Abstract
Growing evidence implicates the dentate gyrus in temporal lobe epilepsy (TLE). Dentate granule cells limit the amount of excitatory signaling through the hippocampus and exhibit striking neuroplastic changes that may impair this function during epileptogenesis. Furthermore, aberrant integration of newly-generated granule cells underlies the majority of dentate restructuring. Recently, attention has focused on the mammalian target of rapamycin (mTOR) signaling pathway as a potential mediator of epileptogenic change. Systemic administration of the mTOR inhibitor rapamycin has promising therapeutic potential, as it has been shown to reduce seizure frequency and seizure severity in rodent models. Here, we tested whether mTOR signaling facilitates abnormal development of granule cells during epileptogenesis. We also examined dentate inflammation and mossy cell death in the dentate hilus. To determine if mTOR activation is necessary for abnormal granule cell development, transgenic mice that harbored fluorescently-labeled adult-born granule cells were treated with rapamycin following pilocarpine-induced status epilepticus. Systemic rapamycin effectively blocked phosphorylation of S6 protein (a readout of mTOR activity) and reduced granule cell mossy fiber axon sprouting. However, the accumulation of ectopic granule cells and granule cells with aberrant basal dendrites was not significantly reduced. Mossy cell death and reactive astrocytosis were also unaffected. These data suggest that anti-epileptogenic effects of mTOR inhibition may be mediated by mechanisms other than inhibition of these common dentate pathologies. Consistent with this conclusion, rapamycin prevented pathological weight gain in epileptic mice, suggesting that rapamycin might act on central circuits or even peripheral tissues controlling weight gain in epilepsy.
Introduction
Status epilepticus (SE) selectively disrupts the integration of adult-generated hippocampal granule cells (Parent et al., 2006; Jessberger et al., 2007; Walter et al., 2007; Kron et al., 2010; Santos et al., 2011). Changes include mossy fiber axon sprouting, formation of hilar basal dendrites and ectopic migration of newly-generated cells. These phenomena have been implicated in promoting hyperexcitability in the temporal lobe and may facilitate epileptogenesis (Jung et al., 2004; 2006; Cho et al., 2015). Hilar ectopic granule cells, for example, are more excitable in rodent epilepsy models (Zhan et al., 2010; Althaus et al., 2015) and can exhibit both spontaneous and evoked bursting (Scharfman et al., 2000; Cameron et al., 2011), distinguishing them from normal granule cells. In addition to ectopically-located cells, granule cells with mossy fiber axon sprouting to the dentate inner molecular layer and hilar-projecting basal dendrites mediate the formation functional granule cell to granule cell synapses (Okazaki et al., 1999; Thind et al., 2008). These synaptic connections may mediate increased excitatory flow through the dentate gyrus. Despite the evidence that these abnormalities may contribute to epileptogenesis and associated comorbidities, no current FDA-approved treatments can prevent the aberrant integration of adult-generated neurons in epilepsy.
Recently, the mTOR pathway has emerged as a promising molecular target that may mediate aberrant granule cell integration. mTOR signaling in the dentate is enhanced in chemical, injury-induced and genetic models of epilepsy (Wong, 2013; LaSarge and Danzer, 2014), suggesting the pathway could be involved in many different forms of the disease. Treatment with rapamycin, an inhibitor of mTOR complex 1, has been shown to reduce seizures (Zeng et al., 2009; Huang et al., 2010; van Vliet et al., 2012), prevent mossy fiber sprouting (Zeng et al., 2009; Huang et al., 2010; Buckmaster et al., 2009; Buckmaster and Lew, 2011; Tang et al., 2012; van Vliet et al., 2012; Heng et al., 2013; Shima et al., 2015), mitigate cell loss (Zeng et al., 2009; van Vliet et al., 2012; Guo et al., 2013; Butler et al., 2015) and reduce reactive astrogliosis (Shima et al., 2015) in rodent modes of acquired epilepsy. Conversely, recent work from our lab demonstrates that genetically enhancing mTOR signaling by PTEN deletion in adult-generated hippocampal granule cells causes mossy fiber axon sprouting, formation of hilar basal dendrites and ectopic cell migration (Pun et al., 2012). We hypothesized, therefore, that mTOR hyperactivation underlies the cellular abnormalities evident in the hippocampus following status epilepticus.
To test our hypothesis we utilized rapamycin to directly inhibit mTOR activity in mice following pilocarpine-induced status epilepticus. A genetic fate-mapping strategy was used to determine the effectiveness of rapamycin at mitigating cellular abnormalities among adult-generated granule cells. In addition, mossy cell survival and reactive astrocytosis were examined in the animals, since these changes have also been implicated in epileptogenesis. These experiments provide new insights into the role of mTOR signaling in the formation of epilepsy-induced anatomical changes within the dentate gyrus.
Methods
Animals
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital Research Foundation and conform to NIH guidelines for the care and use of animals. Two animal breeding schemes were followed: (1) In order to generate Gli1-CreERT2::GFP mice on a pure C57BL/6NCrl background for the study, hemizygous Gli1-CreERT2 mice (Ahn and Joyner, 2004, 2005) on a C57BL/6NCrl background were crossed to homozygous CAG-CAT-EGFP (GFP) mice (Nakamura et al., 2006) on a C57BL/6NCrl background (B6-GFP, n=77). (2) In order to generate Gli1-CreERT2::tdTomato positive mice on a 50:50 mix C57BL/6NCrl::FVB N background for the study, hemizygous Gli1-CreERT2 mice on an FVB N background were crossed to mice heterozygous for a CAG promoter-driven red fluorescent protein (tdTomato) reporter construct (Jax; stock 007914) on a C57BL/6NCrl background (B6/FVB-Tomato, n=9). Gli1-CreERT2::tdTomato negative mice produced from this cross were also used (B6/FVB, n=5). All animals were analyzed for phosphorylated S6 protein (pS6), hilar ectopic dentate granule cell density, mossy fiber sprouting, mossy cell density, reactive astrocytosis and microgliosis. Basal dendrite frequency and granule cell hypertrophy were analyzed only in B6-GFP and B6/FVB-Tomato mice, because GFP or tdTomato expression is needed to visualize granule cell somas and dendrites. All animals were maintained as a colony in the vivarium at CCHMC. Animals were provided with food and water ad libitum with a 14/10 hour light/dark cycle to optimize breeding (Fox et al., 2006).
Postnatal tamoxifen treatment of Gli1-CreERT2 mice activates CreERT2 in granule cell progenitors (Ahn and Joyner, 2005), resulting in continuous expression of GFP or tdTomato in the progenitor cells and subsequent daughter cells in B6-GFP and B6/FVB-Tomato mice. All mice were given injections of tamoxifen (250 mg/kg, s.c.) at 3, 4, 5, 6 and 7 weeks of age, limiting labeling to granule cells born after three weeks of age. At eight weeks of age, mice received methyl scopolamine nitrate in sterile saline (1 mg/kg, s.c.) followed by pilocarpine (420 mg/kg, s.c.) ten minutes later. Animals were monitored for behavioral seizures and the onset of status epilepticus (SE). Following three hours of SE mice were given two injections of diazepam ten minutes apart (10 mg/kg, s.c.) to alleviate seizure activity. Mice were given sterile Ringers as needed to maintain pretreatment body weight. Animals not designated to undergo SE received saline instead of pilocarpine, but were given scopolamine and diazepam. Rapamycin (LC Laboratories) was dissolved in 5% Tween 80, 5% polyethyleneglycol and 4% ethanol in PBS and was administered daily (6 mg/kg, i.p; or vehicle control) for six weeks beginning one day after pilocarpine SE. The final treatment groups were: (1) SE+rapamycin, (2) SE+vehicle, (3) rapamycin only and (4) vehicle only. At 14 weeks of age mice were anesthetized with pentobarbital (100 mg/kg, i.p.) and perfused with 1U/ml heparin, 2.5% paraformaldehyde and 4% sucrose in PBS (pH 7.4). Brains were extracted, stored overnight at 4°C in the same fixative, cryoprotected in ascending sucrose series (10, 20, 30%) in PBS and snap-frozen in isopentane at −25°C. Brains were sectioned coronally at 60 µm, and sections were affixed to gelatin coated slides and stored at −80°C.
Immunohistochemistry
Slide mounted brain sections (two per slide) from dorsal hippocampus were processed for histological studies. Sections were immunostained with the following antibodies: rabbit anti-pS6 (1:500, Cell Signaling), chicken anti-GFP (1:500, Abcam, Boston, MA), rabbit anti-zinc transporter 3 (ZnT3) (1:3000, Synaptic Systems, Gottingen, Germany), rabbit anti-Glutamate receptor 2/3 (1:100, Millipore, Temecula, CA), rabbit anti-prox1 (1:1000, Sigma-Aldrich, St. Louis, MO), rabbit anti-GFAP (1:500, Millipore) and rabbit anti-Iba1 (1:1000, Synaptic Systems). AlexaFluor488 goat anti-chicken, AlexaFluor488 goat anti-rabbit, AlexaFluor594 goat anti-rabbit and AlexaFluor647 goat anti-rabbit secondary antibodies were used (Invitrogen, Eugene, OR). Sections were dehydrated in alcohols, cleared in xylenes and coverslips were attached with Krystalon mounting-media (Harleco, Darmstadt, Germany).
Confocal imaging and quantification of histological measures
Imaging for pS6, hilar basal dendrites, mossy fiber sprouting, dentate granule cell soma area and mossy cell density analyses was conducted using a Leica SP5 confocal system set up on a DMI 6000 inverted microscope equipped with a 63× oil immersion objective (NA 1.4; image size 240 × 240 µm in the XY plane). Images for hilar ectopic dentate granule cell density were generated using a 3024 Nikon A1Rsi inverted microscope with a 40× water objective (NA 1.15, image size 317 × 317 µm in the XY plane). Images for analyses of reactive astrocytosis and microgliosis were generated using the 3024 Nikon A1Rsi microscope with a 60× water objective (NA 1.27, image size 210 × 210 um in the XY plane). For measures analyzing immunohistochemical staining intensity, including dentate and cortical pS6 expression, images were all collected with identical parameters. For soma areas, cell density measures and measures of cellular and neuronal process, image collection parameters were set to optimize cellular detail. For these latter measures, accurately identifying the boundaries of cellular structures is critical, so image setting were adjusted to avoid “over or underexposure”; conditions which can artifactually increase or decrease structure size, respectively. Images were collected and analyzed by an investigator blind to treatment group to avoid bias. For each parameter, dentate gyri were analyzed from dorsal hippocampus (1.6–2.0 mm posterior to bregma; Paxinos and Franklin, 2001). All cell counts were conducted using a variation of the optical dissector method (Howell et al., 2002; Hofacer et al., 2013).
1) Phosphorylated S6 protein (pS6)
To quantify pS6 immunostaining, single confocal optical sections were collected from the midpoint of the upper blade of the dentate gyrus and parietal association cortex. Images were taken 3 µm below the surface of the tissue section for all samples to control for antibody penetration. Tissue sections were imaged using 633 nm light with 10.0% laser intensity with the argon lamp set to 25.0%. For all images the PMT gain was set to 1200, PMT offset was set to −18.0, and pinhole was set to 95.2 µm. Images were all collected with 2048 × 2048 voxels at 200 Hz. Optical sections were analyzed for average pixel intensity within the upper blade of the granule cell body layer of the hippocampus using LAS AF software (Leica Microsystems). Average pixel intensity was measured on a scale of 0–255, with 0 indicating no pS6 staining, and 255 indicating complete saturation.
2) Dentate granule cell mossy fiber axon sprouting
Granule cell mossy fiber sprouting was evaluated using ZnT3-labeling. Single confocal optical sections were collected at the midpoint of the upper and lower blades of the dentate gyrus to capture portions of the dentate inner molecular layer. Optical sections were taken 3 µm below the surface of the tissue for all samples. The area occupied by ZnT3-immunoreactive puncta within the dentate inner molecular layer was quantified using Neurolucida automated detection software (version 3.4.2). Puncta were defined as ZnT3-immunoreactive regions with a diameter greater than 0.5 µm. The degree of mossy fiber sprouting was defined as [(total area occupied by ZnT3 puncta/total inner molecular layer area) × 100].
3) Hilar ectopic dentate granule cells
The density of ectopic granule cells in the hilus was assessed from confocal z-series image stacks of sections immunostained with the granule cell marker Prox1 (1 µm step through the top 10 µm of tissue). Prox1-immunolabeled cells were considered ectopic if they were in the hilus and at least two cell body diameters [~20µm] from the granule cell layer/hilar border. One hippocampal section for each animal was analyzed to determine hilar ectopic granule cell density [Prox1-expressing hilar granule cells/mm3 of hilus].
4) Dentate granule cells with basal dendrites
To assess basal dendrite frequency, confocal z-series image stacks of GFP or tdTomato labeled granule cells were collected (1 µm step through the top 10 µm of the tissue). One dentate gyrus/animal from dorsal hippocampus was imaged. Image stacks were imported into Neurolucida software, and cells were scored for the presence of basal dendrites projecting into the dentate hilus. Inclusion criteria for granule cells with basal dendrites were as follows: 1; Granule cells were morphologically mature, defined as cells with an axon extending into the hilus and spiny apical dendrites extending to the hippocampal fissure, 2; Cell bodies were fully contained within the tissue section, 3; Cell bodies were located within the granule cell layer and 4; Cells were well-labeled with GFP or tdTomato, such that processes could be followed to their natural endings. Data are presented as percentages (number of granule cells with basal dendrites/total number of granule cells scored).
5) Reactive astrocytosis and microgliosis
Changes in astrocytes and microglia were assessed by measuring the soma areas and total volume of GFAP- and Iba1-expressing cells located in the hilus. Image stacks were collected from within the dentate hilus (0.25 µm step through the top 5 µm of tissue). To measure soma areas, stacks were imported into Neurolucida software for measuring the maximum profile area of each respective cell type. Only cells whose somas were completely contained within the image stack were used. A total of five astrocytes and five microglia per animal were chosen at random for analysis. Soma areas presented for each animal are averages. To estimate total hilar volume occupied by astrocytes and microglia, 2 µm and 4 µm stacks (for GFAP- and Iba1-stained tissue, respectively) were imported into Imaris software. Stack size was determined so that the greatest number of sections with optimal antibody penetration for all brain preparations was used. Surfaces with a staining intensity of at least 4× local background with a minimum detail level of 0.414 µm were automatically identified. Surfaces were added and removed manually following the automatic detection. Total volume occupied by the surfaces was determined and data is represented as [(total volume GFAP- or Iba1-stained regions/total volume of hilus) × 100].
6) Dentate granule cell soma area
Changes in granule cell soma area were assessed by measuring the maximum profile area of GFP- or tdTomato-expressing adult-born granule cells. Image stacks used to analyses the presence of basal dendrites on GFP or tdTomato expressing cells were also used to determine dentate granule cell soma area. Measurements were completed using Neurolucida software. Granule cells with partially visible cell bodies were excluded (i.e. cut at the tissue surface or edge). Five granule cells per animal were chosen at random for analysis. For each animal, soma areas were averaged for statistical analysis.
7) Loss of hilar mossy cells
The density of mossy cells in the hilus was determined from confocal image stacks using GluR2/3-labeling (1 µm step through the top 10 µm of the tissue). Images were collected from the center of the hilus. Small, hilar GluR2/3-immunoreactive cells (8–12 µm diameter) were considered to be ectopic granule cells and were excluded, while larger GluR2/3-expressing neurons (30–40 µm diameter) localized to the hilus were counted as mossy cells (Fujise and Kosaka, 1999; Jiao and Nadler, 2007; Scharfman and Myers, 2013). Mossy cell hilar density is presented as [GluR2/3-expressing hilar mossy cells/mm3 of hilus].
Statistics
Statistical analyses were performed using Sigma Stat software (version 13.0). No differences were found between male and female animals, so data were pooled for further analysis (pilocarpine/rapamycin (n=4M, 2F), pilocarpine/vehicle (n=3M, 4F), saline/rapamycin (n=3M, 5F), saline/vehicle (n=3M, 4F). To account for differences between mouse strains, a two-way ANOVA was employed for each measure, controlling for strain (pure C57BL/6NCrl background vs. 50:50 mix C57BL/6NCrl::FVB N background) and treatment. Data that failed tests of normality or equal variance were normalized using either square root or rank transformations. Pairwise multiple comparison procedures were conducted using the Holm-Sidak method. Values are presented as least square means of raw (non-transformed) data ± standard error of the mean (SEM).
Figure preparation
Figures were prepared using Microsoft Excel and Adobe Photoshop (CS5-Extended). Brightness and contrast were adjusted to optimize cellular detail. Identical adjustments were made to all images meant for comparison. All error bars in graphs are SEM.
Results
To induce the development of epilepsy, mice were treated with the cholinergic agonist pilocarpine to provoke status epilepticus (SE), which was allowed to proceed for three hours before seizures were mitigated by treatment with diazepam. Control animals received saline instead of pilocarpine. Studies were begun using C57BL/6NCrl mice. Because of high mortality during the 24 hour period following SE, however, mice on a 50:50 background of C57BL/6NCrl::FVB N (B6/FVB) were also used. B6/FVB mice showed significantly improved survival one day after pilocarpine treatment relative to C57BL/6NCrl mice (C57BL/6NCrl survival, 27.7% [18/65]; B6/FVB survival, 100% [10/10]; p<0.001, Mann-Whitney rank sum test), consistent with prior work showing significant strain differences in seizure susceptibility (Schauwecker, 2012). 24 hours after SE, mice were randomly assigned to receive daily treatments with either six mg/kg rapamycin (Rap) or vehicle (Veh), producing four treatment groups (SE+Rap; SE+Veh; Rap only; Veh only). Mice were killed six weeks later; a time point when spontaneous seizures are typically occurring in this model (Castro et al., 2012; Hester and Danzer, 2013). Mortality was not altered by rapamycin treatment (p=1, z test, SE+Rap vs. SE+Veh). Specifically, 66.7% of SE+Rap (6 of 9 mice; 4 of 7 B6, 2 of 2 B6/FVB), 50.0% of SE+Veh (7 of 14 mice; 2 of 6 B6, 5 of 8 B6/FVB), 100% of rapamycin only (8 of 8; 6 of 6 B6, 2 of 2 B6/FVB) and 87.5% of vehicle only (7 of 8 mice; 5 of 6 B6, 2 of 2 B6/FVB) mice survived the period from one day to six weeks after pilocarpine (or saline) treatment. Not included in this count are five SE+Rap mice implanted with cortical EEG electrodes for 24/7 seizure monitoring either one or three weeks after SE (data not shown). 100% of these mice died between four and seven days after surgery.
Rapamycin reduces pS6 in the granule cell layer and the cortex
To confirm that systemic rapamycin was able to reduce mTOR activity we immunostained brain sections with antibodies against pS6, a downstream product of mTOR activation. SE+Veh-treated mice displayed increased pS6 immunoreactivity in the granule cell layer and cortex (Figure 1), confirming previous reports (Zeng et al., 2009). Systemic administration of rapamycin significantly reduced pS6 immunoreactivity in the granule cell layer of status epilepticus [SE+Rap] and control [Rap only] mice. Two-way ANOVA revealed an interaction (p<0.001; square root-transformed data) between strain [B6 vs. B6/FVB mice] and treatment [Veh, Rap, SE+Veh, and SE+Rap]. Specifically, rapamycin reduced pS6 more efficiently in the dentate of SE-exposed B6/FVB mice than SE-exposed B6 mice (p<0.001, Holm-Sidak). However, pS6 was significantly reduced in the dentate of both strains following rapamycin treatment (B6: SE+Veh vs. SE+Rap, p<0.001; B6/FVB: SE+Veh vs. SE+Rap, p<0.001, Holm-Sidak). In cortex, rapamycin decreased the amount of pS6 expression (SE+Veh vs. SE+Rap, p=0.003; Veh only vs. Rap only, 0=0.014). A significant strain effect was observed as well, with less pS6 expression observed in the cortex of B6/FVB mice compared to B6 mice (p<0.001, two-way ANOVA). These results demonstrate the ability of systemic rapamycin to effectively block phosphorylation of S6.
Figure 1.
Rapamycin decreases pS6 levels in epileptic and non-epileptic mice. Confocal maximum projections of pS6 immunolabeling in the hippocampus of mice receiving the following treatments: vehicle (Veh), vehicle following status epilepticus (SE+Veh), rapamycin (Rap) and rapamycin following status epilepticus (SE+Rap). The graph shows optical density quantification of pS6 intensity in the granule cell layer (left) and cortex (right). Scale bar, 100 µm.
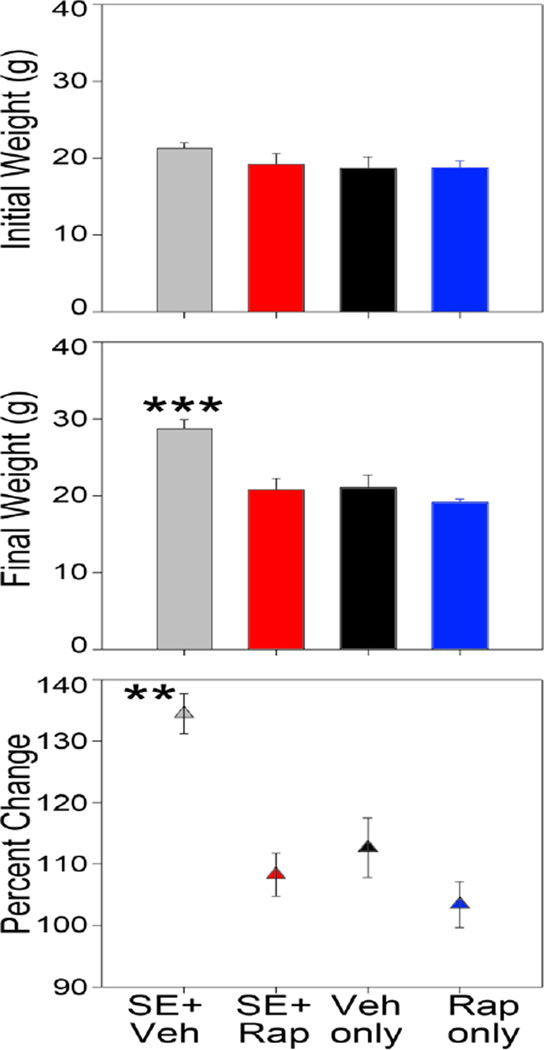
Rapamycin reduces status epilepticus-induced weight gain
Pilocarpine epileptogenesis leads to obesity in rodents (Scharfman et al., 2008; Ruiz et al., 2011) and a similar phenomenon has been described in patients with epilepsy (Daniels et al., 2009). In the present study, SE+Veh mice exhibited a significant 35% increase in body weight over the course of the experiment (p<0.001, one-way RM ANOVA). Both male and female mice exhibited increases (Figure 2, top). Vehicle-treated mice also grew, weighing 12% more at the end of the experiment (p<0.034, one-way RM ANOVA). By contrast, neither SE+Rap mice (p<0.066, one-way RM ANOVA) nor Rap only mice (p<0.456, one-way RM ANOVA) exhibited significant weight gain (Figure 2). When normalized to percent change in body weight, SE+Veh mice gained significantly more weight relative to all other groups (Figure 2, bottom; p<0.001 vs SE+Rap and Rap only, p=0.003 vs. Vehicle; Holm-Sidak). No sex or strain differences were found among the treatment groups (data not shown). Furthermore, rapamycin-treated and vehicle-treated mice that did not undergo SE exhibited identical outcomes for all additional measures in the study. Therefore, these animals were pooled into a common control group for the remainder of the manuscript.
Figure 2.
Rapamycin blocks weight gain following SE. Bar graphs show animal weights at the beginning of the experiment (top) and prior to sacrifice (middle). The lower graph shows the percent change in body weight for animals in each group. **, p<0.01 vs. all other groups. ***, p<0.001 vs all other groups.
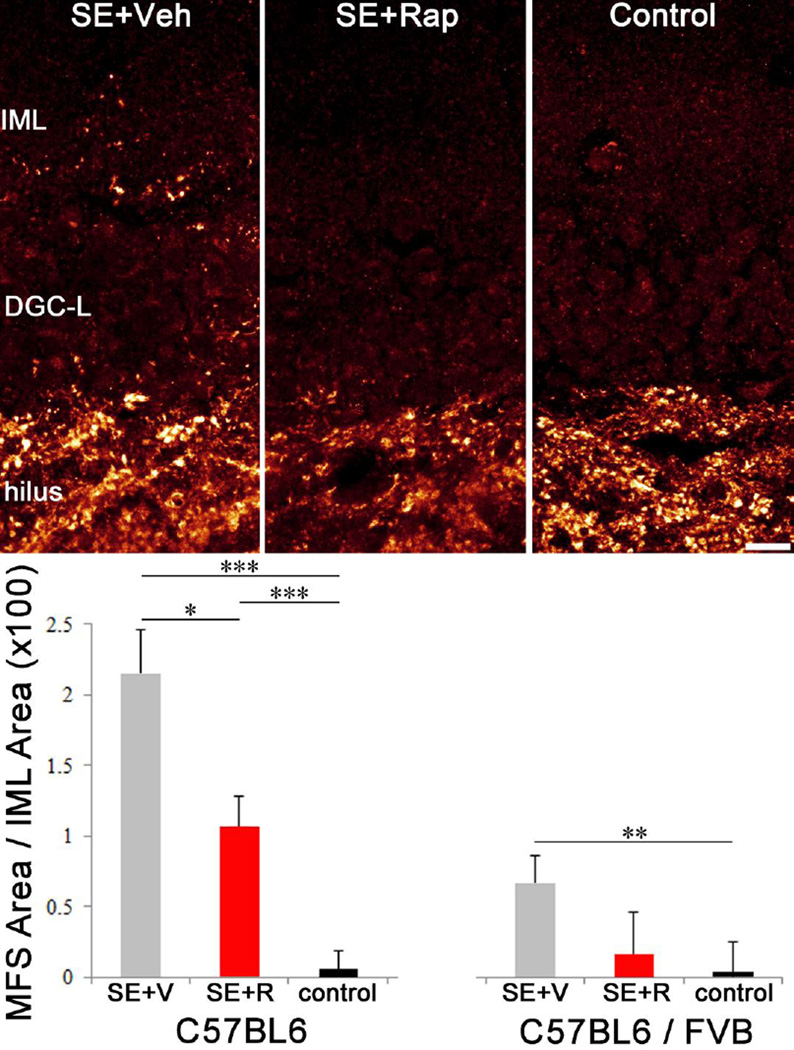
Mossy fiber sprouting is reduced in rapamycin treated mice
Mossy fiber sprouting in the dentate inner molecular layer was assessed in each animal by ZnT3-immunohistochemistry. ZnT3 is localized to the axon terminals of granule cells, providing a reliable measure of mossy fiber sprouting (Wenzel et al., 1997; McAuliffe et al., 2011). Two-way ANOVA revealed a significant strain effect between B6 and B6/FVB mice (p=0.016). B6 mice in the SE+Veh group exhibited robust mossy fiber sprouting compared to controls (p<0.001, square root-transformed data) and rapamycin treatment significantly reduced this sprouting (p=0.042, Figure 3). Pilocarpine treatment also significantly increased mossy fiber sprouting in B6/FVB strain relative to controls (p=0.003, Holm-Sidak), however, the effect was not as dramatic as that observed in B6 mice, producing a significant difference between strains for within SE+Veh (p=0.003, Holm-Sidak) and within SE+Rap comparisons (p=0.008, Holm-Sidak). In addition, rapamycin did not significantly reduce mossy fiber sprouting in the B6/FVB line (SE+Veh vs. SE+Rap, p=0.120, Holm-Sidak), perhaps because the reduced sprouting overall in this line limited statistical power. Notably, however, rapamycin-treated B6/FVB mice were also statistically indistinguishable from controls (p=0.307, Holm-Sidak), suggesting that the drug has some efficacy.
Figure 3.
Rapamycin reduces mossy fiber sprouting. Confocal maximum projections of ZnT3 immunolabeling (a marker of granule cell axon terminals) in the hippocampi of C57BL6 SE+Veh, SE+Rap and control (Veh only) mice. Graphs show quantification of ZnT3-expressing puncta density in the inner molecular layer (IML) of C57BL6 and C57BL6/FVB mice. DGC-L, dentate granule cell layer. *, p<0.05; **, p<0.01; ***, p<0.001. Scale bar = 10 µm.
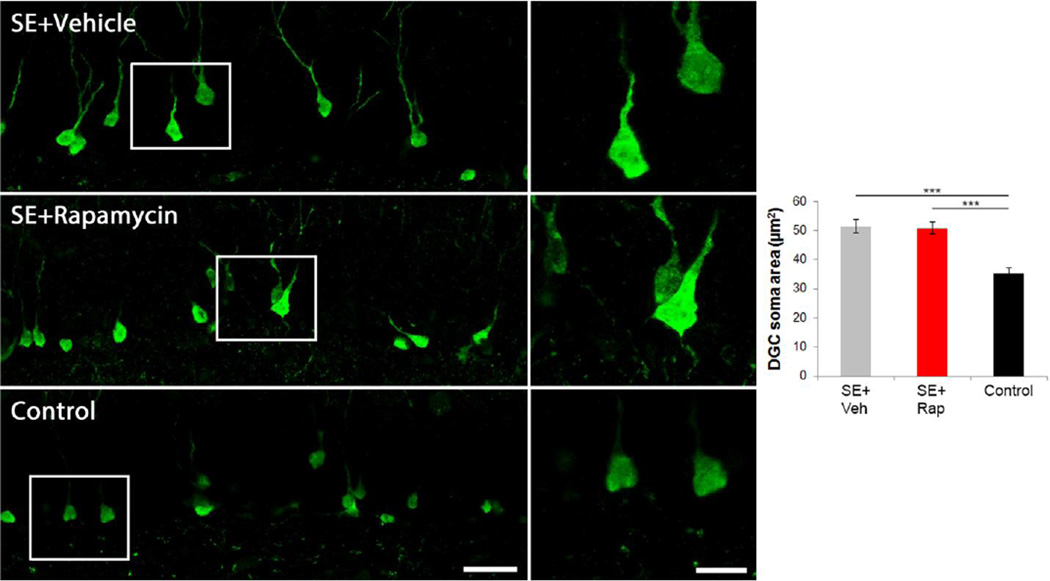
Rapamycin does not affect dentate granule cell soma size
mTOR is a known regulator of cell growth (Fingar and Blenis, 2004). Hyperactivation of the mTOR pathway has been shown to increase soma size (Kwon et al., 2001), while suppression of mTOR reduces soma size (Thomanetz et al., 2013). Status epilepticus also results in increased granule cell soma size (Suzuki et al., 1995; Young et al., 2009; Murphy et al., 2011; 2012). To see if rapamycin reduces the soma size of adult-born granule cells following SE we measured maximum profile area of GFP or tdTomato positive cell bodies. We found increased soma size in vehicle-treated mice following pilocarpine induced SE (51.7±2.6µm2) compared to control mice (34.5±2.7µm2; p<0.001; two-way ANOVA, Holm-Sidak, Figure 4). However, rapamycin treatment did not reduce soma size following pilocarpine treatment (50.6±2.6µm2; p=0.773). Mean values between mouse backgrounds were not different (p=0.705, two-way ANOVA). These findings suggest that increased soma size following SE may be mediated by mTORC2, which is rapamycin insensitive. mTORC2 has been shown to control actin cytoskeleton (Jacinto et al., 2004) and soma size (Thomanetz et al., 2013) in other models.
Figure 4.
Rapamycin does not prevent SE-induced granule cell enlargement. Confocal maximum projections of GFP-expressing granule cells. Mice that underwent SE and received daily vehicle injections contained granule cells with enlarged somas. Mice receiving rapamycin treatment following SE also possessed large granule cell somas. Non-epileptic mice (control) contained granule cells with normal cell bodies (~10 µm diameter). Micrographs on the right are enlargements of the boxed regions to the left. The graph shows quantification of granule cell soma size. ***, p<0.001. Scale bars: Left panels = 25 µm; Right panels = 10 µm.
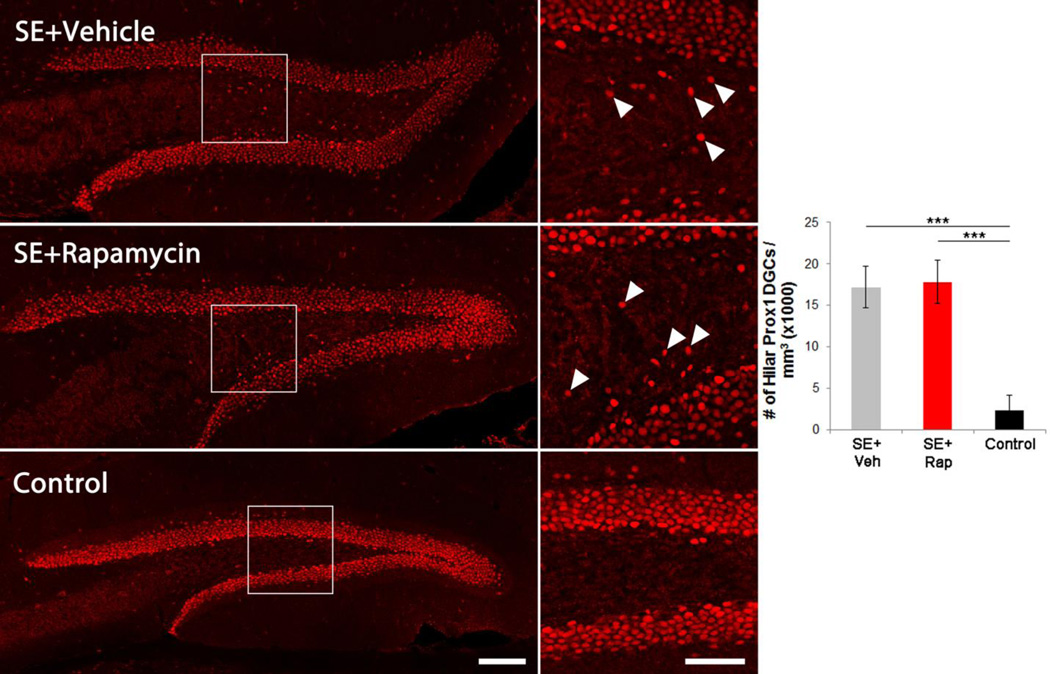
Rapamycin does not alter the number of hilar ectopic granule cells
Granule cells born after an epileptic insult commonly migrate to ectopic locations in the dentate hilus (Parent et al., 2006; Jessberger et al., 2007; Walter et al., 2007; Kron et al., 2010). Ectopic granule cells are rare in non-epileptic animals. Here, we used the granule cell-specific marker prox1 to label hilar ectopic granule cells for quantification (Figure 5). Hilar ectopic granule cell density was significantly greater in vehicle-treated epileptic mice (17.2±2.6 × 103 cells/mm3) relative to controls (2.3±1.8 × 103 cells/mm3; p<0.001; two-way ANOVA on rank-transformed data, Holm-Sidak). Rapamycin treatment following SE had no effect on ectopic granule cell accumulation (17.8±2.6 × 103 cells/mm3; p=0.566) and treated animals continued to exhibit significantly more ectopic granule cells than controls (p<0.001), confirming previously reported data (Buckmaster and Lew, 2011). No differences were found between mouse strains (p=0.092, two-way ANOVA on rank-transformed data).
Figure 5.
Rapamycin has no effect on ectopic granule cell migration. Confocal maximum projections of Prox1 immunolabeling (a mature granule cell marker) in the hippocampus of SE+Veh, SE+Rap and control (Rap only) brains. Right panels are enlargements of the boxed regions on the left. Arrowheads denote hilar ectopic granule cells. The graph shows quantification of ectopic granule cell densities in the hilus. ***, p<0.001. Scale bars: Left panels = 100 µm; Right panels = 50 µm.
Rapamycin may reduce the number of granule cells with basal dendrites
GFP and tdTomato expression was used to score granule cells for the presence of basal dendrites, which form recurrent excitatory currents and are implicated in the development of epilepsy (Ribak et al., 2000; Austin and Buckmaster, 2004; Thind et al., 2008; Hester and Danzer, 2013). The total numbers of granule cells scored in each treatment group were as follows: SE+Veh (180 cells, 15–49 cells/animal); SE+Rap (300, 8–105) and control (321, 8–71). The percentage of adult-born granule cells possessing basal dendrites was significantly increased in vehicle-treated epileptic mice (33.3±4.3%) relative to controls (3.5±4.4%; p<0.001; two-way ANOVA on square root transformed data, Holm-Sidak; Figure 6). Rapamycin-treatment of epileptic mice produced a trend towards fewer granule cells with basal dendrites; however, this reduction did not reach significance (15.9±4.3%; p=0.066). This suggests that basal dendrite formation may be partially mediated through mTOR signaling, but other pathways are likely involved. No differences in basal dendrite frequency were found between mouse strains (p=0.963, two-way ANOVA on square root transformed data).
Figure 6.
Rapamycin does not significantly affect basal dendrite formation. Confocal maximum projections of GFP-expressing granule cells. SE+Veh mice contained granule cells that possessed basal dendrites (arrowheads) extending across the granule cell layer-hilar border (white dashed lines). SE+Rap treatment produced a non-significant trend towards reduced basal dendrite frequency. Mice that did not undergo SE rarely contained basal dendrites (control). The graph shows the percentage of GFP-expressing granule cells that contained basal dendrites. *, p<0.05; ***, p<0.001. Scale bar, 20 µm.
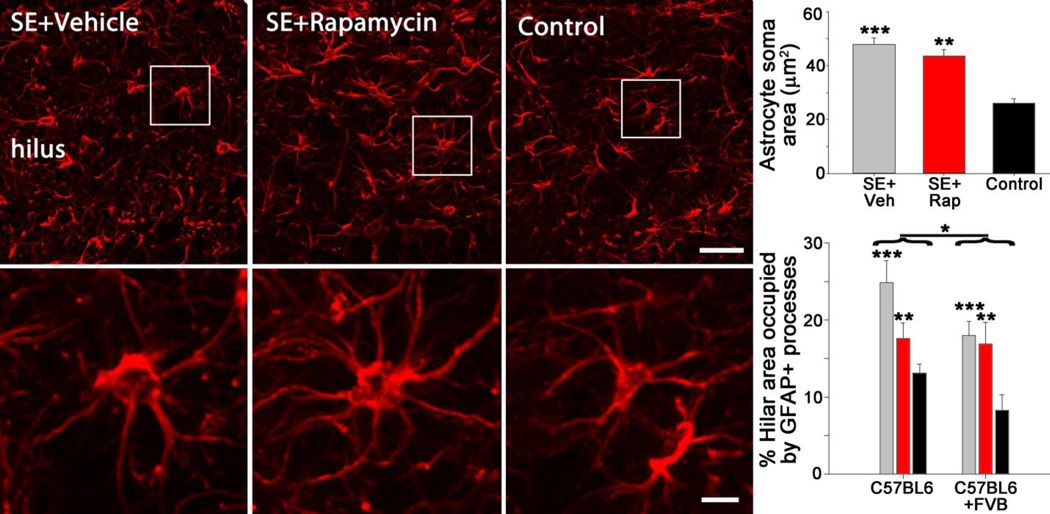
Rapamycin treatment does not affect reactive astrocytosis or microgliosis
Reactive astrocytosis may facilitate the progression of epileptogenesis (Gibbons et al., 2013). Rapamycin has been shown to inhibit immune responses in normal animals (Lu et al., 2015) and can attenuate epilepsy-associated inflammation (Brewster et al., 2013; Nguyen et al., 2015; Shima et al., 2015), leading us to ask whether rapamycin affected the activation state of astrocytes following SE. Astrocytic activation was first assessed by measuring the soma area of astrocytes in the dentate hilus. As astrocytes become reactive, they lose their fine processes and their cell bodies become enlarged. Consistent with prior work (Shapiro et al., 2008), we observed an increase in astrocytic soma size in vehicle-treated epileptic mice (47.8±2.5µm2) compared to control mice (26.1±1.7µm2; p<0.001; two-way ANOVA on square root transformed data, Holm-Sidak, Figure 7). Rapamycin treatment of epileptic mice did not reduce soma size (43.6±2.5µm2; p=0.240), indicating that the drug had no effect on this measure of inflammation. No differences in astrocyte soma area were found between mouse backgrounds (p=0.089, two-way ANOVA on square root transformed data). As a second test of astrocyte activation, we determined the total volume of tissue occupied by astrocytic somata and processes in a sample of the dentate hilus. SE+Veh and SE+Rap mice exhibited greater hilar volume occupied by astrocytes than control mice (p<0.001 and p=0.010, respectively, two-way ANOVA, Holm-Sidak, Figure 7). Rapamycin treatment, however, did not significantly reduce the volume of tissue occupied by astrocytes following status epilepticus (SE+Veh vs. SE+Rap, p=0.097). Two-way ANOVA revealed a strain difference between B6 and B6/FVB mice, with B6/FVB exhibiting less hilar volume occupied by astrocytes than B6 mice (p=0.031).
Figure 7.
Rapamycin does not reduce reactive astrocytosis. Confocal maximum projections of GFAP immunolabeling of astrocytes in the hippocampus. Mice that underwent SE and received daily vehicle injections contained astrocytes with enlarged somas, indicative of astrocyte activation. Mice receiving rapamycin treatment following SE also possessed activated astrocytes. Non-epileptic mice (control) contained astrocytes with smaller cell bodies that did not display an activated morphology. Lower panels are enlargements of the boxed regions in the top panels. The top graph depicts astrocyte soma area, while the lower graph depicts the percentage of the hilar region occupied by astrocytic somata and processes. Strains were graphed separately in the lower panel to show differences. *, p<0.05; **, p<0.01; ***, p<0.001. Scale bars: Upper panels = 25 µm; Lower panels = 5 µm.
We also assessed the impact of rapamycin on microglial activation in the hilus. Microglial soma area was increased following SE (SE+Veh vs. control, p=0.001, two-way ANOVA, Holm-Sidak, Figure 8), but again, rapamycin did not block this increase (SE+Veh vs. SE+Rap, p=0.710). Percent hilar volume occupied by microglial processes was statistically equivalent among groups (SE+Veh, SE+Rap, or control, p=0.161, Figure 8). No strain effects were observed for either measure (microglial soma area, p=0.100; microglial volume/hilar volume, p=0.736).
Figure 8.
Rapamycin does not affect reactive microgliosis. Confocal maximum projections of Iba1 immunolabeling of microglia in the hippocampus. SE+Rap and SE+Veh mice exhibited enlarged soma areas relative to non-epileptic (Control) mice, suggestive of increased microglial activation. Lower panels are enlargements of the boxed regions in the top panels. The top graph depicts microglial soma area, while the lower graph depicts the percentage of the hilar region occupied by Iba1 positive microglia. **, p<0.01 vs. control. Scale bars: Upper panels = 25 µm; Lower panels = 10 µm.
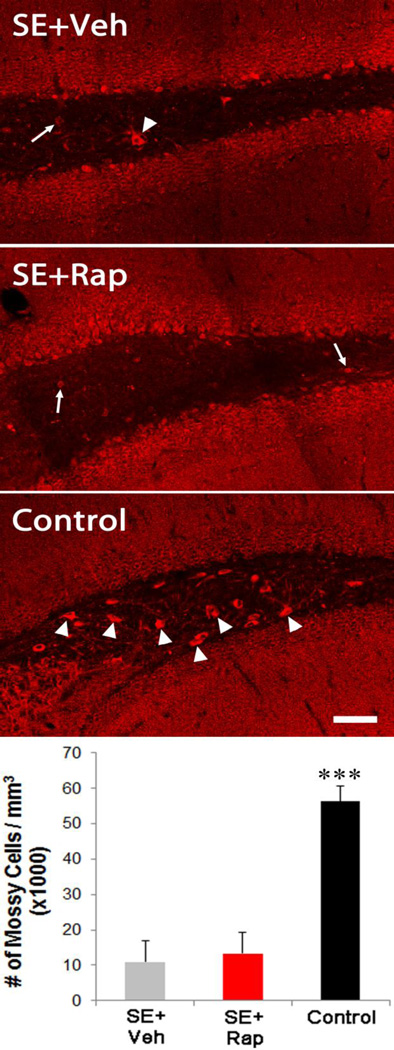
Mossy cell loss is unaffected by rapamycin treatment
Hilar mossy cell death following seizures has been reported for decades (Sloviter, 1987) and has been suggested to play a role in epileptogenesis (Sloviter et al., 2003; Jinde et al., 2013). To quantify mossy cell numbers, sections were immunostained for GluR2/3, a receptor expressed by glutamatergic mossy cells. We confirmed that hilar mossy cell density was significantly reduced in vehicle-treated epileptic mice (10.9±6.1 × 103 cells/mm3) compared to controls (56.4±4.2 × 103 cells/mm3; p<0.001; Figure 9). Rapamycin treatment after SE had no effect on mossy cell density (13.1±6.3 × 103 cells/mm3), with SE+Rap mice exhibiting significantly lower values than controls. Mossy cell loss occurs hours to days after SE, so it was expected that rapamycin treatment would have limited effects on this measure. No differences in mossy cell density were found between mouse strains (p= 0.516, two-way ANOVA).
Figure 9.
Mossy cell loss is not affected by rapamycin. Confocal maximum projections of GluR2/3 expressing cells. SE leads to hilar mossy cell death, as shown by the lack of large cell bodies within the hilus of SE+Veh mice. Rapamycin treatment did not reduce mossy cell loss (SE+Rap). Mossy cells were prominent in control mice that did not undergo SE (arrowheads). Mossy cells are distinguished from ectopic dentate granule cells (arrows) based on their larger soma size. The graph shows quantification of mossy cell density. Scale bar, 50µm.
Discussion
mTOR signaling is enhanced during the development of acquired temporal lobe epilepsy (Figure 1), and rapamycin treatment has been shown to reduce seizures frequency in a variety of epilepsy models, including kainate-SE (Zeng et al., 2009), pilocarpine-SE (Huang et al., 2010), angular bundle electrical stimulation (van Vliet et al., 2012), neonatal hypoxia (Talos et al., 2012), traumatic brain injury (Guo et al., 2013), models of TSC (Zeng et al., 2008; Goto et al., 2011; Zeng et al., 2011) and PTEN inactivation (Ljungberg et al., 2009; Sunnen et al., 2011; Pun et al., 2012). Negative findings have also been reported (Buckmaster and Lew, 2011; Heng et al., 2013; Sliwa et al., 2012). In addition to improving seizure outcome, animal studies have demonstrated that rapamycin improves cognitive and memory deficits following SE (Brewster et al., 2013). The mechanisms by which rapamycin produces these positive effects, however, are unclear. For the present study, we examined several hippocampal abnormalities implicated in the development of temporal lobe epilepsy (Parent and Kron, 2012; Hester and Danzer, 2014), including mossy fiber sprouting (Buckmaster, 2014), hilar ectopic granule cell accumulation (Scharfman and Pierce, 2012), basal dendrite formation (Ribak et al., 2012), reactive astrogliosis and microgliosis (Sharma et al., 2008; Gibbons et al., 2013) and mossy cell loss (Jinde et al., 2013). Consistent with previous studies (Zeng et al., 2009; Buckmaster et al., 2009), we observed a significant reduction in mossy fiber sprouting with rapamycin treatment. Rapamycin was ineffective, however, at significantly mitigating any of the other pathological changes. With the exception of mossy fiber sprouting, our findings suggest that it is unlikely that rapamycin targets these common dentate pathologies to produce its positive effects in animal models of epilepsy.
Rapamycin was highly efficient at blocking mTOR pathway signaling
The negative results presented here cannot be explained by a failure of the drug regimen to inhibit the mTOR pathway in the dentate, since staining for pS6 (a kinase that is one of the primary mediators of mTOR pathway signaling) was dramatically reduced in treated animals. Replication of the effect on mossy fiber sprouting provides additional evidence that the drug worked as expected. Unfortunately it was not possible to combine the rapamycin treatment regimen used here with chronic EEG recording, because drug treatment dramatically increased mortality following electrode implantation surgery. We suspect that the poor survival of SE+Rap mice reflects combined effects of seizure-induced stress, and reduced weight gain. The radio telemetry system used for 24/7 EEG monitory requires implanting a transmitter (1.6 g, 1.1 cc) under the skin of the back. Animals weighing less than 18 g do not tolerate the implant, and animals greater than 20 g do best. In our past studies, both healthy control and epileptic mice weighing >18 g have tolerated the transmitters well (Castro et al., 2012; Pun et al., 2012; Hester and Danzer, 2013; Jiang et al., 2015). The combination of SE and reduced weight gain in SE+Rap mice, however, may have been too great a stressor for successful use of the radio telemetry system. While weight gain is considered to be a comorbidity of epilepsy, it likely enhances the animal’s ability to tolerate the transmitters. Investigators conducting similar studies in the future should consider using larger animals, or less invasive recording systems.
Significance of reduced weight gain in epileptic mice treated with rapamycin
Pilocarpine status epilepticus leads to obesity in rodents (Scharfman et al., 2008; Ruiz et al., 2011). Obesity is also a common comorbidity of human epilepsy (Ladino et al., 2014; Janousek et al., 2013). The present findings indicate that the mTOR pathway is critical for SE-induced weight gain in rodents. Rapamycin could act as a non-specific growth retardant (van Vliet et al., 2016), or it could act more specifically to mitigate dysfunction of brain circuits regulating the neuroendocrine system, which are known to be disrupted in epilepsy (for review see Luef and Rauchenzauner, 2009). Alternatively, rapamycin could act directly on adipose tissue by inhibiting mTOR signaling in these cells. mTOR signaling plays a major role in regulating a variety of adipose tissue functions, including adipogenesis (for review see Cai et al., 2015). Notably, the pronounced ability of rapamycin to block weight gain following SE raises the possibility that the drug’s anti-epileptogenic effects may be mediated by tissues outside the CNS. Changes in metabolism can alter neuronal excitability, as evidenced by the success of the ketogenic diet in treating epilepsy (Nangia et al., 2012; Neal et al., 2008). Indeed, rapamycin treatment can increase ketone body levels in mice (Fang et al., 2013). The possibility that rapamycin produces some of its positive effects in epilepsy by altering metabolism deserve further investigation.
Rapamycin reduced mossy fiber sprouting, but had no effect on other granule cell pathologies
Mossy fiber sprouting is a common hallmark of temporal lobe epilepsy. This sprouting creates recurrent excitatory circuits in the dentate gyrus, promoting local hyperexcitability (Sutula and Dudek, 2007). Mossy fiber sprouting occurs following gene mutations that enhance mTOR signaling (LaSarge and Danzer, 2014; LaSarge et al., 2015). Inhibiting the pathway with rapamycin has consistently been demonstrated to block sprouting in acquired epilepsy models (Zeng et al., 2009; Buckmaster et al., 2009). It remains unclear, however, whether preventing mossy fiber sprouting will have any effect on epileptogenesis (Buckmaster, 2014). Numerous lines of evidence suggest that mossy fiber sprouting may not play an important role in epileptogenesis, including work with rapamycin, which has produced a dissociation between mossy fiber sprouting and seizure occurrence (Buckmaster and Lew, 2011; Heng et al., 2013). Why some groups see reductions in seizure frequency following rapamycin treatment and others do not will require additional studies, but differences among animal models, drug dosing regimens or drug metabolism among rodent species/strains could all contribute to the conflicting findings. Regardless, it is clear that mossy fiber sprouting is not necessary for the development of epilepsy, and the ability of rapamycin to block mossy fiber sprouting is unlikely to be responsible for its effects on seizure prevention.
Rapamycin did not reduce hippocampal astrogliosis or microgliosis
Given the absence of rapamycin-effects on granule cell morphology, we queried whether the drug mitigates inflammatory changes in epilepsy (Russo et al., 2013). Inflammatory changes are a prominent feature of the epileptogenic process (Vezzani et al., 2013). Moreover, rapamycin has robust anti-inflammatory properties (Soliman, 2013). It prevents reactive gliosis following a variety of injuries (Carson et al., 2012; Goldshmit et al., 2015; Li et al., 2015), including seizures (Brewster et al., 2013; Nguyen et al., 2015; Shima et al., 2015). Finally, rapamycin has long been used clinically as an immune suppressant following organ transplants (Geissler and Schlitt, 2010).
Status epilepticus leads to reactive astrocytosis and microgliosis (Garzillo and Mello, 2002; Borges et al., 2003). During this inflammatory process, reactive astrocytes and microglia change their morphology from a ramified structure, with many fine processes, to a more amoeboid morphology, with enlarged somas and shorter, thicker processes (Sofroniew, 2009; Lee and MacLean, 2015). Increased phosphorylation of S6 protein is evident in astrocytes in the kainic acid model of epilepsy (Sha et al., 2012; Macias et al., 2013), suggesting that the mTOR pathway might mediate these changes. Nevertheless, despite the compelling evidence linking mTOR signaling to reactive astrocytosis, rapamycin treatment had no effect on reactive astrocytosis or reactive microgliosis in the dentate gyrus. Enlarged astrocyte and microglia somas were evident in both vehicle and rapamycin treated epileptic animals. These findings suggest that in the pilocarpine model of epilepsy, hippocampal astrogliosis and microgliosis is likely a result of other cellular mechanisms and is not mediated by the mTOR signaling pathway. Inflammation may occur though other pathways which were not analyzed in the present study, including cytokine activation. Notably, these findings contrast with recent work by Shima and colleagues (2015), in which they did demonstrate reduced astrogliosis with rapamycin treatment in the intrahippocampal kainic acid mouse model of epilepsy. Much higher doses of rapamycin were used (40 vs. 6 mg/kg/day), however, increasing the likelihood of mTORC 2 inhibition (Sarbassov et al., 2006; Urbanska et al., 2012).
Alternate mechanisms by which rapamycin might mitigate epileptogenesis
In the present study, we tested several mechanisms by which rapamycin might act to mitigate epileptogenesis, including reducing aberrant neurogenesis, blocking hippocampal inflammatory changes and preventing mossy cell loss. Rapamycin had modest effects on mossy fiber sprouting, and was ineffective in preventing changes among the other parameters, strongly suggesting that the beneficial effects of the drug in epilepsy are not mediated by changes in any of these processes.
Notably, the specific pathological changes required to produce epilepsy have yet to be fully defined, so it is a formal possibility that the processes examined here are not integral to epileptogenesis, and the drug acts on brain regions or by mechanisms not yet assessed. In addition to hippocampus, pilocarpine SE causes neuropathological changes in a variety of other brain regions, including olfactory cortex, amygdala, thalamus, neocortex and substantia nigra (Turski et al., 1983). Systemically-administered rapamycin has the potential to impact all these brain regions, and therefore the drug might act anywhere in the CNS. It is also conceivable that rapamycin might act outside the nervous system, with direct effects on the immune system being the most likely target with the potential to modulate seizure incidence (Soliman, 2013; Vezzani and Viviani, 2015).
In addition to the effects on cell morphology and survival examined here, rapamycin might act by a variety of alternate mechanisms. One unlikely scenario, however, is that it acts like an anticonvulsant, akin to currently available anti-epileptic drugs. When evaluated through a battery of acute seizure tests, rapamycin exhibited minimal acute anticonvulsant properties (Chachua et al., 2012; Hartman et al., 2012; Siebel et al., 2015) and may exhibit some pro-convulsant effects (Huang et al., 2012; Macias et al., 2013). This is not surprising, since the majority of current anti-epileptic drugs directly inhibit or activate ion channels (excitatory or inhibitory, respectively) -- mechanisms not attributed to rapamycin (Daoud et al., 2007; Ruegg et al., 2007). The mTOR pathway is, however, a key regulator of synaptogenesis (Li et al., 2010), synaptic strength (Weston et al., 2014) and long-term potentiation (Stoica et al., 2011). The pathway also modulates the expression of a large number of genes, including voltage-gated sodium, potassium and calcium channels (Huang et al., 2013; Jiang et al., 2013; Sosanya et al., 2015). Changes in ion channel expression could significantly impact seizure occurrence (Snowball and Schorge, 2015) and changes in synaptic strength are likely important for epileptogenesis (Casillas-Espinosa et al., 2012). Recent work by Yamawaki and colleagues (2015) demonstrated that rapamycin reduces synapse density in the dentate gyrus following epileptogenesis. Such changes in synaptic strength and number are not detectable with the methodologies used in the current study, but could be fruitful avenues for investigation of mTOR signaling in epilepsy.
Highlights.
Rapamycin prevents pathological weight gain in epileptic rodents
Rapamycin reduces mossy fiber sprouting in epilepsy
Rapamycin does not block ectopic granule cell accumulation or somatic hypertrophy
Rapamycin does not block hippocampal inflammatory changes
Rapamycin does not prevent hilar mossy cell death
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (SCD, Award Numbers R01NS065020 and R01NS062806). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. We thank Keri Kaeding for assistance with earlier versions of this manuscript. We thank the CCHMC Confocal Core for providing access the 3024 Nikon A1Rsi inverted microscope used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: The authors declare no competing financial interests
References
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118(4):505–516. doi: 10.1016/j.cell.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437(7060):894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- Althaus AL, Sagher O, Parent JM, Murphy GG. Intrinsic neurophysiological properties of hilar ectopic and normotopic dentate granule cells in human temporal lobe epilepsy and a rat model. J Neurophysiol. 2015;113(4):1184–1194. doi: 10.1152/jn.00835.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JE, Buckmaster PS. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol. 2004;476(3):205–218. doi: 10.1002/cne.20182. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182(1):21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Brewster AL, Lugo JN, Patil VV, Lee WL, Qian Y, Vanegas F, Anderson AE. Rapamycin reverses status epilepticus-induced memory deficits and dendritic damage. PLoS One. 2013;8(3):e57808. doi: 10.1371/journal.pone.0057808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29(25):8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31(6):2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS. Does mossy fiber sprouting give rise to the epileptic state? Adv Exp Med Biol. 2014;813:161–168. doi: 10.1007/978-94-017-8914-1_13. [DOI] [PubMed] [Google Scholar]
- Butler CR, Boychuk JA, Smith BN. Effects of Rapamycin Treatment on Neurogenesis and Synaptic Reorganization in the Dentate Gyrus after Controlled Cortical Impact Injury in Mice. Front Syst Neurosci. 2015;9:163. doi: 10.3389/fnsys.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Dong LQ, Liu F. Recent Advances in Adipose mTOR Signaling and Function: Therapeutic Prospects. Trends Pharmacol Sci. 2015 doi: 10.1016/j.tips.2015.11.011. pii: S0165-6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MC, Zhan RZ, Nadler JV. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2011;519(11):2175–2192. doi: 10.1002/cne.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson RP, Van Nielen DL, Winzenburger PA, Ess KC. Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin. Neurobiol Dis. 2012;45(1):369–380. doi: 10.1016/j.nbd.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillas-Espinosa PM, Powell KL, O’Brien TJ. Regulators of synaptic transmission: roles in the pathogenesis and treatment of epilepsy. Epilepsia. 2012;53(Suppl 9):41–58. doi: 10.1111/epi.12034. [DOI] [PubMed] [Google Scholar]
- Castro OW, Santos VR, Pun RY, McKlveen JM, Batie M, Holland KD, Gardner M, Garcia-Cairasco N, Herman JP, Danzer SC. Impact of corticosterone treatment on spontaneous seizure frequency and epileptiform activity in mice with chronic epilepsy. PLoS One. 2012;7(9):e46044. doi: 10.1371/journal.pone.0046044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachua T, Poon KL, Yum MS, Nesheiwat L, DeSantis K, Velíšková J, Velíšek L. Rapamycin has age-, treatment paradigm-, and model specific anticonvulsant effects and modulates neuropeptide Y expression in rats. Epilepsia. 2012;53:2015–2025. doi: 10.1111/j.1528-1167.2012.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG, Scharfman HE, Eisch AJ, Hsieh J. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels ZS, Nick TG, Liu C, Cassedy A, Glauser TA. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology. 2009;73(9):658–664. doi: 10.1212/WNL.0b013e3181ab2b11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud D, Scheld HH, Speckmann EJ, Gorji A. Rapamycin: brain excitability studied in vitro. Epilepsia. 2007;48(4):834–836. doi: 10.1111/j.1528-1167.2006.00976.x. [DOI] [PubMed] [Google Scholar]
- Fang Y, Westbrook R, Hill C, Boparai RK, Arum O, Spong A, Wang F, Javors MA, Chen J, Sun LY, Bartke A. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17(3):456–462. doi: 10.1016/j.cmet.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby, Smith AL. The Mouse in Biomedical Research: Normative Biology, Husbandry, and Models. Academic press; 2006. [Google Scholar]
- Fujise N, Kosaka T. Mossy cells in the mouse dentate gyrus: identification in the dorsal hilus and their distribution along the dorsoventral axis. Brain Res. 1999;816(2):500–511. doi: 10.1016/s0006-8993(98)01202-5. [DOI] [PubMed] [Google Scholar]
- Garzillo CL, Mello LE. Characterization of reactive astrocytes in the chronic phase of the pilocarpine model of epilepsy. Epilepsia. 2002;43(Suppl 5):107–109. doi: 10.1046/j.1528-1157.43.s.5.40.x. [DOI] [PubMed] [Google Scholar]
- Geissler EK, Schlitt HJ. The potential benefits of rapamycin on renal function, tolerance, fibrosis, and malignancy following transplantation. Kidney Int. 2010;78(11):1075–1079. doi: 10.1038/ki.2010.324. [DOI] [PubMed] [Google Scholar]
- Gibbons MB, Smeal RM, Takahashi DK, Vargas JR, Wilcox KS. Contributions of astrocytes to epileptogenesis following status epilepticus: opportunities for preventive therapy? Neurochem Int. 2013;63(7):660–669. doi: 10.1016/j.neuint.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Kanner S, Zacs M, Frisca F, Pinto AR, Currie PD, Pinkas-Kramarski R. Rapamycin increases neuronal survival, reduces inflammation and astrocyte proliferation after spinal cord injury. Mol Cell Neurosci. 2015;68:82–91. doi: 10.1016/j.mcn.2015.04.006. [DOI] [PubMed] [Google Scholar]
- Goto J, Talos DM, Klein P, Qin W, Chekaluk YI, Anderl S, Malinowska IA, Di Nardo A, Bronson RT, Chan JA, Vinters HV, Kernie SG, Jensen FE, Sahin M, Kwiatkowski DJ. Regulable neural progenitor-specific Tsc1 loss yields giant cells with organellar dysfunction in a model of tuberous sclerosis complex. Proc Natl Acad Sci U S A. 2011;108(45):E1070–E1079. doi: 10.1073/pnas.1106454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Zeng L, Brody DL, Wong M. Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One. 2013;8(5):e64078. doi: 10.1371/journal.pone.0064078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman AL, Santos P, Dolce A, Hardwick JM. The mTOR inhibitor rapamycin has limited acute anticonvulsant effects in mice. PLoS One. 2012;7(9):e45156. doi: 10.1371/journal.pone.0045156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng K, Haney MM, Buckmaster PS. High-dose rapamycin blocks mossy fiber sprouting but not seizures in a mouse model of temporal lobe epilepsy. Epilepsia. 2013;54(9):1535–1541. doi: 10.1111/epi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33(21):8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy - a possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014;38:105–116. doi: 10.1016/j.yebeh.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofacer RD, Deng M, Ward CG, Joseph B, Hughes EA, Jiang C, Danzer SC, Loepke AW. Cell age-specific vulnerability of neurons to anesthetic toxicity. Ann Neurol. 2013;73(6):695–704. doi: 10.1002/ana.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K, Hopkins N, Mcloughlin P. Combined confocal microscopy and stereology: a highly efficient and unbiased approach to quantitative structural measurement in tissues. Exp Physiol. 2002;87:747–756. doi: 10.1113/eph8702477. [DOI] [PubMed] [Google Scholar]
- Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40(1):193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, McMahon J, Yang J, Shin D, Huang Y. Rapamycin down-regulates KCC2 expression and increases seizure susceptibility to convulsants in immature rats. Neuroscience. 2012;219:33–47. doi: 10.1016/j.neuroscience.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Ko ML, Ko GY. A new functional role for mechanistic/mammalian target of rapamycin complex 1 (mTORC1) in the circadian regulation of L-type voltage-gated calcium channels in avian cone photoreceptors. PLoS One. 2013;8(8):e73315. doi: 10.1371/journal.pone.0073315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6(11):1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Janousek J, Barber A, Goldman L, Klein P. Obesity in adults with epilepsy. Epilepsy Behav. 2013;28(3):391–394. doi: 10.1016/j.yebeh.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27(35):9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Pang X, Niu Q, Hua L, Cheng M, Ji Y. Activation of mammalian target of rapamycin mediates rat pain-related responses induced by BmK I, a sodium channel-specific modulator. Mol Pain. 2013;9:50. doi: 10.1186/1744-8069-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Pun RYK, Peariso K, Holland KD, Lian Q, Danzer SC. Olfactory bulbectomy leads to the development of epilepsy in mice. PLoS One. 2015;10(9):e0138178. doi: 10.1371/journal.pone.0138178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinde S, Zsiros V, Nakazawa K. Hilar mossy cell circuitry controlling dentate granule cell excitability. Front Neural Circuits. 2013;7:14. doi: 10.3389/fncir.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19(12):3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;23(2):237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kron MM, Zhang H, Parent JM. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. Neurobiology of Disease. 2010;30(6):2051–2059. doi: 10.1523/JNEUROSCI.5655-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat Genet. 2001;29(4):404–411. doi: 10.1038/ng781. [DOI] [PubMed] [Google Scholar]
- Ladino LD, Hernández-Ronquillo L, Téllez-Zenteno JF. Obesity and its association with generalised epilepsy, idiopathic syndrome, and family history of epilepsy. Epileptic Disord. 2014;16(3):343–353. doi: 10.1684/epd.2014.0677. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Danzer SC. Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation. Front Mol Neurosci. 2014;7:18. doi: 10.3389/fnmol.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSarge CL, Santos VR, Danzer SC. PTEN deletion from adult-generated dentate granule cells disrupts granule cell mossy fiber axon structure. Neurobiol Dis. 2015;75:142–150. doi: 10.1016/j.nbd.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, MacLean AG. New advances on glial activation in health and disease. World J Virol. 2015;4(2):42–55. doi: 10.5501/wjv.v4.i2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Li X, Liu SF, Qu WS, Wang W, Tian DS. Inhibition of mTOR pathway restrains astrocyte proliferation, migration and production of inflammatory mediators after oxygen-glucose deprivation and reoxygenation. Neurochem Int. 2015;83–84:9–18. doi: 10.1016/j.neuint.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2(7–8):389–398. doi: 10.1242/dmm.002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Liu F, Chen L, Zhang H, Ding Y, Liu J, Wong M, Zeng LH. Effect of Chronic Administration of Low Dose Rapamycin on Development and Immunity in Young Rats. PLoS One. 2015;10(8):e0135256. doi: 10.1371/journal.pone.0135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luef G, Rauchenzauner M. Epilepsy and hormones: a critical review. Epilepsy Behav. 2009;15(1):73–77. doi: 10.1016/j.yebeh.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Macias M, Blazejczyk M, Kazmierska P, Caban B, Skalecka A, Tarkowski B, Rodo A, Konopacki J, Jaworski J. Spatiotemporal Characterization of mTOR Kinase Activity Following Kainic Acid Induced Status Epilepticus and Analysis of Rat Brain Response to Chronic Rapamycin Treatment. PLoS One. 2013;8(5):e64455. doi: 10.1371/journal.pone.0064455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuliffe JJ, Bronson SL, Hester MS, Murphy BL, Dahlquist-Topala R, Richards DA, Danzer SC. Altered patterning of dentate granule cell mossy fiber inputs onto CA3 pyramidal cells in limbic epilepsy. Hippocampus. 2011;21(1):93–107. doi: 10.1002/hipo.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Pun RY, Yin H, Faulkner CR, Loepke AW, Danzer SC. Heterogeneous integration of adult-generated granule cells into the epileptic brain. J Neurosci. 2011;31(1):105–117. doi: 10.1523/JNEUROSCI.2728-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Hofacer RD, Faulkner CN, Loepke AW, Danzer SC. Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced post-injury neurogenesis. Epilepsia. 2012;53(5):908–921. doi: 10.1111/j.1528-1167.2012.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98(12):1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Nangia S, Caraballo RH, Kang HC, Nordli DR, Scheffer IE. Is the ketogenic diet effective in specific epilepsy syndromes? Epilepsy Res. 2012;100(3):252–257. doi: 10.1016/j.eplepsyres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomized controlled trial. Lancet Neurol. 2008;7(6):500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Nguyen LH, Brewster AL, Clark ME, Regnier-Golanov A, Sunnen CN, Patil VV, D’Arcangelo G, Anderson AE. mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia. 2015;56(4):636–646. doi: 10.1111/epi.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki MM, Molnár P, Nadler JV. Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol. 1999;81(4):1645–1660. doi: 10.1152/jn.1999.81.4.1645. [DOI] [PubMed] [Google Scholar]
- Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59(1):81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- Parent JM, Kron MM. Neurogenesis and Epilepsy. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. SourceJasper’s Basic Mechanisms of the Epilepsies [Internet] 4th edition. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. London: Academic; 2001. [Google Scholar]
- Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75(6):1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428(2):240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Shapiro LA, Yan XX, Dashtipour K, Nadler JV, Obenaus A, Spigelman I, Buckmaster PS. Seizure-induced formation of basal dendrites on granule cells of the rodent dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet] 4th. Bethesda (MD): National Center for Biotechnology Information (US); 2012. [PubMed] [Google Scholar]
- Ruegg S, Baybis M, Juul H, Dichter M, Crino PB. Effects of rapamycin on gene expression, morphology, and electrophysiological properties of rat hippocampal neurons. Epilepsy Res. 2007;77(2–3):85–92. doi: 10.1016/j.eplepsyres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N, Pacheco LF, Farrell B, Cox CB, Ermolinsky BS, Garrido-Sanabria ER, Nair S. Metabolic gene expression changes in the hippocampus of obese epileptic male rats in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2011;1426:86–95. doi: 10.1016/j.brainres.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Donato G, Camastra C, Iuliano R, Cuzzocrea S, Constanti A, De Sarro G. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology. 2013;69:25–36. doi: 10.1016/j.neuropharm.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Santos VR, de Castro OW, Pun RY, Hester MS, Murphy BL, Loepke AW, Garcia-Cairasco N, Danzer SC. Contributions of mature granule cells to structural plasticity in temporal lobe epilepsy. Neuroscience. 2011;197:348–357. doi: 10.1016/j.neuroscience.2011.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20(16):6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Kim M, Hintz TM, MacLusky NJ. Seizures and reproductive function: insights from female rats with epilepsy. Ann Neurol. 2008;64(6):687–697. doi: 10.1002/ana.21518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012;53(Suppl 1):109–115. doi: 10.1111/j.1528-1167.2012.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2013;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiol Dis. 2012;45(1):297–304. doi: 10.1016/j.nbd.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha LZ, Xing XL, Zhang D, Yao Y, Dou WC, Jin LR, Wu LW, Xu Q. Mapping the Spatio-Temporal Pattern of the Mammalian Target of Rapamycin (mTOR) Activation in Temporal Lobe Epilepsy. PLoS One. 2012;7(6):e39152. doi: 10.1371/journal.pone.0039152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Jordan WH, Reams RY, Hall DG, Snyder PW. Temporal profile of clinical signs and histopathologic changes in an F-344 rat model of kainic acid-induced mesial temporal lobe epilepsy. Toxicol Pathol. 2008;36(7):932–943. doi: 10.1177/0192623308326093. [DOI] [PubMed] [Google Scholar]
- Shima A, Nitta N, Suzuki F, Laharie AM, Nozaki K, Depaulis A. Activation of mTOR signaling pathway is secondary to neuronal excitability in a mouse model of mesio-temporal lobe epilepsy. Eur J Neurosci. 2015;41(7):976–988. doi: 10.1111/ejn.12835. [DOI] [PubMed] [Google Scholar]
- Siebel AM, Menezes FP, da Costa Schaefer I, Petersen BD, Bonan CD. Rapamycin suppresses PTZ-induced seizures at different developmental stages of zebrafish. Pharmacol Biochem Behav. 2015 doi: 10.1016/j.pbb.2015.05.022. pii: S0091-3057(15)30005-8. [DOI] [PubMed] [Google Scholar]
- Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett. 2012;509(2):105–109. doi: 10.1016/j.neulet.2011.12.051. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235(4784):73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459(1):44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- Snowball A, Schorge S. Changing channels in pain and epilepsy: Exploiting ion channel gene therapy for disorders of neuronal hyperexcitability. FEBS Lett. 2015;589(14):1620–1634. doi: 10.1016/j.febslet.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman GA. The role of mechanistic target of rapamycin (mTOR) complexes signaling in the immune responses. Nutrients. 2013;5(6):2231–2257. doi: 10.3390/nu5062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosanya NM, Brager DH, Wolfe S, Niere F, Raab-Graham KF. Rapamycin reveals an mTOR-independent repression of Kv1.1 expression during epileptogenesis. Neurobiol Dis. 2015;73:96–105. doi: 10.1016/j.nbd.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108(9):3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunnen CN, Brewster AL, Lugo JN, Vanegas F, Turcios E, Mukhi S, Parghi D, D’Arcangelo G, Anderson AE. Inhibition of the mammalian target of rapamycin blocks epilepsy progression in NS-Pten conditional knockout mice. Epilepsia. 2011;52(11):2065–2075. doi: 10.1111/j.1528-1167.2011.03280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Junier MP, Guilhem D, Sørensen JC, Onteniente B. Morphogenetic effect of kainate on adult hippocampal neurons associated with a prolonged expression of brain-derived neurotrophic factor. Neuroscience. 1995;64(3):665–674. doi: 10.1016/0306-4522(94)00463-f. [DOI] [PubMed] [Google Scholar]
- Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ. Joseph A, Jensen FE. The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTORC1) pathway. PLoS One. 2012;7(5):e35885. doi: 10.1371/journal.pone.0035885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Long H, Zeng C, Li Y, Bi F, Wang J, Qian H, Xiao B. Rapamycin suppresses the recurrent excitatory circuits of dentate gyrus in a mouse model of temporal lobe epilepsy. Biochem Biophys Res Commun. 2012;420(1):199–204. doi: 10.1016/j.bbrc.2012.02.143. [DOI] [PubMed] [Google Scholar]
- Thind KK, Ribak CE, Buckmaster PS. Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J Comp Neurol. 2008;509(2):190–202. doi: 10.1002/cne.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomanetz V, Angliker N, Cloëtta D, Lustenberger RM, Schweighauser M, Oliveri F, Suzuki N, Rüegg MA. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol. 2013;201(2):293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9(3):315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Urbanska M, Gozdz A, Swiech LJ, Jaworski J. Mammalian target of rapamycin complex 1 (mTORC1) and 2 (mTORC2) control the dendritic arbor morphology of hippocampal neurons. J Biol Chem. 2012;287(36):30240–30256. doi: 10.1074/jbc.M112.374405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, de Vries HE, Aronica E, Gorter JA. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood-brain barrier leakage but not microglia activation. Epilepsia. 2012;53(7):1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, de Vries HE, Dijkhuizen RM, Gorter JA. Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats I: Magnetic resonance imaging. Epilepsia. 2016;57(1):59–69. doi: 10.1111/epi.13246. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2013;69:16–24. doi: 10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. 2015;96(Pt A):70–82. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Walter C, Murphy BL, Pun RY, Spieles-Engemann AL, Danzer SC. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J Neurosci. 2007;27(28):7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich J, Löb S, Löffler M, Königsrainer I, Zieker D, Königsrainer A, Coerper S, Beckert S. Rapamycin-induced impaired wound healing is associated with compromised tissue lactate accumulation and extracellular matrix remodeling. Eur Surg Res. 2011;47(1):39–44. doi: 10.1159/000327972. [DOI] [PubMed] [Google Scholar]
- Wenzel HJ, Cole TB, Born DE, Schwartzkroin PA, Palmiter RD. Ultrastructural localization of zinc transporter-3 (ZnT-3) to synaptic vesicle membranes within mossy fiber boutons in the hippocampus of mouse and monkey. Proc Natl Acad Sci U S A. 1997;94(23):12676–12681. doi: 10.1073/pnas.94.23.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MC, Chen H, Swann JW. Loss of mTOR repressors Tsc1 or Pten has divergent effects on excitatory and inhibitory synaptic transmission in single hippocampal neuron cultures. Front Mol Neurosci. 2014;7:1. doi: 10.3389/fnmol.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. A critical review of mTOR inhibitors and epilepsy: from basic science to clinical trials. Expert Rev Neurother. 2013;13(6):657–669. doi: 10.1586/ern.13.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki R, Thind K, Buckmaster PS. Blockade of excitatory synaptogenesis with proximal dendrites of dentate granule cells following rapamycin treatment in a mouse model of temporal lobe epilepsy. J Comp Neurol. 2015;523(2):281–297. doi: 10.1002/cne.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CC, Stegen M, Bernard R, Müller M, Bischofberger J, Veh RW, Haas CA, Wolfart J. Upregulation of inward rectifier K+ (Kir2) channels in dentate gyrus granule cells in temporal lobe epilepsy. J Physiol. 2009;587(Pt 17):4213–4233. doi: 10.1113/jphysiol.2009.170746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Timofeeva O, Nadler JV. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol. 2010;104(6):3293–3304. doi: 10.1152/jn.00663.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63(4):444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29(21):6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Zhang B, Gutmann DH, Gambello MJ, Wong M. Tsc2 gene inactivation causes a more severe epilepsy phenotype than Tsc1 inactivation in a mouse model of tuberous sclerosis complex. Hum Mol Genet. 2011;20(3):445–454. doi: 10.1093/hmg/ddq491. [DOI] [PMC free article] [PubMed] [Google Scholar]