Abstract
Extracellular vesicles present an attractive delivery vehicle for therapeutic proteins. They intrinsically contain many proteins which can provide information to other cells. Advantages include reduced immune reactivity, especially if derived from the same host, stability in biologic fluids, and ability to target uptake. Those from mesenchymal stem cells appear to be intrinsically therapeutic, while those from cancer cells promote tumor progression. Therapeutic proteins can be loaded into vesicles by overexpression in the donor cell, with oligomerization and membrane sequences increasing their loading. Examples of protein delivery for therapeutic benefit in pre-clinical models include delivery of: catalase for Parkinson’s disease to reduce oxidative stress and thus help neurons to survive; prodrug activating enzymes which can convert a prodrug which crosses the blood–brain barrier into a toxic chemotherapeutic drug for schwannomas and gliomas; and the apoptosis-inducing enzyme, caspase-1 under a Schwann cell specific promoter for schwannoma. This therapeutic delivery strategy is novel and being explored for a number of diseases.
Keywords: Exosomes, Extracellular vesicles, Parkinson’s disease, Glioma, Schwannoma, Therapeutic protein delivery
Overview of EVs for Therapeutic Delivery of Proteins
EV Protein Load: Variations Between Cells, Proteomic Analysis, and Common Proteins
Extracellular vesicles (EVs) span a range of vesicle types, including exosomes, microvesicles (MVs or ectosomes), apoptotic bodies, and oncosomes (for review see Abels and Breakefield (2016)). Exosomes and MVs have been among the most highly studied types of EVs (Henderson and Azorsa 2012; Cocucci and Meldolesi 2015). These two classes of EVs are typically distinguished on the basis of their size, with exosomes being in the range of 30–100 nm in diameter, whereas MVs are greater than 100 nm (i.e., 100 nm−1 μm). Exosomes are generated as an outcome of the trafficking of multi-vesicular bodies to the cell surface where they fuse with and are released from the plasma membrane. MVs are produced by plasma membrane budding at the cell surface (Yoon et al. 2014; El Andaloussi et al. 2013). The different EV subtypes are thought to contain a wide range of proteins. These proteins themselves carry important information both as ligands that are aligned along the EV surface or are present within the lumen of the vesicles. Some proteins commonly found in EVs and often used to characterize them include heat shock proteins (HSP70, HSP90), export molecules (Alix, RAb27a/b, TSG101, flotillin), tetraspanins (CD63, CD81 and CD9), and antigen presenting proteins, MHCI and MHCII (Chaput and Théry 2011); however, it is still not clear which subsets of EV subtypes harbor which proteins (For review of the proteomics of vesicles see Kreimer et al. 2015; Simpson et al. 2008; Kalra et al. 2012—Vesiclepedia).
The exact extent to which the protein cargo is specific to the different classes of EVs (i.e., MVs versus exosomes or other subtypes) remains to be determined, as definitive methods for resolving and purifying each of these types of EVs are still being developed. Indeed, in many cases, it is likely that EV preparations contain some mixtures of several vesicle subtypes, as well as ribonuclear protein particles and high/low density lipoproteins (Vickers et al. 2011). Similarly, the manner and degree to which different cell types package their vesicles with unique cargo have not been clearly established, although there are good reasons to suspect that MVs and exosomes from different cellular sources contain some unique cargos. MVs isolated from human breast cancer cells have been reported to contain the EGF receptor ligand, amphiregulin (Higginbotham et al. 2011), as well as the EGF receptor family member, ErbB2/HER-2 (Martins et al. 2013). MVs obtained from glioblastoma cells have been shown to contain EGF receptors and the oncogenic EGFRvIII (Graner et al. 2009; Antonyak et al. 2011; Al-Nedawi et al. 2008; Skog et al. 2008), while exosomes isolated from pancreatic cancers have recently been suggested to serve as diagnostic markers based on the enriched presence of the cell surface protein glypican-1 (Melo et al. 2015).
The protein content of EVs determines to a large extent their therapeutic potential, and in fact EVs from some cell types are intrinsically therapeutic, e.g., mesenchymal stem cells can promote repair of damaged tissues and regression of tumors (Gnecchi et al. 2006; for review see El Andaloussi et al. 2013; Nakano et al. 2015; see Katakowski and Chopp 2016). Other EVs derived from dendritic cells can assume therapeutic potential, for example, improve vaccination outcome by treatment of these cells with antigens prior to EV isolation (Cheng and Schorey 2013). However, in the case of cancer cell-derived EVs, there have been a number of reports indicating that these vesicles can contribute in aggressive ways to different key aspects of cancer progression and metastasis. For example, EVs shed from glioblastoma cells carrying the oncogenic EGF receptor variant, EGFRvIII, were shown to activate signaling pathways supporting cell proliferation upon their engagement with neighboring glioblastoma cells (Al-Nedawi et al. 2008). Cancer cell-derived EVs containing the receptor tyrosine kinase MET were reported to promote metastasis (Peinado et al. 2012), whereas exosomes derived from pancreatic adenocarcinomas have been shown to ‘educate’ bone-marrow-derived cells to initiate pre-metastatic niche formation in the liver (Costa-Silva et al. 2015). EVs containing CD147 appear to stimulate angiogenesis (Millimaggi et al. 2007) and can contain tissue factors associated with increased coagulation in cancer patients (Tesselaar et al. 2007). Cancer cell-derived EVs also contain metalloproteinases and proteins that activate matrix metalloproteinases, e.g., EMMPRIN (Sidhu et al. 2004) which digest the extracellular matrix and thereby facilitate the migration of cancer cells (Di Vizio et al. 2012; Shimoda and Khokha 2013), as well as promote tumor cell invasion (Zomer et al. 2015). Moreover, EVs shed by aggressive cancer cell lines, such as MDAMB231 breast cancer cells or U87 glioblastoma cells, contain fibronectin and the acyl transferase enzyme transglutaminase-2, which catalyzes crosslinking of fibronectin, thereby enabling the vesicles to engage integrins on recipient non-transformed cells and markedly alter their signaling properties, such that they exhibit transformed characteristics (Antonyak et al. 2011; Li et al. 2012). This latter aspect, the potential for normal cell transformation by cancer cell EVs, and potentially by EVs from transformed cell lines, is a major issue to resolve in the design of therapeutic vesicles.
Viruses also use EVs to transmit proteins; for example, HIV can transfer the CCR5 receptor to normally resistant cells making them susceptible to infection (Mack et al. 2000). Epstein Barr virus incorporates the viral oncogene latent membrane protein LMP1 into EVs as a transforming factor (Verweij et al. 2011). Herpes simplex virus type 1 incorporates STING (stimulator of IFN genes), an innate immune sensor, into exosomes released by infected cells to modulate viral latency (Kalamvoki et al. 2014). EVs can also carry immunosuppressive ligands, for example interleukin-4 (IL-4) and IL-10, which can be protective under inflammatory conditions (Robbins and Morelli 2014), or Fas ligand and TRAIL to suppress activated T cells against the fetus in pregnancy (Stenqvist et al. 2013). Another important role for EVs is to serve as a means to discard excess proteins, as in the transferrin receptors during maturation of red blood cells (Harding et al. 1983), or proteins that are limiting growth, as PTEN in prostate cancer cells (Gabriel et al. 2013).
Examples of Delivery Schemes, Including Methods for Loading Proteins in EVs and Assessing Uptake into Recipient Cells
Methods for loading specific proteins into vesicles are still being developed (for review see György et al. 2015; Fig. 1). One approach to label vesicles has been to fuse GFP or other fluorescent proteins in-frame with a protein known to be in vesicles, e.g., CD63 (Amano et al. 2001), TSG101, ARRDC1 (Nabhan et al. 2012) or a peptide which targets the protein to the plasma membrane, e.g., palmitoylated tdTomato (Lai et al. 2015). Others have fused cargo proteins to EV proteins, e.g., the C1C2 domain of lactadherin (Hartman et al. 2011) or epidermal growth factor VIII (Zeelenberg et al. 2008), or the platelet-derived growth factor receptor (PDGFR) transmembrane domain (Lai et al. 2014), to the cargo they want to deliver. Transfection of cells with an expression cassette or vector that produces high levels of a protein will lead to more incorporation into vesicles. Presumably, proteins in the cytoplasm, and not those in the nucleus or membrane compartments of the cells, such as the endoplasmic reticulum and Golgi, are more readily incorporated into EVs. After experimenting with a number of protein modifications, Gould and co-workers tested a combination of a membrane targeting myristoylation peptide combined with an oligomerizing protein, in their case yeast TyA, which markedly enriched proteins in EVs (Shen et al. 2011).
Fig. 1.
Loading protein into EVs. Several strategies have emerged for increasing the content of specific proteins in EVs: 1. Overexpression of a cytoplasmic protein (red swirl) will increase its relative content in EVs generated through natural processes; 2. Overexpression of a protein can be combined with artificial generation of vesicles by passage of cells through devices (triangles) of different pore sizes; 3. The protein of interest can be fused in-frame with a protein component of the vesicles, such as CD63 (Singh et al. 2014); 4. Proteins can be linked to the inner surface of the plasma membrane by palmitoylation or myristoylation (Shen et al. 2011) peptides fused in-frame to the N-terminal, or by fusion with a transmembrane motif, such that they are presented on the surface of the vesicles (Lai et al. 2014). Linking membrane attachment with an oligomerization signal increases loading of the protein into the vesicle even further (Shen et al. 2011)
Advantages of EV Delivery
For clinical use it will be important to identify normal cell types which can be grown under appropriate sterile, non-toxic conditions in large numbers with release of EVs which can be loaded and collected for patient administration. Several cell types look promising, such as mesenchymal stem cells, dendritic cells, and embryonic or induced pluripotent stem cells; in some cases, it may be possible to generate EVs from autologous cells for treatment. Efforts are also underway to generate therapeutic vesicles from plants (Ju et al. 2013) to which humans are normally exposed and which do not fall into the classification of natural products, and thus are not under FDA regulations. In general, EVs are considered quite stable and capable of being frozen for later use. If obtained from the same patient or possibly from embryonic cells, they should have low immunogenicity. It may also be possible to target them for cell specific uptake by genetically engineering the cells to express ligands on their surface that will be transferred to the surface of the vesicles and bind to specific receptors on target cells. This would include, for example, peptide ligands which can cross the blood–brain barrier (BBB), such as the rabies virus glycoprotein which targets acetylcholine receptors (Alvarez-Erviti et al. 2011; Wiklander et al. 2015). The mode of uptake of vesicles by recipient cells may prove critical to the effectiveness of their cargo, with endocytosis, believed to be the most common route, potentially leading to some lysosomal degradation of the cargo. Methods to promote direct fusion of vesicles with recipient cells in order to dump cargo directly into the cytoplasm, or to promote the exit of cargo from endosomes, may further enhance the effectiveness of these therapeutic vehicles.
Examples of Therapeutic Delivery of Proteins via EVs
Catalase for Neurodegeneration
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by microglia stimulation, swelling of the brain, and the release of neurotoxic reactive oxygen species (ROS) (Mcgeer et al. 1988; Busciglio and Yankner 1995; Ebadi et al. 1996; Wu et al. 2003). Proteins that reverse these oxidative phenomena, such as catalase and superoxide dismutase, are observed in decreased amounts in PD patient brains undergoing neurodegeneration (Ambani et al. 1975; Riederer et al. 1989; Abraham et al. 2005). While catalase is an attractive therapeutic protein for PD treatment, the BBB restricts its access to the brain following intravascular delivery (Peng et al. 2013). While nanoparticle transportation techniques are able to carry molecules across the BBB, once traversal has been completed, delivery is further hindered by clearance through the mononuclear phagocyte system (MPS), which destroys the nanoparticles and their cargo (Peng et al. 2013).
Exosomes have the potential to cross the BBB and transport cargo, while avoiding the MPS (Lai and Breakefield 2012; Tian et al. 2014; Vlassov et al. 2012). Taking advantage of this phenomenon, Haney et al. (2015) incorporated the PD therapeutic protein catalase into exosomes through four different methods: co-incubation, freeze/thaw, sonication, and extrusion—with sonication and extrusion producing the most efficient loading. Nanoparticle tracking analysis (NTA) and catalase activity experiments indicated that there were ~940 ± 15 catalase molecules per exosome. Catalase loading was confirmed by hyperspectral microscopy, which allows accurate optical evaluation of the protein. These researchers demonstrated that when macrophages, which had been activated by incubation with lipopolysaccharides (LPS) and tumor necrosis factor-alpha (TNF-α), were administered catalase-exosomes, ROS levels were significantly decreased when compared to non-exosome exposed macrophages.
This therapeutic strategy was also tested in vivo using C57BL/6 mice injected with 6-hydroxydopamine (6-OHDA) into the substantia nigra as a model of PD (Haney et al. 2015). Intravascular injections of catalase-containing exosomes were administered 10 times per day every other day. Free catalase was injected as a control. 6-OHDA injections caused significant brain inflammation as showed by the increased production of CD11b. However, the delivery of catalase-exosomes significantly reduced CD11b production (p < 0.005), with free catalase not being able to reduce brain inflammation. This study supports the ability of exosomes to effectively transport a therapeutic protein across the BBB and produce a measurable treatment response.
Suicide Genes for Tumors
Schwannomas are benign tumors derived from de-differentiated Schwann cells that form along peripheral nerves, with compression of the nerves causing pain, weakness, paralysis and/or hearing loss (Lu-Emerson and Plotkin 2009). In the dominantly inherited, tumor suppressor syndrome, neurofibromatosis type 2 (NF2), patients inherit one mutant allele of the merlin gene and schwannoma formation is instigated by somatic loss of the normal allele (James et al. 2009). The most common treatment for schwannomas is surgery, which can be invasive and cause nerve damage (Stanuszek et al. 2014). Mizrak et al. (2013) demonstrated the packaging of an mRNA encoding a prodrug activating enzyme (suicide protein) into EVs, with direct injection of these EVs into the tumor, followed by treatment with an inactive pro-drug leading to regression of schwannomas in a mouse sciatic nerve model. This suicide gene is a fusion cDNA encoding cytosine deaminase (CD) and uracil phosphoribosyltransferase (UPRT) (Invitrogen, Grand Island, NY) which act synergistically to convert the prodrug, 5-fluorocytosine (5-FC), to the chemotherapeutic drug, 5-fluorouracil (5-FU) (Porosnicu et al. 2003). CD converts 5-FC into 5-FU and UPRT converts 5-FU into 5-fluoro-deoxyuridine monophosphate (5-FdUMP). This compound inhibits thymidine synthase, restricting the production of dTMP and dTTP, thereby inhibiting DNA synthesis and causing cell death (Huber et al. 1994; Mullen et al. 1994).
In order to load CD-UPRT into EVs, cultured human embryonic kidney cells (293T) were transfected with an expression cassette for CD-UPRT-EGFP, and EVs released into the medium were harvested by ultracentrifugation (Mizrak et al. 2013). The EVs were tested for the presence of CD-UPRT-EGFP mRNA using qRT-PCR to prove proper cargo loading. RNA was shown to be completely encapsulated within the EVs by virtue of its RNase resistance. Treatment of human NF2 schwannoma cells (HEI-193; Hung et al. 2002) in culture with these loaded EVs followed by 5-FC treatment resulted in 80 % cell death, while mock treated cells showed no toxicity. These vesicles were also tested for their therapeutic potential in vivo in a xenograft model in which schwannoma cells expressing firefly luciferase were implanted into the sciatic nerve of nude mice (Saydam et al. 2011). Once the tumors had developed, EVs carrying CD-UPRT mRNA and presumably CD-UPRT protein were injected once per week for two months followed by intraperitoneal delivery of 5-FC. Tumor growth was monitored using in vivo bioluminescence at 28-day intervals. Tumors were completely eliminated by this therapy in 6/9 mice, while tumors in the PBS injected controls continued to grow. It was hypothesized that improved CD-UPRT loading into EVs would increase tumor toxicity.
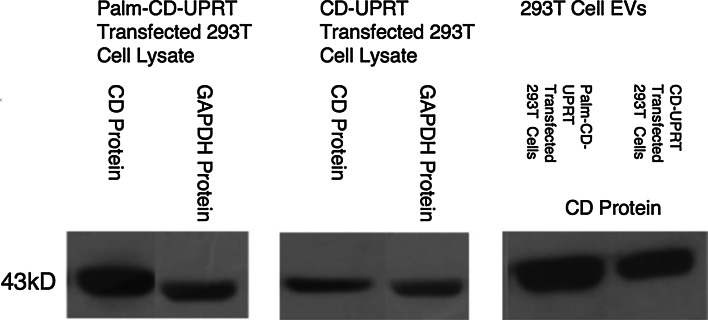
The CD-UPRT-GFP expression constructs were redesigned so that they contained a palmitoylation sequence at the N-terminal. This short hydrophobic peptide inserts into the plasma membrane and into EVs produced by cells, as demonstrated for palmitoylated GFP and tdTomato (Lai et al. 2015). To see if this membrane signal increased the levels of CD-UPRT protein in EVs, 293T cells were transfected with expression constructs for CD-UPRT with and without the palmitoylation signal. Proteins in cells and EVs derived from them were resolved by SDS-PAGE gel (10–14 % Bis Tris) electrophoresis and immunoblotted using antibodies to CD and to control proteins—GAPDH for cell lysates and HSP90 for EVs. The presence of the palmitoylation signal did not substantially increase the amount of CD-UPRT in the vesicles (Fig. 2). EVs from CD-UPRT transfected cells sensitized mouse glioma cells (GL261; Ausman et al. 1970) in culture to death induced by 5-FC (Fig. 3), indicating that the palmitoylation signal did not decrease the activity of CD-UPRT. Comparable CD-UPRT EV enrichment between palmitoylated and unpalmitoylated samples was also confirmed by GL261 viability experiments. Palmitoylated samples did not cause any difference in toxicity levels, suggesting that activity of these fused enzymes was not compromised by their presumed association with membranes in recipient cells.
Fig. 2.
Palmitoylation does not markedly increase loading of CD-UPRT protein into vesicles. 293T cells were either transfected with Palm-CD-UPRT-GFP or CD-UPRT-GFP expression cassettes using polyethylimine (PEI). High level transfection (>80 % of cells) was confirmed by GFP fluorescence. EVs were harvested from 48-h conditioned medium through ultracentrifugation. Cells and EVs were lysed using RIPA buffer, loaded at 30 μg of protein/well and resolved by SDS-PAGE, followed by Western blotting for CD and GAPDH antibodies. A representative blot is shown from one of two experiments
Fig. 3.
Delivery of 293T EVs carrying CD-UPRT combined with 5-FC causes glioma cell death in culture. 293T cells were transfected with either CD-UPRT-GFP or Palm-CD-UPRT-GFP plasmids through PEI transfection (as in Fig. 2). EVs were harvested by ultracentrifugation 72 h later from 2, 15 cm plates per plasmid type. GL261 cells cultured on 96-well plates at 2–6 × 105 EVs/cell were untreated or treated with 5-FC (150 μg/mL) after exposure to CD-UPRT EVs or Palm-CD-UPRT EVs. Viability was measured 72 h later using CellTiter-Glo assay (Promega, Madison, WI). (*p < 0.0001, n = 2 experiments and 3 wells for each experimental condition)
EV Delivery of Caspase-1 for Schwannoma Treatment
Direct intratumoral injection of an adeno-associated virus (AAV) vector was used to induce regression of a human NF2 xenograft schwannoma in a mouse sciatic nerve model (Prabhakar et al. 2013). The AAV vector encoded caspase-1 (ICE), which can trigger cell death (Croker et al. 2015), under the control of a P0 promoter, which is active in myelinating Schwann cells (Shen et al. 2011). Considering that only a fraction (i.e., about 20 %) of schwannoma cells were transduced by the vector injection, it was surprising to achieve almost complete regression of the tumors (Prabhakar et al. 2013). One explanation for this may be found in studies showing that ICE is incorporated into EVs, or travel by nanotubes, which can then be taken up by and kill surrounding cells (Sarkar et al. 2009). This hypothesis is illustrated for AAV-P0-ICE gene therapy of schwannomas in Fig. 4.
Fig. 4.
Theoretical combined AAV-ICE and EV-ICE regression of schwannomas. a Mature Schwann cells form a myelin sheath along peripheral nerve fibers. In adults, myelination is completed and the P0 promoter is no longer active in the Schwann cells. b Loss of merlin in Schwann cells can lead to their de-differentiation and proliferation, causing formation of a growing schwannoma which can compromise nerve function by compression. Injection of the AAV-ICE vector into the schwannoma results in transduction of a portion of the cells with expression of ICE, which can also be incorporated into EVs. Other non-transduced cells take up ICE carried in these EVs. The myelin sheath of normal Schwann cells is thought to block the uptake of EVs. c The presence of ICE in schwannoma cells, either through AAV infection or uptake of EV containing ICE, leads to their death, resulting in regression of a large portion of the tumor mass
To test this hypothesis, the DNA expression construct encoding P0-ICE (Prabhakar et al. 2013) was transfected into human NF2 schwannoma cells (HEI-193; Hung et al. 2002) or human embryonic kidney 293T fibroblastic cells as a negative control. Twelve hours after transfection, cells were rinsed to remove free plasmid DNA and the media were replaced with DMEM (5 % EV depleted FBS, 1 % P/S) (Corning Life Sciences, Tewksbury, MA). Forty-eight hrs after media replacement (prior to notable cell death of HEI-193 cells), EVs were isolated from the conditioned medium by two low-speed centrifugations to remove cells and debris (7 min at 300×g and 10 min at 2000×g) and a high-speed centrifugation (2 h at 100,000×g) to separate the supernatant (EV-free media) from pelleted EVs (Mizrak et al. 2013). Remarkably the EVs from medium conditioned by P0-ICE-transfected schwannoma cells were highly toxic to non-transfected schwannoma cells, while those from P0-ICE-transfected 293T cells were not, with about 4 times more EVs being produced by transfected HEI-193 cells compared to transfected 293T cells (Fig. 5). This is consistent with the P0 promoter being active in the schwannoma cells and not in the 293T cells, and with ICE (protein and/or RNA) being incorporated in the EVs, which in this case may have included apoptotic vesicles from dying schwannoma cells. The higher transfection efficiency of 293T cells compared to the schwannoma cells, and the fact that EVs from 293T cells were not toxic to schwannoma cells, support the schwannoma cell specificity of the P0 promoter and indicate that this effect was not due to the transport of cDNA in EVs (Kanada et al. 2015), but rather the mRNA or protein. The overall loss of viability in EV-free conditioned medium compared to the unconditioned medium is assumed to be due to a reduction in nutrients used by cells during the 48-h period necessary for the generation of EVs, as this difference was seen in all transfected and non-transfected cell types. In addition to the presumed presence of ICE protein in these EVs from P0-ICE-transfected schwannoma cells, there may also be mRNA encoding this protein. From other studies, we know that overexpressed protein and mRNAs of about 1 kb (ICE cDNA is 1.2 kb) can be packaged to some extent intact in EVs (Dr. Bence György, unpublished data), and that mRNA delivered by EVs can be translated in recipient cells (Skog et al. 2008; Lai et al. 2015). Overall, these data support the hypothesis that the schwannoma by-stander killing mechanism mediated by AAV-P0-ICE vectors may be due, at least in part, to the transport of ICE protein and/or mRNA from transduced to non-transduced schwannoma cells within the tumor microenvironment via EVs.
Fig. 5.
EVs from AAV-P0-ICE transfected schwannoma cells are toxic to non-transfected schwannoma cells. EVs and EV-free conditioned medium (supernatant) were harvested from HEI-193 schwannoma cells and human kidney 293T cells which had been transfected (t) with P0-ICE plasmid DNA or non-transfected (nt). EVs, EV-free conditioned medium or unconditioned fresh medium was added to the culture medium of HEI-193 cells cell viability was determined 96 h later using the CellTiter-Glo assay (Promega). a Viability of HEI-193 cells 96 h after treatment with vesicles from P0-ICE transfected HEI-193 cells (*p ≤ 0.00005; or supernatant p ≤ 0.00003); b Viability of HEI-193 cells 96 h after treatment with vesicles from non-transfected HEI-193 cells; c Viability of HEI-193 cells 96 h after treatment with vesicles harvested from P0-ICE transfected 293T cells; d Viability of HEI-193 cells 96 h after treatment with vesicles from non-transfected 293T cells; EVs added to cells ranged from 2.0 to 6.0 x 105 EVs per HEI-193 cell
Other Proteins and Disease Targets
The use of EV protein therapy is still under development. There have been reports where exosomes have been examined as a form of cancer immunotherapy (Tan et al. 2010). In particular, studies have been performed evaluating the potential application of dendritic cell-derived exosomes to promote immune responses against tumors (Taieb et al. 2006). Likewise, there have been studies examining whether exosomes derived from cancer cells might actually induce an immune response (Zhang et al. 2010; Lee et al. 2011). EVs from some cell types, e.g., mesenchymal stem cells, appear to have restorative effects in cardiac, lung, and kidney injury (Zhao et al. 2015; Heldring et al. 2015; Katakowski and Chopp 2016). But there may be a downside to the use of exosomes as therapeutic delivery vehicles, in that given the many proteins and other cargos within them, it is difficult to pinpoint the critical components, and those, especially from cancer cells may promote the transformation of normal cells in the tumor environment (e.g., Paggetti et al. 2015).
Acknowledgments
We thank Suzanne McDavitt for skilled editorial assistance and Dr. Bence György for insights into packaging of different length mRNAs into EVs. This work was supported by the NIH Common Fund through the Office of Strategic Coordination/Office of the NIH Director, NCI U19 CA179563 (XOB) and Voices Against Brain Cancer (XOB & CPL).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abels ER, Breakefield XO (2016) Introduction into extracellular vesicles—biogenesis, secretion, uptake and RNA cargo loading. Cell Mol Neuobiol (in press) [DOI] [PMC free article] [PubMed]
- Abraham S, Soundararajan CC, Vivekanandhan S, Behari M (2005) Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res 121(2):111–115 [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10(5):619–624. doi:10.1038/ncb1725 [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29(4):341–345. doi:10.1038/nbt.1807 [DOI] [PubMed] [Google Scholar]
- Amano T, Furuno T, Hirashima N, Ohyama N, Nakanishi M (2001) Dynamics of intracellular granules with CD63-GFP in rat basophilic leukemia cells. J Biochem 129(5):739–744 [DOI] [PubMed] [Google Scholar]
- Ambani LM, Van Woert MH, Murphy S (1975) Brain peroxidase and catalase in Parkinson disease. Arch Neurol 32(2):114–118 [DOI] [PubMed] [Google Scholar]
- Antonyak MA, Li B, Boroughs LK, Johnson JL, Druso JE, Bryant KL, Holowka DA, Cerione RA (2011) Cancer cell-derived microvesicles induce transformation by transferring tissue transglutaminase and fibronectin to recipient cells. Proc Natl Acad Sci U S A 108(12):4852–4857. doi:10.1073/pnas.1017667108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausman JI, Shapiro WR, Rall DP (1970) Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res 30:2394–2400 [PubMed] [Google Scholar]
- Busciglio J, Yankner BA (1995) Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature 378(6559):776–779 [DOI] [PubMed] [Google Scholar]
- Chaput N, Théry C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33(5):419–440. doi:10.1007/s00281-00010-00233-00289 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Schorey JS (2013) Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol 43(12):3279–3290. doi:10.1002/eji.201343727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocucci E, Meldolesi J (2015) Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol 25(6):364–372. doi:10.1016/j.tcb.2015.1001.1004 [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, García-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17(6):816–826. doi:10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker BA, Silke J, Gerlic M (2015) Fight or flight: regulation of emergency hematopoiesis by pyroptosis and necroptosis. Curr Opin Hematol 22(4):293–301. doi:10.1097/MOH.0000000000000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR (2012) Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 181(5):1573–1584. doi:10.1016/j.ajpath.2012.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M, Srinivasan SK, Baxi MD (1996) Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog Neurobiol 48(1):1–19 [DOI] [PubMed] [Google Scholar]
- El Andaloussi S, Mäger I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12(5):347–357. doi:10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- Gabriel K, Ingram A, Austin R, Kapoor A, Tang D, Majeed F, Qureshi T, Al-Nedawi K (2013) Regulation of the tumor suppressor PTEN through exosomes: a diagnostic potential for prostate cancer. PLoS One 8(7):e70047. doi:10.71371/journal.pone.0070047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ (2006) Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 20(6):661–669 [DOI] [PubMed] [Google Scholar]
- Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA, Bigner DD (2009) Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23(5):1541–1557. doi:10.1096/fj.1508-122184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- György B, Hung ME, Breakefield XO, Leonard JN (2015) Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmcol Tox 55:439–464. doi:10.1146/annurev-pharmtox-010814-124630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30. doi:10.1016/j.jconrel.2015.1003.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97(2):329–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang XY, Osada T, Hobeika A, Delcayre A, Le Pecq JB, Morse MA, Clay TM, Lyerly HK (2011) Increasing vaccine potency through exosome antigen targeting. Vaccine 29(50):9361–9367. doi:10.1016/j.vaccine.2011.9309.9133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Mäger I, Wood M, Le Blanc K, El Andaloussi S (2015) Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther 26(8):506–517 [DOI] [PubMed] [Google Scholar]
- Henderson MC, Azorsa DO (2012) The genomic and proteomic content of cancer cell-derived exosomes. Front Oncol 2:38. doi:10.3389/fonc.2012.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ (2011) Amphiregulin exosomes increase cancer cell invasion. Curr Biol 21(9):779–786. doi:10.1016/j.cub.2011.1003.1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber BE, Austin EA, Richards CA, Davis ST, Good SS (1994) Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A 91(17):8302–8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung G, Li X, Faudoa R, Xeu Z, Kluwe L, Rhim JS, Slattery W, Lim D (2002) Establishment and characterization of a schwannoma cell line from a patient with neurofibromatosis 2. Int J Oncol 20(3):475–482 [PubMed] [Google Scholar]
- James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, Gusella JF, Ramesh V (2009) NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol 29(15):4250–4261. doi:10.1128/MCB.01581-01508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, Roth M, Welti R, Mobley J, Jun Y, Miller D, Zhang HG (2013) Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther 21(7):1345–1357. doi:10.1038/mt.2013.1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamvoki M, Du T, Roizman B (2014) Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc Natl Acad Sci U S A 111(46):E4991–E4996. doi:10.1073/pnas.1419338111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-’t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S (2012) Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol 10(12):e1001450. doi:10.1001371/journal.pbio.1001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH (2015) Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A 112(12):E1433–E1442. doi:10.1073/pnas.1418401112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakowski M, Chopp M (2016) Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol. doi:10.1007/s10571-015-0280-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer S, Belov AM, Ghiran I, Murthy SK, Frank DA, Ivanov AR (2015) Mass-spectrometry-based molecular characterization of extracellular vesicles: lipidomics and proteomics. J Proteome Res 14(6):2367–2384. doi:10.1021/pr501279 [DOI] [PubMed] [Google Scholar]
- Lai CP, Breakefield XO (2012) Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol 3:228. doi:10.3389/fphys.2012.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire CA, Chen JW, Tannous BA, Breakefield XO (2014) Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8:483–494. doi:10.1021/nn404945r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CP, Kim EY, Badr CE, Weissleder R, Mempel TR, Tannous BA, Breakefield XO (2015) Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun 6:7029. doi:10.1038/ncomms8029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim SH, Cho JA, Kim CW (2011) Introduction of the CIITA gene into tumor cells produces exosomes with enhanced anti-tumor effects. Exp Mol Med 43:281–290. doi:10.3858/emm.2011.3843.3855.3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Antonyak MA, Zhang J, Cerione RA (2012) RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene 31:4740–4749. doi:10.1038/onc.2011.4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu-Emerson C, Plotkin SR (2009) The Neurofibromatoses. Part 1: NF1. Rev Neurol Dis 6:E47–E53 [PubMed] [Google Scholar]
- Mack M, Kleinschmidt A, Bruhl H, Klier C, Nelson PJ, Cihak J, Plachy J, Stangassinger M, Erfle V, Schlondorff D (2000) Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 6:769–775 [DOI] [PubMed] [Google Scholar]
- Martins VR, Dias MS, Hainaut P (2013) Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol 25:66–75. doi:10.1097/CCO.1090b1013e32835b32837c32881 [DOI] [PubMed] [Google Scholar]
- Mcgeer PL, Itagaki S, Boyes BE, Mcgeer EG (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291 [DOI] [PubMed] [Google Scholar]
- Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R (2015) Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523(7559):177–182. doi:10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millimaggi D, Mari M, D’Ascenzo S, Carosa E, Jannini EA, Zucker S, Carta G, Pavan A, Dolo V (2007) Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 9:349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, Ströbel T, Breakefield XO, Saydam O (2013) Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 21:101–108. doi:10.1038/mt.2012.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen CA, Coale MM, Lowe R, Blaese RM (1994) Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res 54:1503–1506 [PubMed] [Google Scholar]
- Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q (2012) Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A 109:4146–4151. doi:10.1073/pnas.1200448109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Garnier D, Minata M, Rak J (2015) Extracellular vesicles in the biology of brain tumour stem cells—Implications for inter-cellular communication, therapy and biomarker development. Semin Cell Dev Biol 40:17–26. doi:10.1016/j.semcdb.2015.1002.1011 [DOI] [PubMed] [Google Scholar]
- Paggetti J, Haderk F, Seiffert M, Janji B, Distler U, Ammerlaan W, Kim YJ, Adam J, Lichter P, Solary E, Berchem G, Moussay E (2015) Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood 126(9):1106–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18:883–891. doi:10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, Zhang CL, Chen QM, Zhang ZR, Lin YF (2013) Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 34:8521–8530. doi:10.1016/j.biomaterials.2013.8507.8102 [DOI] [PubMed] [Google Scholar]
- Porosnicu M, Mian A, Barber GN (2003) The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res 63(23):8366–8376 [PubMed] [Google Scholar]
- Prabhakar S, Taherian M, Gianni D, Conlon TJ, Fulci G, Brockmann J, Stemmer-Rachamimov AO, Sena-Esteves M, Breakefield XO, Brenner GJ (2013) Regression of schwannomas induced by adeno-associated virus-mediated delivery of caspase-1. Hum Gene Ther 24:152–162. doi:10.1089/hum.2012.1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52:515–520 [DOI] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE (2014) Regulation of immune responses by extracellular vesicles. Nat Rev Immunol 14:195–208. doi:10.1038/nri3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Mitra S, Mehta S, Raices R, Wewers MD (2009) Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One 4:e7140. doi:10.1371/journal.pone.0007140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saydam O, Ozdener GB, Senol O, Mizrak A, Prabhakar S, Stemmer-Rachamimov AO, Breakefield XO, Brenner GJ (2011) A novel imaging-compatible sciatic nerve schwannoma model. J Neurosci Methods 195:75–77. doi:10.1016/j.jneumeth.2010.1010.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Wu N, Yang JM, Gould SJ (2011) Protein targeting to exosomes/microvesicles by plasma membrane anchors. J Biol Chem 286:14383–14395. doi:10.11074/jbc.M14110.208660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda M, Khokha R (2013) Proteolytic factors in exosomes. Proteomics 13(10–11):1624–1636. doi:10.1002/pmic.201200458 [DOI] [PubMed] [Google Scholar]
- Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C (2004) The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene 23:956–963 [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Jensen SS, Lim JW (2008) Proteomic profiling of exosomes: current perspectives. Proteomics 8:4083–4099. doi:10.1002/pmic.200800109 [DOI] [PubMed] [Google Scholar]
- Singh R, Pochampally R, Watabe K, Lu Z, Mo YY (2014) Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer 13:256. doi:10.1186/1476-4598-1113-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Würdinger T, van Rijn S, Meijer D, Gainche L, Curry WTJ, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476. doi:10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanuszek A, Piątek P, Kwiatkowski S, Adamek D (2014) Multiple faces of children and juvenile meningiomas: a report of single-center experience and review of literature. Clin Neurol Neurosurg 118:69–75. doi:10.1016/j.clineuro.2013.1012.1019 [DOI] [PubMed] [Google Scholar]
- Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L (2013) Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J Immunol 191:5515–5523. doi:10.4049/jimmunol.1301885 [DOI] [PubMed] [Google Scholar]
- Taieb J, Chaput N, Schartz N, Roux S, Novault S, Ménard C, Ghiringhelli F, Terme M, Carpentier AF, Darrasse-Jèze G, Lemonnier F, Zitvogel L (2006) Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J Immunol 176:2722–2729 [DOI] [PubMed] [Google Scholar]
- Tan A, De La Peña H, Seifalian AM (2010) The application of exosomes as a nanoscale cancer vaccine. Int J Nanomedicine 5:889–900. doi:10.2147/IJN.S13402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S (2007) Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost 5:520–527 [DOI] [PubMed] [Google Scholar]
- Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35:2383–2390. doi:10.1016/j.jneumeth.2010.2310.2021 [DOI] [PubMed] [Google Scholar]
- Verweij FJ, van Eijndhoven MA, Hopmans ES, Vendrig T, Wurdinger T, Cahir-McFarland E, Kieff E, Geerts D, van der Kant R, Neefjes J, Middeldorp JM, Pegtel DM (2011) LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J 30(11):2115–2129. doi:10.1038/emboj.2011.2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–433. doi:10.1038/ncb2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820:940–948. doi:10.1016/j.bbagen.2012.1003.1017 [DOI] [PubMed] [Google Scholar]
- Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CE, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Ves 4:26316. doi:10.23402/jev.v26314.26316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S (2003) NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A 100:6145–6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YJ, Kim OY, Gho YS (2014) Extracellular vesicles as emerging intercellular communicasomes. BMB Rep 47:531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadière B, Amigorena S, Théry C (2008) Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 68:1228–1235. doi:10.1158/0008-5472.CAN-1207-3163 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Luo CL, He BC, Zhang JM, Cheng G, Wu XH (2010) Exosomes derived from IL-12-anchored renal cancer cells increase induction of specific antitumor response in vitro: a novel vaccine for renal cell carcinoma. Int J Oncol 36:133–140 [PubMed] [Google Scholar]
- Zhao Y, Sun X, Cao W, Ma J, Sun L, Qian H, Zhu W, Xu W (2015) Exosomes derived from human umbilical cord mesenchymal stem cells relieve acute myocardial ischemic injury. Stem Cells Int 2015:761643. doi:10.761155/762015/761643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schäfer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SI, Wurdinger T, Pegtel DM, van Rheenen J (2015) In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161:1046–1057. doi:10.1016/j.cell.2015.1004.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]