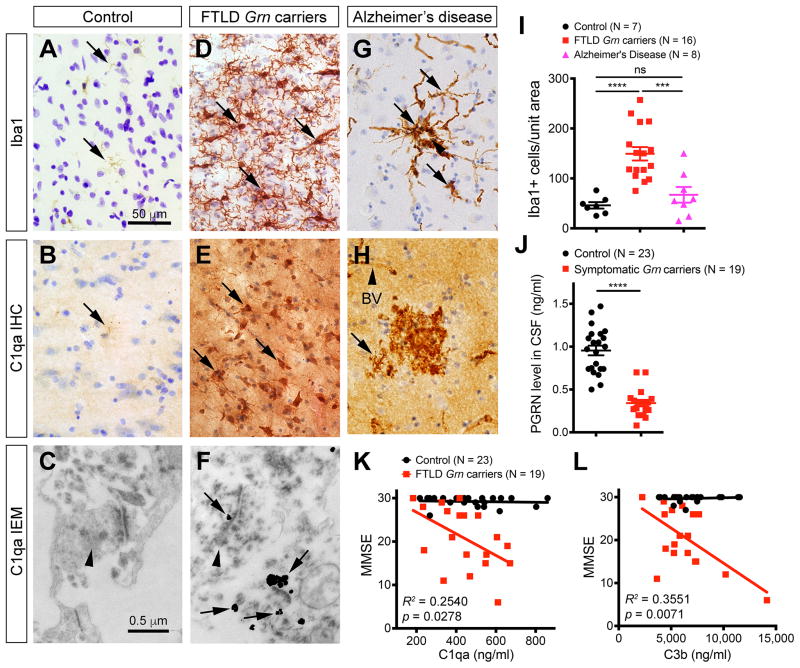
Finally, we asked whether microglial and complement activation also contribute to neurodegeneration in FTD patients with GRN mutations, and if so, whether complement activation could be a potential disease-specific biomarker. To this end, we examined microglial density in the frontal cortex of FTLD patients with GRN mutations (see Supplemental Information and Table S2 for details). In addition, we included patients with sporadic Alzheimer’s disease (AD) and age-matched control cases with no evidence of neurodegeneration (Table S2). In control frontal cortex, only few microglia with quiescent morphology were identified (Figure 7A). Most microglia in control individuals expressed very low levels of C1qa by immunohistochemistry and no C1qa was identified near synapses by IEM (Figure 7B–C). In contrast, FTLD GRN carriers showed a marked increase in microglial density, most prominently affecting layers I–III of the frontal cortex (Figure 7D). Similar to the microglia Grn−/− mouse brain, microglia in FTLD GRN carriers exhibited prominent reactive features and abundant C1qa deposits (Figure 7E). Furthermore, immunogold EM identified prominent C1qa signals near or at synapses in the frontal cortex of GRN mutation carriers (Figure 7F). To determine whether microglial infiltration in the frontal cortex of FTLD GRN carriers is disease-specific, we examined frontal cortex of AD patients and found that the majority of microglia in these cases appeared to surround the amyloid plaques, where abundant C1qa deposits were found (Figure 7G–H). On average, the microglial density in the frontal cortex of FTLD GRN carriers showed a 3–4 fold increase, whereas the microglial density in AD patients was similar to controls (Figure 7I).
Figure 7. Microglial pathology and CSF complement levels in FTLD Grn mutation carriers.
(A–H) Immunostains of frontal cortex from control, FTLD Grn carriers and AD patients detect the presence of microglia and C1qa (A–B, D–E, G–H). In addition, immunogold EM detects the presence of C1qa deposits at the synapses in FTLD Grn mutation carriers (C, F). Arrows in A, B, D, E, G and H indicate microglia. Arrowhead in G indicates an amyloid plaque, and in H indicates a blood vessel (BV). Arrowheads in C and F indicate presynaptic terminals, and arrows in F indicate C1qa-positive immunogold particles in synapses. (I) Quantification of Iba-1+ microglia in the frontal cortex of controls (n = 7), FTLD Grn carriers (n = 16), and AD patients (n = 8). *** p < 0.005, **** p < 0.001, ns, not significant, Student’s t test. (J) Quantification of CSF PGRN levels in controls and FTLD GRN carriers. Student’s t test, **** indicates p < 0.0001. (K–L) ELISA assays for C1qa and C3b protein levels in the CSF of controls (n = 23) and FTLD Grn carriers (n = 19). Chi Square Goodness of Fit test to calculate R2 and p values. See also Figure S7, Tables S2 and Table S3.
The results from the frontal cortex of FTD GRN carriers raise the possibility that complement proteins might be released into the cerebrospinal fluid (CSF) and could serve as biomarkers to predict disease onset and/or progression. Indeed, the utility of CSF was underscored by the ~70% drop in PGRN levels in FTLD patients with GRN mutations (Figure 7J). Interestingly, the levels of C1qa and C3b showed extensive overlapping in controls and FTLD GRN carriers, indicating that complement protein levels in CSF can be quite variable. However, as disease progressed in FTLD GRN carriers, the levels of C1qa and C3b progressively increased as cognitive functions declined (as determined by mini-mental status exam [MMSE] score)(Figure 7K–L). In contrast, analyses of previously published CSF data from AD patients (Smyth et al., 1994) showed that C1qa levels in AD patients were significantly lower than those in control and FTLD GRN carriers (controls: 479.5 ± 35.6, FTLD GRN carriers: 442.3 ± 33.0, AD: 268.0 ± 14.2, p < 0.0001, unpaired t test)(Figure S7A). Interestingly, as the MMSE declined in AD patients, the CSF C1qa levels showed progressive decrease (Figure S7B). Together, these results highlight the distinct differences in the microglia pathology and CSF complement protein levels between FTLD GRN carriers and AD patients.
DISCUSSION
PGRN Deficiency and Excessive Microglia Activation in the Aging Brain
Although PGRN has been implicated in lysosomal functions (Belcastro et al., 2011; Zhou et al., 2015), the exact mechanism remains poorly understood. Several lines of evidence from our study provide key mechanistic insights that PGRN regulates the formation and functions of lysosomes in the microglia. First, transcriptome profiling in Grn+/+, Grn+/− and Grn−/− aging brains shows that loss of PGRN leads to progressive up-regulation of genes that control lysosomal functions and the innate immunity response (Figure 1 and Figure S1). Second, confocal microscopic analyses of cultured microglia show that abundant PGRN protein is expressed in the microglia and localized primarily in the Golgi apparatus, Sortilin+ vesicles and lysosomes, suggesting that PGRN might regulate intracellular trafficking and the formation of lysosomes. In support of this idea, Grn−/− microglia exhibit profound lysosomal defects that facilitate more efficient processing via the endolysosomal pathway. Such lysosomal phenotypes, working in conjunction with the upregulation of complement protein C3, promote proteolytic cleavage of C3 by C3 convertase in the lysosomes and lead to a marked increase in the release of biologically active C3 products, including C3b and iC3d (Figure 2 and Figures S2-3)(Liszewski et al., 2013; Naito et al., 2012). Finally, western blot analyses show age-dependent accumulation of C1qa and cleaved C3 products in the synaptosomes of Grn−/− brain. Consistent with these results, Grn−/− microglia show a marked increase in synaptic pruning activity in microglia-neuron co-cultures and in the ventral thalamus of Grn−/− brain (Figures 3–6). Together, these results support the idea that PGRN serves as an important “brake” to suppress excessive microglia activation in the aging brain by facilitating phagocytosis and endolysosomal trafficking in microglia. Given the robust microglial activation in young mice exposed to the neurotoxin MPTP (Figure 2)(Martens et al., 2012), it is very likely that PGRN deficiency may have a broader role in suppressing microglia activation in other injury paradigms.
The identification of aberrant complement activation in Grn−/− microglia provides key mechanistic insights into the pathogenesis of neurodegeneration due to PGRN deficiency. The classical complement pathway serves as an important and well-recognized arm of the innate immunity surveillance system (Walport, 2001). Several previous studies indicate that complement-mediated synaptic pruning by microglia plays a critical role in the refinement of neural circuits during early postnatal development and in the normal aging process (Schafer et al., 2012; Shi et al., 2015; Stephan et al., 2013; Stevens et al., 2007). Although it has been postulated that excessive microglial activation may promote neurodegeneration (Aguzzi et al., 2013), the exact mechanisms remain unclear. Remarkably, our results show that loss of C1qa reduces synaptic pruning activity in Grn−/− microglia, reduces synapse loss, protects against hyperexcitability in the thalamocortical circuit, reduces behavioral abnormalities and improves survival in Grn−/−;C1qa−/− mice. These results support that lysosomal dysfunctions and complement activation in Grn−/− microglia are the main drivers that directly promotes neurodegeneration in a mouse model of FTLD.
Circuit-Specific Synaptic Pruning by Grn−/− Microglia
One surprising neurodegenerative feature in Grn−/− brains is the selective loss of inhibitory synapses in the ventral thalamus, despite the fact that the accumulation of C1qa can be detected in both excitatory and inhibitory synapses (Figure 6 and Figure S5). Such preferential elimination of inhibitory synapses by Grn−/− microglia is unprecedented in other neurodegenerative models. It is possible that differential expression of complement receptors or other recognition molecules in different synaptic subtypes may contribute to this selective phenotype. Alternatively, intrinsic properties of excitatory and inhibitory synapses may determine the efficacy of pruning by Grn−/− microglia. Regardless of the mechanism, the selective loss of inhibitory synapses in Grn−/− brain leads to increased excitability in the thalamocortical circuits.
Another remarkable finding in Grn−/− mice is the much more robust increase in microglia density in ventral thalamus, compared to other brain regions (Yin et al., 2010). This finding provides a neuroanatomical basis for the observed excessive grooming and OCD-like behaviors, and is further supported by the well-established role of the ventral thalamus in integrating sensory inputs, frontostriatal circuits and motor learning (Burguiere et al., 2015). While it remains unclear why Grn−/− microglia show a preferential effect on the thalamocortical circuit, given the highly evolutionarily conserved function of this circuit in sensorimotor integration in mammals (Petersen, 2007), it is possible that similar pathology may contribute to the clinical manifestations in FTLD patients. Consistent with this idea, perseverative and compulsive behaviors are among the most important diagnostic criteria for FTLD (Rascovsky et al., 2011). Together, the results reveal previously unrecognized mechanisms of PGRN in microglia function and how loss of PGRN may affect behaviors in a circuit-specific manner.
Despite the robust effects of deleting C1qa in mitigating several key neurodegenerative phenotypes in the aging Grn−/− mice, it is important to note that loss of C1qa does not completely rescue all the Grn−/− phenotypes. Although the microglial infiltration is significantly reduced in the ventral thalamus of Grn−/−;C1qa−/− mice, the microglial density is still significantly higher than age-matched Grn+/+ mice (Figure 5). Many microglia in Grn−/−;C1qa−/− mice continue to exhibit morphological features similar to those seen in Grn−/− mice. Furthermore, despite the significant improvement in survival, Grn−/−;C1qa−/− mice still show a modest increase in mortality compared to control Grn+/+ littermates. These results suggest that additional pathogenic factors, such as over-production of pro-inflammatory cytokines, other intrinsic microglia and/or neuronal defects, or intricate microglia-neuron interactions, may contribute to disease progression in Grn−/−;C1qa−/− mice in the absence of complement activation. For instance, the interacting network of up-regulated genes in Grn−/− brain also identifies Trem2 as a node that connects many other genes in the lysosomal and innate immunity pathways (Figure 1). Since the recent study indicates that TREM2 loss-of-function and TREM2-R47H variant attenuate lipid-sensing ability in microglia to mount a sustained response to neuronal injury in mouse AD models (Wang et al., 2015), these results raise the possibility that the elevated level of TREM2 in Grn−/− microglia may promote their synaptic pruning activity.
Microglia and Complement Activation As Critical Biomarkers for FTLD
One important neuropathologic feature in the frontal cortex of FTLD patients with GRN mutations is microglial activation, characterized by reactive morphology and excessive complement production (Figure 7). These findings are strikingly similar to those observed in Grn−/− mouse brain, and raise the possibility that GRN mutations in FTLD patients may lead to chronic PGRN deficiency in brain tissues, resulting in a de facto PGRN loss-of-function phenotype similar to Grn−/− mice (Baker et al., 2006; Cruts et al., 2006). Another interesting finding is that CSF samples from FTLD patients with Grn mutations show progressive increases in C1qa and C3b that correlate with cognitive decline (Figure 7). In contrast, the histopathologic characteristics of C1qa distribution in the frontal cortex and the positive correlation of complement elevation in the CSF with the decline of MMSE in FTLD GRN carriers are distinctly different from those in AD patients. These results support the disease specificity of complement activation in FTLD (Figure 7 and Figure S7)(Smyth et al., 1994), and further indicate that the underlying mechanisms that regulate the complement activation pathway in these two neurodegenerative diseases may be fundamentally different. These results underscore the importance of microglia and complement activation in human FTLD caused by GRN mutations, and support the feasibility of developing strategies that target complement proteins as biomarkers to track disease progression and as valid therapeutic targets to mitigate neurodegeneration in FTLD.
EXPERIMENTAL PROCEDURES
Brain Region-Specific Microarray and Bioinformatics Analyses
An aging cohort of Grn+/+, Grn+/− and Grn−/− mice, ranging from 2, 6, 9, 12 and 18 month old, was used to characterize age-dependent changes in transcriptomes caused by PGRN deficiency. Mice were perfused with ice-cold PBS, and brains were removed and further dissected to isolate cerebral cortex, hippocampus and cerebellum. Tissues were homogenized using the Bullet Blender (Next Advance, Averill Park, NY) in Trizol (Invitrogen, Grand Island, NY). RNA integrity was measured by running samples on a Bioanalyzer (Agilent, Santa Clara, CA). RNA samples were hybridized to Illumina (San Diego, CA) Mouse8 version 2 microarray chips as previously described (Rosen et al., 2011). Network analyses were performed as previously described, and gene module merging was accomplished using the WGCNA package in R (Langfelder and Horvath, 2008). Differentially expressed genes within a given module were compared against the murine background for enrichment within gene ontology (GO) analysis (Table S1). These modules were further subjected to system-level functional analyses by determining their topographical overlap.
Human Brain Tissues & Cerebrospinal Fluid Samples
Frozen frontal lobe tissues were procured from controls with no known neurodegenerative diseases, FTLD patients with GRN mutations and Alzheimer Disease (AD) patients. All cases were clinically and neuropathologically evaluated at the University of California San Francisco, Northwestern University and University of British Columbia. In addition, cerebrospinal fluid (CSF) samples were collected from controls and patients with GRN mutations and clinical diagnosis of FTD at UCSF and Italy. All human tissues and CSF samples were collected with informed consents and institutional IRB approvals. The demographic information, GRN mutations and pertinent clinical data of these cases are provided in Tables S2 and S3
Supplementary Material
Acknowledgments
We thank Ivy Hsieh for immunogold EM and Dr. Jennifer Cotter for critical comments on the manuscript. This work has been supported by NIH AG013854 (E.H.B.), P50 AG023501 and P01 AG019724 (B.L.M.), Italian Ministry of Helath (Ricerca Corrente, R.G.), JPB Foundation (B.A.B.), and Consortium for Frontotemporal Dementia Research (CFR) and the Bluefield Project (E.J.H.). Special thanks to Dr. Laura Mitic and Dr. Rodney Pearlman for their unwavering support.
Footnotes
ACCESSION NUMBERS
All microarray data have been deposited at GEO (GSE75083).
Supplemental information includes Supplemental Experimental Procedures & References, seven figures, three tables and one video can be found with this article online.
AUTHOR CONTRIBUTIONS
H.L., J.Z., H.Y.H., M.K.C., and Y.S. designed and performed the experiments, and analyzed data with E.J.H.. S.R.M. performed electrophysiology recordings, and S.R.M. and J.T.P. analyzed data. L.M.H. and G.C. performed and analyzed microarray data, J.Z. and C.L.H. performed FACS sorting of microglia, and F.G., G.C., K.W.K., S.A.S., M.C.O. and C.C.K. provided bioinformatics analyses. E.H.B., S.W., M-M.M., R.R., I.R.M., W.W.S., A.K., B.L.M., B.B., R.G., R.V.F. and B.A.B. provided human samples and reagents. E.J.H. supervised the project and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Belcastro V, Siciliano V, Gregoretti F, Mithbaokar P, Dharmalingam G, Berlingieri S, Iorio F, Oliva G, Polishchuck R, Brunetti-Pierri N, et al. Transcriptional gene network inference from a massive dataset elucidates transcriptome organization and gene function. Nucleic Acids Res. 2011;39:8677–8688. doi: 10.1093/nar/gkr593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguiere E, Monteiro P, Mallet L, Feng G, Graybiel AM. Striatal circuits, habits, and implications for obsessive-compulsive disorder. Curr Opin Neurobiol. 2015;30:59–65. doi: 10.1016/j.conb.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda JA, Lim MJ, Cooper JD, Pearce DA. Immune system irregularities in lysosomal storage disorders. Acta Neuropathol. 2008;115:159–174. doi: 10.1007/s00401-007-0296-4. [DOI] [PubMed] [Google Scholar]
- Cotman SL, Karaa A, Staropoli JF, Sims KB. Neuronal ceroid lipofuscinosis: impact of recent genetic advances and expansion of the clinicopathologic spectrum. Curr Neurol Neurosci Rep. 2013;13:366. doi: 10.1007/s11910-013-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- Finch N, Baker M, Crook R, Swanson K, Kuntz K, Surtees R, Bisceglio G, Rovelet-Lecrux A, Boeve B, Petersen RC, et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain. 2009;132:583–591. doi: 10.1093/brain/awn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF. Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:938–948. e933. doi: 10.1016/j.jaac.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidoni R, Benussi L, Glionna M, Franzoni M, Binetti G. Low plasma progranulin levels predict progranulin mutations in frontotemporal lobar degeneration. Neurology. 2008;71:1235–1239. doi: 10.1212/01.wnl.0000325058.10218.fc. [DOI] [PubMed] [Google Scholar]
- Gotzl JK, Mori K, Damme M, Fellerer K, Tahirovic S, Kleinberger G, Janssens J, van der Zee J, Lang CM, Kremmer E, et al. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014;127:845–860. doi: 10.1007/s00401-014-1262-6. [DOI] [PubMed] [Google Scholar]
- Kao AW, Eisenhut RJ, Martens LH, Nakamura A, Huang A, Bagley JA, Zhou P, de Luis A, Neukomm LJ, Cabello J, et al. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc Natl Acad Sci U S A. 2011;108:4441–4446. doi: 10.1073/pnas.1100650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens LH, Zhang J, Barmada SJ, Zhou P, Kamiya S, Sun B, Min SW, Gan L, Finkbeiner S, Huang EJ, et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J Clin Invest. 2012;122:3955–3959. doi: 10.1172/JCI63113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Bryant AS, Peng K, Fenno L, Yizhar O, Frankel WN, Deisseroth K, Huguenard JR. A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nat Neurosci. 2011;14:1167–1173. doi: 10.1038/nn.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz JT, Davidson TJ, Frechette ES, Delord B, Parada I, Peng K, Deisseroth K, Huguenard JR. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Rosen EY, Wexler EM, Versano R, Coppola G, Gao F, Winden KD, Oldham MC, Martens LH, Zhou P, Farese RV, Jr, et al. Functional genomic analyses identify pathways dysregulated by progranulin deficiency, implicating Wnt signaling. Neuron. 2011;71:1030–1042. doi: 10.1016/j.neuron.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Colodner KJ, Matousek SB, Merry K, Hong S, Kenison JE, Frost JL, Le KX, Li S, Dodart JC, et al. Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J Neurosci. 2015;35:13029–13042. doi: 10.1523/JNEUROSCI.1698-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Van Damme P, Engelborghs S, Gijselinck I, van der Zee J, Peeters K, Mattheijssens M, Cruts M, Vandenberghe R, et al. Serum biomarker for progranulin-associated frontotemporal lobar degeneration. Ann Neurol. 2009;65:603–609. doi: 10.1002/ana.21621. [DOI] [PubMed] [Google Scholar]
- Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MD, Cribbs DH, Tenner AJ, Shankle WR, Dick M, Kesslak JP, Cotman CW. Decreased levels of C1q in cerebrospinal fluid of living Alzheimer patients correlate with disease state. Neurobiol Aging. 1994;15:609–614. doi: 10.1016/0197-4580(94)00055-7. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Madison DV, Mateos JM, Fraser DA, Lovelett EA, Coutellier L, Kim L, Tsai HH, Huang EJ, Rowitch DH, et al. A Dramatic Increase of C1q Protein in the CNS during Normal Aging. J Neurosci. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Vazquez CL, Colombo MI. Assays to assess autophagy induction and fusion of autophagic vacuoles with a degradative compartment, using monodansylcadaverine (MDC) and DQ-BSA. Methods Enzymol. 2009;452:85–95. doi: 10.1016/S0076-6879(08)03606-9. [DOI] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Banerjee R, Thomas B, Zhou P, Qian L, Jia T, Ma X, Ma Y, Iadecola C, Beal MF, et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J Exp Med. 2010;207:117–128. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4 doi: 10.2202/1544-6115.1128. Article17. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Sun L, Bastos de Oliveira F, Qi X, Brown WJ, Smolka MB, Sun Y, Hu F. Prosaposin facilitates sortilin-independent lysosomal trafficking of progranulin. J Cell Biol. 2015;210:991–1002. doi: 10.1083/jcb.201502029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.