Abstract
Background
Over 70 million Americans inherit the strongest genetic risk factor for Alzheimer’s disease (AD), apolipoprotein E4 (APOE4), but have no course for reducing their risk. The association of non-steroidal anti-inflammatory drug (NSAID) use with reduced risk of AD for APOE4-carriers suggests that NSAIDs may be useful in AD prevention.
Methods
We identified phenotypes associated with APOE4 in APOE knock-in mice in order to define modifiable measures that correlate with risk of AD.
Results
APOE4 mouse brains showed altered post-translational modifications and biochemical distribution of APOE compared to APOE3 mice; these differences were also observed in brains of human APOE4 carriers. Two-month treatment with ibuprofen significantly altered the expression pattern of APOE in APOE4 mice to that of APOE3 mice; PPAR-γ agonist pioglitazone also had a significant effect. APOE4 mice also show deficits in dendritic spine density, and ibuprofen and pioglitazone significantly increased dendritic spine density.
Conclusions
We report new phenotypes associated with APOE4 in control human and APOE knock-in mice and their mitigation with NSAID treatment, through COX-2 inhibition and PPAR-γ activation.
Keywords: NSAID, ibuprofen, pioglitazone, celecoxib, APOE, preventative treatment
Introduction
Unsuccessful clinical trials for Alzheimer’s disease (AD) have led to the suggestion that successful therapies may need to be preventative in nature (Imbimbo et al., 2010; Sperling et al., 2014). Clinical trials for these approaches rely in part on altering AD biomarkers. Biomarkers related to AD neuropathological hallmarks include Aβ and tau in the cerebrospinal fluid (Tapiola et al., 2009) and brain (Klunk et al., 2004; Maruyama et al., 2013); these change years before clinical symptoms of AD (Bateman et al., 2012). Prevention studies for AD are now being pursued for individuals with causative AD mutations (St George-Hyslop et al., 1987) or two copies of the apolipoprotein E (APOE) risk allele, APOE-ε4 (Reiman et al., 2011).
Most of the genetic risk for late onset AD is contained in the alleles of APOE (Farrer et al., 1997). Thus, APOE genotypes could help identify new phenotypes for AD risk not dependent on AD neuropathological changes, particularly through examination of young individuals. For example, in young control individuals, APOE-ε4 is associated with brain structure and activity alterations (DiBattista et al., 2014; Green et al., 2014; Stevens et al., 2014), particularly within the medial temporal lobe (Kunz et al., 2015). Similarly, AD risk phenotypes could be identified in mice expressing different APOE alleles, but not displaying the plaques and tangles diagnostic of AD. Indeed, APOE4 knock-in mice have several differences compared to APOE3 mice, including pre-synaptic metabolic abnormalities (Dumanis et al., 2013), reduced post-synaptic neuronal complexity (Dumanis et al., 2009), and spatial learning impairments (Rodriguez et al., 2013), corroborating the human studies showing that APOE genotype impacts normal brain function independent of AD pathology (Caselli et al., 2004; Filippini et al., 2009; Scarmeas et al., 2005). Effective preventative treatments would be expected to alter these measures such that APOE4 mice would appear more like APOE3 mice.
One potential AD preventative treatment is the class of non-steroidal anti-inflammatory drugs (NSAIDs). Epidemiological studies have repeatedly shown that early NSAID use is associated with reduced AD risk in humans (Cornelius et al., 2004; in t’ Veld et al., 2001; Lindsay et al., 2002; Stewart et al., 1997; Zandi et al., 2002), but NSAIDs have not been successful at treating AD in clinical trials (Pasqualetti et al., 2009), or preventing AD in short term prevention trials of the elderly (Breitner et al., 2011). Interestingly, the preventative effect of NSAIDs may be most powerful in those with the APOE4 risk genotype (Cornelius et al., 2004; Hayden et al., 2007; Szekely et al., 2008; Yip et al., 2005). These findings suggest that NSAIDs are protective against AD, but only before accumulation of the neuropathological changes associated with AD (Breitner et al., 2011).
The impact of the findings on NSAID use to prevent AD has been limited due to the lack of an experimental model to demonstrate a biologically plausible mechanism for how NSAIDs could reduce risk of AD in the absence of pathological changes. The association of chronic NSAID use with higher rates of cardiovascular events provides further motivation to identify the mechanism for its protective effect in AD in order to develop a preventative treatment that eliminates this harmful side effect (Trelle et al., 2011). Here, we report new measures associated with the APOE risk genotype in the human APOE knock-in mouse model without gross AD pathology, including measures of the APOE protein maturation and distribution. We interrogated the response of APOE4 mice to treatment with the NSAID ibuprofen, and tested whether ibuprofen affected brain APOE distribution and neuronal dendritic spine density. For each of these measures, ibuprofen, which acts as both a COX-2 inhibitor and PPPAR-γ agonist, modified the APOE4 phenotypes to levels seen in APOE3 mice. A selective COX-2 inhibitor (celecoxib) and a PPAR-γ agonist (pioglitazone) also partially mitigated these APOE4 phenotypes, suggesting that both targets of ibuprofen (COX and PPAR-γ) are important for its effects on APOE4 phenotypes. These findings identify new APOE-associated phenotypes, demonstrate that APOE4 phenotypes can be modified by drug treatment, and demonstrate mechanisms by which ibuprofen could reduce AD risk.
Materials and Methods
Animals
Male and female mice expressing human APOE3 or APOE4 under the control of the endogenous murine APOE promoter were used (APOE3 and APOE4 mice (Sullivan et al., 1997)). These mice were backcrossed to a C57BL/6J background. Analyses of brain phenotypes associated with APOE4 were performed in age-matched mice at four ages: 5–6 months, 8 months, 12 months, and 22 months. For analysis of the effects of ibuprofen treatment, additional cohorts of animals were fed a control diet (Purina Rodent Chow, #5001, C11000; Research Diets Inc., New Brunswick, NJ, USA) or the identical diet containing 375 ppm ibuprofen (C12694) for 2 months beginning at 4 months of age, or 1 week beginning at 23 weeks of age. Other cohorts were also fed the same control diet, or an identical diet containing 240 ppm pioglitazone (C13418) for 1 week beginning at 23 weeks of age, or 120 ppm celecoxib (C12693) for 2 months beginning at 4 months of age. The dosages and durations were chosen based on previously published studies indicating that they were able to reduce amyloid load and inflammation in a mouse model of AD (Heneka et al., 2005; Varvel et al., 2009). All experiments were carried out in compliance with the Institutional Animal Care and Use Committee of Georgetown University.
Human Brain Tissue
Post-mortem human brain tissue from patients with Alzheimer’s disease was obtained from Johns Hopkins University (Braak Scores=5–6; CERAD=C). Brain tissue was resected from the medial frontal gyrus. Samples were genotyped, and divided into three groups: APOE3/APOE3 (n=7, mean age=81 years, 2 males, mean post-mortem duration=7.1 hours), APOE3/APOE4 (n=13, mean age=82.7 years, 2 males, mean post-mortem duration=10.3 hours), and APOE4/APOE4 (n=8, mean age=69.1 years, 4 males, mean post-mortem duration=17.1 hours).
Tissue Homogenization
Animals were euthanized and transcardially perfused with phosphate-buffered saline solution. Brain cortex and hippocampi were dissected and homogenized using a dounce homogenizer in Tris-buffered saline (TBS) buffer (50mM Tris-HCl, 150 mM NaCl, 1× protease and phosphatase inhibitor cocktails, pH 7.4) at 4°C. Homogenates were centrifuged at 100,000 × g at 4°C for 45 min, and the supernatant solutions were collected as the TBS-soluble fractions. The insoluble pellets were sonicated following resuspension in TBS-X buffer (50mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1× and phosphatase inhibitor cocktails, pH 7.4) at 4°C, and centrifuged at 100,000 × g at 4°C for 45 min. These supernatant solutions were collected as the TBSX-soluble fractions. Total protein concentrations were measured using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
Western blot and Isoelectric Focusing
For immunoblots, 20 μg of TBS-soluble or TBSX-soluble brain samples and Laemmli buffer with 10% β-mercaptoethanol were heated to 95°C for 5 min. Samples were subjected to gel electrophoresis and separated by size (Western blot) or isoelectric point and size (Isoelectric Focusing). Separated proteins were transferred to nitrocellulose (Western blot) or PVDF (Isoelectric Focusing) membranes and incubated in blocking buffer (5% non-fat dry milk in TBS with 0.05% Tween 20 (TBS-T)) for 1 hr. The following primary antibodies were diluted 1:1000 in blocking buffer and incubated overnight at 4°C: mouse anti-APOE (D6E10, ab1906, Abcam, Cambridge, MA, USA), mouse anti-IκB-α (L35A5, 4814, Cell Signaling, Danvers, MA, USA), rabbit anti-COX2 (ab15191, Abcam, Cambridge, MA, USA), rabbit anti-tubulin (Sigma, St. Louis, MO, USA), mouse anti-β-actin (Sigma, St. Louis, MO, USA). Membranes were washed 3× in TBS-T. The appropriate horseradish peroxidase-conjugated secondary antibody was diluted 1:5000 in blocking buffer and incubated at room temperature for 1 hr. Membranes were then washed 3× in TBS-T. SuperSignal West DURA luminol/enhancer solution (Pierce, Rockford, IL, USA) was added to membranes for 5 min. The density of bands was quantified using ImageJ software (NIH, Bethesda MD, USA).
Dendritic Spine Quantification
Dendritic spines were counted in Golgi-stained brains in the medial entorhinal cortex (MEC) along apical oblique (AO) dendrites (20 micron sections) and basal shaft (BS) dendrites (10 micron sections). Dendritic spine density was measured as dendritic spine count divided by the length of the dendritic segment. A minimum of 30 dendritic segments were analyzed per dendritic region (AO vs BS)/treatment group. When analyzing dendritic segments, at least five neurons were measured and averaged per animal, and 6 animals were used from each cohort.
Statistical Analyses
Unless otherwise specified, data are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism software (San Diego, CA, USA). Two-way ANOVAs were used to analyze the effect of age and APOE genotype on biochemical markers. One-way ANOVAs and Student’s t-tests were used to determine the effect of treatments on APOE-associated phenotypes of interest. Non-parametric statistical analyses were substituted for parametric tests when the data did not follow a Gaussian distribution.
Results
Differences in APOE solubility are associated with its post-translational modifications
Several relatively subtle biochemical differences have been identified between brains of APOE3 and APOE4 mice (Dumanis et al., 2013; Sullivan et al., 2011); we sought to identify other brain phenotypes associated with APOE genotype. We measured APOE levels in the brains of APOE3 or APOE4 mice using a sequential brain fractionation method to isolate different forms of APOE: APOE soluble in Tris buffered saline (TBS) and APOE soluble in TBS with 1% Triton X (TBSX). APOE was easily detected in both brain fractions (Figure 1A), suggesting that there are subtypes of APOE with different solubility characteristics. Interestingly, the APOE extracted by TBS was a higher molecular weight species compared to APOE extracted in TBSX (Figure 1A). We hypothesized that differences in post-translational modifications of APOE were responsible for this difference. Although other types of modifications could account for this effect, we hypothesized that this is due to O-linked glycosylation of APOE that allows for the addition of sialic acid groups, which would both increase the molecular weight and decrease the isoelectric point (pI) of APOE (Mailly et al., 1990; Rebeck et al., 1998; Zannis et al., 1986). Thus, we used 2D gel electrophoresis to analyze TBS- and TBSX-soluble APOE from APOE3 and APOE4 mice. We found that in addition to a higher molecular weight, TBS-soluble APOE had a lower pI compared TBSX-soluble APOE (Figure 1B). As a control, we also measured levels of a known cytosolic protein (IκB) and known membrane-bound protein (COX2) in these samples. As anticipated, the TBS fraction was enriched in cytosolic protein IκB while the TBSX fraction was enriched in membrane-bound protein COX2 (Figure 1C). These results demonstrate that the TBS- and TBSX-soluble fractionations represent different forms of APOE: TBS-soluble APOE is larger and more acidic than TBSX-soluble APOE.
Figure 1. Differences in APOE solubility are associated with its post-translational modifications.
(A) Western blot for hippocampal brain fractions demonstrating a higher molecular weight for TBS-soluble APOE compared to TBSX-soluble APOE from 6-month-old mice. (B) 2D gel electrophoresis for TBS- and TBSX-soluble hippocampal brain fractions in APOE3 (top panels) and APOE4 (bottom panels) mice shows that TBS-soluble APOE has a lower pI than TBSX-soluble APOE. (C) Western blot of hippocampal TBS and TBSX fractionations shows that the TBS fraction is enriched in cytosolic protein IκB while the TBSX fraction is enriched in membrane-bound protein COX2.
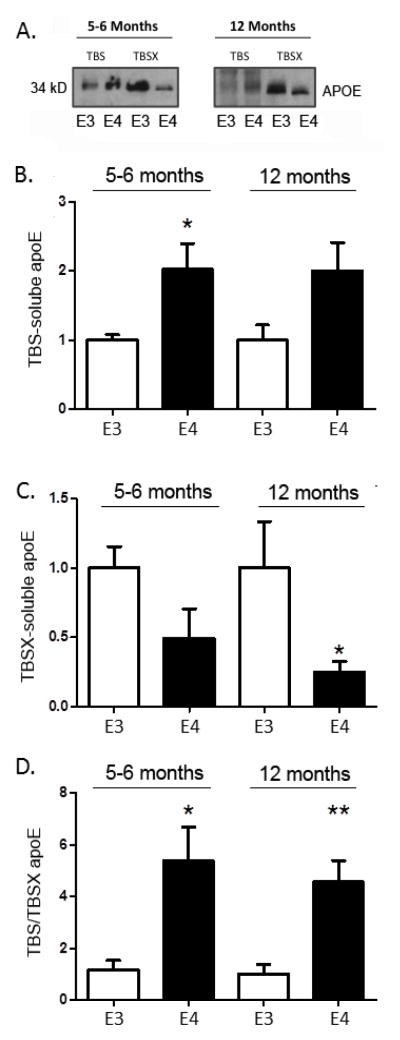
Levels of TBS-soluble APOE are higher, and levels of TBSX-soluble APOE are lower, in APOE4 mouse cortex
We next measured whether there were differences in TBS- and/or TBSX-soluble APOE levels in the cortices of the APOE3 and APOE4 mice during aging. APOE levels in the TBS fraction of brain cortex were significantly higher in the APOE4 mice at 5–6 months, 8 months, 12 months and 22 months of age compared to APOE3 mice (Two (age) × two (genotype) ANOVA, n=4 brains/genotype/age group, F(1, 24)=86.23, p<0.0001 (Figure 2A, C)). Conversely, APOE levels in the TBSX cortical brain fractions were significantly lower in APOE4 mice at 5–6 months, 8 months, 12 months and 22 months of age compared to APOE3 mice (F(1, 24)=19.93, p=0.0002 (Figure 2B, D)). We did not observe a significant effect of aging on the levels of APOE in the TBS or TBSX cortical brain fractions in APOE3 mice (Figure 2C, D). Levels of TBS-soluble APOE were significantly affected by aging in the APOE4 mice, with the lowest levels in the 5–6 month old mice, and the highest levels in the 12 month old mice (F(2, 24)=12.23, p<0.0001 (Figure 2C)). To generate a single indicator of APOE distribution between these brain fractions, we defined the ratio of TBS-soluble APOE over TBSX-soluble APOE; as expected, this ratio was significantly affected by age (F(3, 24)=3.28, p=0.0381) and APOE genotype (F(1, 24)=20.03, p=0.0002) (Figure 2E). Previous work also revealed an age-dependent decrease in cortical dendritic spine density with aging in APOE4 mice compared to APOE3 mice (Dumanis et al., 2009). These data demonstrate a significant and robust difference in the distribution of APOE between the TBS- and TBSX-soluble cortical fractions in APOE4 mice compared to APOE3 mice.
Figure 2. TBS-soluble APOE is higher, while TBSX-soluble APOE is lower, in APOE4 mouse cortex.
Representative Western blot images for APOE levels in APOE3 mice (E3) and APOE4 mice (E4) in the TBS-soluble (A) and TBSX-soluble cortical fractions (B) at 5–6 months, 8 months, 12 months, and 22 months of age. (C) Quantification of Western blots in A, ***p<0.001 main effects of genotype and age. (D) Quantification of Western blots in B, ***p<0.001 main effect of genotype. (E) Quantification of the ratio of TBS-soluble and TBSX-soluble APOE in the cortex. ***p<0.001 main effect of genotype and age. Levels were normalized to β-actin, expressed as the mean percentage of the 5–6 month old APOE3 group ± SEM, and compared using a 2 (age) x 2 (genotype) factor ANOVA, ***p<0.001 main effects; #p<0.01, ###p<0.001 Bonferroni multiple comparisons post-hoc analyses within each age group, n = 4 brains/genotype/age group.
APOE genotype similarly affects APOE distribution in mouse hippocampus
We next investigated whether there was also a similar effect of APOE genotype on the distribution of APOE between the TBS- and TBSX-soluble fractions in the hippocampus, a region affected early in AD. We measured APOE levels in the hippocampus of APOE3 and APOE4 mice at 5–6 months of age and 12 months of age (Figure 3), and found results consistent with data from the cortex (Figure 2). In the TBS-soluble fraction, APOE levels were significantly higher in APOE4 mice compared to APOE3 mice at 5–6 months of age (one-way ANOVA, n=4 brains/group, F(3, 12)=4.188, p=0.03 main effect; t=2.616, p<0.05, post-hoc); a similar non-significant trend (t=2.375, p=0.067, post-hoc) was observed in hippocampal samples from 12 month old mice (Figure 3A, B). APOE levels in the TBSX-soluble hippocampal fraction were significantly lower in APOE4 mice compared to APOE3 mice at 12 months of age (F(3, 12)= 6.93, p=0.0058 main effect; t=1.653, p<0.05, post-hoc), and a similar but not statistically significant trend (t=1.653, p=0.072, post-hoc) was observed in samples from mice at 5–6 months of age (Figure 3A, C). As in the cortex, the ratio of APOE in the TBS-soluble fraction to the TBSX-soluble fraction in the hippocampus was significantly higher (four to five fold) in APOE4 mice compared to APOE3 mice at 5–6 months of age (F(3, 12)= 7.688, p=0.004 main effect; t=3.635, p<0.01, post-hoc) and 12 months of age (t=3.081, p<0.01, post-hoc) (Figure 3D). In total, we have observed the effect of APOE genotype on brain APOE distribution in 22 APOE3 mice and 22 APOE4 mice (Figure 2, n=16/APOE genotype, with n=4/genotype/age group; Figure 3, n=6/APOE genotype).
Figure 3. APOE genotype similarly affects APOE distribution in mouse hippocampus.
(A) Representative Western blot images for APOE levels in APOE3 mice (E3) and APOE4 mice (E4) in the TBS- and TBSX-soluble hippocampal fractions. Quantification of 5–6 month and 12 month old hippocampal APOE as TBS-soluble APOE (B), TBSX-soluble APOE (C), and (D) the ratio of TBS to TBSX extracted samples, n = 6 brains/genotype/age group. Levels were normalized to β-actin, expressed as the mean percentage of the APOE3 control group ± SEM, and compared using one-way ANOVA with Bonferroni multiple comparison post-hoc analyses, *p<0.05, **p<0.01, ***p<0.001.
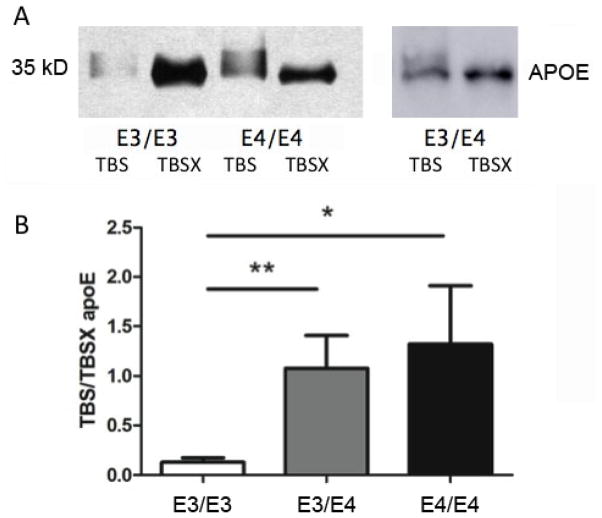
APOE genotype also affects APOE distribution in human brain samples
We next tested whether the difference in APOE distribution was also present in human brain samples. We genotyped human AD brain samples (E3/E3: n=7, E3/E4: n=13, E4/E4: n=8) and processed those tissues in the same manner as the APOE mouse brains. Western blot analysis revealed an increased molecular weight corresponding to TBS-soluble APOE compared to TBSX-soluble APOE (Figure 4A), as previously observed for the APOE knock-in mice (Figure 1). The ratio of TBS to TBSX soluble APOE significantly higher in APOE4/APOE4 carriers compared to APOE3/APOE3 carriers (Kruskal-Wallis nonparametric test, p=0.0046; post-test, p<0.05) (Figure 4B). Heterozygous APOE3/APOE4 carriers had a significantly different APOE distribution compared to APOE3/APOE3 carriers (p<0.01) (Figure 4B). Although it is possible that disease state impacted the observed APOE phenotype, the altered APOE distributions were consistent between control APOE4 mice (E4>E3), and human AD APOE4 carriers (E4/E4 > E3/E4 > E3/E3).
Figure 4. APOE genotype affects APOE distribution in human brain samples.
(A) Representative Western blot of human brain samples probed with the D6E10 APOE antibody in APOE3/APOE3 (E3/E3), APOE4/APOE4 (E4/E4), and APOE3/APOE4 (E3/E4) samples. (B) Ratio of TBS to TBSX soluble APOE in APOE3/APOE3 (n=7), APOE3/APOE4 (n=13), and APOE4/APOE4 (n=8) carriers (Kruskal-Wallis nonparametric test, p=0.0046; APOE3/APOE3 vs. APOE4/APOE4 post-test, p<0.05; APOE3/APOE3 vs. APOE3/APOE4, p<0.01).
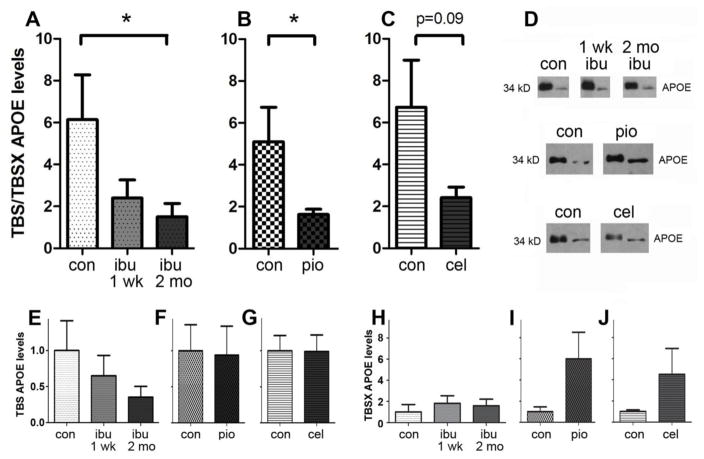
APOE distribution can be modified with drug treatment in 6-month-old APOE4 mice
We then tested whether the difference in APOE distribution in the APOE4 animals would be amenable to rescue via drug treatment. We tested ibuprofen because epidemiological studies suggest that ibuprofen may prevent AD specifically in APOE4 carriers (Cornelius et al., 2004; Hayden et al., 2007; Szekely et al., 2008; Yip et al., 2005). We fed a new cohort of APOE4 mice (n=6 per group) control diets or diets containing 375 ppm ibuprofen for 1 week or 2 months (this dosage was shown to reduce glial inflammation in an animal model of AD (Heneka et al., 2005; Varvel et al., 2009)). We euthanized the 6-month-old animals, and measured APOE in defined brain extracts. Ibuprofen treatment resulted in a redistribution of APOE between TBS and TBSX brain fractions in APOE4 brains, such that there was a significant decrease in the ratio of APOE in the TBS versus TBSX fractions (F=3.172, t=2.374, p<0.05, post-hoc), similar to what we had previously observed in the APOE3 brains (Figure 5A, D, E, H). To determine whether the mechanism of action of ibuprofen relied on its activity as a COX-2 inhibitor or as a PPAR-γ agonist, we fed new cohorts of APOE4 mice with the selective COX-2 inhibitor celecoxib, or the PPAR-γ agonist pioglitazone, based on previously reported dosages to reduce glial inflammation in an animal model of AD (Heneka et al., 2005; Varvel et al., 2009). Specifically, control diets or diets containing 240 ppm pioglitazone were fed to one cohort for 1 week (n=6 per group), and another cohort was fed control diets or diets containing 120 ppm celecoxib for 2 months (n=6 per group). Animals were again euthanized at 6 months of age, and APOE was measured in hippocampal brain extracts. Similar to ibuprofen, pioglitazone treatment resulted in a redistribution of APOE between TBS and TBSX brain fractions, such that there was a significant decrease in the ratio of APOE in the TBS versus TBSX fractions (t=2.076, p=0.032) (Figure 5B, D, F, I). Although celecoxib treatment did not result in a significant redistribution of APOE between TBS and TBSX fractions, there was a trend toward a decrease in the ratio of TBS to TBSX soluble APOE (t=1.87, p=0.09) (Figure 5C, D, G, J). These results suggest that both the COX-2 inhibiting and PPAR-γ activating actions of ibuprofen are contributing to its effects on APOE expression, and that changes to both the TBS- and TBSX-soluble APOE fractions are altered by drug treatments.
Figure 5. APOE distribution can be modified with drug treatment in 6-month-old APOE4 mice.
Ratio of TBS to TBSX soluble hippocampal APOE in APOE4 mice treated with: (A) control diets versus 375 ppm ibuprofen (ibu) for 1 week (1 wk) or 2 months (2 mo) (F=3.172, t=2.374, p<0.05, post-hoc, n=6/group), (B) control diets versus 240 ppm pioglitazone (pio) for 1 week (t=2.076, p=0.032, n=6/group), (C) control diets versus 120 ppm celecoxib (cel) for 2 months (t=1.87, p=0.09, n=6/group). (D) Representative Western blots showing hippocampal TBS-soluble (left band in each pair) and TBSX-soluble (right band in each pair) APOE. (E–G) TBS-soluble APOE in APOE4 mice treated with: (E) ibuprofen (F=0.9583, p=0.4287), (F) pioglitazone (t=0.1081, p=0.9165), (G) celecoxib (t=1.434, p=0.1822), expressed as a percentage of control. (H–J) TBSX-soluble APOE in APOE4 mice treated with: (H) ibuprofen (F=0.3364, p=0.7293), (I) pioglitazone (t=2.162, p=0.0589), (J) celecoxib (t=1.434, p=0.1822), expressed as a percentage of control.
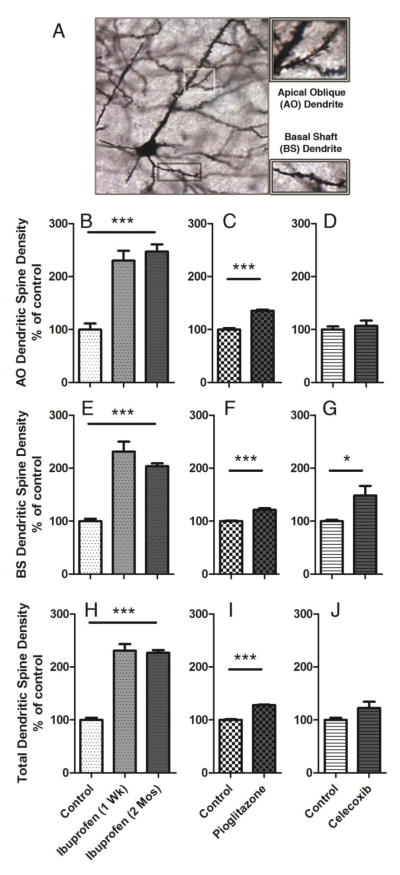
Ibuprofen and pioglitazone promote dendritic spine density in 6-month-old APOE4 mice
APOE4 is associated with reduced dendritic spine density in human hippocampus (Ji et al., 2003), mouse motor cortex (Dumanis et al., 2009) and mouse medial entorhinal cortex (MEC) (Rodriguez et al., 2013). APOE4 is also associated with deficits in grid-cell-like representations in the entorhinal cortex in young adult humans (Kunz et al., 2015). Therefore, in addition to measuring brain biochemical phenotypes and their changes with ibuprofen, we investigated whether ibuprofen could also increase dendritic spine density, focusing on the MEC (Figure 6A).
Figure 6. Ibuprofen and pioglitazone promote dendritic spine density in 6-month-old APOE4 mice.
Six-month old APOE4 mice were treated with control diets, 375 ppm ibuprofen diets for 1 week, 375 ppm ibuprofen diets for 2 months, 240 ppm pioglitazone diets for 1 week, or 140 ppm celecoxib diets for 2 months (n = 4 animals/treatment group), and dendritic spines were quantified. (A) Representative image of Golgi-stained apical oblique (AO) and basal shaft (BS) dendrites in the MEC. (B–D) Quantification of dendritic spine density in the AO dendrites ((B) Ibuprofen: F=29.37, p<0.0001; post-hoc, p<0.001; (C) pioglitazone: t=10.73, p<0.0001; (D) celecoxib: t=0.601, p=0.57) (E–G) Quantification of dendritic spine density in the BS dendrites ((E) Ibuprofen: F=35.66, p<0.0001, post-hoc, p<0.001; (F) pioglitazone: t=6.642, p<0.0006; (G) celecoxib: (t=2.604, p=0.04)). (H–J) Quantification of total dendritic spine density in the AO and BS dendrites. ((H) Ibuprofen: F=86.54, p<0.0001, post-hoc, p<0.001; (I) pioglitazone: t=15.75, p<0.0001; (J) celecoxib: t=1.768, p=0.128)). One-way ANOVA, Bonferroni multiple comparisons post-hoc analyses, *p<0.05, **p<0.01, ***p<0.001.
Treatment with ibuprofen for one week or two months each significantly increased apical oblique (F=29.37, p<0.0001; post-hoc, p<0.001) (Figure 6B) and basal shaft (F=35.66, p<0.0001, post-hoc, p<0.001) (Figure 6E) dendritic spine density in the entorhinal cortex of APOE4 mice compared to those treated with a control diet. Thus, ibuprofen had a significant effect increasing total dendritic spine density (F=86.54, p<0.0001, post-hoc, p<0.001) (Figure 6H). Similarly, pioglitazone also increased apical oblique (t=10.73, p<0.0001) (Figure 6C) and basal shaft (t=6.642, p<0.0006) (Figure 6F) dendritic spine density, causing total dendritic spine density to increase (t=15.75, p<0.0001) (Figure 6I). Celecoxib significantly increased basal shaft (t=2.604, p=0.04) (Figure 6G), but had no significant effect on apical oblique (t=0.601, p=0.57) (Figure 6D) or total (t=1.768, p=0.128) (Figure 6J), dendritic spine density. Overall, these findings suggest that the PPAR-γ activity of ibuprofen may drive its effects on dendritic spine density more than the COX-2 inhibiting effects of ibuprofen.
Discussion
Accumulating evidence suggests that APOE impacts normal brain function, independent of AD pathology (Caselli et al., 2004; Filippini et al., 2009; Scarmeas et al., 2005). A recent study also suggests that human APOE-ε4 carriers have reduced functionality of the entorhinal cortex, an area affected early in AD (Kunz et al., 2015). We examined APOE4 mice to determine whether the APOE-ε4 genotype was associated with phenotypes that could be converted to those associated with lower risk of AD (the APOE-ε3 genotype). It is necessary to identify early markers of AD risk in order to allow testing of potential preventative treatments that reduce the risk of AD without directly affecting the accumulation of the later overt neuropathological changes related to Aβ and tau. By utilizing the APOE KI animal model, which does not display gross AD pathology, we were able to identify such targets for preventative treatments. We report here that APOE4 mouse brains had a different distribution of APOE protein in brain extracts compared to APOE3 mice. The PPAR-γ agonist and COX inhibitor ibuprofen improved APOE and dendritic spine density phenotypes associated with APOE4, suggesting anti-inflammatory approaches could reduce AD risk for individuals identified with the APOE-ε4 allele. Further, these observations are consistent with the model that APOE expression and dendritic spine density are mechanistically related, each altered by ibuprofen, the PPAR-γ agonist pioglitazone, and the selective COX-2 inhibitor celecoxib.
Brain APOE metabolism
By examining a simple mouse model of endogenous expression of different APOE alleles, we have identified differences in the brain that occur in the absence of plaques and tangles, supporting a mechanism by which APOE genotype affects APOE metabolism in the normal, young brain. Compared to APOE3 brains, APOE4 brains showed significantly more APOE in TBS extracted brain fractions compared to TBSX extracted fractions. Taking different ages and brain regions into account, we consistently observed this difference large numbers of animals. Similarly, other studies have found that total APOE levels are decreased in APOE4 mice compared to APOE3 mice (Riddell et al., 2008; Sullivan et al., 2011; Youmans et al., 2012). The amino acid substitution in APOE4 affects APOE folding and stability in astrocytes (Zhong et al., 2009). Our work shows that APOE post-translational modifications are altered in mouse and human APOE4 brains (Figures 1, 4). Although other types of protein modifications could also both increase molecular weight and decrease isoelectric point (pI), it has been widely demonstrated that O-linked glycosylation of APOE allows for the addition of sialic acid groups (Mailly et al., 1990; Rebeck et al., 1998; Zannis et al., 1986). The majority of intracellular and newly secreted APOE is sialylated, while the majority of APOE in the plasma is not sialylated (Zannis et al., 1986). We hypothesize that TBS-soluble APOE from the brain is comprised of more newly translated immature APOE whereas mature APOE is predominantly found in the TBSX-soluble fraction. We speculate that TBSX-soluble APOE may represent a fraction of APOE that is associated with neuronal membranes, a process important for promoting dendritic spine formation. In a related manner, TBS-soluble APOE may represent a fraction of APOE that is not being utilized by neurons for dendritic spine formation. Because APOE4 is associated with the TBS fraction compared to APOE3 (Figure 1–4), and ibuprofen improves this distribution of APOE in mouse hippocampus along with dendritic spine deficits (Figures 5–6), we propose that APOE maturation could be a phenotype associated with promoting dendritic spine density. In a mouse model of brain amyloid, APOE3 was preferentially extracted in TBSX compared to APOE4 (Youmans et al., 2012), supporting a connection between this characteristic of APOE and AD pathological changes. In the human hippocampus, APOE in APOE-ε4 carriers has a lower pI (indicative of increased sialylation) compared to APOE-ε3 carriers (Alzate et al., 2014). APOE-ε3 carriers with AD also had more of this lower pI APOE compared to APOE-ε3 carriers without AD (Alzate et al., 2014), suggesting that APOE modification may represent not only an APOE-ε4-associated biomarker, but also an AD associated biomarker.
Ibuprofen as an AD preventative therapy
Many epidemiological studies tracking thousands of participants have demonstrated that NSAID use is associated with a significant decrease in AD risk (Etminan et al., 2003; in t’ Veld et al., 2001; Stewart et al., 1997; Szekely et al., 2008; Vlad et al., 2008), and that ibuprofen showed the greatest effect compared to other NSAIDs (Vlad et al., 2008). Despite this promising epidemiological evidence, clinical trials of NSAIDs showed no efficacy in treating AD (Pasqualetti et al., 2009). Furthermore, an AD prevention trial of elderly control individuals (average age of 74) showed no evidence of delay by NSAIDs (Breitner et al., 2011). These findings led to the speculation that NSAIDs were useful only as a preventative treatment early in life, before any AD pathological changes have occurred (Breitner et al., 2011; Imbimbo et al., 2010). Low level brain inflammation prior to AD onset may lead to neuronal damage, but higher levels of inflammation after AD onset may be necessary to alleviate it. Thus, decreasing inflammation before disease onset with an NSAID like ibuprofen may be beneficial (as in the epidemiological studies), but detrimental once the disease has begun (as in the elderly patients in clinical trials). Indeed, any AD therapeutic approach targeting neuroinflammation will likely have very different effects before and after brain amyloid deposition, given the very strong inflammatory response to amyloid (Breitner et al., 2011). The beneficial effect of ibuprofen on mitigating AD risk may be specific to APOE4 carriers (Cornelius et al., 2004; Hayden et al., 2007; in t’ Veld et al., 2001; Szekely et al., 2008; Yip et al., 2005). Thus, the effectiveness of NSAIDs as a therapy to prevent AD may be limited to APOE4 carriers, perhaps due to the sensitivity of APOE4 carriers to brain inflammation (Szekely et al., 2008; Yip et al., 2005).
APOE and inflammation
The most widely acknowledged mechanism of action of ibuprofen is cyclooxygenase (COX) inhibition, and the effects observed in our study with ibuprofen may be at least partially due to this mechanism for prevention of inflammation due to celecoxib treatment promoting an APOE3-like phenotype in APOE4 mice (Figures 5–6). In mouse models, APOE4 brains have increased susceptibility to diseases associated with inflammation (Farrer et al., 1997; Tu et al., 2009), or inflammation caused by LPS (Zhu et al., 2012) or Aβ (Rodriguez et al., 2014). Treatment of a mouse model of amyloid with ibuprofen for 6 months led to decreased brain amyloid levels and glial activation (Lim et al., 2000). Protection from AD by anti-inflammatory treatments suggests that low levels of chronic inflammation may encourage amyloid accumulation (for review, see (Krstic and Knuesel, 2013)). Other therapeutic approaches more targeted to specific APOE4-related inflammation pathways may prove successful at preventing APOE4-associated effects without harmful side effects (Ophir et al., 2005).
Ibuprofen also works independently of COX inhibition to affect other signaling pathways. For instance, ibuprofen decreased the number of neurons in aberrant cell cycles in a mouse model of AD (Varvel et al., 2009). Ibuprofen acts as an agonist for PPAR-γ in the brain (Lehmann et al., 1997). PPAR-γ is a nuclear receptor that acts as a transcription factor to promote levels of gene targets, including APOE. In doing so, PPAR-γ reduces inflammation and β-amyloid toxicity (Combs et al., 2001), perhaps due to the anti-inflammatory nature of lipidated APOE (Guo et al., 2004; Pocivavsek et al., 2009). In addition, microglia can be switched from an activated phenotype (promoting further inflammation) to an alternative phenotype (promoting repair) through PPARγ (Perry and Holmes, 2014). The PPAR-γ agonist in our study, pioglitazone, was associated with protection against AD in a large retrospective epidemiological study of individuals treated for diabetes mellitus (Heneka et al., 2015). Another drug, bexarotene, an RXR agonist known stimulate the formation of RXR and PPAR-γ heterodimers, reverses APOE4-related differences in APOE lipidation and behavioral deficits in mice (Boehm-Cagan and Michaelson, 2014). Likewise, PPAR-γ agonist pioglitazone had effects similar to ibuprofen in APOE4 mice, promoting dendritic spine density (Figure 6) and an APOE3-like distribution of TBS and TBSX soluble APOE (Figure 5). Thus, several APOE-related therapeutic approaches could prove successful in reducing the incidence of AD.
Conclusions
We report here a new model for preclinical testing of compounds that may act to decrease risk of AD, independent of effects on neuropathological changes engineered into other mouse models of AD. Now that we have determined that ibuprofen alters the APOE4 phenotype in a mouse model, future studies will investigate the effects of compounds in homozygous APOE3/APOE3 mice, heterozygous APOE3/APOE4 mice, and APOE null mice. Moreover, we will also investigate whether effects are dose-dependent and/or dependent on the duration of treatment. These findings demonstrate a tractable model for analyzing other preventative treatments for AD that may work through effects on APOE, and demonstrate biologically plausible mechanisms for how NSAIDs cause the observed reduction of AD in humans.
Highlights.
Post-translationally modified APOE was associated with brain extraction conditions.
APOE4 brains in humans and mice have an altered biochemical distribution of APOE
Alterations in APOE4 mouse brains were modifiable with ibuprofen and related drugs
These treatments also increased dendritic spine density in APOE4 mouse brains
Acknowledgments
This work was supported by NIH P01 AG030128 (GWR), NIH 5T32NS041218 (AMD), and NIH 1F31AG051308 (AMD).
Footnotes
Financial Disclosures: The authors declare no that they have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alzate O, Osorio C, DeKroon RM, Corcimaru A, Gunawardena HP. Differentially charged isoforms of apolipoprotein E from human blood are potential biomarkers of Alzheimer’s disease. Alzheimer’s research & therapy. 2014;6:43. doi: 10.1186/alzrt273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci. 2014;34:7293–7301. doi: 10.1523/JNEUROSCI.5198-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN, Group AR. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimers Dement. 2011;7:402–411. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Reiman EM, Osborne D, Hentz JG, Baxter LC, Hernandez JL, Alexander GG. Longitudinal changes in cognition and behavior in asymptomatic carriers of the APOE e4 allele. Neurology. 2004;62:1990–1995. doi: 10.1212/01.wnl.0000129533.26544.bf. [DOI] [PubMed] [Google Scholar]
- Combs CK, Bates P, Karlo JC, Landreth GE. Regulation of beta-amyloid stimulated proinflammatory responses by peroxisome proliferator-activated receptor alpha. Neurochem Int. 2001;39:449–457. doi: 10.1016/s0197-0186(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Cornelius C, Fastbom J, Winblad B, Viitanen M. Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology. 2004;23:135–143. doi: 10.1159/000075957. [DOI] [PubMed] [Google Scholar]
- DiBattista AM, Stevens BW, Rebeck GW, Green AE. Two Alzheimer’s disease risk genes increase entorhinal cortex volume in young adults. Frontiers in human neuroscience. 2014;8:779. doi: 10.3389/fnhum.2014.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, DiBattista AM, Miessau M, Moussa CE, Rebeck GW. APOE genotype affects the pre-synaptic compartment of glutamatergic nerve terminals. J Neurochem. 2013;124:4–14. doi: 10.1111/j.1471-4159.2012.07908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Weeber EJ, Turner RS, Xu B, Rebeck GW, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etminan M, Gill S, Samii A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: systematic review and meta-analysis of observational studies. BMJ. 2003;327:128. doi: 10.1136/bmj.327.7407.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AE, Gray JR, Deyoung CG, Mhyre TR, Padilla R, Dibattista AM, William Rebeck G. A combined effect of two Alzheimer’s risk genes on medial temporal activity during executive attention in young adults. Neuropsychologia. 2014;56:1–8. doi: 10.1016/j.neuropsychologia.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, LaDu MJ, Van Eldik LJ. A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004;23:205–212. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Khachaturian AS, Szekely CA, Fotuhi M, Norton MC, Tschanz JT, Pieper CF, Corcoran C, Lyketsos CG, Breitner JC, Welsh-Bohmer KA Cache County I. Does NSAID use modify cognitive trajectories in the elderly? The Cache County study. Neurology. 2007;69:275–282. doi: 10.1212/01.wnl.0000265223.25679.2a. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann Neurol. 2015;78:284–294. doi: 10.1002/ana.24439. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain: a journal of neurology. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Solfrizzi V, Panza F. Are NSAIDs useful to treat Alzheimer’s disease or mild cognitive impairment? Frontiers in aging neuroscience. 2010:2. doi: 10.3389/fnagi.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform-specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer’s disease patients. Neuroscience. 2003;122:305–315. doi: 10.1016/j.neuroscience.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nature reviews Neurology. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- Kunz L, Schroder TN, Lee H, Montag C, Lachmann B, Sariyska R, Reuter M, Stirnberg R, Stocker T, Messing-Floeter PC, Fell J, Doeller CF, Axmacher N. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science. 2015;350:430–433. doi: 10.1126/science.aac8128. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Mailly F, Davignon J, Nestruck AC. Analytical isoelectric focusing with immobilized pH gradients of human apolipoprotein E from very low density lipoproteins and total plasma. Journal of lipid research. 1990;31:149–155. [PubMed] [Google Scholar]
- Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, Zhang MR, Trojanowski JQ, Lee VM, Ono M, Masamoto K, Takano H, Sahara N, Iwata N, Okamura N, Furumoto S, Kudo Y, Chang Q, Saido TC, Takashima A, Lewis J, Jang MK, Aoki I, Ito H, Higuchi M. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-kappaB signaling cascade. Neurobiology of disease. 2005;20:709–718. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Pasqualetti P, Bonomini C, Dal Forno G, Paulon L, Sinforiani E, Marra C, Zanetti O, Rossini PM. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res. 2009;21:102–110. doi: 10.1007/BF03325217. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nature reviews Neurology. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Burns MP, Rebeck GW. Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia. 2009;57:444–453. doi: 10.1002/glia.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeck GW, Alonzo NC, Berezovska O, Harr SD, Knowles RB, Growdon JH, Hyman BT, Mendez AJ. Structure and functions of human cerebrospinal fluid lipoproteins from individuals of different APOE genotypes. Experimental neurology. 1998;149:175–182. doi: 10.1006/exnr.1997.6710. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Quiroz YT, Kosik KS, Lopera F, Tariot PN. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, Reinhart PH. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28:11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learning & memory. 2013;20:256–266. doi: 10.1101/lm.030031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez GA, Tai LM, LaDu MJ, Rebeck GW. Human APOE4 increases microglia reactivity at Abeta plaques in a mouse model of Abeta deposition. J Neuroinflammation. 2014;11:111. doi: 10.1186/1742-2094-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Hilton J, Anderson KE, Flynn J, Park A, Stern Y. APOE related alterations in cerebral activation even at college age. Journal of neurology, neurosurgery, and psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron. 2014;84:608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St George-Hyslop PH, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers RH, Feldman RG, Pollen D, Drachman D, et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science. 1987;235:885–890. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- Stevens BW, DiBattista AM, William Rebeck G, Green AE. A gene-brain-cognition pathway for the effect of an Alzheimers risk gene on working memory in young adults. Neuropsychologia. 2014 doi: 10.1016/j.neuropsychologia.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Han B, Liu F, Mace BE, Ervin JF, Wu S, Koger D, Paul S, Bales KR. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging. 2011;32:791–801. doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Szekely CA, Breitner JC, Fitzpatrick AL, Rea TD, Psaty BM, Kuller LH, Zandi PP. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P, Soininen H, Pirttila T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, Egger M, Juni P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JL, Zhao CB, Vollmer T, Coons S, Lin HJ, Marsh S, Treiman DM, Shi J. APOE 4 polymorphism results in early cognitive deficits in an EAE model. Biochemical and biophysical research communications. 2009;384:466–470. doi: 10.1016/j.bbrc.2009.04.153. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Bhaskar K, Kounnas MZ, Wagner SL, Yang Y, Lamb BT, Herrup K. NSAIDs prevent, but do not reverse, neuronal cell cycle reentry in a mouse model of Alzheimer disease. J Clin Invest. 2009;119:3692–3702. doi: 10.1172/JCI39716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip AG, Green RC, Huyck M, Cupples LA, Farrer LA. Nonsteroidal anti-inflammatory drug use and Alzheimer’s disease risk: the MIRAGE Study. BMC Geriatr. 2005;5:2. doi: 10.1186/1471-2318-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, Kim J, Eimer WA, Estus S, Rebeck GW, Weeber EJ, Bu G, Yu C, Ladu MJ. APOE4-specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. J Biol Chem. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- Zannis VI, vanderSpek J, Silverman D. Intracellular modifications of human apolipoprotein E. J Biol Chem. 1986;261:13415–13421. [PubMed] [Google Scholar]
- Zhong N, Ramaswamy G, Weisgraber KH. Apolipoprotein E4 domain interaction induces endoplasmic reticulum stress and impairs astrocyte function. J Biol Chem. 2009;284:27273–27280. doi: 10.1074/jbc.M109.014464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Nwabuisi-Heath E, Dumanis SB, Tai LM, Yu C, Rebeck GW, LaDu MJ. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 2012;60:559–569. doi: 10.1002/glia.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]