Abstract
Background
Antidepressant treatment failure is a common problem worldwide. In this study, we assess whether or not an important aspect of depression, cognitive impairment, is untreated by antidepressants by studying the effect of acute antidepressant treatment on a range of cognitive domains.
Methods
In this randomised longitudinal study, which is part of the International Study to Predict Optimized Treatment in Depression (iSPOT-D) trial, we assessed the effects of acute antidepressant treatment in a large patient population, across clinical remission outcomes, on a range of cognitive domains: attention, response inhibition, executive function during visuospatial navigation, cognitive flexibility, verbal memory, working memory, decision speed, information processing speed, and psychomotor response speed. We enrolled patients from primary or specialty care clinics in a multicentre, international, open-label, randomised, prospective trial. Eligible patients (aged 18–65 years) were previously untreated or were willing to undergo a 1-week medication washout before the study start, and could not have had inadequate response to study medications in the past. We enrolled a large population of medication-free (ie, untreated) outpatients in a depressive episode and assessed them for cognitive function at enrolment (pre-treatment), and again after 8 weeks of treatment with one of three antidepressant drugs (escitalopram, sertraline, or venlafaxine extended-release). Patients were randomly assigned (1:1:1) to one of the three antidepressants using a blocked randomisation procedure (block size of 12). As a comparison group, we also simultaneously enrolled matched healthy participants. Healthy participants received no medication or intervention, but were assessed for change in cognitive and clinical measures during the same interval and testing protocol. Therefore, this group acts as a test–retest control for the primary outcome measure examined in this study, change in cognitive measures over 8 weeks of treatment in depressed patients. This study is registered with ClinicalTrials.gov, number NCT00693849.
Findings
Between Dec 8, 2008, and Sept 30, 2011, we enrolled 1008 eligible people into the study. Impairment in five domains—attention, response inhibition, verbal memory, decision speed, and information processing—showed no relative improvement with acute treatment (controlling for time or repeated testing), irrespective of antidepressant treatment group, even in patients whose depression remitted acutely according to clinical measures. Broader cognitive impairment was associated with greater illness chronicity (earlier illness onset) but not with symptom severity or previous antidepressant failures.
Interpretation
Depression is associated with impairments in higher-order cognitive functions and information processing, which persist independently of clinical symptom change with treatment. We recorded no difference between the three antidepressants tested, with none showing efficacy for these impairments. Although the 8-week treatment period limits interpretation to acute treatment effects, it does highlight cognitive impairment as an untargeted contributor to incomplete treatment success.
Funding
Brain Resource Company Operations Pty Ltd and NIH.
Introduction
Major depressive disorder is a leading cause of the global burden of disability and disease,1 and carries a persistent risk of relapse even during remission. Neither chronic illness features nor treatment response can be predicted by clinical symptoms in the acutely ill state. Rather, data increasingly point to persistent cognitive impairments being related to the effect of the illness on quality of life and underlying brain network mechanisms.2,3 New medications are also being developed with potential for greater efficacy for cognition.4,5 Therefore, definitive characterisation of the effects of depression and present treatment on cognition can have rapid implications for guiding optimisation of treatment.
Cognition encompasses a hierarchy of dissociable domains, mediated by distinct neurocircuitry, functioning in concert to enable people to carry out behaviour. These domains range from high cognitive load functions coordinating several task demands according to abstract rules and goals, to lower cognitive load psychomotor functions that sequence and enact motor responses based on concrete rules and cues.6 Depression and its treatment can have varying effects across these domains. Therefore, to definitively understand the effects of depression on cognition as a whole or its use as a treatment target, depression needs to be characterised on a wide range of cognitive tests in both the acutely ill and antidepressant-treated states. Unfortunately, the published scientific literature is very inconsistent with respect to whether or not successful antidepressant treatment improves cognition, and if so in which domains—an especially important question in light of previous reports implicating poor cognition as a predictor of worse treatment outcomes.7–9
Cognitive impairments have been recorded in acute depressive episodes in the domains of episodic memory, verbal memory, and varying subsets of executive function,10,11 although some studies instead have reported a more global cognitive impairment across all domains.12 In terms of the effects of treatment, some studies have reported that impairments in executive function generally persist into euthymia13 but that verbal fluency improves with clinical remission.11,14 By contrast, a recent large meta-analysis reported treatment effects most commonly in the domains of verbal memory (a combination of verbal learning or acquisition with verbal recall and memory), working memory, processing speed, and, to a lesser degree, executive function.15 Importantly, however, in the studies that the meta-analysis was based on, these findings were often not corrected for multiple comparisons, used inconsistent methods, and reported quite small improvements compared with the size of the illness-related impairment. These problems were described as substantial limitations by the authors of the meta-analysis. Other authors have cited additional inconsistencies in the existing scientific literature studying cognition in depression that limit definitive conclusions, including small study sizes and variability in cognitive domains assessed, tasks used, task design and reporting, assignment of task impairment to cognitive domain, and clinical assessment methods and criteria.15
Therefore, a large-scale and systematic investigation of cognition using a standardised test battery is needed to ascertain which cognitive domains, if any, are improved by antidepressant treatment.16 We sought to address this major knowledge gap by first studying the effect of depression on cognition in a well powered sample, and second, by assessing the effects of three frequently used antidepressants on cognitive change as a function of clinical treatment outcome. Based on the published literature, we postulated that a subset of cognitive impairments in depression would persist despite acute treatment with conventional antidepressants and attainment of clinical remission—ie, cognition is independent of clinical symptoms.
Methods
Study design and participants
The methods of the International Study to Predict Optimized Treatment in Depression (iSPOT-D) are described in detail elsewhere.17,18 Briefly, this is a multicentre, international, open-label, randomised, prospective trial, measuring change in multiple cognitive and clinical measures in medication-free patients compared with healthy controls.
A complete list of participating institutions and regulations is provided in the original publication of the trial protocol.17,18 Eligible participants were adults (aged 18–65 years) with first-onset or recurrent, non-psychotic major depressive disorder or healthy controls matched in age, sex, and years of education. Patients were non-medicated at the start of the trial (they were either previously untreated or had at least 1 week of medication washout before the start of the trial). The inclusion criteria stipulated that patients had no history of treatment failure to any of the three protocol drugs (escitalopram, sertraline, or venlafaxine extended release [venlafaxine-XR]) and met criteria for single or recurrent non-psychotic major depressive disorder with total score on the 17-item Hamilton Rating Scale for Depression (HRSD-17) less than or equal to 17. Patients were excluded for other psychiatric comorbidities and medical conditions that would interfere with completion of assessments or lead to contraindication to protocol medications. Patients were diagnosed according to DSM-IV criteria and their diagnosis was confirmed by Mini-International Neuropsychiatric Interview (MINI-Plus).19
Institutional review board approval was obtained at each clinical site. Every participant received a verbal and written explanation of study aims, methods, and potential risks and benefits from investigators, and provided written informed consent.
Randomisation and masking
Participants were randomly assigned 1:1:1 to antidepressant treatment with escitalopram, sertraline, or venlafaxine-XR, using a blocked randomisation procedure (block size of 12). An open-label study design was used to match clinical practice and to ensure safety, with treatment managed by usual care clinicians who did not participate in study ratings. Clinically trained study coordinators supervised by clinically qualified investigators carried out ratings masked to both patient treatment and cognitive performance.
Procedures
Clinic visits for all patients took place at study clinical sites (including academic settings and clinical practices) at week 0 (pretreatment) and week 8 (post-treatment) and included an interview with a study coordinator who used the HRSD-17 and the self-report 16-item Quick Inventory of Depression Symptomatology (QIDS-SR16) to assess each patient’s severity of depressive symptoms. Additionally, the QIDS-SR16 was administered via self-report assessments using standardised web-based infrastructure at clinical sites at 1, 2, 4, and 6 weeks into treatment. Since some of the discrepancies in the published literature are attributed to use of different assessments of clinical remission, we did our analysis using these two different assessments to define clinical remission, which enabled us to ask whether our findings would differ depending on the definition of clinical remission used.
Antidepressant medication dosing was adjusted by the usual treating clinicians according to standard clinical practice. The mean dose of each treatment given to the patients was 12·3 mg per day (range 5–20 mg) for escitalopram, 61·1 mg per day (range 12·5–200 mg) for sertraline, and 83·4 mg per day (range 18·75–225 mg) for venlafaxine-XR.18
Clinical remission was defined by an HRSD-17 score less than or equal to 7 or a QIDS-SR16 score less than or equal to 5. Cognitive testing was done with the IntegNeuro battery, derived from and validated against well established neuropsychological constructs,20 and delivered at the pretreatment timepoint (week 0) and at week 8 as parallel versions through a computerised infrastructure allowing standardised acquisition. Nine cognitive domains were tested at each timepoint, which were chosen to cover a gradient of increasing cognitive load and complexity. These domains were: attention (1–back continuous performance test); response inhibition (go–no go task); verbal memory (verbal interference task); executive function (Austin maze); cognitive flexibility (Stroop task); working memory (forward digit span); decision speed (choice reaction time); information processing (switching of attention between numbers and letters in a trails A and B task); and motor coordination (finger tapping). In total, the computerised task battery took around 40 min to complete, and is used in patients with depression,9 and in cognitively impaired patient groups with schizophrenia21 and distractible groups with attention-deficit hyperactivity disorder (ADHD).22
Outcomes
We measured changes in performance in patients with depression after 8 weeks of treatment with one of the three antidepressants compared with the healthy controls who were retested at the same interval with no other intervention. Second, we analysed change in cognitive performance across domains in the subset of patients whose clinical symptoms remitted with treatment, compared with healthy controls tested during the same intervals and patients with depression whose symptoms did not remit. We assessed changes in cognition both across the group as a whole and at the individual level (to ensure that group effects were not countered or driven by a subpopulation of patients). We postulated that the effects on cognition would be similar across the three different treatment groups. We therefore studied the effects of treatment group on change in cognitive performance across domains at both the group and individual level. We did additional post-hoc analyses to assess differences in clinical severity and chronicity associated with different individual cognitive courses.
Statistical analysis
For each task, we recorded measures of accuracy, reaction time, and task completion time. We created summary-normalised performance indices for each task (appendix pp 1–2). Since our primary analysis compared change in cognitive measures over time and repeat testing, and therefore needed data from both pre-treatment and post-treatment timepoints to assess change, we included only patients who completed both the week 0 and week 8 clinical and cognitive testing in our analysis (712 [71%] of 1008 patients). We used median replacement for outliers, which were defined as results that were more than 4 SDs away from the mean, after initial confirmation that findings are consistent irrespective of whether median replacement or exclusion of outliers is used. Median replacement is done by replacing the outlier value with the value of the median of the group (with the outliers excluded) to avoid introducing skew in the distribution of the variable. To account for the effects of time and test– retest practice, all our analyses compared the change in cognitive performance in healthy controls against the change in performance in patient groups using repeated measures ANOVAs (at a group level) or reliable change index (on an individual level), or compared the same single timepoint in all groups (eg, week 8 patient group performance is compared with week 8 healthy control performance). We considered inter-individual variability in cognitive change with treatment by assessing individual longitudinal change in cognitive performance. We did this using the reliable change index—a statistical device that establishes whether or not the change in each individual’s cognitive performance scores is significantly greater than expected from test–retest variation or practice effects. The reliable change index is defined as ([X2 - X1] – [M2 – M1]) / SDD, in which X1 is the recorded pre-test score for a patient in the depressed group, X2 is the recorded post-test score for a patient in the depressed group, SDD is the SD of the healthy control group test–retest difference, M1 is the healthy control group mean pre-test score, and M2 is the healthy control group mean post-test score (see appendix p 4 for detail).
Role of the funding source
Brain Resource was involved in study design, central coordination, and quality control of the raw data. Brain Resource provided a publication committee to manage preparation of the report and factual check of protocol information. Brain Resource had no role in the acquisition, analysis, or interpretation of the data, nor in the preparation, review, or approval of the report, or the decision to submit for publication. AE had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between Dec 8, 2008, and Sept 30, 2011, we enrolled 1008 patients with depression (mean age 37·8 years [SD 12·6]; 571 [57%] were women), and 336 healthy controls (mean age 37·0 years [SD 13·1]; 191 [57%] were women).18 Enrolled patients were randomly assigned (1:1:1) to escitalopram (n=336), sertraline (n=336), or ven lafaxine-XR (n=336). Appendix p 21 shows the trial profile. Participant demographics, clinical history, and completer rate did not differ by treatment group.18 Completers (patients who completed both the week 0 and week 8 clinical and cognitive testing) did not diff er from non-completers in week 0 clinical measures or cognitive scores (p>0·05, two-tailed t test) except in attention (p=0·002, Cohen’s d=0·21, two-tailed t test). 316 (94%) of the 336 healthy controls were completers (median replaced, appendix p 1).
We initially looked for evidence of persistent cognitive dysfunction after treatment in the patient group as a whole (appendix p 2). We found no additional improvement in cognitive performance in patients with depression after 8 weeks of treatment beyond the effects of time and practice effects noted in healthy controls during the same period (repeated measures ANOVA, group × time interactions; table 1). Cognitive performance in patients remained impaired post-treatment at week 8 compared with healthy controls at week 8 in most domains, with effect sizes similar to those reported in previous large studies (table 1; appendix).23
Table 1.
Cognitive performance impairments across domains in patients with depression compared with healthy controls
| Mean norm performance* (all patients with major depressive disorder/healthy controls) |
Repeated measures ANOVA, main effect of group |
Post-hoc t test, week 0 | Post- hoc t test, week 8 | Repeated measures ANOVA, group × time interaction |
Repeated measures ANOVA, group × time × age interaction |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 mean |
Week 0 SD |
Week 8 mean |
Week 8 SD |
p value | Partial η2 | F (1,1046) | p value | Cohen’s d | t(1046) | p value | Cohen’s d | t(1046) | p value | Partial η2 | F (1,1046) | p value | Partial η2 | F (1,1046) | |
| Attention | −0.37/ 0.00 |
0.85/ 0.73 |
−0.41/ −0.05 |
1.01/ 0.75 |
<0.0001 | 0.05 | 55.72 | <0.0001 | 0.45 | 5.90 | <0.0001 | 0.39 | 5.81 | 0.83 | <0.001 | 0.05 | 0.57 | <0.001 | 0.33 |
| Response inhibition |
−0.38/ 0.00 |
1.10/ 0.71 |
−0.45/ −0.13 |
1.20/ 0.91 |
<0.0001 | 0.03 | 37.07 | <0.0001 | 0.38 | 5.77 | 0.0009 | 0.29 | 4.35 | 0.48 | <0.001 | 0.50 | 0.52 | <0.001 | 0.42 |
| Executive function |
−0.47/ 0.00 |
1.48/ 0.92 |
0.00/ 0.26 |
1.08/ 0.53 |
<0.0001 | 0.03 | 30.31 | <0.0001 | 0.35 | 5.34 | 0.002 | 0.27 | 4.08 | 0.007 | 0.01 | 7.21 | 0.21 | 0.002 | 1.50 |
| Cognitive flexibility |
−0.22/ 0.00 |
0.90/ 0.80 |
0.03/ 0.24 |
0.78/ 0.70 |
<0.0001 | 0.02 | 22.05 | <0.0001 | 0.25 | 3.83 | <0.0001 | 0.29 | 4.37 | 0.98 | <0.001 | 0.001 | 0.002 | 0.009 | 9.24 |
| Verbal memory | −0.31/ 0.00 |
0.93/ 0.93 |
−0.20/ 0.21 |
1.00/ 0.95 |
<0.0001 | 0.04 | 42.54 | <0.0001 | 0.33 | 5.05 | <0.0001 | 0.42 | 5.40 | 0.08 | 0.003 | 3.04 | 0.08 | 0.003 | 3.07 |
| Working memory |
−0.12/ 0.00 |
1.03/ 0.98 |
−0.07/ 0.09 |
1.01/ 0.98 |
0.04 | 0.004 | 4.48 | 0.07 | 0.02 | 1.83 | 0.02 | 0.15 | 2.38 | 0.51 | <0.001 | 0.44 | 0.02 | 0.005 | 5.97 |
| Information processing |
−0.18/ 0.00 |
0.87/ 0.85 |
−0.06/ 0.26 |
0.95/ 0.83 |
<0.0001 | 0.02 | 21.27 | 0.001 | 0.21 | 3.20 | <0.0001 | 0.35 | 5.22 | 0.02 | 0.005 | 5.19 | 0.09 | 0.003 | 2.93 |
| Decision speed | −0.25/ 0.00 |
1.05/ 1.00 |
−0.15/ 0.19 |
0.85/ 0.54 |
<0.0001 | 0.03 | 32.27 | 0.0001 | 0.24 | 3.57 | <0.0001 | 0.44 | 5.50 | 0.11 | 0.002 | 2.54 | 0.47 | <0.001 | 0.51 |
| Psychomotor response speed |
−0.21/ 0.00 |
1.15/ 0.74 |
−0.14/ −0.11 |
0.97/ 0.93 |
0.004 | 0.008 | 8.23 | 0.005 | 0.20 | 2.98 | 0.34 | 0.05 | 0.95 | 0.02 | 0.005 | 5.37 | 0.44 | 0.001 | 0.59 |
Norm score=performance normalised to baseline healthy control group, with negative scores indicating worse performance.
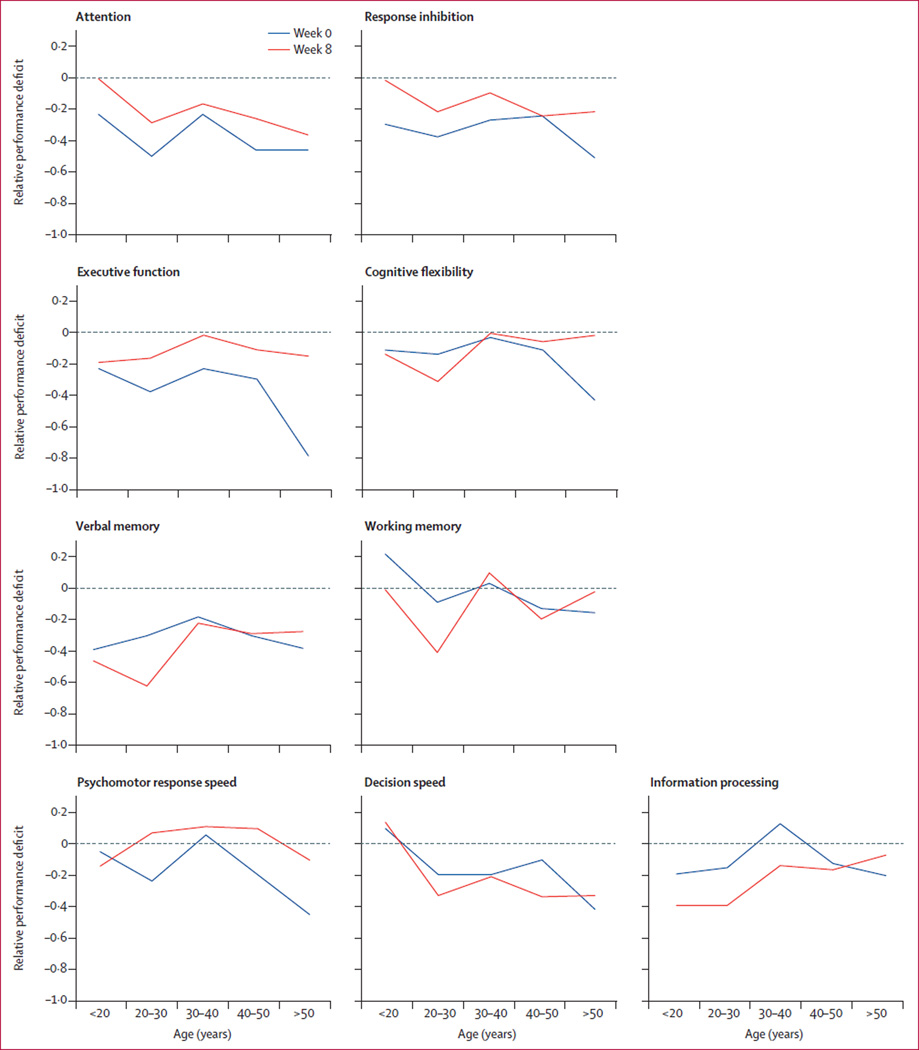
We then analysed the relation of impairments in each cognitive domain to patient factors such as age and subjective cognitive symptoms. Age is not related to cognitive impairment in patients, when studied across the age range of participants (figure 1; appendix p 6). Furthermore, subjective measures of concentration and decision making did not correlate with objective performance (appendix p 7).
Figure 1. Distribution of relative cognitive deficits in depression by age group.
Relative deficits were calculated as average performance in healthy controls subtracted from average performance in patients with major depressive disorder in each age group. Week 0 is pre-treatment and week 8 is post-treatment.
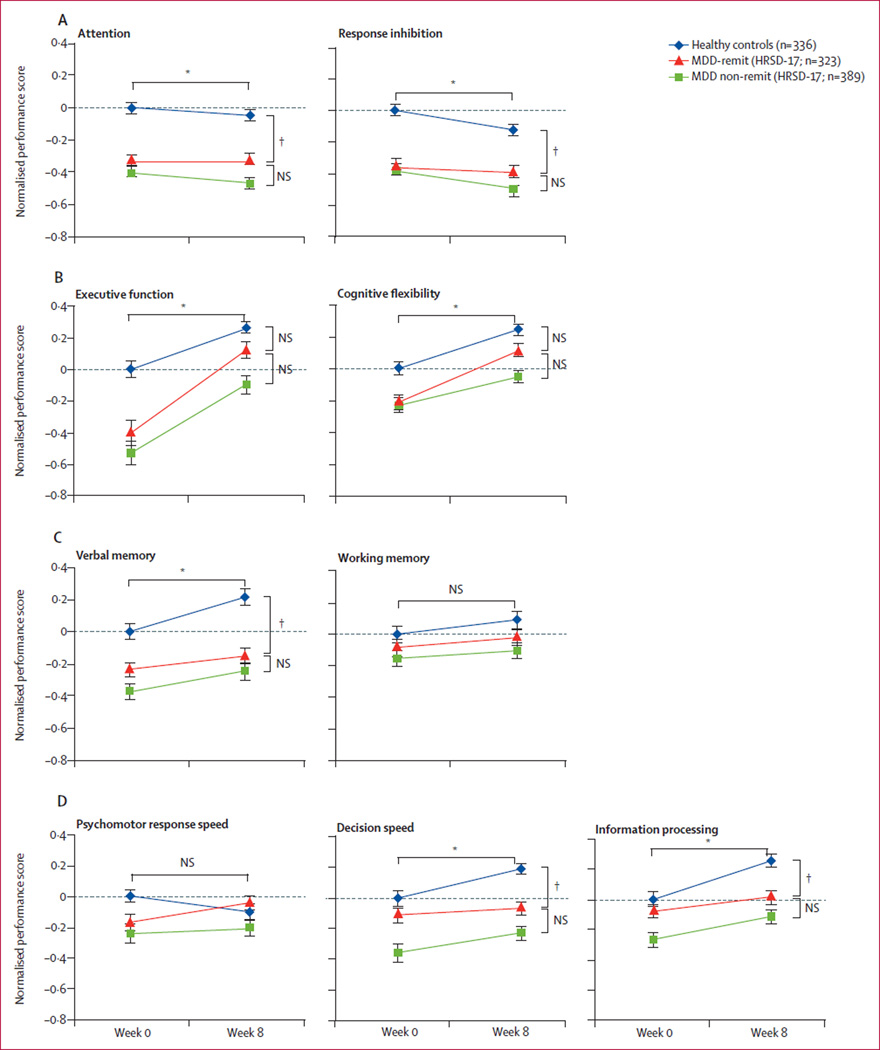
We then studied whether or not depression-related cognitive dysfunction persisted in the subgroup of patients whose clinical symptoms remitted after 8 weeks of antidepressant treatment. Here, we describe the results of our analysis with remission defined according to HRSD-17 scores at week 8, and report similar analyses with remission defined according to week 8 QIDS-SR16 scores in the appendix. We compared change in cognitive performance in patients who achieved clinical remission (major depressive disorder [MDD]-remit group) over the course of treatment with change in cognitive performance in healthy controls during the same time interval and retesting procedure. We did this analysis in cognitive domains in which the MDD-remit group showed impairments compared with healthy controls (repeated measures ANOVA, main effect of group; table 2). Within these domains, cognitive performance in the MDD-remit group between the two timepoints showed no change above that seen in healthy controls (repeated measures ANOVA, group × time interactions; table 2) in attention, response inhibition, cognitive flexibility, verbal memory, decision speed, and information processing (figure 2). We then compared cognition between the MDD-remit group at post-treatment on week 8 and the healthy control group at week 8. At the post-treatment timepoint, the MDD-remit group showed significant deficits (post-hoc t test, table 2) in attention, response inhibition, verbal memory, decision speed, and information processing compared with healthy controls after the same 8-week interval (figure 2). Therefore, these five domains showed persistent impairment in people affected by major depressive disorder, with no change in cognition above that seen in healthy controls, despite clinical improvement.
Table 2.
Course of cognitive impairment with treatment in MDD-remit patients compared with healthy controls
| Mean norm performance* (MDD-remit patients/ healthy controls) |
Repeated measures ANOVA, main effect of group |
Post-hoc t test, week 0 | Post-hoc t test, week 8 | Repeated measures ANOVA, group × time inter action |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 8 | p value | F (1,657) | Partial η2 | p value | Cohen’s d | t (657) | p value | Cohen’s d | t (657) | p value | F (1,657) | Partial η2 | |
| Attention | −0.34/ 0.00 | −0.33/ −0.05 | <0.0001 | 3346 | 0.05 | <0.0001 | 0.42 | −5.36 | <0.0001 | −0.34 | −4.41 | 0.46 | 0.54 | 0.001 |
| Response inhibition | −0.37/ 0.00 | −0.40/ −0.13 | <0.0001 | 27.93 | 0.04 | <0.0001 | 0.40 | −5.13 | 0.0005 | −0.28 | −3.53 | 0.27 | 1.24 | 0.002 |
| Executive function | −0.40/ 0.00 | 0.13/ 0.26 | 0.0001 | 15.61 | 0.02 | <0.0001 | 0.33 | −4.24 | 0.03 | −0.17 | −2.16 | 0.003 | 9.19 | 0.014 |
| Cognitive flexibility | −0.21/ 0.00 | 0.11/ 0.24 | 0.001 | 10.47 | 0.001 | 0.002 | 0.24 | −3.13 | 0.02 | −0.19 | −2.39 | 0.23 | 1.43 | 0.002 |
| Verbal memory | −0.23/ 0.00 | −0.15/ 0.21 | <0.0001 | 22.40 | 0.03 | 0.001 | 0.26 | −3.37 | <0.0001 | −0.38 | −4.91 | 0.06 | 3.46 | 0.005 |
| Working memory | −0.09/ 0.00 | −0.02/ 0.09 | 0.14 | 2.21 | 0.003 | .. | .. | .. | .. | .. | .. | 0.74 | 0.11 | <0.001 |
| Information processing | −0.08/ 0.00 | 0.01/ 0.26 | 0.004 | 8.20 | 0.01 | 0.23 | 0.09 | −1.21 | 0.0004 | −0.28 | −3.56 | 0.02 | 0.008 | 5.34 |
| Decision speed | −0.11/ 0.00 | −0.07/ 0.19 | 0.0003 | 13.07 | 0.02 | 0.13 | 0.12 | −1.53 | <0.0001 | −0.36 | −4.66 | 0.07 | 3.33 | 0.005 |
| Psychomotor response speed |
−0.17/ 0.00 | −0.05/ −0.11 | 0.16 | 1.95 | 0.003 | .. | .. | .. | .. | .. | .. | 0.007 | 7.30 | 0.01 |
MDD-remit=patients with depression whose clinical symptoms remit (defined by the 17-item Hamilton Rating Scale for Depression).
Norm score=performance normalised to baseline healthy control group, with negative scores indicating worse performance.
Figure 2. Impairment in cognition before and after treatment in patients, compared across clinical outcome groups.
Normalised performance scores shown as group mean (SE) in the domains of (A) attention and response inhibition, (B) visuospatial navigation and cognitive flexibility, (C) verbal memory and working memory, and (D) psychomotor function, decision speed, and information processing. MDD-remit=patients with major depressive disorder whose clinical symptoms remitted (defined by HRSD-17). HRSD-17=17-item Hamilton Rating Scale for Depression. MDD-non-remit=patients with depression whose clinical symptoms did not remit. NS=non-significant. *Denotes significance of repeated measures ANOVA main effect of group, for the comparison of MDD-remit patients vs healthy controls. †Denotes significance of post-hoc t test between indicated groups at week 8.
Cognitive flexibility and executive function (figure 2B) showed impairments in the acutely ill phase, but week 8 performance scores did not differ significantly between the MDD-remit group and healthy controls (post-hoc week 8 t test; table 2). Change in cognition in the MDD-remit group over the 8-week interval did not differ across treatment groups in either executive function (repeated measures ANOVA, time×treatment group p=0·43, F[2,320]=0·85) or cognitive flexibility (repeated measures ANOVA, remission time×treatment group, p=0·21, F[2,320]=1·55). Causes of improvement in these two domains cannot be distinguished between effects of treatment, placebo, and practice effects (which would have to be greater in patients than controls). However, within cognitive flexibility, the data suggest a benefit from practice effects with retesting in the MDD-remit group because no change in cognitive performance occurred above that found in healthy controls over the 8 weeks and the test–retest procedure.
In summary, although this pattern was not detected in assessment of the patient group as a whole, analysis only of patients with a successful treatment course showed improvement in executive function (in this case, assessed by a visuospatial planning task) and cognitive flexibility (figure 2B). By contrast, even when treatment led to clinical remission, no change occurred in cognitive impairments in attention, response inhibition, verbal memory, decision speed, or information processing. Furthermore, the severity of impairments in these five domains did not differ significantly between remitters and non-remitters (appendix pp 7–8). These findings remain robust irrespective of whether remission is defined by objective (HRSD-17) or subjective (QIDS-SR16) measures of clinical symptoms, or timing of clinical change (appendix pp 8–9, 12).
Next, we assessed treatment group effects, to ascertain whether any one of the three antidepressant treatments led to a greater change in cognition than the others during the 8 weeks of treatment and retesting, in either the MDD-remit or the MDD-non-remit group. We did not record a significant effect of treatment group on changes in cognition in either patient subgroup (repeated measures ANOVA, group×time×treatment; table 3, appendix p 13). Individual drug-level data, specifically cognitive change in each domain in MDD-remit and MDD-non-remit groups with each drug, are shown in appendix p 13. Overall, we recorded no change in the course of cognitive symptoms over time, testing, or treatment; this situation remained the same irrespective of antidepressant treatment choice between venlafaxine-XR, escitalopram, or sertraline.
Table 3.
Course of cognitive impairment with treatment in MDD-remit patients compared with the MDD-non-remit group, in domains where MDD-remit group showed persistent impairment compared with healthy controls*
| Mean norm performance† (MDD-remit/ MDD-non-remit) |
Repeated measures ANOVA group × time interaction |
Repeated measures ANOVA, main effect of group |
Post-hoc t test, week 0 | Post-hoc t test, week 8 | Repeated measures ANOVA group × time × treatment interaction |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 8 | p value | F (1,710) | Partial η2 | p value | Cohen’s d | t (710) | p value | F (1710) | Partial η2 | p value | Cohen’s d | t (710) | p value | F (2,709) | Partial η2 | |
| Attention | −0.34/ −0.47 |
−0.33/ −0.36 |
0.39 | 0.73 | 0.001 | 0.08 | 0.13 | −1.77 | 0.08 | 2.99 | 0.004 | 0.29 | 0.08 | −1.05 | 0.28 | 1.29 | 0.004 |
| Response inhibition |
−0.38/ −0.39 |
−0.44/ −0.49 |
0.47 | 0.52 | 0.001 | 0.29 | 0.08 | −1.06 | 0.40 | 0.72 | 0.001 | 0.78 | 0.02 | −0.28 | 0.53 | 0.63 | 0.002 |
| Executive function |
−0.14/ −0.53 |
−0.31/ −0.10 |
0.36 | 0.85 | 0.001 | 0.01 | 0.21 | −2.74 | 0.04 | 4.43 | 0.01 | 0.25 | 0.09 | −1.15 | 0.28 | 1.30 | 0.004 |
| Cognitive flexibility |
−0.05/ −0.23 |
−0.14/ −0.04 |
0.05 | 3.96 | 0.006 | 0.01 | 0.20 | −2.66 | 0.10 | 2.72 | 0.004 | 0.76 | 0.02 | −0.31 | 0.27 | 1.32 | 0.004 |
| Verbal memory |
−0.19/ −0.37 |
−0.31/ −0.25 |
0.59 | 0.29 | <0.001 | 0.18 | 0.10 | −1.33 | 0.06 | 3.67 | 0.01 | 0.05 | 0.15 | −2.00 | 0.61 | 0.50 | 0.001 |
| Information processing |
−0.03/ −0.27 |
−0.19/ −0.11 |
0.42 | 0.65 | 0.001 | 0.07 | 0.14 | −1.81 | 0.005 | 8.07 | 0.01 | 0.003 | 0.22 | −2.94 | 0.71 | 0.34 | 0.001 |
| Decision speed |
−0.09/ −0.36 |
−0.30/ −0.23 |
0.34 | 0.92 | 0.001 | 0.01 | 0.19 | −2.53 | 0.001 | 12.14 | 0.02 | 0.002 | 0.24 | −3.09 | 0.16 | 1.81 | 0.005 |
| Working memory |
.. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 0.61 | 0.50 | 0.001 |
| Psychomotor response speed |
.. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | .. | 0.41 | 0.89 | 0.003 |
MDD-remit=patients with depression whose clinical symptoms remit (defined by the 17-item Hamilton Rating Scale for Depression).
There was no significant difference in cognition change in response to treatment and testing paradigm across treatment groups. f Norm score=performance normalised to baseline healthy control group, with negative scores indicating worse performance.
For our evaluation of inter-individual variability in cognitive change with treatment, we assessed individual longitudinal change in cognitive performance. The percentage of patients showing improvement, worsening, or no change in performance in each cognitive domain over the course of treatment is tabulated (appendix p 14). In all domains, most patients (82·0–95·1%) showed no significant change, irrespective of clinical remission status (appendix p 14). Furthermore, improvement in any one cognitive domain was not consistently associated with improvement in any other domain. Only 4·5% of patients showed improvement in more than two areas of cognition, and these patients showed similar clinical symptom severity (appendix p 15) but less clinical chronicity (appendix p 15). The proportion of individuals who showed improvement in more than two cognitive domains was no higher in any of the treatment groups versus the others (nine patients in the escitalopram group, 12 in the sertraline group, and 11 in the venlafaxine-XR group, χ² [2 degrees of freedom; n=712]=0·33, p=0·85, no significant deviation from equal distribution across all three groups). The percentage of patients with previously unsuccessful antidepressant treatment did not differ significantly between patients whose cognition improved in more than two domains and all other patients (appendix p 15, χ² [1 degree of freedom, n=712]=0·22, p=0·64).
Discussion
In this report, we present a large-scale and definitive study that broadly assesses the profile of the cognitive effects of depression and antidepressant treatment, longitudinally across ill and euthymic states (encompassing successful and unsuccessful treatment) and across three different, frequently used antidepressants. With this approach, we reconcile inconsistencies in the published literature that have restricted our understanding of the cognitive effects of depression and antidepressant treatment, and the relevance of cognition as an endophenotype or as a treatment target. Until now, the question of whether cognitive impairments in depression are trait-like or state-like phenomena had not been answered conclusively. Here, we show a trait-like persistence of and absence of anti depressant treatment efficacy for impairments in attention, response inhibition, verbal memory, information processing, and decision speed, even in the context of clinical remission and independent of age. Furthermore, we show state-like improvement in the domains of cognitive flexibility and executive function (here, measured in visuospatial planning and self-monitoring task). Although we cannot definitively say if improvement in executive function above that seen in healthy controls is due to benefit from practice, placebo effects, or active treatment, our data for cognitive flexibility suggest improvement caused by intact practice effects in this domain. By virtue of assessing a broad set of cognitive domains over the course of treatment in a large sample, our data overcome difficulties of previous work that has produced inconsistent results, and clearly delineate which subsets of domains show more trait-like and which show more state-like impairments. Interestingly, the pattern of trait-like and state-like cognitive impairments is not directly indicative of cognitive load, which is consistent with previous studies.11,16 One area probed by our tasks with more traitlike impairments,24 the orbitofrontal cortex, also shows trait-like abnormalities in activity during a functional imaging study of a mood challenge in the clinically remitted state of depression.25 Perhaps tasks showing trait-like impairments probe an underlying persistent neural circuit dysfunction associated with depression.
Our objective test battery captured objective task-based cognitive impairments that persisted beyond improvement in subjective reports of impaired concentration. A poor correlation between subjective and objective measures of cognitive performance has long been recorded in depression, across different assessment methods.26 Subjective report (either broadly, or of concentration symptoms specifically) therefore provides a poor assessment of cognitive function in the setting of depression, where it can be affected by mood symptoms and self-evaluation biases. Additionally, poor subjective insight into cognitive accuracy is reported in healthy patients, in part due to expectancy effects,27 and similarly might contribute to a poor correlation between subjective and objective assessment of cognition in depression. Our work emphasises the usefulness of objective cognitive measures in capturing the complete range of impairments associated with depression during acute episodes and upon remission.
Our study assessed the effects of three different commonly prescribed antidepressants on cognitive impairments that occur in depression. All three antidepressants were prescribed according to standard clinical practice: two selective serotonin reuptake inhibitors (escitalopram and sertraline) and a serotonin– norepinephrine reuptake inhibitor (venlafaxine-XR). Cognitive change, however, does not differ across treatment groups in any cognitive domain, including for those cognitive domains in which improvement was recorded at week 8. Therefore, if any true difference between treatment groups were to exist, it would have to have a very small effect size relative to the severity of cognitive impairment in patients, since our study is powered to detect changes with effect sizes greater than 0·009 (partial η², based on power analysis for repeated measures ANOVA examining time×remission status×treatment group effects), which is well below what is accepted as a small effect size (0·2–0·3). Consistent with this idea, only a small proportion of people show broad cognitive improvement in any treatment group (4·5%, based on the individual reliable change index analysis), and the average cognitive change in any treatment group is small compared with the total cognitive impairment in all domains (based on the group analysis). Interestingly, cortical activity during cognitive task performance (response inhibition) predicts outcome to venlafaxine-XR in a neuroimaging subsample from this study,28 but cognitive performance itself does not change with venlafaxine treatment at the behavioural level.
Our analysis of individuals grouped by their course of cognitive symptoms shows that improvement in cognition rarely occurs in more than two cognitive domains in any individual. However, the small subgroup of patients (32 [4·5%] of all patients with depression) showing broader improvement across more cognitive domains also has later age of illness onset, although interestingly not a decrease in the number of lifetime depressive episodes. Furthermore, where improvement is recorded at the group level in cognitive flexibility and executive function, greater improvement is associated with less clinical chronicity.
Several limitations of this study should be noted. Since the remitted group still has a small but still statistically significant increase in symptoms after 8 weeks of treatment compared with healthy controls, we cannot rule out that complete elimination of symptoms might be necessary before cognitive dysfunction improves. Second, the 8-week treatment period is quite short, with sustained remission defined by a period of 8 weeks since last depressive episode in published literature.23 Therefore, further studies assessing patients over longer periods of treatment will be important follow-up to the present findings. Additionally, patients have a variable clinical history before entry into this study. Neither clinical history nor demographics predicted treatment response or differed across treatment groups in this study;18 however, we cannot generalise our findings here to patients earlier in their illness course without previous acute episodes or treatment history. Again, follow-up studies in this population might address whether or not cognitive impairment is less persistent earlier in illness or lifetime treatment course. Finally, although unlikely, cognitive dysfunction post-treatment might be related to medication use at week 8 in all patients (whereas cognitive impairments at baseline were due to the illness itself).
Future studies can address these limitations and analyse cognitive dysfunction as a distinct treatment target, aimed at improving long-term outcomes and addressing domains not already targeted by anti-depressant medication. Antidepressants with alternative mechanisms to those tested in this study are available and can be assessed for efficacy in targeting cognitive impairments in domains that we find remain untreated using the medications tested here. Early studies of these medications have reported efficacy for cognitive domains that we found to be unresponsive to selective serotonin reuptake inhibitor and serotonin–norepinephrine reuptake inhibitor antidepressants used in this study. For example, 8 weeks of treatment with vortioxetine (a pharmacodynamically novel multimodal antidepressant that exerts effects across several neurotransmitter systems in addition to acting at serotonin receptors and inhibiting the serotonin transporter) was shown to improve verbal memory (measured using the Rey auditory verbal learning test), information processing (using simple reaction time), decision speed (using the trail-making test), and cognitive flexibility (using Stroop) in adult patients with recurrent major depressive disorder.4 Although cognitive flexibility was improved by antidepressants in our study too, the first three domains were not improved in our study of the effects of antidepressants and therefore might be domains where vortioxetine shows improved efficacy for cognition. Adjunct lisdexamfetamine (a pharmacologically inactive prodrug of d-amphetamine currently approved for treatment of ADHD) was also shown to improve executive function in patients with mild depression above that reported with monotherapy with a con ventional antidepressant.5 However, here we also found executive function to improve with selective serotonin reuptake inhibitor or serotonin–norepinephrine reuptake inhibitor treatment. In future work, it will be important to examine whether adjunct lisdexamfetamine also improves function in the five cognitive domains unaffected by common antidepressant treatment in our study. Consistent with our findings here, studies of these agents suggest that additional mechanisms besides those used by selective serotonin reuptake inhibitor or serotonin– norepinephrine re uptake inhibitor anti depressants are needed to target the cognitive impairments associated with depression. Finally, our finding that patients still benefit from intact practice-related learning in some domains is also consistent with early evidence for the efficacy of cognitive training approaches in depression.29
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed from Jan 1, 1975, through to Nov 30, 2015, with the terms “cognition”, “unipolar depression”, and “antidepressant” for articles published in English characterising change in cognitive impairment in unipolar depression with antidepressant treatment. Despite published studies showing overwhelming consensus for the importance of assessment and treatment of cognition in depression, no conclusion has been reached about the full profile of cognitive domains impaired in unipolar depression, whether this profile differs between acutely ill and euthymic patients, and the effect of treatment with typical antidepressants on cognition. Rather, the existing literature is fractured by conflicting findings from small studies using inconsistent methods, examining different subgroups of cognitive domains and often without appropriate controls for multiple comparisons, assessing patients with mild to severe clinical symptoms rather than those in remission, and often focusing only on older adults. Therefore, whether or not cognition is affected by treatment with typical antidepressants and whether this differs by type of antidepressant or clinical outcome, remain unknown. This major knowledge gap has important clinical implications: no guidelines exist regarding assessment of cognitive impairment in depression or treatment strategies for patients with impaired cognition. What is therefore needed is a definitive determination of the effects of antidepressants on the entire cognitive profile associated with depression, across the full non-elderly adult age range (18–65 years).
Added value of this study
To the best of our knowledge, this study is the first large-scale, primary, prospective analysis of cognitive change with typical antidepressant medication treatment. It is also the first study to systematically probe a broad range of cognitive functions, longitudinally, in patients across the full adult age range, allowing a comprehensive determination of the efficacy of three commonly used antidepressants on the cognitive profile of depression. Furthermore, we assess the effects of both successful and unsuccessful treatment on cognition, compared with a large sample of healthy controls reassessed at the same interval, and do analyses at both the group and individual level. This approach ensures a robust result that is not dependent on analysis method, outcome definitions, or the potential for false-positive results common in small studies. Our findings show that at the population level, patients with depression have robust cognitive impairments in attention, response inhibition, verbal memory, decision speed, and information processing, which show no change despite antidepressant treatment when compared with controls tested at the same intervals, and are notably similar irrespective of clinical remission. Other cognitive domains (executive function and cognitive flexibility) do improve over the course of acute treatment. These findings create a robust foundation for understanding previous studies that were not able to assess all domains in the same patient population, adding a characterisation of relative impairments and change across domains. Our findings are also notable for the analysis of the course of cognitive impairments on an individual level, which is rarely done but is of major clinical importance. We found that impairments persist despite treatment in more than 95% of patients and broader cognitive impairments occur in individuals with greater illness chronicity. Importantly, we also add to the literature a comparison across treatments, and report that none of the three commonly used antidepressants tested showed better efficacy for cognition compared with the others, irrespective of clinical outcome or age.
Implications of all the available evidence
Impairments in higher order cognitive operations are representative of trait-like features of depression that commonly used antidepressants do not effectively improve in the overwhelming majority of patients, even when clinical remission is achieved. These specific cognitive domains are an untreated aspect of depression that probably contributes to high rates of treatment failure and risk of relapse. Our findings therefore support the importance of cognition as an unmet treatment need, and therefore a potential therapeutic target, and highlight the need for careful assessment of cognition in trials of new antidepressants.
Acknowledgments
This study is funded by Brain Resource Company Operations Pty Ltd and the NIH. LMW was the overall academic principal investigator for iSPOT-D (2008–13) and Claire Day (Brain Resource [Sydney, Australia]) was the global coordinator for iSPOT-D (2008–15). We acknowledge the iSPOT-D Investigators Group, the principal investigators at each site, and Scoring Server management by Donna Palmer (Brain Resource).
Footnotes
See Online/Comment http://dx.doi.org/10.1016/S2215-0366(16)00095-X
See Online for appendix
Contributors
CS, LMW, and AE developed the analysis plan for the hypotheses tested in this paper. LMW designed the overall iSPOT-D study. CS, LMW, AG, TU, and AE did the research and analysed the data. AH was the principal investigator for the Sydney Brain Dynamics Center site and oversaw the clinical ratings and training of personnel in clinical assessments. All authors wrote the report.
Declaration of interests
AE and LMW have received research funding from Brain Resource for iSPOT-D. LMW previously served as a consultant to Brain Resource and was previously a stockholder in Brain Resource. LMW is a now a consultant for Humana. CS received support from the APA/Eli Lily resident research award for this work, and is supported by NIH grant 2T32MH019938-21. AG, AH, and TU declare no competing interests.
Contributor Information
Carrie Shilyansky, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA.
Prof Leanne M Williams, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Anett Gyurak, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Anthony Harris, Brain Dynamics Center, University of Sydney Medical School and Westmead Millennium Institute for Medical Research at Westmead Hospital, Sydney, NSW, Australia.
Prof Timothy Usherwood, Department of General Practice, Sydney Medical School, Westmead, University of Sydney, Sydney, NSW, Australia.
Amit Etkin, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
References
- 1.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 2.Bain L, Stroud C. Enabling discovery, development, and translation of treatments for cognitive dysfunction in depression: workshop summary. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 3.Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs. 2010;24:267–284. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17:1557–1567. doi: 10.1017/S1461145714000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madhoo M, Keefe RSE, Roth RM, et al. Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology. 2014;39:1388–1398. doi: 10.1038/npp.2013.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruder GE, Alvarenga JE, Alschuler D, et al. Neurocognitive predictors of antidepressant clinical response. J Affect Disord. 2014;166:108–114. doi: 10.1016/j.jad.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60:13–23. doi: 10.1016/s0165-0327(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 9.Etkin A, Patenaude B, Song YJC, et al. A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacology. 2015;40:1332–1342. doi: 10.1038/npp.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 11.Elliott R. The neuropsychological profile in unipolar depression. Trends Cogn Sci. 1998;2:447–454. doi: 10.1016/s1364-6613(98)01235-2. [DOI] [PubMed] [Google Scholar]
- 12.Veiel HO. A preliminary profile of neuropsychological deficits associated with major depression. J Clin Exp Neuropsychol. 1997;19:587–603. doi: 10.1080/01688639708403745. [DOI] [PubMed] [Google Scholar]
- 13.Reppermund S, Ising M, Lucae S, Zihl J. Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med. 2009;39:603–614. doi: 10.1017/S003329170800411X. [DOI] [PubMed] [Google Scholar]
- 14.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keefe RSE, McClintock SM, Roth RM, Doraiswamy PM, Tiger S, Madhoo M. Cognitive effects of pharmacotherapy for major depressive disorder: a systematic review. J Clin Psychiatry. 2014 doi: 10.4088/JCP.13r08609. [DOI] [PubMed] [Google Scholar]
- 16.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- 17.Williams LM, Rush AJ, Koslow SH, et al. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials. 2011;12:4. doi: 10.1186/1745-6215-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saveanu R, Etkin A, Duchemin A-M, et al. The International Study to Predict Optimized Treatment in Depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2015;61:1–12. doi: 10.1016/j.jpsychires.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 20.Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of ‘integneuro’: a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115:1549–1567. doi: 10.1080/00207450590957890. [DOI] [PubMed] [Google Scholar]
- 21.Williams LM, Whitford TJ, Flynn G, et al. General and social cognition in first episode schizophrenia: identification of separable factors and prediction of functional outcome using the IntegNeuro test battery. Schizophr Res. 2008;99:182–191. doi: 10.1016/j.schres.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Williams LM, Hermens DF, Thein T, et al. Using brain-based cognitive measures to support clinical decisions in ADHD. Pediatr Neurol. 2010;42:118–126. doi: 10.1016/j.pediatrneurol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Hasselbalch BJ, Knorr U, Hasselbalch SG, Gade A, Kessing LV. Cognitive deficits in the remitted state of unipolar depressive disorder. Neuropsychology. 2012;26:642–651. doi: 10.1037/a0029301. [DOI] [PubMed] [Google Scholar]
- 24.Boone KB, Pontón MO, Gorsuch RL, González JJ, Miller BL. Factor analysis of four measures of prefrontal lobe functioning. Arch Clin Neuropsychol. 1998;13:585–595. [PubMed] [Google Scholar]
- 25.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 26.Miller WR. Psychological deficit in depression. Psychol Bull. 1975;82:238–260. doi: 10.1037/h0076367. [DOI] [PubMed] [Google Scholar]
- 27.Schwarz KA, Büchel C. Cognition and the placebo effect—dissociating subjective perception and actual performance. PLoS One. 2015;10:e0130492. doi: 10.1371/journal.pone.0130492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A. Frontoparietal activation during response inhibition predicts remission to antidepressants in patients with major depression. Biol Psychiatry. 2016;79:274–281. doi: 10.1016/j.biopsych.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 29.Iacoviello BM, Wu G, Alvarez E, et al. Cognitive-emotional training as an intervention for major depressive disorder. Depress Anxiety. 2014;8:1–8. doi: 10.1002/da.22266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.