Abstract
Objectives
Surgical resection for locally-advanced rectal adenocarcinoma commonly occurs 6–10 weeks after completion of neoadjuvant chemoradiation (nCRT). We sought to determine the optimal timing of surgery related to the pathological complete response (pCR) rate and survival endpoints.
Methods
Retrospective analysis of 92 patients treated with nCRT followed by surgery from 2004 – 2011 at our institution. Univariate and multivariate analysis was performed to assess the impact of timing of surgery on local regional control (LRC), distant failure (DF), disease-free survival (DFS), and overall survival (OS).
Results
Time-to-surgery was ≤8 wks (group A) in 72% (median 6.1 weeks) and >8 weeks (group B) in 28% (median 8.9 weeks) of patients. No significant differences in patient characteristics, LRC, or pCR rates were noted between groups. Univariate analysis revealed that group B had significantly shorter time to DF (group B median 33 months; group A median not reached, p=0.047) and shorter OS compared to group A (group B median 52 months; group A median not reached, p=0.03). Multivariate analysis revealed that increased time-to-surgery showed a significant increase in DF (HR 2.96, p=0.02) and trends towards worse OS (HR 2.81, p=0.108) and DFS (HR 2.08, p=0.098).
Conclusions
We found that delaying surgical resection longer than 8 weeks after nCRT was associated with an increased risk of DF. This study, in combination with a recent larger study, question the recent trend in promoting surgical delay beyond the traditional 6–10 weeks. Larger, prospective databases or randomized studies may better clarify surgical timing following nCRT in rectal adenocarcinoma.
Keywords: Rectal cancer, neoadjuvant therapy, chemotherapy, radiation, time to surgery
INTRODUCTION
Colorectal cancer is the fourth most common cancer worldwide and the third most common in the US. In locally-advanced rectal adenocarcinoma (cT3-4N0, or N+ disease), chemoradiation (CRT) has long been a standard component of care. In the last decade, most oncologists have shifted their practice to deliver chemoradiation preoperatively, based on the results of multiple randomized trials comparing pre-operative versus post-operative approaches, including the German Rectal Cancer Trial, as well as NSABP-R03, FFCD 9203, and EORTC 22921 [1–5]. These trials informed us that preoperative chemoradiation results not only in improved outcomes such as locoregional control (LRC) and disease-free survival (DFS), but also reduces toxicity compared with postoperative CRT.
Pathologic complete response (pCR) after neoadjuvant chemoradiation (nCRT) appears to be associated with improved outcomes (i.e. decreased locoregional failure, improved disease-free, and overall survival), in rectal cancer as well as other types of gastrointestinal cancer, including esophageal cancer [6]. A systemic review and meta-analysis was done by Martin et al. on 16 studies involving 3,363 patients and showed that pCR after nCRT is associated with increased LRC, distant control, DFS, and OS [7]. Thus, investigators have speculated that one strategy to prolong survival might be to improve pCR rates after neoadjuvant therapy. Others have proposed that achieving pCR might also allow patients to forgo adjuvant chemotherapy [6]. Furthermore, achieving a clinical complete response (cCR) in selected patients might allow for non-operative management with close observation, and surgery reserved for recurrent disease [8–10].
Retrospective analyses have suggested that delaying surgical resection longer than the commonly planned 6–8 weeks after nCRT appears to result in improved pCR rates with increased tumor downstaging [11–17]. Thus, several investigators have proposed that delaying surgical resection beyond 8 weeks could increase pCR rates as a means to improving prognostication, tumor control and outcomes. Recently, an analysis was performed on 1,593 patients from a Dutch series which demonstrated that delaying surgery to 10–11 weeks after radiation results in higher pCR rates [18]. However, they noted incidentally that higher metastasis rates were observed when surgery was delayed beyond 9 weeks. In this study, we performed a single institution retrospective analysis of patients treated with nCRT and surgery to assess the role of timing of surgery after nCRT in order to determine whether delaying surgery increases rates of pCR or alters clinical outcomes such as metastasis.
MATERIALS AND METHODS
Patient population
Retrospective analysis was done on 118 consecutive patients at The Ohio State University Wexner Medical Center treated with nCRT followed by surgical resection for Stage II–III locally-advanced, rectal adenocarcinoma from 2004 to 2011. The median follow-up time is 28 months. Patient information was obtained after approval from The Ohio State University institutional review board (IRB). Of the 118 patients treated from 2004–2011, 26 were excluded due to lack of radiation or chemotherapy details due to being treated outside our institution, leaving 92 patients for the analysis. Clinical staging was performed with physical exam, endoscopy, endorectal ultrasonography, abdominopelvic CT, and/or MRI. All patients received neoadjuvant chemoradiation with the majority receiving 5-FU or capecitabine-based chemotherapy with a median dose of 50.4 Gy radiation in 1.8 Gy daily fractions. Tumors were staged according to AJCC guidelines, based on tumor invasiveness, nodal involvement, and metastasis. Pathologic examination of the surgical specimens was done by institutional pathologists using AJCC criteria to assess response to neoadjuvant therapy. pCR is defined as no residual adenocarcinoma in the surgical specimen.
Given that the time frame typically recommended as the interval between completion of nCRT and surgical resection is 6–10 weeks and based on the recent Dutch analysis [18], we divided patients into two groups midway between our recommended institutional interval: ≤8 weeks (group A) and >8 weeks (group B) between completion of nCRT and surgery. This dichotomization is consistent with prior published studies [12,16,17]. Furthermore, performing the analysis at time points other than 8 weeks was limited by loss of statistical power. Time to surgery was ≤8 weeks (group A) in 66 patients (72%; median 6.1 weeks; range 2.7–8 weeks) and >8 weeks (group B) in 26 patients (28%; median 8.9 weeks; range 8.1 to 17.6 weeks) of patients. Ninety-one percent (84/92) of patients underwent either low anterior resection or abdominoperineal resection, however, 7% (6/92) underwent other procedures such as transanal excision or total pelvic exenteration. There were only 2 patients for whom the type of surgery was not recorded. Sixty-one percent (56/92) of patients received adjuvant chemotherapy, including FOLFOX, Capecitabine, XELOX, or other chemotherapies. Factors analyzed to ensure balanced characteristics between groups A & B included pre-treatment variables (gender, age at diagnosis, clinical T- and N-stage, abnormal pre-treatment CEA level), treatment variables (time interval between completion of nCRT and surgery, radiation dose), and post-treatment variables (pCR, pathologic T- and N-stage, adjuvant chemotherapy). Clinical and pathologic T and N stages were treated as dichotomized variables for the analysis (T1-2 vs T3-4; pN0 vs N1-2). We also grouped patients according to a previously published risk stratification scheme based on a pooled analysis of NCCTG 79-47-51, NCCTG 86-47-51, INT 0114, NSABP R-01, and NSABP R-02 [19]. These pathologic risk groupings include low risk T1-2N0 (L), intermediate risk T1-2N1/T3N0 (I), moderately high risk T1-2N2/T3N1/T4N0 (MH), and high risk T3N2/T4N1/T4N2 (H). The risk groupings were then dichotomized as low-intermediate risk (“LI”) versus moderately high-high risk (“MHH”). Follow-up information was collected including recurrences (local, regional, and distant) as well as survival data.
Statistical analysis
Categorical variables between group A and group B were compared using the Fisher’s exact test. Continuous variables between group A and group B were compared using Student’s t-test. For each patient, time to locoregional recurrence, distant recurrence, and OS was determined from the date of diagnosis until the date of disease recurrence or death from any cause. Patients lost to follow-up were censored. Kaplan-Meier estimates, log-rank tests, and Cox proportional regression analyses were used to analyze time to locoregional or distant recurrence, DFS, and OS. SPSS, version 20 (SPSS Inc., Chicago, IL) was used to perform the statistical analyses.
RESULTS
Patient characteristics
A total of 92 consecutive patients with available radiation and chemotherapy data underwent nCRT followed by surgical resection for Stage II-III locally-advanced rectal adenocarcinoma. Median follow-up time is 28 months for all patients, as well as for groups A and B. There were 55 (60%) males and 37 (40%) females and the median age was 58.5 years (range 22–88 years). We systematically reviewed the charts for all patients in group B to determine the reasons for delay, and all of the delays except one patient appeared to be due to scheduling factors and our institutional acceptance that 6–10 weeks is an satisfactory time interval between end of chemoradiation and surgery. One patient in group B had toxicity attributed to nCRT requiring a delay in surgery to 17.6 weeks after nCRT. Another 6 patients in group B had delays beyond 10 weeks only attributable to scheduling issues (range 10.3–16.1 weeks). Overall, these two groups share similar baseline characteristics (Table 1). Groups A and B had no significant differences in gender, elevated pre-treatment CEA whether dichotomized by 5 ng/ml or by the mean, radiation dose dichotomized by the median, clinical or pathologic T-stage, clinical or pathologic N-stage, pathologic risk groupings (LI versus MHH), tumor location, positive/close margin resection rate, type of surgical resection, and percent of patients receiving adjuvant chemotherapy. Due to limited reporting, we were unable to compare the number of cycles of adjuvant chemotherapy between the two groups (or the chemo subgroups) since the numbers of patients in each group (or chemo sub-group) were too small. Finally, there was no significant difference in the pCR rate between the two groups, with a total of 16 patients (17.4%) achieving a pCR (18% in group A and 15% in group B, p=1.0).
Table 1.
Clinical, Pathologic, and Treatment-Related Characteristics of Patients in Group A (≤8 weeks between completion of nCRT and surgery) and Group B (> 8 weeks)
| Characteristic | Group A n=66 |
Group B n=26 |
p-value |
|---|---|---|---|
| Gender | 0.49 | ||
| Female | 25 (38%) | 12 (46%) | |
| Male | 41 (62%) | 14 (54%) | |
| Median age(range) | 59 (22–84) | 59 (30–88) | 0.48 |
|
Pre-treatment CEA > 5ng/mL |
19 (29%) | 12 (45%) | 0.26 |
|
Pre-treatment CEA >10 ng/mL (mean) |
13 (19%) | 8 (30%) | 0.52 |
|
RT dose > 50.4 Gray (median) |
13 (20%) | 7 (27%) | 0.55 |
| cT stage | 0.16 | ||
| 1–2 | 12 (18%) | 1 (4%) | |
| 3–4 | 54 (82%) | 25 (96%) | |
| cN stage | 0.14 | ||
| 0 | 37 (56%) | 20 (76%) | |
| 1–2 | 29 (44%) | 6 (24%) | |
| pT stage | 0.19 | ||
| 1–2 | 31 (47%) | 7 (28%) | |
| 3–4 | 35 (53%) | 19 (72%) | |
| pN stage | 0.64 | ||
| 0 | 42 (63%) | 15 (58%) | |
| 1–2 | 24 (37%) | 11 (42%) | |
| Risk Groupings | 0.31 | ||
| Low + Intermediate (LI) | 44 (67%) | 14 (52%) | |
| Moderately-high + High (MHH) |
22 (33%) | 12 (48%) | |
|
Pathologic complete response |
12 (18%) | 4 (15%) | 1.0 |
| R1/CM | 15 (23%) | 5 (18%) | 0.76 |
| Location of tumor | 0.58 | ||
| Low (≤5 cm from anal verge) |
23 (35%) | 6 (23%) | |
| Mid/Upper (>5 cm from anal verge) |
29 (44%) | 11 (42%) | |
| Unknown | 14 (21%) | 9 (35%) | |
| Type of resection | 0.21 | ||
| Low anterior | 40 (60%) | 11 (42%) | |
| Abdominoperineal | 21 (32%) | 12 (46%) | |
| Other | 3 (5%) | 3 (12%) | |
| Unknown | 2 (3%) | 0 (0%) | |
| Adjuvant chemotherapy | 1.0 | ||
| Yes | 40 (61%) | 16 (62%) | |
| FOLFOX | 18 | 9 | |
| Capecitabine | 10 | 3 | |
| XELOX | 4 | 2 | |
| Unknown | 8 | 2 | |
| No | 16 (24%) | 6 (23%) | |
| Unknown | 10 (15%) | 4 (15%) |
Abbreviations: CEA= carcinoembryonic antigen; cT=clinical tumor stage; cN=clinical nodal stage; pT= pathologic tumor stage; pN= pathologic nodal stage; R1/CM= microscopically positive or close margin; XELOX= capecitabine, oxaliplatin; FOLFOX= leucovorin, fluorouracil, oxaliplatin
Treatment-related death and toxicities
In order to determine whether differences in survival could potentially be related to differences in peri-operative or post-operative toxicities/complications, we retrospectively reviewed the charts for deaths in the postoperative period, deaths attributable to surgery, number of hospitalizations, timing of hospitalizations, and reasons for hospitalizations (Table 2). First, we found no significant differences in the total number of hospitalizations or hospitalizations attributable to therapy between Groups A and B. Also, no significant differences were detected in the number of pre-operative hospitalizations (during or after chemoradiation), which occurred in 3% of patients in Group A, and 7.7% of patients in Group B. In terms of surgery-related mortality, no deaths were directly attributable to surgery. The 30-day mortality after surgery was 0% for both Groups A and B indicating no differences between the two groups. Likewise, the 90-day mortality rate was 0% for Group A and 3.8% (1/26) for Group B, which was not statistically significant (p=0.29). Likewise, no significant differences were identified between Groups A and B for the number of post-operative hospitalizations within 30 days, 90 days, within 1 year, between 1–3 years, or greater than 3 years. Finally, no significant differences were noted for the indications for hospitalizations amongst the patients. The most common reasons for hospitalizations occurring at a frequency of 5% or more between both groups were infection (related to urinary tract infection/urosepsis), small bowel obstruction, renal obstruction or acute kidney injury, fistula, abscess, anastomotic complications, and need for hernia repair (Table 2).
Table 2.
Hospitalizations of patients during/after nCRT and after resection compared between Group A (≤8 weeks between completion of nCRT and surgery) and Group B (>8 weeks).
| Characteristic | Groups A + B (n=92) |
Group A (n=66) |
Group B (n=26) |
p-value |
|---|---|---|---|---|
| Deaths attributable to Surgery |
0 | 0 | 0 | -- |
| Hospitalizations (Any) | 215 | 150 | 65 | 0.755 |
| Hospitalizations (From Toxicity) |
116 | 81 | 35 | 0.818 |
|
Timing of Hospitalization: |
||||
| Pre-operative | 4 | 2 | 2 | 0.846 |
| Post-operative (30 days) |
8 | 7 | 1 | 0.298 |
| Post-operative (90 days) |
7 | 3 | 4 | 0.240 |
| < 1 year from surgery | 17 | 10 | 7 | 0.418 |
| 1–3 years from surgery | 43 | 28 | 15 | 0.586 |
| >3 years from surgery | 40 | 33 | 7 | 0.485 |
|
Percent of Patients Hospitalized for: |
||||
| Urinary tract infection/urosepsis |
17.4 % | 15.1 % | 23.1 % | 0.367 |
| Ileus/Small Bowel Obstruction |
10.9 % | 13.6 % | 3.8 % | 0.175 |
| Renal Complications (obstruction/ hydronephrosis, stent placement, acute kidney injury) |
9.8 % | 7.6 % | 15.4 % | 0.255 |
| Fistula | 7.6 % | 7.6 % | 7.7 % | 0.987 |
| Abscess | 7.6 % | 7.6 % | 7.7% | 0.987 |
| Anastomotic Complications (e.g. leaks, repair) |
5.4 % | 6.1 % | 3.8 % | 0.667 |
| Hernia repair | 5.4 % | 3 % | 11.5 % | 0.102 |
| Stoma Complication/Revision |
3.3 % | 3 % | 3.8 % | 0.838 |
| Incontinence | 2.2 % | 1.5 % | 3.8 % | 0.487 |
| Diverticulitis | 2.2 % | 1.5 % | 3.8 % | 0.487 |
Oncologic outcomes
Of the 92 patients, 11 died during the analysis period (5 patients or 7.5% of group A; 6 patients or 22% in group B). We performed univariate analysis for clinical and pathologic T and N staging which are the major established prognostic variables in rectal cancer. We found that clinical T- and N-staging obtained prior to therapy (comparing cT1-2 vs cT3-4 or cN0 vs cN+) was not significantly associated with differences in LRC, DF, DFS or OS. However, consistent with the improved prognostic power of pathologic staging, lower pathologic T-stage (pT1-2 versus pT3-4) was significantly associated with increased LRC (p=0.01), increased DFS (p<0.01), decreased DF (p<0.01), but no difference in OS. In addition, lower nodal pathologic stage (pN0 vs pN+) was significantly associated with increased DFS (p<0.01), decreased DF (p<0.01), but no significant differences were noted for LRC or OS. We then applied previously published risk classifications from a large pooled analysis which groups patients into low, intermediate, moderately high, and high risk categories [19], and provides very strong prognostic power compared to traditional T and N staging. Specifically, we dichotomized risk groupings of low and intermediate (LI) versus moderately high and high risk (MHH) which incorporates both T and N stage and performed univariate analysis. Using these risk groupings, we found strong associations with outcomes, including OS which was not observed even with separate pT and pN stage analysis. LI patients experienced improved DFS (p<0.05), improved OS (p=0.01), a trend for decreased DF (p=0.06), but no difference in LRC compared to MHH patients.
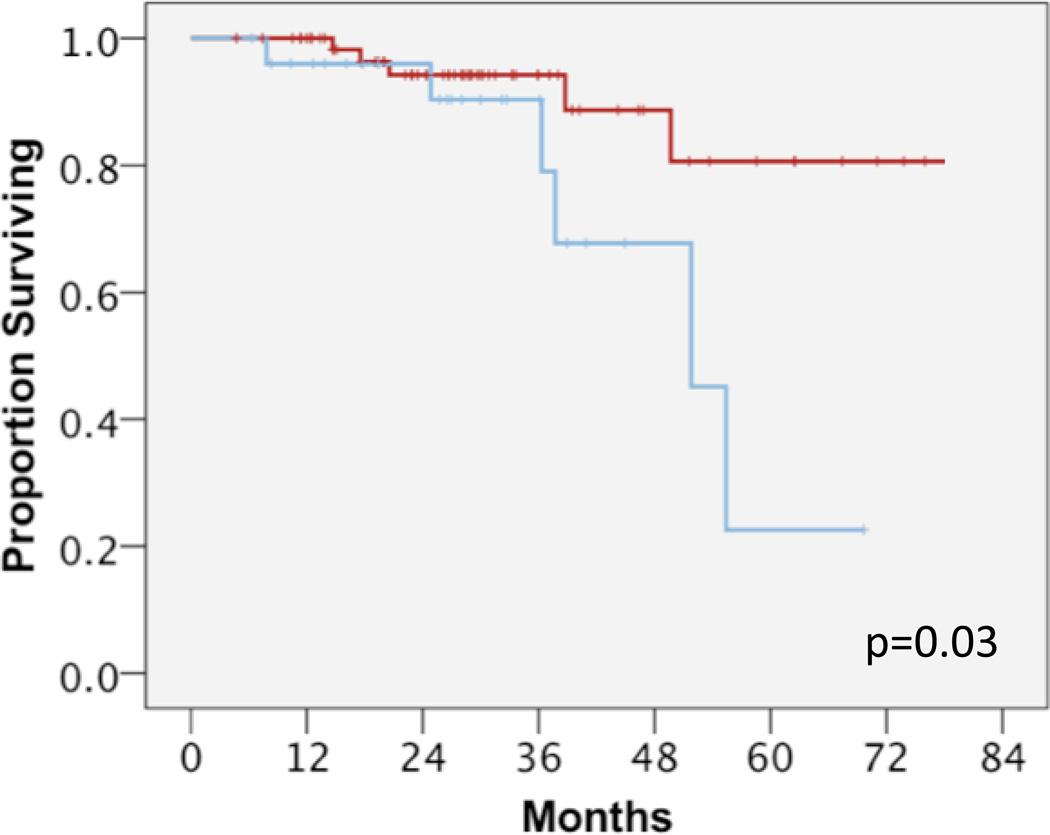
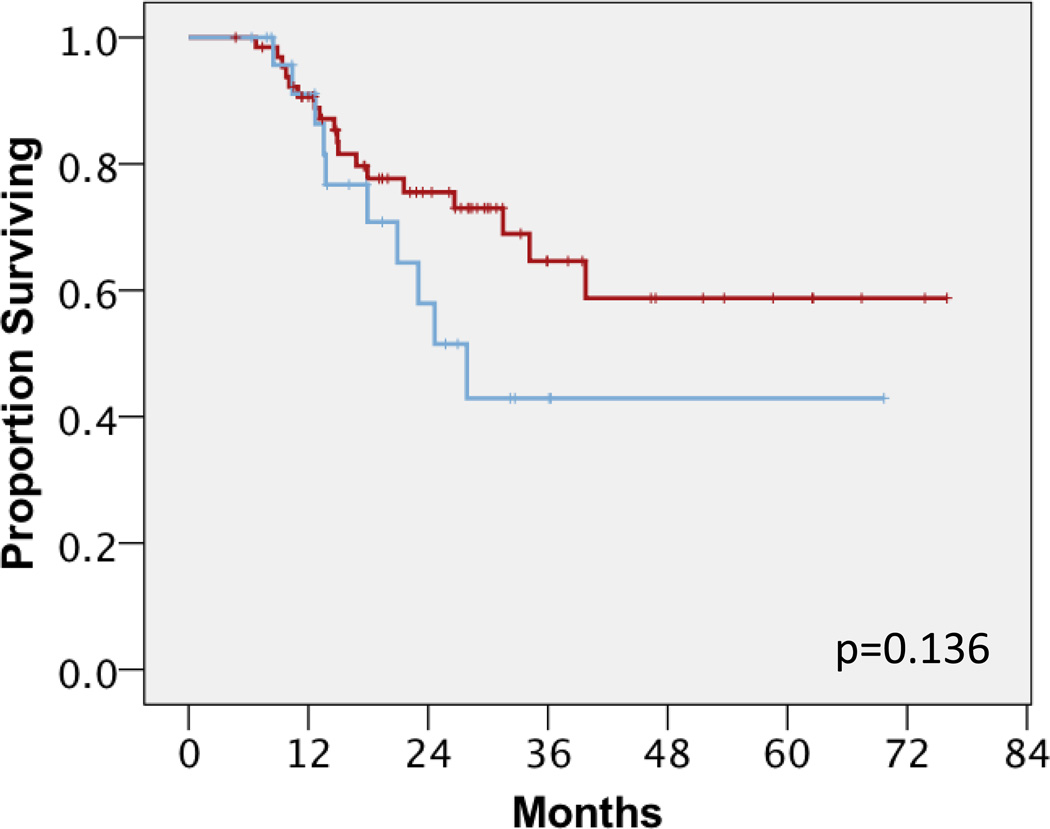
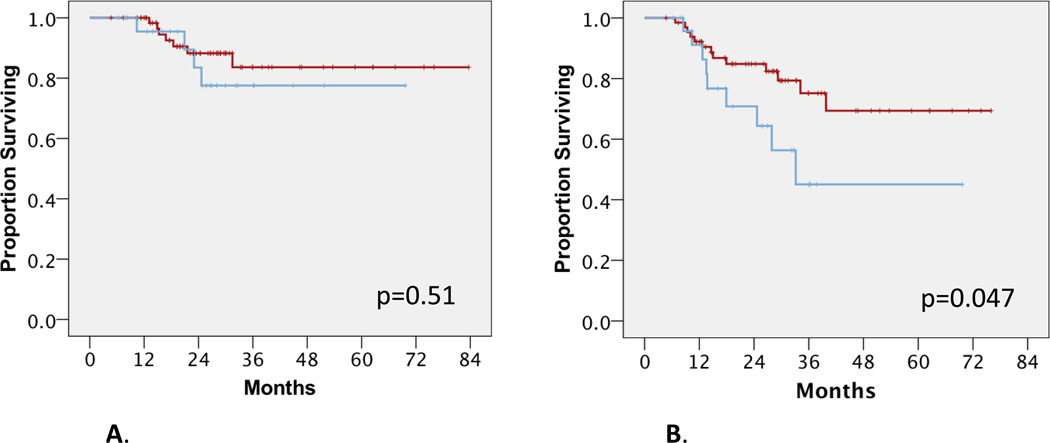
We next performed clinical outcomes analysis of groups A and B to investigate the impact of surgical timing after nCRT. Univariate analysis showed that group B had statistically significantly shorter OS compared to group A (median not reached for group A versus 52 months for group B; mean 75 and 50 months respectively, p=0.03) (Figure 1). While two year OS for Groups A and B were similar at 93–94%, four year OS for Groups A and B were substantially different at 88% and 68%, respectively. Univariate analysis also showed that group B had a trend towards shorter DFS compared to group A (median not reached for group A vs 28 months for group B; mean 41 and 54 months respectively, p=0.136) (Figure 2). When disease relapse was more specifically analyzed as local regional recurrences versus distant metastases, we found that there was no difference in LRC between both groups. In group A, 7 patients (11%) failed local-regionally; in group B, 4 patients (15%) failed local-regionally (p=0.51) (Figure 3A). However, we detected a statistically significant difference in DF between groups A and B. In group A, 13 patients (20%) failed distantly, compared to group B where 8 patients (31%) failed distantly, (median not reached for group A versus 33 months for group B, p=0.047) (Figure 3B). In terms of 2 year distant-metastasis free failure, 83% and 71% of patients in Groups A and B respectively were free of distant metastasis at 2 years, in contrast to 2 year OS numbers. This difference became even more apparent at 4 years, with 70% and 42% of patients in Groups A and B being free of DF.
Figure 1.
Overall survival between group A (red, ≤8 weeks) and group B (blue, >8 weeks). Log rank p-value, p=0.03.
Figure 2.
Disease free survival between group A (red line) and group B (blue). Log rank p-value, p=0.136.
Figure 3.
A. Locoregional control between group A (red) and group B (blue). Log rank p-value, p=0.51.
B. Distant failure between group A (red) and group B (blue). Log rank p-value, p<0.05.
Finally, we performed Cox regression multivariate analysis with risk groupings (LI versus MHH) and time-to-surgery (Group A vs B) in relation to OS, DFS and DF. We found that with increased time to surgery (Group B), there is a trend towards decreased OS (p=0.108, HR 2.81 [95% CI 0.80–9.89]), and decreased disease-free survival (p=0.098, HR 2.08 [95% CI 0.87–4.94]). Finally, in the multivariable model, Group B had a significantly decreased time to DF (p=0.02, HR 2.96 [95% CI 1.17–7.50]).
DISCUSSION
The current standard of care for locally advanced rectal adenocarcinoma is nCRT followed by surgical resection that typically occurs 6–10 weeks after completion of nCRT at many institutions. This has been shown to improve LRC, disease control, and be associated with reduced toxicity based on phase III data [1,2,5]. Despite improvements in LRC with additional perioperative treatments, studies show that the survival rate for patients with locally-advanced rectal cancer has not improved with a 5-yr OS of ~75% [1]. Current research efforts are seeking novel therapeutic approaches, including different regimens of chemotherapy and radiotherapy or the addition of molecularly-targeted therapy, to optimize disease control and improve survival rate [20]. Recently, numerous retrospective studies have suggested that prolonging the interval from nCRT to surgical resection beyond 7–8 weeks improves pCR rate, which has been shown in other series to improve outcomes in those receiving nCRT. These studies mostly examined single-institution cohorts in a retrospective fashion, and analyzed patients who received nCRT followed by surgical resection [11–17,21]. Most of these studies have shown that pCR and downstaging rates increase with surgical delays beyond 7–8 weeks. Specifically, pCR rates are 12–18% in patients with a shorter time interval to surgery (<7–8 weeks), and are 19–35% in patients with delayed surgery (>8weeks). Some of these studies have also suggested that patients with longer time intervals to surgery had better local control, disease-free survival, and cause-specific survival [11,12,14]. Furthermore, a few of these studies retrospectively evaluated toxicity during and after surgery, and cited no increased rates of blood loss, blood transfusions, operative time, post-operative complications, length of hospital stay, or overall morbidity/toxicity with longer interval to surgery, arguing for the safety of this approach [11,13,14,21].
In contrast to prior studies, we did not detect a difference in pCR rates between patients who had surgical resection before or after 8 weeks after completion of nCRT. While our rates of pCR (15–18%) are consistent with the published literature, the inability to detect a difference between our two patient groups could potentially be explained by the small sample size in this analysis, or confounding variables. Further limitations of our study include potential selection bias and imbalances that are inherent to retrospective reviews. Indeed, group B seemed to have higher T-stage than group A, but this was counterbalanced by a lower N-stage in group B compared with group A (and the differences were not statistically significant). The strengths of our study include the comparison between patient and tumor characteristics of groups A and B, the inclusion of multivariate modeling, the use of well-validated risk groupings that incorporate both T and N stage established by Gunderson et al. [19], and that careful determination of the causes of delay in group B were scheduling-related, and due to our institutional acceptance that a standard interval from end of nCRT to surgery is 6–10 weeks.
A major concern in delaying surgery after nCRT is potentially worse outcomes by increasing the risk of tumor dissemination as tumor repopulates. While none of the afore-mentioned studies reported a detriment to longer interval to surgery, many of them did not perform an outcomes analysis or measure rates of distant failure. While many of them examined DFS, this outcome measures both locoregional and distant control. Since we found no differences in LRC between both groups, it is possible that the reason why these studies did not find statistical differences in DFS were due to loss of statistical power from inclusion of LRC data into DFS. We speculate that if the studies separated out distant failure, this difference might be more commonly noted. That being said, one particular retrospective study [12] did report on distant failure rates and found no differences between the groups examined when using a very similar dichotimization as ours (8 weeks). It is not clear to these authors why the disparate findings between our study and theirs. One potential explanation is that most reports suffer from being retrospective analyses, which again have inherent problems with patient selection and potential unidentified imbalances between study groups. In our study, we validated that higher pathologic T stage, N stage, and LI and MHH risk groupings were significantly associated with worse outcomes. Our outcomes analysis not only included DFS, but sub-divided the analysis into LRC and DF. We found that delaying surgical resection beyond 8 weeks is associated with an increased rate of DF, which is perhaps the reason for decreased OS and DFS in patients with delayed surgery beyond 8 weeks, as LRC is similar between our two groups. In support of this, we observe differences in the development of metastases at 2 years between the 2 groups which don’t translate to significant differences in survival until 3–4 years after therapy. This is likely due to effective systemic (and other therapies) which enable continued survival despite development of metastatic disease.
The lack of difference in local-regional control between both groups is not unexpected. Adequate resection will remove any residual disease in the primary tumor as well as regional lymph nodes, likely making differences in the interval to surgery less relevant with regards to LRC. In the multivariate model, we observed that increased time to surgery beyond 8 weeks results in significantly increased DF with trends for decreased survival and DFS being statistically significant in the multivariate analysis. We speculate that the lack of significance in this analysis for DFS may be due to the contributions of LRC to assessing DFS (and hence diluting differences due primarily to DF), relatively effective salvage therapy, relatively low patient numbers, or may be due to imbalances or unseen biases between groups that were not accounted for (and are inherent to retrospective studies). Nevertheless, our data suggest that there might be a potential transition point at about 8–10 weeks when cancer cells begin to disseminate from the primary tumor or regional lymphatics after nCRT.
We are not the first to report that delaying surgical resection impacts distant failure. Recently, using a large, prospectively-collected database of almost 1,600 patients from the Dutch Surgical Colorectal Audit, Sloothaak et al. found that delaying surgery past 10–11 weeks after nCRT resulted in the highest rates of pCR (18%) and nodal downstaging (58.6%) which were significantly improved compared to earlier timepoints of less than 8 weeks, or 8–9 weeks [18]. However, they found that delaying time to surgery resulted in significantly increased rates of metastatic disease and T4 disease resected beyond 10–11 weeks. In fact, the lowest rates of metastatic disease occurred in individuals whose timing to surgery was less than 9 weeks (rates of 4.4–4.8%), while at 10–11 weeks the rate of metastasis was 8.9%, and further worsened at 14.9% for 12 weeks or beyond (p<0.001). Unfortunately, the low sample size of our dataset did not permit us to explore similar statistical cutpoints of 9, 10, 11, or 12 weeks. In addition, in comparison to the Dutch study, our data demonstrated rather large differences in DF and potentially OS, which may be more a function of chance in a smaller sample size, or unaccounted for imbalances in T/N stage and risk groupings (despite these not being statistically different). Overall, our data is very consistent with this high-quality, prospective study and argues that the benefit of waiting for further tumor response may at some point be outweighed by the increased development of distant disease beyond 9 weeks. Recently, an oral abstract was presented by Huntington et al. at the 2015 Gastrointestinal Cancers Symposium (abstract #510), which showed that in over 6,800 rectal cancer patients from the National Cancer Database, delaying surgery resulted in increased rates of pCR. Despite this, delaying surgery beyond 60 days (~8.6 weeks) or 75 days (~10.7 weeks) was associated with a 20% and 28% increased risk of mortality, respectively, which perhaps is related to the same phenomenon observed in this study. However, these results are preliminary and we await peer-reviewed publication of these findings.
As an alternative explanation of our findings, it could be that the time interval from end of chemoradiation or surgery to the start of receiving adjuvant chemotherapy could impact on the development of metastasis by destroying micrometastatic disease or delaying growth/progression of these microscopic cells. Unfortunately, due to inconsistent and limited reporting on details of adjuvant chemotherapy including the start date of chemotherapy (due in large part to many patients receiving adjuvant chemotherapy closer to home), we are unable to perform this analysis. Overall, the frequency of patients receiving adjuvant chemotherapy was similar between both groups (61–62%). Furthermore, multiple randomized trials, including EORTC22921, Chronicle, PROCTOR/SCRIPT, examining the role of adjuvant chemotherapy for rectal cancer after curative resection have failed to demonstrate significant clinical improvements [22–25]. Thus, it seems unlikely that differences in adjuvant chemotherapy start date, type, or number of cycles will account for our observed findings given the benefit of adjuvant chemotherapy in rectal cancer still remains in question.
In terms of future studies, our data may provide support to recommending that surgery occur at 8–9 weeks or sooner after neoadjuvant chemoradiation. Alternatively, our data could lend support to the addition of neoadjuvant chemotherapy after the completion of nCRT followed by a short interval (e.g. 4 weeks) to surgical resection as another way to decrease the risk of distant metastases, while allowing for increased rates of pCR. Indeed, in a phase II trial that explored the addition of FOLFOX chemotherapy after standard nCRT found that rates of pCR increased from 18% to 25%, although the trial was not randomized [21]. Additionally, our study and others highlight the need for identifying pre-therapy biomarkers that will predict which patients will develop a pCR and/or have a higher likelihood of local or distant failure, allowing us to better determine which patients may or may not benefit from delaying surgery. As a word of caution, however, it is not clear that delaying surgery in order to improve rates of pCR will translate to improved outcomes. Achieving a pCR most likely simply reflects the inherent favorable biology of a tumor, which would result in improved outcomes, regardless of whether a pCR is achieved prior to surgical resection. Until a causal relationship between delaying resection to achieve a pCR and improved outcomes is established, achieving a pCR likely simply represents a pathologic prognostic biomarker of favorable disease.
In summary, we confirmed the prognostic utility of pT stage, pN stage, and risk groupings that incorporate T&N staging. In addition, we found that delaying surgical resection beyond 8 weeks is associated with increased rates of distant failure despite no differences in LRC, with trends towards worse DFS and overall survival. The findings that delaying surgical resection can result in worse outcomes are corroborated by 2 recent large studies. Given the retrospective nature of our study, we suggest the need for larger prospectively collected data or randomized studies to better clarify the role of the timing of surgery in the setting of nCRT, before delaying surgical resection further to achieve a pCR becomes common practice.
Acknowledgments
This research was presented at the American Society of Radiation Oncology Annual Meeting (ASTRO) 2013.
Funding: This work had no specific funding.
REFERENCES
- 1.Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. The New England journal of medicine. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rodel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the german cao/aro/aio-94 randomized phase iii trial after a median follow-up of 11 years. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 3.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. The New England journal of medicine. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 4.Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in t3–4 rectal cancers: Results of ffcd 9203. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 5.Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ, Wolmark N. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: Nsabp r-03. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5124–5130. doi: 10.1200/JCO.2009.22.0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan AK, Wong A, Jenken D, Heine J, Buie D, Johnson D. Posttreatment tnm staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. International journal of radiation oncology, biology, physics. 2005;61:665–677. doi: 10.1016/j.ijrobp.2004.06.206. [DOI] [PubMed] [Google Scholar]
- 7.Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. The British journal of surgery. 2012;99:918–928. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 8.Habr-Gama A, Perez RO, Proscurshim I, Campos FG, Nadalin W, Kiss D, Gama-Rodrigues J. Patterns of failure and survival for nonoperative treatment of stage c0 distal rectal cancer following neoadjuvant chemoradiation therapy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2006;10:1319–1328. doi: 10.1016/j.gassur.2006.09.005. discussion 1328-1319. [DOI] [PubMed] [Google Scholar]
- 9.Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, van Dam RM, Jansen RL, Sosef M, Leijtens JW, Hulsewe KW, Buijsen J, Beets GL. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 10.Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Annals of surgery. 2012;256:965–972. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 11.Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, D'Hoore A. Impact of interval between neoadjuvant chemoradiotherapy and tme for locally advanced rectal cancer on pathologic response and oncologic outcome. Annals of surgical oncology. 2012;19:2833–2841. doi: 10.1245/s10434-012-2327-1. [DOI] [PubMed] [Google Scholar]
- 12.de Campos-Lobato LF, Geisler DP, da Luz Moreira A, Stocchi L, Dietz D, Kalady MF. Neoadjuvant therapy for rectal cancer: The impact of longer interval between chemoradiation and surgery. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2011;15:444–450. doi: 10.1007/s11605-010-1197-8. [DOI] [PubMed] [Google Scholar]
- 13.Moore HG, Gittleman AE, Minsky BD, Wong D, Paty PB, Weiser M, Temple L, Saltz L, Shia J, Guillem JG. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Diseases of the colon and rectum. 2004;47:279–286. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 14.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Annals of surgical oncology. 2008;15:2661–2667. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 15.Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, Cecconello I. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: Does delayed surgery have an impact on outcome? International journal of radiation oncology, biology, physics. 2008;71:1181–1188. doi: 10.1016/j.ijrobp.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, Fazio VW. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Annals of surgery. 2009;250:582–589. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 17.Evans J, Tait D, Swift I, Pennert K, Tekkis P, Wotherspoon A, Chau I, Cunningham D, Brown G. Timing of surgery following preoperative therapy in rectal cancer: The need for a prospective randomized trial? Diseases of the colon and rectum. 2011;54:1251–1259. doi: 10.1097/DCR.0b013e3182281f4b. [DOI] [PubMed] [Google Scholar]
- 18.Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, Tanis PJ. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. The British journal of surgery. 2013;100:933–939. doi: 10.1002/bjs.9112. [DOI] [PubMed] [Google Scholar]
- 19.Gunderson LL, Sargent DJ, Tepper JE, Wolmark N, O'Connell MJ, Begovic M, Allmer C, Colangelo L, Smalley SR, Haller DG, Martenson JA, Mayer RJ, Rich TA, Ajani JA, MacDonald JS, Willett CG, Goldberg RM. Impact of t and n stage and treatment on survival and relapse in adjuvant rectal cancer: A pooled analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 20.Aklilu M, Eng C. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol. 2011;8:649–659. doi: 10.1038/nrclinonc.2011.118. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Aguilar J, Smith DD, Avila K, Bergsland EK, Chu P, Krieg RM. Optimal timing of surgery after chemoradiation for advanced rectal cancer: Preliminary results of a multicenter, nonrandomized phase ii prospective trial. Annals of surgery. 2011;254:97–102. doi: 10.1097/SLA.0b013e3182196e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavere P, Glanzmann C, Cellier P, Collette L Group ERO. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the eortc 22921 randomised study. The Lancet Oncology. 2014;15:184–190. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 23.Sainato A, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (larc): Long term results of a randomized trial (i-cnr-rt) Radiother Oncol. 2014 doi: 10.1016/j.radonc.2014.10.006. http://dx.doi.org/10.1016/j.radonc.2014.1010.1006. [DOI] [PubMed] [Google Scholar]
- 24.Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, Ledermann J, Sebag-Montefiore D. Chronicle: Results of a randomised phase iii trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (xelox) versus control. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:1356–1362. doi: 10.1093/annonc/mdu147. [DOI] [PubMed] [Google Scholar]
- 25.Breugom AJ, van Gijn W, Muller EW, Berglund A, van den Broek CB, Fokstuen T, Gelderblom H, Kapiteijn E, Leer JW, Marijnen CA, Martijn H, Meershoek-Klein Kranenbarg E, Nagtegaal ID, Pahlman L, Punt CJ, Putter H, Roodvoets AG, Rutten HJ, Steup WH, Glimelius B, van de Velde CJ Cooperative Investigators of the Dutch Colorectal Cancer G, the Nordic Gastrointestinal Tumour Adjuvant Therapy G. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A dutch colorectal cancer group (dccg) randomized phase iii trialdagger. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014 doi: 10.1093/annonc/mdu560. [DOI] [PubMed] [Google Scholar]