Abstract
Deciphering the molecular basis for guiding specific aspects of neocortical development remains a challenge due to the complexity of histogenic events and the vast array of protein interactions that mediate these events. The Eph family of receptor tyrosine kinases is implicated in a number of these neurodevelopmental activities. Eph receptors have been known to be capable of responding to several ephrin ligands within their respective subgroups, often eliciting similar downstream effects. However, several recent studies have reported specificity between receptor-ligand pairs within each subfamily, the functional relevance of which is not defined. Here, we show that a receptor of the EphA subfamily, EphA4, has distinct effects from its close relative EphA7 in the developing brain. Both EphA4 and EphA7 interact similarly with corresponding ligands expressed in the developing neocortex. However, only EphA7 shows strong interaction with ligands in the somatosensory thalamic nuclei; EphA4 affects only cortical neuronal migration with no visible effects on the guidance of CT axons, while EphA7 affects both cortical neuronal migration and CT axon guidance. Our data provide new evidence that Eph receptors in the same subfamily are not simply interchangeable, but functionally specified through selective interactions with distinct ligands in vivo.
Keywords: Eph receptor, Ephrin, Cortex, Thalamus, Corticothalamic Projections, AB_221569, AB_10015282, AB_777699, AB_514496, AB_2313608, nif-0000-30467, rid_000042
Graphical Abstract
Using tissue-binding analysis of EphA-Fc fusion proteins and in vivo overexpression analysis, the authors show that two closely related receptors of the EphA subfamily, EphA4 and EphA7, do not have interchangeable effects in the targeting of corticothalamic projections. The data provide new evidence for functional specification of Eph receptors of the same subfamily through selective interactions with distinct ligands in vivo.
INTRODUCTION
Development of the nervous system requires sensitive and precise regulatory mechanisms to ensure both the proper positioning of neurons and targeting of their projections. Amongst the molecules implicated in these processes are the Eph family, a large group of signaling proteins comprised of the Eph receptors and their respective ephrin ligands. The Ephs are the largest family of receptor tyrosine kinases, playing key roles in a wide array of biological functions in the nervous system, vascular networks, ocular tissues, and cancers (Pasquale, 2008; 2010). These molecules have been extensively studied in neural development (Flanagan and Vanderhaeghen, 1998; O’Leary and Wilkinson, 1999; Klein, 2004; Lackmann and Boyd, 2008; North et al., 2013; Klein and Kania, 2014), and have been shown to mediate both the distribution of neocortical neurons (Steinecke et al., 2014; Dimidschstein et al., 2013; Sentürk et al., 2011; Zimmer et al., 2008; Torii et al., 2009) and the formation of neural circuits, including projections that connect the neocortex with thalamic structures (Vanderhaeghen et al., 2000; Dufour et al., 2003; Torii and Levitt, 2005; Dufour et al., 2006; Uziel et al., 2006; Torii et al., 2013a; Torii et al., 2013b; Tai and Kromer, 2014).
Eph receptors are divided into two subfamilies (EphA and EphB receptors) which primarily interact with their corresponding subfamily of ligands (ephrin-A and ephrin-B ligands, respectively). One key feature of this molecular family is their proposed extensive receptor-ligand promiscuity within (Flanagan and Vanderhaeghen, 1998) and, in some cases, between subfamilies (Holland et al., 1996; Kullander et al., 2001; Yokoyama et al., 2001; Blits-Huizinga et al., 2004; Himanen et al., 2004). This wide range of interactions has functional consequences, as several studies on retinotopic map formation have reported that molecules within the same subfamily can be interchangeable while eliciting similar effects (Gale et al., 1996; Brown et al., 2000; Reber et al., 2004; Bevins et al., 2011; McLaughlin et al., 2014). As a result, a prevailing view within the field has been that the bindings and functions within subclasses of the Eph-ephrin family are largely identical and redundant (Orioli and Klein, 1997; Yokoyama et al., 2001; Himanen and Nikolov, 2003; Pasquale, 2004; Lackmann and Boyd, 2008).
However, several studies have suggested distinct specificities in interactions within members of each subclass. Preferential binding between certain receptor-ligand pairs have been observed between both EphA receptors and ephrin-A ligands (Gale et al., 1996; Monschau et al., 1997; Orioli and Klein, 1997) and between EphB receptors and ephrin-B ligands (Sakano et al., 1996; Bergemann et al., 1998; Munthe et al., 2000). In addition, ligand–receptor binding assays in brain tissues have provided compelling evidence of binding selectivity between respective EphA/ephrin-A pairs (Janis et al., 1999; Tai et al., 2013). Several in vitro studies have also provided functional evidence of differential biological activities between specific Eph receptor and ephrin ligand pairs, including within retinal ganglion cells (Monschau et al., 1997), hippocampal neurons (Stein et al., 1999), and epidermal keratinocytes (Walsh and Blumenberg, 2011). Yet, there is still a question of whether functional specificity between Eph receptors subtypes with their respective ephrin ligands has relevance in vivo during development.
In this present study, we examined whether EphA4 and EphA7, two closely related receptors of the EphA subfamily with distinct binding affinities to different ephrin-A ligands, can mediate unique developmental outcomes in vivo. We analyzed this activity through the positioning of cortical neurons and the formation of corticothalamic (CT) projections, both of which are highly dependent on the Eph family for their precision (Bolz et al., 2004; Torii and Levitt, 2005; Flanagan, 2006; Price et al., 2006; Grant et al., 2012; Molnar et al., 2012; Leyva-Diaz and Lopez-Bendito, 2013; Garel and Lopez-Bendito, 2014; Tai and Kromer, 2014). Here, we show that EphA4 has a dissimilar functionality from EphA7 in the targeting of CT projections, while retaining similar functions in cortical neuronal migration.
MATERIALS AND METHODS
Animal Care
All mouse experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committees of Yale University and Children’s National Medical Center. CD-1 wild-type mice (Charles River) were used for all studies. Embryonic day 0.5 (E0.5) and postnatal day 0 (P0) were designated as noon of the day on which the presence of a vaginal plug was observed and the day of birth, respectively.
Immunohistochemistry
Brains were dissected and immersion-fixed into 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS, pH 7.4) overnight at 4 °C and sectioned coronally at 75 μm via vibratome (Leica). Slices were incubated with rabbit polyclonal anti-green fluorescent protein (GFP) antibody (1:1000; Cat#A11122; RRID:AB_221569; Invitrogen) overnight at 4 °C, washed extensively in PBS, and incubated with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit secondary antibody (1:1000; Cat#711-035-152; RRID:AB_10015282; Jackson Immunoresearch). Sections were then rinsed and visualized using the TSA Plus Fluorescence system (Perkin Elmer). For double staining, HRP activity was inactivated with 3% hydrogen peroxide in methanol after the first staining with the GFP antibody. After washing in PBS, slices were incubated with a biotin-conjugated rabbit polyclonal anti-red fluorescent protein (RFP) antibody (1: 1000; Cat#ab34771; RRID:AB_777699; Abcam) overnight at 4 °C, washed in PBS, and incubated with VECTASTAIN Elite ABC Kit (Vector Laboratories). Sections were then rinsed and visualized using the TSA Plus Cy3 system (Perkin Elmer). Sections were counterstained with 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) (Invitrogen) to detect nuclei. Images were captured using a confocal LSM 510 NLO system or Axioplan2 microscope (Zeiss). Images were not modified other than to balance brightness and contrast.
Antibody Characterization
The anti-GFP (Cat#A11122; RRID:AB_221569; Invitrogen) and anti-RFP (Cat#ab34771; RRID:AB_777699; Abcam) antibodies are from the IgG fraction of rabbit serum raised against full-length GFP isolated from Aequorea victoria and RFP fusion protein corresponding to the full-length amino acid sequence (234aa) derived from mushroom polyp coral Discosoma, respectively. These antibodies react with EYFP (a GFP variant) and DsRed2 (an RFP variant), respectively. In control experiments, we confirmed the lack of staining with these antibodies on sections of mouse brains that were not electroporated (data not shown).
In Situ Hybridization
Methods for in situ hybridization on tissue cryosections have been previously described (Torii et al., 2009). Probes for EphA4, EphA7, ephrin-A2, ephrin-A3 and ephrin-A5 have been previously described (Torii and Levitt, 2005; Torii et al., 2009). The plasmids to generate probes for ephrin-A1 (mouse, 646 base-pairs [bps]) and ephrin-A4 (rat, 440 bps) were gifts from L. F. Kromer (Georgetown University School of Medicine). Digoxigenin-labeled sense or antisense probes were transcribed from linearized plasmids containing the corresponding cDNA fragment using the DIG RNA Labeling Kit (Roche Diagnostics) according to the manufacturer’s protocol. Brains were dissected and immersion-fixed into 4% PFA in PBS overnight and cryopreserved in 30% sucrose. Sections were then cut on a cryostat (Leica) at 14 μm, treated with proteinase K (1Ug/ml in 100 mM Tris-HCl pH 8.0, 50mM EDTA) for 10 min at room temperature (RT), followed by fixation with 4% PFA in PBS. Sections were hybridized overnight at 60°C in hybridization buffer (50% formamide, 750 mM NaCl, 75 mM Na citrate, 1% SDS, 50 Ug/ml tRNA, 50 Ug/ml heparin) containing 0.5 Ug/ml of probe. Following hybridization, the slides were washed 3 times with washing solution (50% formamide, 300 mM NaCl, 30 mM Na citrate, 1% SDS) at 60 °C for 45 min, and 3 times with TBST (250 mM Tris-HCl pH 7.5, 1.36 M NaCl, 26.8 mM KCl, 10% Tween-20) at RT for 15 min. Sections were then incubated in anti-digoxigenin antibody (1:5000; #11333089001; RRID:AB_514496; Roche Diagnostics) in 1.5% Blocking Reagent (Roche Diagnostics) within B1 buffer (100 mM Tris-HCl pH 7.5, 150 mM NaCl) overnight at 4 °C. After washing in TBST, detection was made possible using nitroblue tetrazolium chloride (NBT; Roche Diagnostics) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP; Roche Diagnostics) as per the instructions by the manufacturer, with images captured using a BX61 microscope (Nikon).
Ligand-Binding and Receptor-Binding Histochemistry
Minor modification of previously described ligand- and receptor-binding histochemistry on cryosections was used (Janis et al., 1999; Torii and Levitt, 2005; Torii et al., 2013a). Briefly, mouse embryos and brains were immersion-fixed in 4% PFA in PBS for 1 hour and cryopreserved in 30% sucrose overnight. Sections were then cut on a cryostat (Leica) at 14 μm. Sections were incubated with recombinant EphA-Fc or ephrin-A5-Fc protein (2 μg/ml; #SMPK1, #7396-EA; R&D Systems) for 90 minutes at room temperature, rinsed, and post-fixed with 4% PFA in PBS for 30 minutes. Samples were rinsed and then incubated with biotin- or HRP-conjugated anti-human IgG secondary antibody (1:1000; #715-035-150; RRID:AB_2313608; Jackson Immunoresearch). For alkaline phosphatase (AP) detection, samples were incubated with the VECTASTAIN ABC-AP kit (Vector Laboratories) with detection made possible using NBT (Roche Diagnostics) and BCIP (Roche Diagnostics) as per the instructions by the manufacturer, with images captured using a BX61 microscope (Nikon). For fluorescence, samples were visualized with the TSA Plus Cy3 System (Perkin Elmer), with images captured using an Axioplan2 microscope (Zeiss).
In Utero Electroporation
EphA4 (pCAG-EphA4-IRES-Venus, containing the full-length cDNA of EphA4), EphA7 (pCAG-EphA7-IRES-Venus, containing the full-length cDNA of EphA7), control (pCAG-IRES-EGFP), DsRed2 (pCAG-DsRed2) and EYFP (pCAG-EYFP) plasmids have been described previously (Torii and Levitt, 2005; Hashimoto-Torii et al., 2008; Torii et al., 2009; Torii et al., 2013a; Torii et al., 2013b). In utero electroporation-mediated gene transfer was performed as previously described (Saito and Nakatsuji, 2001; Tabata and Nakajima, 2001; Torii et al., 2013b). Briefly, E12.5 pregnant mice were anesthetized with ketamine/xylazine (100/10 mg/kg) and the uterine horns were exposed. DNA was injected via a pulled glass pipette into the lateral ventricle of each embryo, followed by electrodes placed onto either side of the head parallel to the sagittal plane. An electrical current (five 50 ms pulses of 30 V at 950 ms intervals) was then passed to drive the DNA into parietal cortical areas using an ECM 830 Square Wave Electroporator (BTX-Harvard Apparatus). Following electroporation, the uterine horns were replaced, and the mouse was allowed time to recover and give birth normally. Electroporated mice were screened visually at P0 for EYFP expression though a stereo fluorescent microscope. Animals were selected for EYFP expression located within the somatosensory cortex prior to analysis for CT axons.
Quantitative Analysis of the Distribution of CT Axons
For analysis of CT projections via centroid analysis, the ventrobasal complex (VB) and medial division of the posterior nuclear group (POm) were outlined. The centroids of the area covered by DsRed2+ and EYFP+ CT axons within these nuclei were calculated by measuring the average X and Y coordinates of all pixels of DsRed2+ and EYFP+ projections, respectively, using ImageJ (http://rsb.info.nih.gov/ij/index.html, RRID:nif-0000-30467). The relative position of the centroid of EYFP+ axons from that of DsRed2+ axons along the ventro-medial to dorso-lateral (VM-DL) axis (X-axis) and the ventro-lateral to dorso-medial (VL-DM) axis (Y-axis) (see Fig. 7I) was determined. The data are graphed by setting the relative position of the centroid of EYFP+ projections in control-electroporated brains at (0.0). Statistical analysis was performed using one-way ANOVA/Tukey-Kramer’s multiple comparison with SPSS (http://www-01.ibm.com/software/uk/analytics/spss/, RRID:rid_000042).
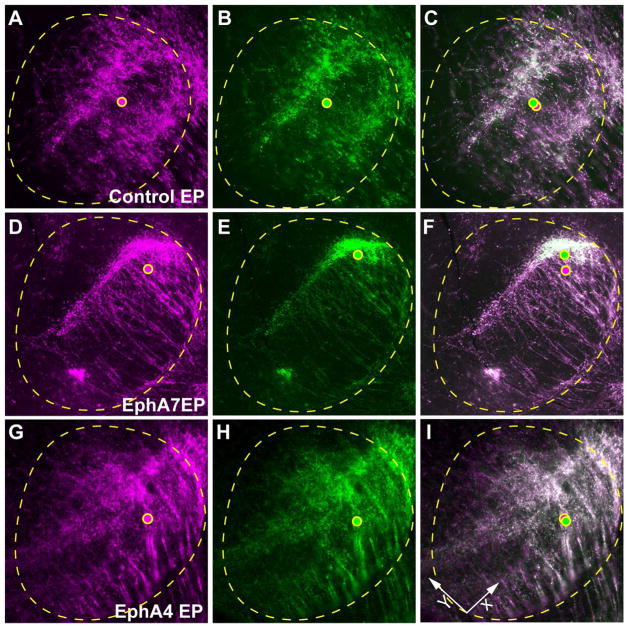
Figure 7. Centroid analysis of control, EphA7- and EphA4-overexpressing CT projections within the VB.
(A–C) The VB/POm of control electroporated brains. The centroids of the area covered by DsRed2+ and EYFP+ CT axons (dots in magenta and green, respectively) are closely positioned. (D–F) The VB/POm of EphA7 electroporated brains. The centroid of EYFP+ CT axons are shifted dorsally from that of DsRed2+ CT axons compared to controls. (G–I) The VB/POm of EphA4 electroporated brains. The centroids of DsRed2+ and EYFP+ CT axons are closely positioned.
RESULTS
Different binding patterns of EphA receptors to the ephrin-As expressed in the developing thalamus
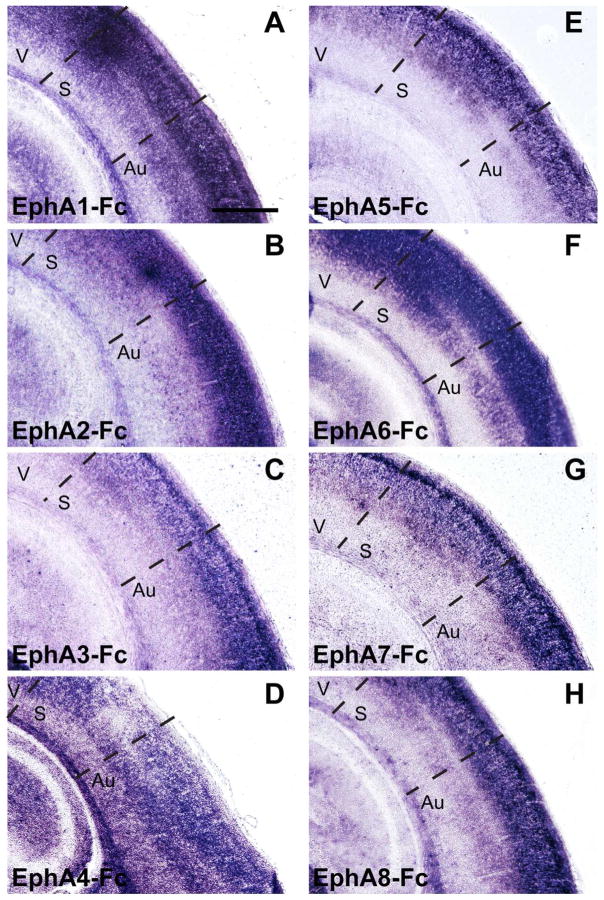
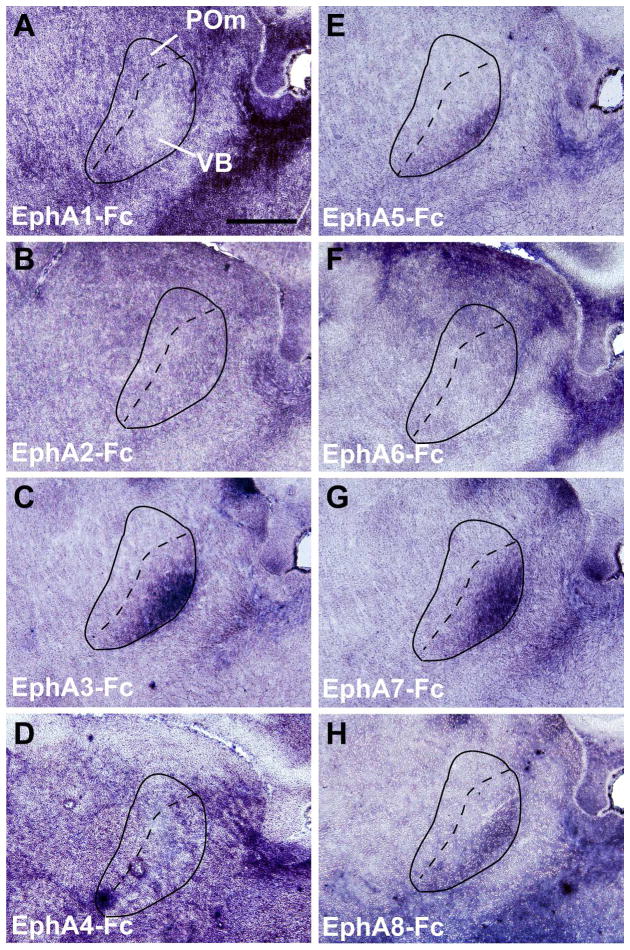
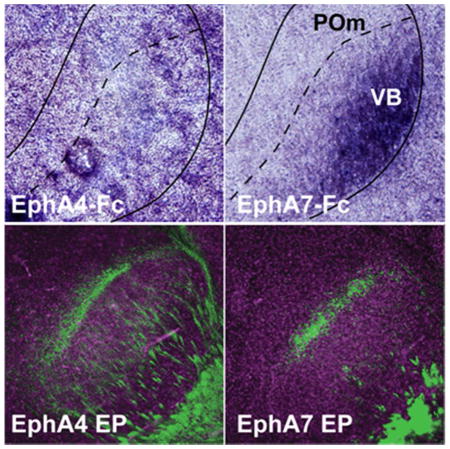
We have previously shown that the interaction between EphA7 expressed in the neocortex and ephrin-A ligands expressed in the thalamus participates in the topographic targeting of primary sensory CT projections within each corresponding thalamic nucleus (Torii and Levitt, 2005; Torii et al., 2013b). To determine the relationship of other EphA subfamily members with ephrin-As in CT circuits, we compared the binding patterns of Fc-tagged EphA receptors within the cortex and thalamus at P4 (Figs. 1 and 2). This age was chosen as it corresponds to the time during which CT axons are topographically established within their appropriate terminal zones (Torii et al., 2013b). Binding of EphA-Fc fusion proteins was observed throughout the early postnatal brain, with all EphA-Fc fusion proteins examined (EphA1-Fc – EphA8-Fc) showing significant and specific binding in cortical regions (Fig. 1). However, we found variable binding of the different Eph receptor fusion proteins in the somatosensory thalamic nuclei, namely in the ventrobasal complex (VB) and the medial division of the posterior nuclear group (POm) (Fig. 2). EphA1-Fc and EphA2-Fc displayed relatively non-specific binding patterns throughout the thalamus (Fig. 2A and B). EphA3, A5, A7, and A8 had similar binding patterns in the VB and POm, with specifically high levels of binding in the ventrolateral region of VB (Fig. 2C, E, G, H). The binding pattern of EphA4 and A6 lacked a unique labeling distribution within the VB (Fig. 2D and F).
Figure 1. Patterns of EphA receptor binding in the cortex of the perinatal brain.
(A–H) Binding patterns of Fc-tagged EphA receptors (EphA1 – EphA8) within the cortex at P4. All EphA-Fc receptor displayed binding in the cortex, with significant binding observed in the upper cortical layers. V = Visual Cortex, S = Somatosensory Cortex, Au = Auditory Cortex. Scale bar = 500 μm.
Figure 2. Patterns of EphA receptor binding in the VB and POm thalamic nuclei in the perinatal brain.
(A–H) Binding patterns of Fc-tagged EphA receptors (EphA1 – EphA8) within the VB and POm at P4. Note that the labeling by EphA3-Fc, A5-Fc, A7-Fc and A8-Fc binding shows a distinct gradient in the VB with the strongest labeling at the ventro-lateral region (C, E, G, H), whereas EphA1-Fc, A2-Fc, EphA4-Fc, and EphA6-Fc binding in the VB (A, B, D, F) does not. Scale bar = 500 μm.
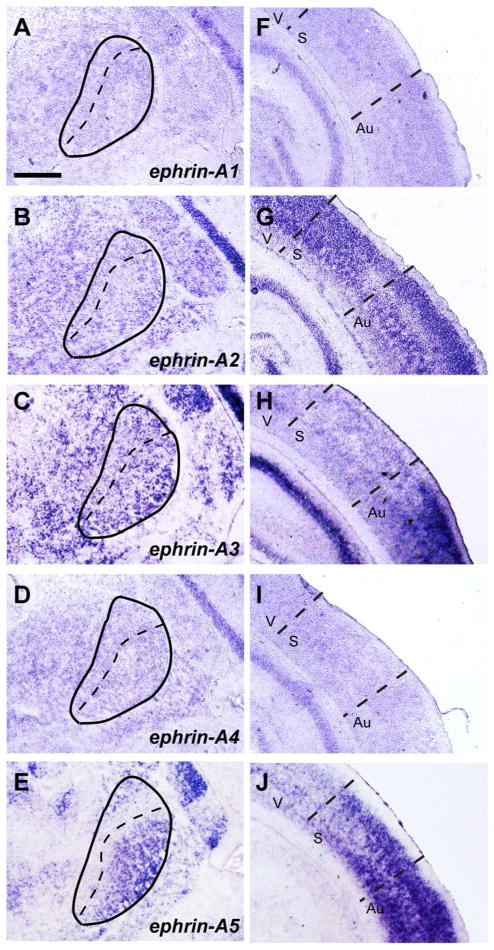
We next determined ephrin-A ligand expression within the VB/POm (Fig. 3A–E) and the neocortex (Fig. 3F–J) at P4. Of particular interest was ephrin-A5, which exhibited strong expression in the ventrolateral region of the VB (Fig. 3E) similar to the gradient observed in the binding of EphA3, A5, A7 and A8 (Fig. 2). Ephrin-Bs have not been detected within this region at this stage (Allen Brain Atlas and GENSAT), suggesting that the binding patterns of each EphA receptor in the VB and POm are mediated through ephrin-A ligands.
Figure 3. Patterns of ephrin-A ligand expression in the VB/POm and cortex of the perinatal brain.
(A–E) In situ hybridization of ephrin-A ligands within the VB/POm at P4. Expression of ephrin-A2 and -A3 (B and C) is observed throughout the VB/POm in a uniform fashion. In contrast, the expression of ephrin-A5 is observed in a distinct ventro-lateral gradient within the VB/POm (E). Expression of ephrin-A1 and –A4 was low/not expressed (A and D). (F–J) In situ hybridization of ephrin-A ligands within the cortex. Strong expression of ephrin-A2, -A3, and –A5 (G, H and J) are observed in the cortex, while ephrin-A1 and –A4 expression was low/not expressed. V = Visual Cortex, S = Somatosensory Cortex, Au = Auditory Cortex. Scale bar = 500 μm.
EphA7 expression, and not EphA4, is similar to ephrin-A5 ligand binding pattern
Given the unique binding patterns in the thalamus (Fig. 2), the distinct ligand interactions observed in previous studies (Janis et al., 1999; Tai et al., 2013), and our previous analysis on the effects of EphA7 on CT projections (Torii and Levitt, 2005; Torii et al., 2013b), we focused our analysis on comparing the in vivo biological functions of EphA4 in comparison to EphA7. Both EphA4 and EphA7 were expressed throughout the cortex, thalamus and striatum, in overlapping but distinct patterns at P4 (Fig. 4A–D). Consistent with the similarity of the expression of ephrin-A5 in the VB to the binding of EphA7-Fc but not to that of EphA4-Fc, the binding pattern of Fc-tagged ephrin-A5 was similar to EphA7 but not to EphA4 in the thalamus (Fig. 4B, D, F), striatum (Fig. 4A, C, E) and neocortex (Fig. 4A–F). These data support previous reports of ephrin-A5 exhibiting preferential binding to EphA7 and not EphA4 (Janis et al., 1999; Tai et al., 2013). Together, this suggests that EphA4 has little interaction with ephrin-A ligands, including ephrin-A5, within the thalamus and, unlike EphA7 (Torii and Levitt, 2005), may not affect the topographic targeting of CT projections.
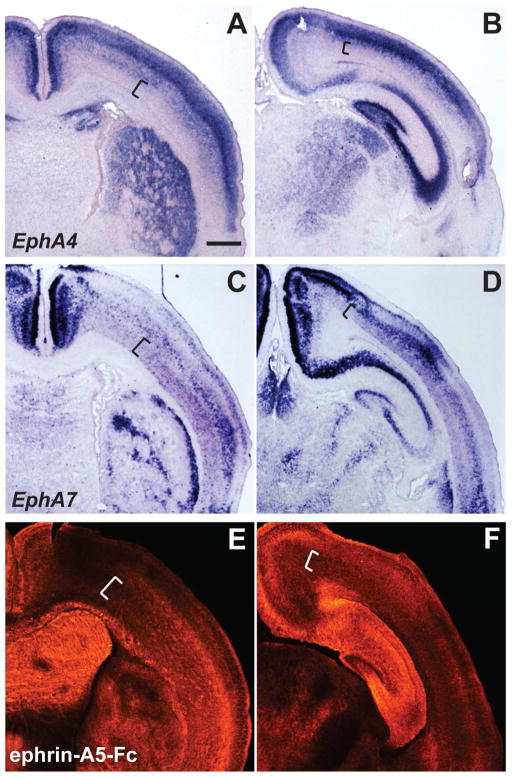
Figure 4. Distinct expression patterns of EphA4 and EphA7, in comparison to ephrin-A5 binding in the early postnatal brain.
(A–D) In situ hybridization of EphA4 (A and B) and EphA7 (C and D) in anterior (A, C) and posterior (B, D) sections of the P4 mouse brain. In both anterior and posterior sections, EphA4 is observed predominantly in upper cortical layers, with lower expression in deeper layers (A and B, brackets). In contrast, EphA7 labeling is evident in distinct gradients within deeper layers, including layer VI (C and D, brackets). Both EphA4 and EphA7 have distinct expression patterns in the striatum (A and C) and thalamus (B and D). (E and F) Binding patterns of ephrin-A5-Fc at anterior (E) and posterior (F) levels. The relative labeling intensity of ephrin-A5-Fc binding displays an overall similarity to EphA7 expression, including the gradients in layer VI (see brackets), striatum, and thalamus. Scale bar = 500 μm.
Overexpression of EphA4 causes columnar segregation of neurons within the neocortex
The differential expression of EphA4 and EphA7 and the unique binding patterns exhibited by EphA4-Fc and EphA7-Fc fusion proteins in P4 brains suggest that these receptors may exhibit specific biological responses. To determine whether functional differences exist in vivo, we overexpressed EphA4 and EphA7 within the neocortex using the in utero electroporation-mediated gene transfer method at E12.5. The most striking phenotype observed was the strong columnar segregation of EYFP–labeled EphA overexpressing neurons in the cortex (Fig. 5B–D, n ≥ 20) compared to the even distribution in the control-electroporated brain (Fig. 5A, n ≥ 20). The distinct columnar patterns of EphA4-overexpressing neurons (Fig. 5C and D) are very similar to those generated by EphA7 overexpression (Fig. 5B) (Torii et al., 2009). Given that the segregation occurs within the intermediate zone (IZ) during the embryonic period (Torii et al., 2009), we performed binding experiments using EphA4-Fc and EphA7-Fc fusion proteins at E14.5. Although each exhibited differential binding patterns in the hippocampus, thalamus and hypothalamus, the binding patterns of both EphA receptors were similar within the cerebral wall at this age (Fig. 5E–H).
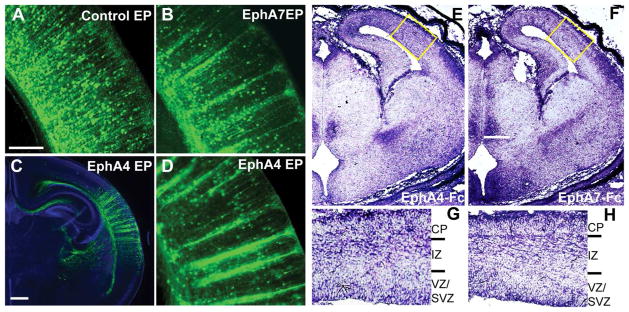
Figure 5. Columnar segregation of EphA4-overexpressing neurons in the neocortex.
(A–D) Effects of EphA overexpression in cortical neurons. E12.5 mice were co-electroporated (EP) with EYFP and an overexpression or control vector, with brains collected at P4. Brains were immunostained for EYFP and counterstained with DAPI (blue, C). Overexpression of EphA7 (B) or EphA4 (C and D) in cortical neurons resulted in distinct columnar segregation, which was not observed in the control (A). (E-H) EphA4-Fc and EphA7-Fc binding of E14.5 brains. Binding of both EphA4-Fc (E) and EphA7-Fc (F) receptors are observed in the developing cortex in similar patterns. G and H are higher magnification views of the boxed areas in E and F, respectively, showing EphA4-Fc and EphA7-Fc binding in the ventricular/subventricular zones (VZ/SVZ), intermediate zone (IZ) and cortical plate (CP). Scale bars = 200 μm (A, B, D), 500 μm (C) and 500 μm (E, F).
Overexpression of EphA4 does not have the same impacts on topographic targeting of CT projections as EphA7
We next examined the effects of EphA4 in the topographic targeting of CT projections in comparison to EphA7 (Fig. 6). We had previously reported that EphA7 overexpression in cortical neurons results in significant shifts on CT projections compared to controls (Torii and Levitt, 2005; Torii et al., 2013b). In addition, our data indicates that EphA4-Fc showed little binding in the VB (Fig. 2D) in contrast to EphA7-Fc (Fig. 2G). To compare the actions between CT axons overexpressing higher and lower levels of EphA4 or EphA7 originating from the same cortical region, control or Eph receptor expression plasmids were co-transfected in the somatosensory cortex with a high concentration of DsRed2 expression plasmid and a low concentration of EYFP expression plasmid (Fig. 6A). This indirect approach to identify the expression levels of electroporated EphA4 and EphA7 was used since prior studies indicate that the expression level of each of the transfected genes in individual cells strongly correlates with that of other co-transfected genes (Hatanaka et al., 2004). In this experiment, neurons and their axons expressing both EYFP and DsRed2 (EYFP+/DsRed2+; designated EYFP+ axons) should have higher levels of the electroporated EphA receptor, while CT axons in which only DsRed2 is detectable (EYFP−/DsRed2+; designated EYFP− axons) should express lower levels of the EphA receptor (Fig. 6B), as previously confirmed by differential levels of ephrin-A5-Fc binding between these neuronal populations (Torii and Levitt, 2005).
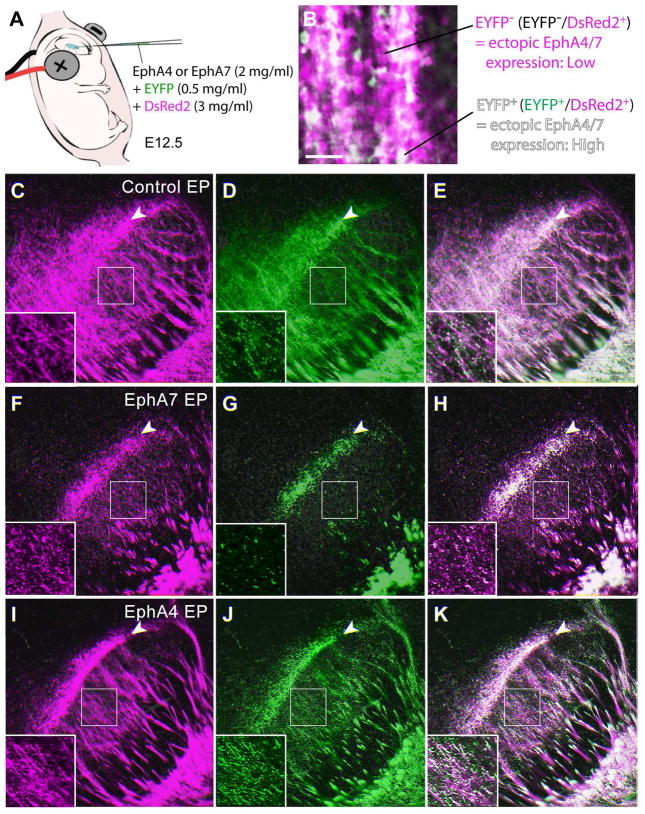
Figure 6. Overexpression of EphA4 in the somatosensory cortex does not result in a shift of CT projections in the VB/POm at P4.
(A) Schema for in utero electroporation with two fluorescent markers at different concentrations. The control, EphA4-, or EphA7-expression plasmid together with 0.5 mg/ml (low concentration) of EYFP-expression plasmid and 3 mg/ml (high concentration) of DsRed2-expression plasmid was injected into the lateral ventricle of E12.5 brains, followed by electroporation. (B) P4 cortical neurons in the somatosensory cortex electroporated with low concentration of EYFP (green) and high concentration of DsRed2 (magenta). EYFP+ cells (white; EYFP+/DsRed2+) displays neurons with higher levels of ectopic EphA expression, while EYFP− cells (magenta; EYFP−/DsRed2+) shows cells with lower levels of ectopic EphA expression. (C–E) The VB/POm of control electroporated (EP) brains. EYFP+ and EYFP− CT axons are distributed similarly in the center of the VB (insets show higher magnification views of the boxed areas) as well as at the border region between the VB and POm (arrowheads). (F–H) The VB/POm of EphA7 electroporated brain. CT axons with high levels of EphA7 expression (EYFP+) show a restricted distribution at the border region between the VB and POm (arrowheads) and are segregated from axons with low levels of ectopic EphA7 expression (EYFP−) that distribute also in the body of the VB (insets show higher magnification views of the boxed areas). (I–K) The VB/POm of EphA4 electroporated brain. Unlike EphA7 overexpression, EphA4 has no effect on CT axons in this region similar to that of controls, with high (EYFP+) and low (EYFP−) levels of expression distributed similarly at the VB and POm (insets show higher magnification views of the boxed areas). Scale bar = 100 μm (for B), 200 μm (for C–K).
In the control, the EYFP+ and EYFP− CT axons displayed broadly similar distributions in the VB and VB/POm border. Overexpression of EphA7 displayed a markedly different effect, resulting in distinct targeting of EYFP+ and EYFP− CT axons, with strong accumulation of EYFP+ axons at the border region between the VB and POm (Fig. 6G, H) and broader distribution of EYFP− axons in the VB (Fig. 6F,H), corroborating with our previous results (Torii and Levitt, 2005). However, in stark contrast to EphA7, EphA4 overexpression showed broad distribution of EYFP+ and EYFP− CT axons within the VB, a pattern very similar to controls (Fig. 6I–K). Increasing the concentration of EphA4 expression plasmid up to 5 mg/ml did not alter axon distribution (data not shown). The VB/POm border is the region at which some EphA4-overexpressing brains show certain levels of restrictions in the distribution of labeled CT axons (Fig. 6K), though this is also the case for some control-electroporation cases, and the effects appears limited and variable (see Fig. 7I). Quantification of the distribution of EYFP+ CT axons along the dorso-medial to ventro-lateral axis of the POm and VB, by which strong shift of EYFP+ CT axons to the VB-POm border region by EphA7 overexpression was revealed in our previous study (Torii and Levitt, 2005), indicated that the effect of EphA4 overexpression on topographic targeting of CT axons is statistically insignificant comparing to the control-electropoerated brains (Mixed design ANOVA, F(29,290) = 1.06, p = 0.39, K-S test, p = 0.30, n=5 brains per group) (data not shown). This may reflect interactions between EphA4 and other more evenly expressed ephrin-A ligands in the VB/POm aside from ephrin-A5 (see Fig. 3). These results are consistent with the binding data of high binding of EphA7-Fc and low binding of EphA4-Fc in the VB (see Fig. 2).
To quantitatively determine the extent of the changes after EphA-receptor overexpression, we performed centroid analysis of CT projections in the VB/POm (Fig. 7). The shift in the central mass of EYFP+ (Fig. 7B, E, H) axons compared to that of DsRed2+ (Fig. 7A, D, G) axons within the VB/POm were respectively averaged. This shift was then plotted along the axis perpendicular to dorsomedial-to-ventrolateral axis, with (0.0) denoting the average shift observed in controls (Fig. 8). As expected, EphA7 over-expression resulted in a statistically significant dorsomedial shift in CT targeting compared to controls (Fig. 7D–F, Fig. 8). In contrast, no significant topographic shift was found in thalamus containing EphA4-overexpressing axons (Fig. 7G–I, Fig. 8). Together, these data indicate that EphA4 and EphA7 do not have interchangeable function on controlling topographic targeting of CT projections in the somatosensory thalamus.
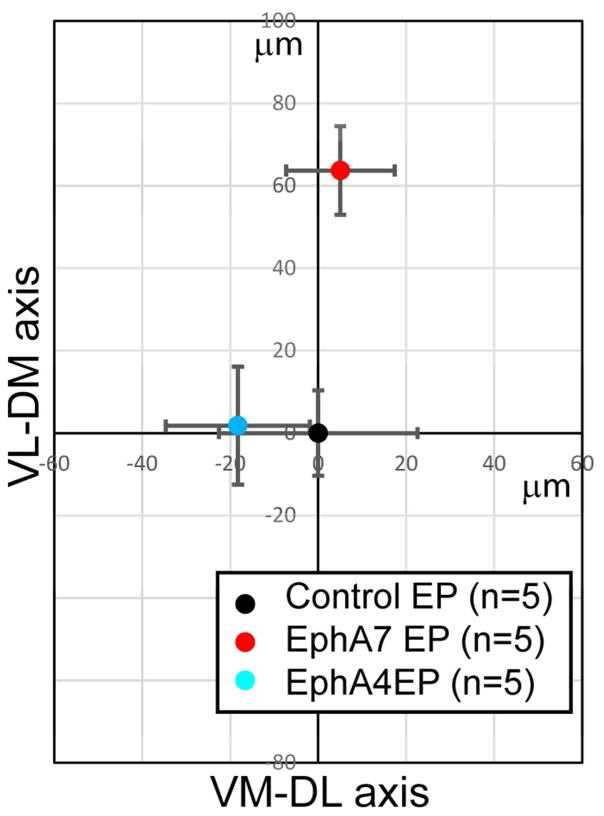
Figure 8. No significant shifts in CT projections within the VB by overexpression of EphA4.
The relative position of the centroid of EYFP+ axons from that of DsRed2+ axons along the ventro-medial to dorso-lateral (VM-DL) axis (X-axis) and the ventro-lateral to dorso-medial (VL-DM) axis (Y-axis) (arrows in Fig. 7I). The data for EphA4-, EphA7- and control-electroporated brains are graphed by setting the relative position of the centroid of EYFP+ projections in control-electroporated brains at (0.0). The differences only along the VL-DM axis (Y-axis) are significant between control- and EphA7-electroporated brains, and between EphA4- and EphA7-electroporated brains (p < 0.01, ANOVA/Tukey-Kramer’s multiple comparison). Error bars show the SEM, and n = the number of brains analyzed in each condition.
DISCUSSION
The present study demonstrates that EphA4 and EphA7 receptors, in spite of their similar structure, have distinct in vivo effects on CT projections into the VB/POm. EphA4 and EphA7 exhibit both overlapping and non-identical expression and binding patterns in the neocortex and thalamus. It is therefore possible that the receptors have the ability to respond to distinct sets of ligands with differing affinities. Ephrin-A5 appears to be the preferred ligand for EphA7 but not EphA4 in the developing forebrain during early postnatal development, based on similarities of EphA7 expression and ephrin-A5 binding patterns, and of ephrin-A5 expression and EphA7 binding patterns. This finding has been documented previously in relation to receptor-ligand binding in the striatum (Janis et al., 1999). Perhaps most important, we found that cell context is a key determinant of EphA4 and EphA7 neurodevelopmental activities. Thus, overexpression of EphA4 and EphA7 in CT axons have distinct effects on projection patterning, but play a very similar role in regulating the dispersion patterns of migrating neocortical neurons. Each of the EphA receptors, under specific conditions, may therefore respond similarly or differently to ligands. It is not possible to conclude a priori, that ligands are interchangeable (or not) unless tested specifically in a developmentally relevant context.
It should be noted that while the present study shows the difference in biological activities of overexpressed EphA4 and EphA7 in the context of CT axon targeting, the experiments do not address directly the different in vivo roles for these EphA receptors. Our previous studies used both EphA7 knockdown and overexpression approaches by in utero electroporation to manipulate EphA7-mediated topographic targeting of CT projections (Torii and Levitt, 2005; Torii et al., 2013a; Torii et al., 2013b). Such approaches that manipulate EphA4 will be required in the future to determine whether EphA4 activation can mediate any aspect of CT targeting.
EphA Promiscuity versus Specificity
One unique property of the Eph family is the promiscuity in the interaction between the receptor and ligand pairs both within and, in some occasions, between subclasses (Flanagan and Vanderhaeghen, 1998; Himanen and Nikolov, 2003; Blits-Huizinga et al., 2004). Several studies, particularly those focusing on retinotopic map formation, have reported that this wide range of interaction within the Eph-ephrin system is open to high levels of redundancy, and that molecules within the same subfamily can be largely interchangeable (Gale et al., 1996; Brown et al., 2000; Reber et al., 2004; Bevins et al., 2011; McLaughlin et al., 2014).
There may, however, be fundamental differences in promiscuity driven by context. For example, studies involving hippocampal neurons (Henkemeyer et al., 2003), cortical pyramidal neurons (Kayser et al., 2006), striatal tissue (Janis et al., 1999; Tai et al., 2013), and retinal ganglion cell projections at the optic chiasm (Petros et al., 2009; Chenaux and Henkemeyer, 2011) have indicated more defined specificities between Eph receptor-ephrin ligand subfamily members. What remains to be determined is whether a physiological consequence exists as a result of this difference in binding affinity.
Binding specificity of Eph receptors within the same subfamily may be due to differences in their affinities for specific ephrin ligands within specific cell types. Although the mechanisms responsible for such intraclass differences remain unknown, small amino acid changes in the ligand binding domain and/or interactions with specific adaptor proteins may define the structural features that confer the ligand selectivity of each Eph receptor (Chrencik et al., 2006). As a result of the distinctions in interactions with respective ligands, the differences within subclasses may elicit different biological effects when comparing Eph receptors. Overall homology of the shared sequences in the extracellular domains, however, may still allow for EphA receptors to interact with most ephrin-A ligands if applied in high enough concentrations.
Given the present findings and those reported in other developing systems (Bevins et al., 2011), the degree of interchangeability between Eph receptors in vivo remains complex to determine and is likely dependent upon the spatial and temporal aspects of characterization. Based on our current data, we believe this is partially due to the availability of ephrin ligands within a region capable of interacting with EphA4 or EphA7. However, in many of these cases, the specific ligands are likely to be expressed in similar patterns within several regions within the nervous system, with similar downstream signaling events producing redundant effects. For example, the presence of several ephrin-A ligands within the neocortex has been reported previously (Mackarehtschian et al., 1999; Depaepe et al., 2005; Torii et al., 2009; Lodato et al., 2014). As a result, specific ligands may interact with EphA4 and EphA7 with different affinities in order to elicit similar effects in neuronal positioning. In contrast, the ephrin-A ligands within the VB and POm may be those only capable of interacting with EphA7 efficiently and not EphA4. It also may be possible that binding competition between other Eph receptors mediates specific interactions and biological activities, with the most preferable receptor-ligand interactions resulting in specific effects. Together, this specificity of binding within Eph/ephrin subfamilies would provide greater control in the manipulation for axonal guidance, while maintaining some level of promiscuity in their function.
Finally, it is possible that differential interactions and signaling properties exhibited by EphA4 and EphA7 may contribute to their different effector activities (Richter et al., 2007; Petros et al., 2009; Demyanenko et al., 2011; Seiradake et al., 2013). Future studies using chimeric receptors (Petros et al., 2009), in which protein domains of both EphA4 and EphA7 are combined, or the switching of alleles between EphA4 and EphA7 (Spitzweck et al., 2010), whereby each of EphA receptors are expressed in each other’s pattern, will provide further information about the molecular basis for functional specializations among EphA receptors.
Acknowledgments
Supporting Grant: Avery Translational Research Career Development Program Award/Children’s National Medical Center (MT), NARSAD Young Investigator Grant/Brain & Behavior Research Foundation (MT and KH-T), Scott-Gentle Foundation (KH-T), Kavli Institute for Neuroscience at Yale (MT and PR), NIH grants NS014841 (PR) and DA022785 (PL).
We thank Drs. L.F. Kromer, T. Saito, A. Miyawaki, and N.Y. Ip for providing materials. We are grateful to M. Pappy and S. Rodriguez for their technical assistance.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
LITERATURE CITED
- Bergemann AD, Zhang L, Chiang MK, Brambilla R, Klein R, Flanagan JG. Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene. 1998;16(4):471–480. doi: 10.1038/sj.onc.1201557. [DOI] [PubMed] [Google Scholar]
- Bevins N, Lemke G, Reber M. Genetic dissection of EphA receptor signaling dynamics during retinotopic mapping. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(28):10302–10310. doi: 10.1523/JNEUROSCI.1652-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blits-Huizinga CT, Nelersa CM, Malhotra A, Liebl DJ. Ephrins and their receptors: binding versus biology. IUBMB life. 2004;56(5):257–265. doi: 10.1080/15216540412331270076. [DOI] [PubMed] [Google Scholar]
- Bolz J, Uziel D, Muhlfriedel S, Gullmar A, Peuckert C, Zarbalis K, Wurst W, Torii M, Levitt P. Multiple roles of ephrins during the formation of thalamocortical projections: maps and more. Journal of neurobiology. 2004;59(1):82–94. doi: 10.1002/neu.10346. [DOI] [PubMed] [Google Scholar]
- Brown A, Yates PA, Burrola P, Ortuno D, Vaidya A, Jessell TM, Pfaff SL, O’Leary DD, Lemke G. Topographic mapping from the retina to the midbrain is controlled by relative but not absolute levels of EphA receptor signaling. Cell. 2000;102(1):77–88. doi: 10.1016/s0092-8674(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Chenaux G, Henkemeyer M. Forward signaling by EphB1/EphB2 interacting with ephrin-B ligands at the optic chiasm is required to form the ipsilateral projection. The European journal of neuroscience. 2011;34(10):1620–1633. doi: 10.1111/j.1460-9568.2011.07845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrencik JE, Brooun A, Kraus ML, Recht MI, Kolatkar AR, Han GW, Seifert JM, Widmer H, Auer M, Kuhn P. Structural and biophysical characterization of the EphB4*ephrinB2 protein-protein interaction and receptor specificity. The Journal of biological chemistry. 2006;281(38):28185–28192. doi: 10.1074/jbc.M605766200. [DOI] [PubMed] [Google Scholar]
- Demyanenko GP, Siesser PF, Wright AG, Brennaman LH, Bartsch U, Schachner M, Maness PF. L1 and CHL1 Cooperate in Thalamocortical Axon Targeting. Cerebral cortex. 2011;21(2):401–412. doi: 10.1093/cercor/bhq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435(7046):1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Dimidschstein J, Passante L, Dufour A, van den Ameele J, Tiberi L, Hrechdakian T, Adams R, Klein R, Lie DC, Jossin Y, Vanderhaeghen P. Ephrin-B1 controls the columnar distribution of cortical pyramidal neurons by restricting their tangential migration. Neuron. 2013;79(6):1123–1135. doi: 10.1016/j.neuron.2013.07.015. [DOI] [PubMed] [Google Scholar]
- Dufour A, Egea J, Kullander K, Klein R, Vanderhaeghen P. Genetic analysis of EphA-dependent signaling mechanisms controlling topographic mapping in vivo. Development. 2006;133(22):4415–4420. doi: 10.1242/dev.02623. [DOI] [PubMed] [Google Scholar]
- Dufour A, Seibt J, Passante L, Depaepe V, Ciossek T, Frisen J, Kullander K, Flanagan JG, Polleux F, Vanderhaeghen P. Area specificity and topography of thalamocortical projections are controlled by ephrin/Eph genes. Neuron. 2003;39(3):453–465. doi: 10.1016/s0896-6273(03)00440-9. [DOI] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specification by gradients. Current opinion in neurobiology. 2006;16(1):59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annual review of neuroscience. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, Ryan TE, Henkemeyer M, Strebhardt K, Hirai H, Wilkinson DG, Pawson T, Davis S, Yancopoulos GD. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron. 1996;17(1):9–19. doi: 10.1016/s0896-6273(00)80276-7. [DOI] [PubMed] [Google Scholar]
- Garel S, Lopez-Bendito G. Inputs from the thalamocortical system on axon pathfinding mechanisms. Current opinion in neurobiology. 2014;27C:143–150. doi: 10.1016/j.conb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Grant E, Hoerder-Suabedissen A, Molnar Z. Development of the corticothalamic projections. Frontiers in neuroscience. 2012;6:53. doi: 10.3389/fnins.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Torii M, Sarkisian MR, Bartley CM, Shen J, Radtke F, Gridley T, Sestan N, Rakic P. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60(2):273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Hisanaga S, Heizmann CW, Murakami F. Distinct migratory behavior of early- and late-born neurons derived from the cortical ventricular zone. The Journal of comparative neurology. 2004;479(1):1–14. doi: 10.1002/cne.20256. [DOI] [PubMed] [Google Scholar]
- Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. The Journal of cell biology. 2003;163(6):1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Chumley MJ, Lackmann M, Li C, Barton WA, Jeffrey PD, Vearing C, Geleick D, Feldheim DA, Boyd AW, Henkemeyer M, Nikolov DB. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nature neuroscience. 2004;7(5):501–509. doi: 10.1038/nn1237. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB. Eph receptors and ephrins. The international journal of biochemistry & cell biology. 2003;35(2):130–134. doi: 10.1016/s1357-2725(02)00096-1. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T. Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383(6602):722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- Janis LS, Cassidy RM, Kromer LF. Ephrin-A binding and EphA receptor expression delineate the matrix compartment of the striatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(12):4962–4971. doi: 10.1523/JNEUROSCI.19-12-04962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(47):12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Current opinion in cell biology. 2004;16(5):580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Klein R, Kania A. Ephrin signalling in the developing nervous system. Current opinion in neurobiology. 2014;27C:16–24. doi: 10.1016/j.conb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes & development. 2001;15(7):877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackmann M, Boyd AW. Eph, a protein family coming of age: more confusion, insight, or complexity? Science signaling. 2008;1(15):re2. doi: 10.1126/stke.115re2. [DOI] [PubMed] [Google Scholar]
- Leyva-Diaz E, Lopez-Bendito G. In and out from the cortex: development of major forebrain connections. Neuroscience. 2013;254:26–44. doi: 10.1016/j.neuroscience.2013.08.070. [DOI] [PubMed] [Google Scholar]
- Lodato S, Molyneaux BJ, Zuccaro E, Goff LA, Chen HH, Yuan W, Meleski A, Takahashi E, Mahony S, Rinn JL, Gifford DK, Arlotta P. Gene co-regulation by Fezf2 selects neurotransmitter identity and connectivity of corticospinal neurons. Nature neuroscience. 2014 doi: 10.1038/nn.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackarehtschian K, Lau CK, Caras I, McConnell SK. Regional differences in the developing cerebral cortex revealed by ephrin-A5 expression. Cerebral cortex. 1999;9(6):601–610. doi: 10.1093/cercor/9.6.601. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Lim YS, Santiago A, O’Leary DD. Multiple EphB receptors mediate dorsal-ventral retinotopic mapping via similar bi-functional responses to ephrin-B1. Molecular and cellular neurosciences. 2014 doi: 10.1016/j.mcn.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Garel S, Lopez-Bendito G, Maness P, Price DJ. Mechanisms controlling the guidance of thalamocortical axons through the embryonic forebrain. The European journal of neuroscience. 2012;35(10):1573–1585. doi: 10.1111/j.1460-9568.2012.08119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monschau B, Kremoser C, Ohta K, Tanaka H, Kaneko T, Yamada T, Handwerker C, Hornberger MR, Loschinger J, Pasquale EB, Siever DA, Verderame MF, Muller BK, Bonhoeffer F, Drescher U. Shared and distinct functions of RAGS and ELF-1 in guiding retinal axons. The EMBO journal. 1997;16(6):1258–1267. doi: 10.1093/emboj/16.6.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe E, Rian E, Holien T, Rasmussen A, Levy FO, Aasheim H. Ephrin-B2 is a candidate ligand for the Eph receptor, EphB6. FEBS letters. 2000;466(1):169–174. doi: 10.1016/s0014-5793(99)01793-7. [DOI] [PubMed] [Google Scholar]
- North HA, Clifford MA, Donoghue MJ. ‘Til Eph do us part’: intercellular signaling via Eph receptors and ephrin ligands guides cerebral cortical development from birth through maturation. Cerebral cortex. 2013;23(8):1765–1773. doi: 10.1093/cercor/bhs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DD, Wilkinson DG. Eph receptors and ephrins in neural development. Current opinion in neurobiology. 1999;9(1):65–73. doi: 10.1016/s0959-4388(99)80008-7. [DOI] [PubMed] [Google Scholar]
- Orioli D, Klein R. The Eph receptor family: axonal guidance by contact repulsion. Trends in genetics : TIG. 1997;13(9):354–359. doi: 10.1016/s0168-9525(97)01220-1. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin promiscuity is now crystal clear. Nature neuroscience. 2004;7(5):417–418. doi: 10.1038/nn0504-417. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133(1):38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros TJ, Shrestha BR, Mason C. Specificity and sufficiency of EphB1 in driving the ipsilateral retinal projection. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(11):3463–3474. doi: 10.1523/JNEUROSCI.5655-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DJ, Kennedy H, Dehay C, Zhou L, Mercier M, Jossin Y, Goffinet AM, Tissir F, Blakey D, Molnar Z. The development of cortical connections. The European journal of neuroscience. 2006;23(4):910–920. doi: 10.1111/j.1460-9568.2006.04620.x. [DOI] [PubMed] [Google Scholar]
- Reber M, Burrola P, Lemke G. A relative signalling model for the formation of a topographic neural map. Nature. 2004;431(7010):847–853. doi: 10.1038/nature02957. [DOI] [PubMed] [Google Scholar]
- Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB. The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(51):14205–14215. doi: 10.1523/JNEUROSCI.2746-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Developmental biology. 2001;240(1):237–246. doi: 10.1006/dbio.2001.0439. [DOI] [PubMed] [Google Scholar]
- Sakano S, Serizawa R, Inada T, Iwama A, Itoh A, Kato C, Shimizu Y, Shinkai F, Shimizu R, Kondo S, Ohno M, Suda T. Characterization of a ligand for receptor protein-tyrosine kinase HTK expressed in immature hematopoietic cells. Oncogene. 1996;13(4):813–822. [PubMed] [Google Scholar]
- Seiradake E, Schaupp A, del Toro Ruiz D, Kaufmann R, Mitakidis N, Harlos K, Aricescu AR, Klein R, Jones EY. Structurally encoded intraclass differences in EphA clusters drive distinct cell responses. Nature structural & molecular biology. 2013;20(8):958–964. doi: 10.1038/nsmb.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk A, Pfennig S, Weiss A, Burk K, Acker-Palmer A. Ephrin Bs are essential components of the Reelin pathway to regulate neuronal migration. Nature. 2011;472(7343):356–360. doi: 10.1038/nature09874. [DOI] [PubMed] [Google Scholar]
- Spitzweck B, Brankatschk M, Dickson BJ. Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell. 2010;140(3):409–420. doi: 10.1016/j.cell.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Stein E, Savaskan NE, Ninnemann O, Nitsch R, Zhou R, Skutella T. A role for the Eph ligand ephrin-A3 in entorhino-hippocampal axon targeting. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(20):8885–8893. doi: 10.1523/JNEUROSCI.19-20-08885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinecke A, Gampe C, Zimmer G, Rudolph J, Bolz J. EphA/ephrin A reverse signaling promotes the migration of cortical interneurons from the medial ganglionic eminence. Development. 2014;141(2):460–471. doi: 10.1242/dev.101691. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103(4):865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Tai AX, Cassidy RM, Kromer LF. EphA7 expression identifies a unique neuronal compartment in the rat striatum. The Journal of comparative neurology. 2013;521(12):2663–2679. doi: 10.1002/cne.23308. [DOI] [PubMed] [Google Scholar]
- Tai AX, Kromer LF. Corticofugal projections from medial primary somatosensory cortex avoid EphA7-expressing neurons in striatum and thalamus. Neuroscience. 2014;274:409–418. doi: 10.1016/j.neuroscience.2014.05.039. [DOI] [PubMed] [Google Scholar]
- Torii M, Hackett TA, Rakic P, Levitt P, Polley DB. EphA signaling impacts development of topographic connectivity in auditory corticofugal systems. Cerebral cortex. 2013a;23(4):775–785. doi: 10.1093/cercor/bhs066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Hashimoto-Torii K, Levitt P, Rakic P. Integration of neuronal clones in the radial cortical columns by EphA and ephrin-A signalling. Nature. 2009;461(7263):524–528. doi: 10.1038/nature08362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Levitt P. Dissociation of corticothalamic and thalamocortical axon targeting by an EphA7-mediated mechanism. Neuron. 2005;48(4):563–575. doi: 10.1016/j.neuron.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Torii M, Rakic P, Levitt P. Role of EphA/ephrin--a signaling in the development of topographic maps in mouse corticothalamic projections. The Journal of comparative neurology. 2013b;521(3):626–637. doi: 10.1002/cne.23195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uziel D, Garcez P, Lent R, Peuckert C, Niehage R, Weth F, Bolz J. Connecting thalamus and cortex: the role of ephrins. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2006;288(2):135–142. doi: 10.1002/ar.a.20286. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh CA, Frostig RD, Flanagan JG. A mapping label required for normal scale of body representation in the cortex. Nature neuroscience. 2000;3(4):358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- Walsh R, Blumenberg M. Specific and shared targets of ephrin A signaling in epidermal keratinocytes. The Journal of biological chemistry. 2011;286(11):9419–9428. doi: 10.1074/jbc.M110.197087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama N, Romero MI, Cowan CA, Galvan P, Helmbacher F, Charnay P, Parada LF, Henkemeyer M. Forward signaling mediated by ephrin-B3 prevents contralateral corticospinal axons from recrossing the spinal cord midline. Neuron. 2001;29(1):85–97. doi: 10.1016/s0896-6273(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Garcez P, Rudolph J, Niehage R, Weth F, Lent R, Bolz J. Ephrin-A5 acts as a repulsive cue for migrating cortical interneurons. The European journal of neuroscience. 2008;28(1):62–73. doi: 10.1111/j.1460-9568.2008.06320.x. [DOI] [PubMed] [Google Scholar]