Abstract
Previous research has shown that hyperactivation in ventral medial prefrontal cortex (VmPFC) and rostral anterior cingulate cortex (rACC) and high cortisol to corticotrophin ratio (cort:ACTH ratio) during neutral-relaxed states predict relapse in alcohol dependent (AD) patients. Other studies have shown that VmPFC/rACC deactivation and blunted cortisol release to stress and alcohol cues are predictive of time to relapse and relapse severity. However, no previous study has assessed the relationship between these markers of central and peripheral nervous system dysfunction in AD participants and their potential joint effects on relapse risk. Forty early abstinent, treatment engaged AD patients underwent a laboratory experiment with exposure to neutral, alcohol, and stress cues and a separate functional magnetic resonance imaging (fMRI) scan with similar cue exposure. Neutral-relaxed state cort:ACTH ratio was significantly associated with VmPFC hyperreactivity to neutral-relaxing cues, and also with hypoactivation in response to alcohol and stress cues in AD patients. Basal heart rate, neutral cort:ACTH ratio, and neutral VmPFC hyperreactivty were each associated with risk of relapse. However, abnormal VmPFC activation and elevated cort:ACTH ratio overlap in predicting risk for relapse, and dysfunctional VmPFC response was the sole significant predictor of odds of relapse in a joint model of relapse risk. These findings suggest that the Cort:ACTH ratio may serve as a peripheral marker of VmPFC brain dysfunction while aberrant VmPFC responses needs further evaluation as a potential biomarker of alcohol relapse risk in clinical outcome studies.
Keywords: alcohol dependence, ANS, relapse, fMRI, HPA, relapse, stress
Introduction
It has been established preclinically and clinically that stress is intricately involved in the development of and relapse to alcohol dependence (AD; Koob, 2008; Sinha, 2001). However, our understanding of how peripheral stress responses relate to neurobiological mechanisms associated with the chronic, relapsing nature of AD is limited (Sinha, 2001). Chronic alcohol abuse and stress have been linked to altered hypothalamic pituitary adrenal axis (HPA) dysfunction and altered basal sympathetic activity (Spanagel, Noori, & Heilig 2014). For example, blunted HPA axis reactivity to stress has been associated with increased craving and risk of relapse in early abstinent AD participants (Kiefer et al., 2002, Junghanns et al., 2003). Recently, work from our laboratory has indicated that AD-associated HPA axis dysfunction may also be reflected in high morning fasting basal levels of cortisol and high adrenal sensitivity in the active neutral-relaxing condition, as measured by a high cortisol: adrenocorticotropic hormone ratio (cort:ACTH ratio; Sinha et al., 2011). A higher than normal morning cort:ACTH ratio is indicative of greater cortisol in the blood and therefore the brain. This upregulated adrenal state may prevent an effective cortisol response to acute stressors, in part by altering autonomic nervous system (ANS) function (Sinha et al, 2008). AD-associated ANS dysfunction may be evidenced by elevated basal heart rate and lower heart rate variability (Fox et al., 2009; Thayer et al., 2009). The effects of increased basal heart rate therefore could be to worsen stress regulation, a potentially significant factor influencing alcoholic binge drinking (Sinha et al, 2008).
In the central nervous system (CNS), overlearned associations between negative affect and alcohol consumption are encoded by striatal reward and habit circuits (Heinz et al., 2009). Recent studies suggest that AD and stress each contribute to a corresponding loss of inhibitory regulation of these basal ganglia circuits by the prefrontal cortex (PFC; Ansell et al., 2012, Seo et al., 2013; Volkow et al., 2011). The repeated cycles of alcohol binges and withdrawal characteristic of AD result in increased glutamatergic activity and decreased GABAergic tone throughout the striatal corticolimbic circuits (Breese, Sinha & Heilig 2011), leading to a hyper-excitable, basal neurophysiological state. The combination of increased basal CRH tone and altered glutamatergic signaling results in altered monoamergic transmission during acute withdrawal that continues into protracted withdrawal (Koob, 2010). Clinically, this altered neurobiology is associated with increased anxiety, negative mood, high alcohol craving, sleep difficulties, and poor impulse control (Sinha, 2001; Koob, 2008). It follows that during early abstinence, alcohol cues are especially salient and in combination with stress, negative mood, and anxiety, are among the most commonly cited reasons for relapse in AD (Heinz et al., 2009). Environmental and interoceptive alcohol and stress cues are therefore especially relevant during protracted withdrawal/early abstinence (Heilig et al., 2010) and may also significantly impact relapse risk (Sinha, 2012).
Our group has developed and validated functional magnetic resonance imaging (fMRI) protocols that assess the central nervous system responses to neutral-relaxed, alcohol, and stress cues and laboratory paradigms that assess the peripheral (endocrine and autonomic) responses to neutral-relaxed, alcohol, and stress cues that are relevant in alcohol relapse (Sinha, 2009, 2012). Using these paradigms, we reported hyperactivation in regulatory regions, i.e., ventral medial prefrontal cortex (VmPFC) and rostral anterior cingulate cortex (rACC), during the neutral-relaxing state, and that response is predictive of shortened time to relapse in AD patients (Seo et al., 2013). Conversely, we reported that blunted neural VmPFC/rACC response to stress is predictive of time to relapse and relapse severity (Seo et al., 2013). In addition, in a different study, we reported both high fasting morning cortisol level and high cort: ACTH ratio, a marker of adrenal sensitivity, were predictive of time to relapse (Sinha et al., 2011). These neural and HPA axis responses also correlate with AD patients’ subjective anxiety and negative affect during neutral, alcohol, and stress cue exposure (Sinha et al., 2011). However, no previous research has assessed the relationship between HPA axis and VmPFC dysfunction and their joint effects on subsequent relapse risk during early abstinence.
Much evidence exists to support an association between these dysfunctional peripheral and central stress response in AD. For example, the VmPFC receives extensive visceral input and in turn, regulates autonomic system activity (Thayer et al.,2012). The VmPFC is also involved in the suppression of negative affect and cognitions related to long term consequences (Cutuli, 2014). Furthermore, the rACC works in conjunction with the VmPFC to suppress prepotent (habit-driven) responses to stimuli and rACC activation is necessary for behavioral flexibility (Garavan et al., 2002). The VmPFC modulates stress-related physiological changes via its influence on the hypothalamic paraventricular nucleus (PVN) and HPA activity (Spencer, Buller, & Day, 2005). Increased HPA axis activity and high glucocorticoids may decrease prefrontal inhibition of peripheral and central stress responses via steroid-induced upregulation of excitatory amino acids, which may interact with pro-inflammatory cytokines to decrease synaptic relay efficacy between the VmPFC and PVN (Blaine, Ansell & Sinha, under review). Additionally, both the VmPFC and rACC influence behavioral and emotional coping responses to alcohol and stress and cues via direct synaptic connections to the extended amygdala (Edkin, Edger, & Kalisch 2011). If these synaptic relays are damaged via glucocorticoid-induced excitotoxicity, then self-directed behavior may be replaced by habit and sensory-driven automatic responding (Bechara et al., 2005, Thayer et al., 2009). Thus, the VmPFC and rACC dysfunction may relate to autonomic and HPA axis dysfunction and therefore play a role in decreased stress adaptation and increased alcohol consumption.
While the relationships we reported between adrenal sensitivity and relapse and VmPFC dysfunction and relapse have not yet been independently replicated, we hypothesized a link between central and peripheral dysfunction in AD, given both the similar pattern of relaxed state hyperreactivity in heart rate, HPA axis response, and the VmPFC in AD, and the role of the VmPFC in regulating visceral and emotional coping. Correlation among these peripheral and central stress response measures would support the hypothesis that prefrontal and HPA axis dysfunction, due to stress and AD, interact with and are potentially causally related to one another. Therefore, we performed an independent analysis on a subgroup of AD participants who each participated in two separate studies of neural fMRI response to stress and alcohol cues related to relapse risk (Seo et al., 2013) and in the peripheral stress response related to relapse risk (Sinha et al., 2011) during the same alcohol abstinence and inpatient treatment episode, reported in our previous papers. Given that these AD patients participated in both studies, it permitted the unique opportunity to examine relationships between peripheral and central prefrontal stress responses and their unique and combined contribution to future relapse. We attempted to determine if statistical associations exist between adrenal sensitivity, basal heart rate, and neural activation in medial prefrontal stress regulation regions of the brain in response to neutral-relaxing, alcohol, and stress cues. We hypothesized that elevated basal heart rate, neutral-relaxed state cort:ACTH ratio, and stress induced cort:ACTH ratio would be positively related to VmPFC and rACC activation in the neutral-relaxing condition. We also hypothesized that these measures of HPA axis and autonomic dysfunction would be inversely correlated with VmPFC and rACC activation in response to stress and alcohol cues in early AD abstinence. Finally, we hypothesized that basal heart rate, neutral-relaxed state cort:ACTH ratio, and VmPFC neutral condition hyperreactivity would each independently increase the risk of future relapse in AD participants when combined in predictive models, but that neural dysfunction would be the strongest predictor of shortened time to future relapse risk.
Methods
Participants
For the current report, we included forty recovering AD patients (9 female; aged 18–50 years) who had abstained from alcohol for 4 to 8 weeks (mean [SD]: 34 (7.6) days; range, 29–59 days). All patients were residing in an inpatient treatment research facility and were actively engaged in substance abuse treatment for at least 4 weeks. Individuals currently using opiates, those who ever met criteria for opiate dependence, and those taking prescribed, over the counter, or homeopathic medications for any current psychiatric or medical condition were excluded. Women were excluded from the study if they were using hormonal contraceptives or were in the follicular or early and mid-luteal phases during laboratory and fMRI sessions. All subjects underwent a complete medical evaluation, including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid functions, to ensure good physical health. All study procedures were approved by the Human Investigation Committee of the Yale University School of Medicine and all participants signed a written informed consent. The laboratory and fMRI studies were conducted 1 month after admission to the inpatient unit to allow for normalization of neurobiological changes associated with acute alcohol withdrawal and to assess stress responses and craving in the early recovery period. Abstinence from drug and alcohol consumption during treatment was verified by urinalysis. Four structured smoking breaks were allowed in the inpatient treatment facility and subjects were permitted to smoke 1–2 cigarettes during each break. The smoke breaks were implemented at the start of inpatient admission at least 4 weeks prior to laboratory and fMRI testing, and thus AD patients had a 4 week period to adapt to this schedule of nicotine consumption. This sample made up about half (43%) of the sample reported on in the Sinha et al., 2011 study and the majority (89%) of the sample reported on in the Seo et al., 2013 study (see Figure S1 for a flowchart of the current study sample utilized from those reported in previous reports). The criteria for inclusion in the present study was any AD patient who met the above described inclusion and exclusion criteria and also completed both the fMRI and the laboratory experiment during the 4–8 week period of their inpatient stay, and also completed the 90-day prospective followup period for assessment of relapse risk.
Individualized imagery method and script development
Lab
All participants underwent an imagery script development session (week 3 of admission) during which they were asked to identify a highly stressful event from their own lives (rated by the subject as greater than 8 on a 10-point Likert scale for stressfulness); a personal alcohol cue– related event that involved people, places, and objects related to alcohol use and led to subsequent alcohol use; and a personal neutral, relaxing event. Details of each situation were elicited using methods reviewed and summarized in Sinha, 2009. Scripts were developed using a standardized format, based on specific stimulus and response details of each situation, and then audiotaped for presentation in the laboratory sessions.
MRI
Two additional personalized scripts for each of the stress, alcohol cue and neutral-relaxing conditions were developed prior to the fMRI session according to the procedure described above. Each 2-minute script was audiotaped and presented in random order during the scanning session. The neutral-relaxing script served as an active control state for the stress and alcohol cues because it has been shown not to increase alcohol craving and it controls for the nonspecific effects of the experimental manipulation.
Laboratory Sessions
On a day prior to the laboratory sessions, participants took part in a habituation and imagery training procedure that involved exposure to the stress of intravenous catheter insertion and specific instructions on progressive relaxation and guided imagery participation. This was followed by 3 experimental sessions conducted at 7:45 AM on each of 3 consecutive days where subjects were exposed to 5-minute audiotaped scripts of stress, alcohol-related, and neutral, relaxing scenarios. Only 1 stimulus script was presented per session and condition order was randomized and counterbalanced across participants.
HPA Axis Measures
To assess basal plasma levels of ACTH and cortisol, 4 mL of blood were collected in a heparinized tube that was placed on ice immediately after the blood was drawn. Within 30 minutes of collection, the blood was centrifuged at 4°C and the plasma was pooled and aliquoted for ACTH and cortisol assays. All tubes were stored at −70°C and analyzed using standard radioimmunoassay procedures as reported previously (Sinha et al., 2011). Because cortisol secretion from the adrenal glands occurs in response to circulating ACTH, the cort: ACTH ratio serves as a measure of adrenal sensitivity.
Functional Magnetic Resonance Imaging Acquisition and Procedure
Magnetic resonance imaging data were collected using a 3-T Siemens Trio MRI system equipped with a standard quadrature head coil, using T2*-sensitive gradient-recalled single shot echo planar pulse sequence (parameters described in Seo et al., 2013). Six fMRI trials (2 per condition) were acquired using a block design. The order of the 6 trials were randomized and counterbalanced across participants. Each script was presented only once for a participant, and scripts in the same condition were not presented consecutively. Each trial lasted 5 minutes, including a 1.5-minute quiet baseline period followed by 2.5-minute imagery period (2 minutes of read imagery and 0.5 minutes of quiet imagery) and a 1-minute quiet recovery. During baseline, participants were instructed to stay still in the scanner without engaging in any mental activity. Between each trial, participants were engaged in 2-minute progressive relaxation to normalize any residual anxiety or craving from the prior trial. This technique was mainly focused on relaxing physiological muscle tension in specific muscle groups and did not involve mental relaxation or imagery.
fMRI Statistical Analysis
Individual Subject Level
Functional MRI data were converted from Digital Imaging and Communication in Medicine format to analyze (nifti) format using XMedCon. To achieve steady-state equilibrium between radiofrequency pulsing and relaxation, the first 10 images of each trial were discarded. Images were slice-time corrected using a custom-designed MATLAB program. Motion correction was implemented using Statistical Parametric Mapping version 5 for 3 translational and 3 rotational directions, removing trials with linear motion greater than 1.5mm and a rotation exceeding 2 mm. The recovery period (1 minute) was excluded from the data analysis to prevent carryover effects from the imagery period. Individual-level analysis was conducted using a general linear model on each voxel in the entire brain volume with a task specific regressor (2.5-minute imagery relative to a 1.5-minute baseline) using Yale BioImageSuite30 (http://www.bioimagesuite.org/). To account for potential variability in baseline fMRI signal, drift correction was included in the general linear model. Each trial was normalized against the immediate baseline period preceding the script and then the 2 trials of the same type were averaged. Functional images were spatially smoothed with a 6-mm Gaussian kernel, resulting in normalized beta maps in the acquired space (3.44 mm_3.44 mm_4 mm). To adjust for individual anatomical differences, 3 registrations were performed using the Yale BioImageSuite program: a linear individual registration of raw functional image into a 2-dimensional anatomical image, 2-dimensional to 3-dimensional (1 mm_1mm_1 mm) linear registration, and a nonlinear registration to the reference 3-dimensional Montreal Neurological Institute image.
Group Level: Whole Brain Correlations
Whole brain voxel-wise correlation analyses were run to determine the relationship between blood oxygen level dependent (BOLD) response to stress, neutral, and alcohol cues and laboratory session hormonal measurements at baseline and in response to neutral, alcohol, and stress cues. A family-wise error correction was applied to correct for multiple comparisons based on a MonteCarlo Simulation run in AFNI AlphaSim (p<0.05, cluster size minimum=168 voxels). To account for extreme values, 90% winsorization was applied to the cort: ACTH ratio of two outliers, whose heart rate and ROI Beta values were beyond 2 standard deviations from the mean. Ninety percent winsorization is the process of determining the value of the 95th and 5th percentiles of a given measure and setting any outliers to these values (Dixon and Yuen, 1974).
Prospective Follow up
All patients were followed up with face-to-face interviews conducted on days 14, 30, and 90 post discharge to evaluate relapse outcomes using both urinalysis and breathalyzer samples, in addition to the Form 90 substance use timeline follow-back calendar based interview, as reported in our previous studies (Seo et al., 2013, Sinha et al., 2011).
Relapse Prediction Analyses
Beta values for the VmPFC ROI reported to be predictive of future relapse risk and heart rates responses during fMRI from Seo et al., 2013 for the subgroup of AD patients included in the current study were extracted for each of the neutral imagery-baseline, stress imagery- neutral imagery, and alcohol imagery-neutral imagery contrasts. Similarly, for the same subgroup of AD individuals, their neuroendocrine responses to stress and alcohol craving were extracted from the laboratory experiment that assessed peripheral stress responses and alcohol craving and relationship to relapse (Sinha et al., 2011). Then independent analyses to test the proposed current analyses were conducted using Cox proportional hazard regressions, with time to alcohol relapse as a censoring variable, to investigate whether the VmPFC Beta values, cort: ACTH ratio, and heart rate predicted the time to first alcohol relapse and time to heavy drinking.
Results
Demographic and clinical characteristics of the early abstinent alcohol dependent sample are presented in table 1. The sample was 77.5% male, 66.67% Caucasian, with a mean age of 37.73 +/− 7.75 years, and average IQ of 108.6 +/− 8.5. The participants drank, on average, 19.38+/−9.75 days of the previous month and had consumed 398.46+/−288.72 drinks in the past month. All participants had been regularly drinking regularly for at least 10 years (mean 18.62+/−8.8 years). While the majority of the participants were current smokers (90%), smoking status and nicotine addiction variables were not independently predictive of relapse to alcoholic drinking in previous reports and were thus not included as a covariate in analyses. Additionally, 13 of the 40 participants (32.5%) met diagnostic criteria for past anxiety or mood disorders, past psychiatric history was also not predictive of relapse and was thus also not included as a covariate in these analyses.
Table 1.
Demographic and Baseline Characteristics of Alcohol Dependent Sample (N=40).
| AD Subject Variable | N = 40 |
|---|---|
|
| |
| Age | 37.73 (7.75) |
| Gender, % male | 31(77.5%) |
| Shipley IQ | 108.6(8.5) |
| Race, % Caucasian | 30 (66.67%) |
| Average Years of Alcohol Use | 18.62 (8.80) |
| Average Days of Alcohol Use/Month | 19.38 (9.75) |
| Average Amount of Alcohol Use/Month | 398.46 (288.72) |
| Smoking status, % current smokers | 39 (86.7%) |
| Lifetime Prevalence of PTSD | 4 (8.89%) |
| Lifetime Other Anxiety Disorders | 3 (6.67%) |
| Lifetime Major Depression | 6 (13.33%) |
Note: Data presented as Mean (standard deviation), unless otherwise noted. AD= Alcohol Dependent. IQ=intelligence quotient. PTSD= Posttraumatic Stress Disorder.
Whole brain correlations: heart rate and hormonal measures
The laboratory session neutral-relaxing condition Cort:ACTH ratio was associated with VmPFC responses during neutral, stress and alcohol cues in the fMRI session. The neutral-relaxed state cort: ACTH ratio predicted VmPFC hyperactivation during neutral-relaxed state, r (39) = 0.58, p<0.001 (Figure 1). It was also correlated with hypoactivation in the VmPFC in response to stress imagery, r (39) = −0.33, p<0.001 (Figure 2) and alcohol imagery, r (39) = −0.45, p<0.001 (Figure 3). Thus, adrenal sensitivity in the neutral-relaxing state accounts for more 10–33% of the variance in VmPFC response to neutral-relaxing, alcohol, and stress cues in alcohol dependent participants. Similarly, the cort: ACTH ratio from the laboratory stress condition was associated with hypoactivation in the VmPFC in response to stress imagery, r (39) = −0.32, p<0.001 (Figure 4). However, the cort: ACTH ratio from the laboratory alcohol cue session was not related to VmPFC activation in response to alcohol cues, r (39) = −0.285, p = 0.0789. Finally, elevated basal heart rate during the fMRI significantly predicted hypoactivity in the ACC in response to stress, r (39) = −0.36, p<0.001 (Figure 5), but heart rate during the alcohol imagery was not significantly associated with neural activation in response to alcohol cues, r (39) =−0.114, p= 0.4831. Similarly, heart rate during the stressful imagery was not significantly associated with neural activation in response to stress, r (39) = 0.246, p=0.1267. The significant results are summarized in Table 2.
Figure 1.
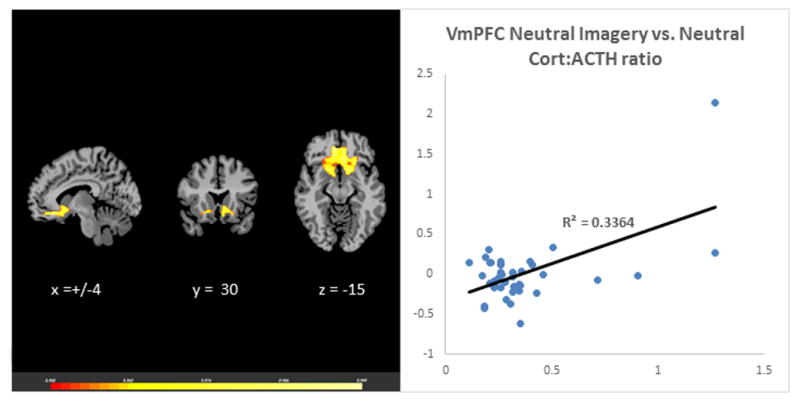
Results of whole brain voxel correlation analysis showing relationship between neutral state cort: ACTH ratio and neutral state VmPFC activation. The cortisol to adrenocorticotropic hormone (cort:ACTH) ratio in response to neutral-relaxing cues during a laboratory session was significantly associated with neural hyperactivation in the Ventromedial Prefrontal Cortex (VmPFC) in response to neutral-relaxing cues during a separate fMRI session, r (39) = 0.58, p<0.001.
Figure 2.
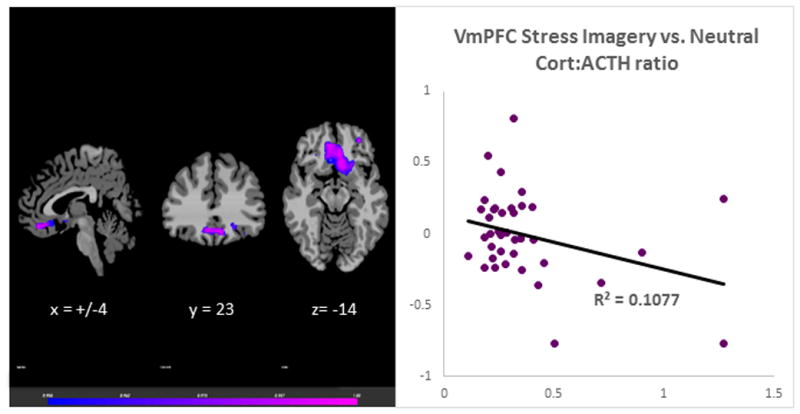
Results of whole brain voxel correlation analysis showing relationship between neutral state cort: ACTH ratio and stress state VmPFC activation The cortisol to adrenocorticotropic hormone (cort: ACTH) ratio in response to neutral-relaxing cues during a laboratory session was significantly associated with neural hyopactivation in the Ventromedial Prefrontal Cortex (VmPFC) in response to stress cues during a separate fMRI session, r (39) = −0.33, p<0.001.
Figure 3.
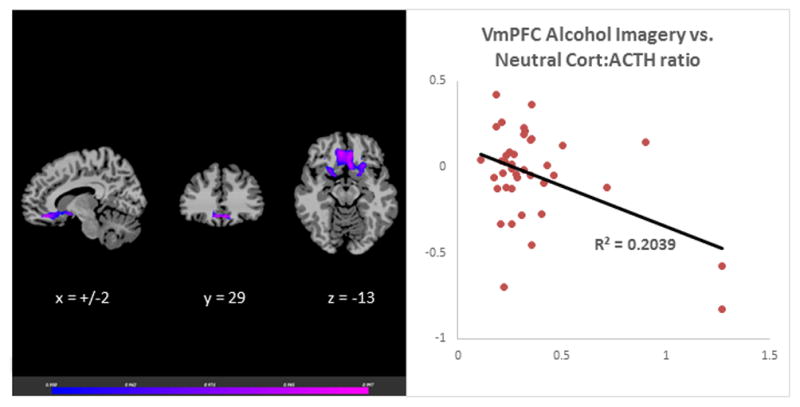
Results of whole brain voxel correlation analysis showing relationship between neutral state cort: ACTH ratio and alcohol cue induced VmPFC activation. The cortisol to adrenocorticotropic hormone (cort:ACTH) ratio in response to neutral-relaxing cues during a laboratory session was significantly associated with neural hyperactivation in the Ventromedial Prefrontal Cortex (VmPFC) in response to alcohol cues during a separate fMRI session, r (39) = −0.45, p<0.001.
Figure 4.
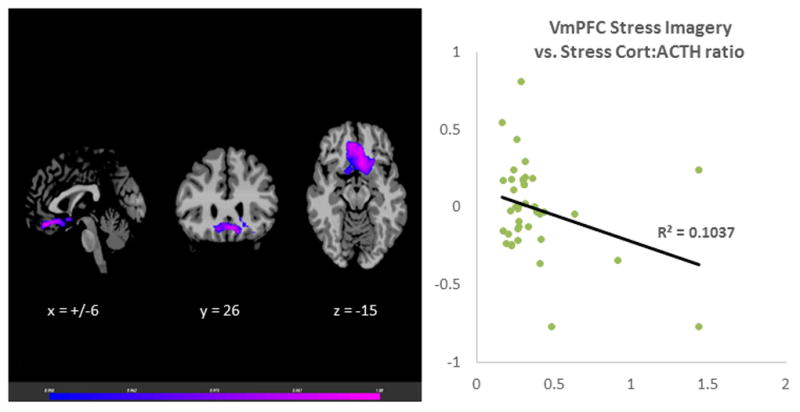
Results of whole brain voxel correlation analysis showing relationship between stress state cort: ACTH ratio and stress state VmPFC activation. The cortisol to adrenocorticotropic hormone (cort:ACTH) ratio in response to stress cues during a laboratory session was significantly associated with neural hyperactivation in the Ventromedial Prefrontal Cortex (VmPFC) in response to stress cues during a separate fMRI session, r (39) = −0.32, p<0.001.
Figure 5.
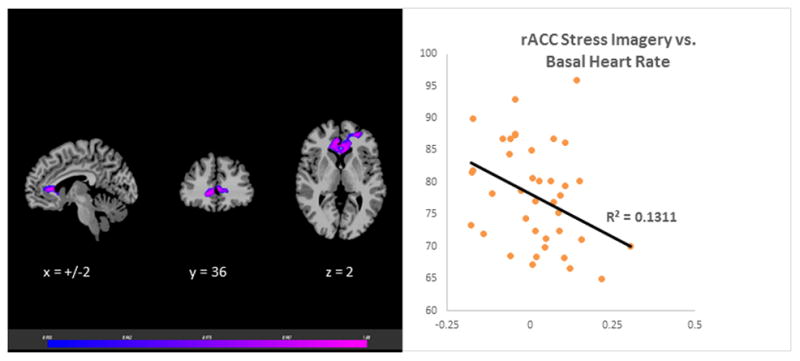
Results of whole brain voxel correlation analysis showing relationship between basal heart rate and stress state VmPFC activation. The Basal heart rate prior to fMRI stress imagery was significantly associated with neural hyperactivation in the Ventromedial Prefrontal Cortex (VmPFC) in response to stress cues during the fMRI session, r (39) = −0.36, p<0.001.
Table 2.
Brain regions showing significant relationships with measures of HPA axis and autonomic nervous system dysfunction.
| HPA/ANS Biomarker | Biomarker Measurement | fMRI condition | Brain Region | Brodmann’s area | Number of Voxels | R2 |
|---|---|---|---|---|---|---|
|
| ||||||
| Cortisol: ACTH Ratio | Laboratory Neutral-relaxing Imagery | Neutral | Bilateral VmPFC | 11 | 320 | 0.336 |
| Stress | Bilateral VmPFC | 25 | 383 | 0.108 | ||
| Alcohol | Bilateral VmPFC | 11 | 316 | 0.204 | ||
|
| ||||||
| Laboratory Stress Imagery | Stress | Bilateral VmPFC | 11 | 314 | 0.104 | |
|
| ||||||
| Heart Rate | fMRI Baseline | Stress | Bilateral rACC | 24 | 271 | 0.131 |
Note: HPA= Hypothalamic Pituitary Adrenal Axis. ANS= Autonomic Nervous System. VmPFC = Ventromedial Prefrontal Cortex. rACC = rostral Anterior Cingulate Cortex. Number of voxels = number of 3 × 3 × 3 mm3 in significant cluster. R2-coefficient of determination. All images were corrected using a whole brain voxel threshold of p <0.05 and minimum cluster size of 168 voxels, all correlations are significant at p<0.001. x, y, z are MNI coordinates of the peak voxel for the reported cluster.
Associations with Future Alcohol Relapse Risk: fMRI heart rate, laboratory session hormonal measures, and neural response to neutral, alcohol, and stress cues
Elevated neutral state cort:ACTH ratio also increased the risk of relapse, hazard ratio=3.015 (95%CI: 1.267–7.173), p=0.0126. Elevated basal heart rate was also associated with relapse, hazard ratio=0.953 (95%CI: 0.912–0.997), p=0.0358. However, in a combined predictive model of peripheral markers of relapse, only the laboratory neutral condition cort:ACTH ratio remained a significant predictor of relapse, hazard ratio= 2.521(95%CI: 1.044–6.091), p=0.0399. As a sole predictor, VmPFC hyperactivation in the neutral-relaxing state was the most influential in increasing the odds of relapse, HR=9.739(95%CI: 2.494–38.028), p=0.0011. When it was combined with the cort:ACTH ratio to assess relapse, the neutral state VmPFC became the sole significant predictor of increase relapse risk, hazard ratio=6.293 (95%CI: 1.341–29.531), p=0.0197, suggesting that the effect of adrenal sensitivity on relapse is significantly mediated by neutral state VmPFC hyperactivation. The results are summarized in Table 3.
Table 3.
Cox proportional hazard regression survival analysis predicting days to heavy alcohol use with alcohol relapse as a censoring variable. Neutral-relaxed state adrenal sensitivity, as indicated by the cortisol: adrenocorticotropic hormone (cort: ACTH) ratio, increases risk of relapse, with a Hazard Ratio (HR) of 2.521 (p=0.0399, 95% confidence interval (CI) = 1.044–6.091). (b). The cort: ACTH ratio accounts for one third of the variance in Ventromedial Prefrontal Cortex (VmPFC) neutral state hyperactivation (R2 = 0.3364, p<0.001) and no longer significantly increases relapse risk when combined with VmPFC neutral state hyperactivation in a predictive model (HR= 1.875, p=0.2956, 95% CI=0.577–6.092). In this combined model, the neutral state VmPFC hyperactivation mediates the effect of high neutral-relaxed state cort: ACTH ratio on relapse risk, (HR=6.293, p=0.0197, 95% CI=1.341–29.531). N.B. We have previously demonstrated that neutral state VmPFC hyperactivation significantly increases risk of relapse, (HR= 9.739, p= 0.0011, 95% CI= 2.494–38.028) in Seo et al., 2013.
| Analysis of Maximum Likelihood Estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | df | Parameter Estimate | Standard Error | Chi-Square | P | Hazard Ratio | 95% Hazard Ratio Confidence Limits | ||
| Model 1 | Neutral Cortisol: ACTH Ratio | 1 | 0.92479 | 0.45005 | 4.2224 | 0.0399 | 2.521 | 1.044 | 6.091 |
| Basal Heart Rate | 1 | −0.04224 | 0.02323 | 3.3073 | 0.069 | 0.959 | 0.916 | 1.003 | |
| Model 2 | Neutral Cortisol: ACTH Ratio | 1 | 0.62876 | 0.60111 | 1.0941 | 0.2956 | 1.875 | 0.577 | 6.092 |
| Neutral VmPFC Hyperreactivity | 1 | 1.83949 | 0.78877 | 5.4386 | 0.0197 | 6.293 | 1.341 | 29.531 | |
Note: df= degrees of freedom, Parameter Estimate= Beta value in equation of the line, p= p value of hazard ratio.
Discussion
In this independent analysis of the relationship between separately assessed peripheral stress and fMRI prefrontal responses, we found that neutral-relaxed state cort:ACTH ratio, as well as elevated basal heart rate, an indication of ANS dysfunction, was significantly associated with aberrant neural activity in the VmPFC and rACC to neutral-relaxing cues, alcohol cues, and stressful cues in AD participants. Remarkably, the difference in neutral-relaxed state cort:ACTH ratio values between participants accounts for 30% of the difference between participants in both VmPFC hyperreactivity under neutral-relaxing conditions and 20% of the difference between participants in VmPFC hypoactivation in response to alcohol cues. Furthermore, the neutral-relaxed state cort:ACTH ratio, along with basal heart rate, and the cort:ACTH ratio under stressful conditions, accounted for a significant portion of the variance in VmPFC hypoactivity during stressful conditions. Furthermore and contrary to our hypothesis, the neutral-relaxed state cort:ACTH ratio did not remain independently predictive of shortened time to relapse when combined in a model with VmPFC neutral state hyperreactivity. These findings suggest the influence of HPA axis dysfunction on increasing relapse risk is mediated by VmPFC dysfunction. While previous research in humans has suggested alcohol-related dysfunction in autonomic, HPA axis, and neural prefrontal responses may each interact to predict alcohol intake and relapse (Seo et al., 2013, Sinha et al., 2011, Thayer et al., 2009), to our knowledge, this is the first study to directly assess associations across these multilevel stress and homeostatic responses in well-controlled experiments in the same sample of early abstinent AD participants during a single inpatient treatment episode.
Our results suggest that just as the clinical manifestation of AD is a vicious cycle of alternating periods of recovery and relapse that worsen over time, VmPFC dysfunction, HPA dysfunction, and ANS dysfunction contribute to and worsen one another, leading to a sensitized subcortical limbic-striatal network that is manifested in elevated morning fasting blood cortisol levels, and greater sympathetic arousal. Heightened physiological arousal in the basal state is associated with a blunted response to stress and also a decreased influence of cortical executive control over striatal and limbic circuits (Breese, Sinha, & Heilig, 2011). This may then result in increased craving and a greater susceptibility to habit-based coping, i.e., a resumption of drinking behavior (Heinz et al., 2009). Continued consumption of alcohol then may worsen autonomic and HPA axis dysfunction, making the next recovery attempt more difficult by weakening the regulatory influence of the VmPFC on persistently sensitized subcortical striatal and limbic circuits (Sullivan & Pfefferbaum, 2014).
Increased cortisol and sympathetic dominance in the autonomic nervous system have direct implications for the clinical experience of protracted withdrawal, acute stress, and environmental and interoceptive alcohol cues (Sinha, 2013). Behaviorally, this altered neurobiological state results in susceptibility to cue reactivity, anxious rumination, poor decision making, and a reliance on habit-based responding (Heilig & Koob 2007). Specifically, the HPA axis dysregulation appears to influence an altered basal state and contribute to an inability to downregulate corticolimbic neural activity in response to relaxation. This may be related to the high morning basal levels of cortisol (Sinha et al., 2008), possibly due to adrenal sensitization in response to repeated cycles of binge intoxication and withdrawal, which are associated with increased basal stress levels and higher sympathetic arousal (Fox et al., 2009). Alternatively, the adrenal sensitization might be secondary to a dysfunction in the ACTH endocrine releasing cells of the pituitary, or the CRH neurosecretory cells of the hypothalamus (Adinoff et al., 2003).
Regardless of the originating source, higher morning circulating cortisol can induce excitotoxicity in the behavior and emotional regulation centers of the brain, i.e., the VmPFC (Hunter, 2012; McEwen & Morrison, 2014). This excitotoxicity, in combination with associated neuroinflammatory processes, may lead to loss of gray matter volume and functional integrity in the VmPFC (Blaine, Ansell, & Sinha, under review). Thus, the VmPFC, the seat of visceral motor regulation and emotion regulation, exerts less control over the HPA axis and autonomic reactivity (Navqi, Tranel, & Bechara 2006). For example, the autonomic dysfunction indicated by increased basal heart rate in AD may be a consequence of VmPFC hypoactivation during stress, an inability to exert executive control over limbic and striatal regions, and the associated emotional dysregulation (Critchley & Harrison, 2013). However, resting heart rate is not only related to sympathetic nervous system dysfunction, but also a dysregulation of parasympathetic activity (Van Eden & Buijs, 2000). This imbalance between the functional branches of the motor division of the peripheral nervous system may, in turn, influence neural activity during acutely stressful states. Given the multitude of factors that influence resting heart rate, it is not surprising that it was not independently predictive of shorted time to relapse in our analyses. Further study on the exact nature of the interactions between VmPFC activity, HPA axis dysregulation, and autonomic disturbances are necessary to elucidate their exact roles in relapse to alcoholic drinking.
Finally, there are a few important limitations of this study. First, the cort:ACTH ratio were not measured during the fMRI session, but rather assessed in a separate laboratory experiment. However, the finding that morning fasting adrenal sensitivity assessed during a laboratory experimental session involving neutral and stress provocation correlated with neural response to similar cue exposure days later in an fMRI experiment suggests there is an enduring relationship between HPA axis sensitization and the inability to regulate basal stress reactivity or to mount an adequate neural prefrontal response to stress and alcohol cues in recovering AD individuals. Second, although elevated heart rate is a biomarker of autonomic dysregulation, lower hear rate variability has been shown to be an important indicator of stress reactivity and dysregulation (Thayer et al., 2012) and the current study did not record heart rate variability during the experiments. Third, given the small sample size we were unable to examine sex differences in these biomarkers of AD. Etiological differences in the course of AD between men and women have been reported, and thus endocrine and neural mechanisms that underlie relapse may differ between the sexes. Additionally, 90% of our sample were current smokers and the potential contribution of nicotine dependence to adrenal sensitivity and/or VmPFC activity was not able to be distinguished in this analysis. Thus, these findings need to replicated and extended in a larger sample. Despite these limitations, to the best of our knowledge, this is the first report of an association between adrenal sensitivity, basal heart rate, and neural response to neutral, alcohol, and stress cues in early abstinent AD patients. The findings suggest clear interactions between the neural response to neutral-relaxing, alcohol, and stress cues and HPA axis and autonomic dysregulation in AD and in their role in alcohol relapse risk. The exact nature and directionality of these interactions may differ during acute and chronic alcohol intake and depend on both context and disease progression (Blaine et al., in press).
These limitations notwithstanding, neutral-relaxed state cort: ACTH ratio appears to be a significant biomarker of VmPFC dysfunction in AD, which itself is a neuromarker that might be used in future studies of AD risk and relapse. Our findings are consistent with previous reports that neuromarkers tend to provide more accurate prognosis than traditional behavioral measures (Gabrieli, Ghosh and Whitfield-Gabrieli, 2015). Treatment providers can benefit from using peripheral biomarkers, including altered adrenal sensitivity, as proxy for neural dysfunction to identify patients at greater risk of relapse. If accessible, fMRI of VmPFC hyperreactivity in the neutral-relaxing state might serve as a more sensitive and specific predictor of relapse risk. Furthermore, normalization of VmPFC dysfunction may be a useful target in medication or behavioral therapy trials. The development of pharmacotherapies for AUDs has sometimes proceeded directly from promising preclinical results to randomized clinical trials, leading to disappointing and conflicting results. For example, medications that directly affect HPA axis functioning, such as CRH antagonists, seem to have little effect on human drinking behavior, despite robust effects in animal models (Kwako et al., 2015). This might be due to the multiple interactions between the HPA axis, the SAM system, the HPG axis, and the PFC in AUDs. We suggest that intermediate human laboratory and neuroimaging paradigms should be used to test the effects of novel therapeutics on alcohol-induced stress system alterations that influence craving and relapse. Specifically, there may be benefit in future a priori tests of these hypotheses to be conducted in simultaneously collected endocrine, autonomic, and neural measures at repeated time points in early abstinence. For example, endocrine and autonomic data could be collected during tests of neurofeedback aimed at reducing craving. As disrupted prefrontal control is emerging as a key aspect of alcohol craving and relapse, multimodal studies core stress systems markers and related psychological variables (affect and behavior regulation) may elucidate novel therapeutic mechanisms.
Supplementary Material
Figure S1. Flowchart showing sample recruitment for the current independent analysis from AD patients who participated in previously reported studies. Inclusion required participation in each of the following procedures and assessments during the same abstinence and treatment episode: stress neuroendocrine laboratory experiment, brain imaging experiment, and prospective longitudinal 90-day follow-up assessment after discharge, in addition to complete, statistically normal data for all measurements.
Acknowledgments
This work was supported by UL1-DE019586, PL1-DA24859, the NIH Common Fund, and R01 013892-11, and T32 DA022975. We also acknowledge the support of the Yale Center for Clinical Investigation (Yale CTSA. U01-RR024139 and the contributions of the staff at the Connecticut Mental Health Center, supported by the State of Connecticut.
Footnotes
Author contributions: DS and RS were responsible for the study concept and design. SKB performed data analysis. DS and RS assisted in interpretation of findings. SKB drafted the manuscript. DS and RS provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcoholism: Clinical and Experimental Research. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biological psychiatry. 2012;72(1):57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Ansell EB, Sinha R. Cumulative Adversity, Brain Structure, and Risk of Psychiatric Illness. Neuroscience & Biobehavioral Reviews under review. [Google Scholar]
- Blaine SK, Milivojevic V, Fox HC, Sinha R. Alcohol Effects on Stress Pathways: Impact on Craving and Relapse Risk. Canadian Journal of Psychiatry. doi: 10.1177/0706743716632512. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacology & therapeutics. 2011;129(2):149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Harrison NA. Visceral influences on brain and behavior. Neuron. 2013;77(4):624–638. doi: 10.1016/j.neuron.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Cutuli D. Cognitive reappraisal and expressive suppression strategies role in the emotion regulation: an overview on their modulatory effects and neural correlates. Frontiers in systems neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ, Yuen KK. Trimming and winsorization: A review. Statistische Hefte. 1974;15(2–3):157–170. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KIA, Siedlarz KM, Bergquist K, Anderson G, Kreek MJ, Sinha R. Sex-specific dissociations in autonomic and HPA responses to stress and cues in alcohol-dependent patients with cocaine abuse. Alcohol and alcoholism. 2009;44(6):575–585. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Ghosh SS, Whitfield-Gabrieli S. Prediction as a Humanitarian and Pragmatic Contribution from Human Cognitive Neuroscience. Neuron. 2015;85(1):11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RAP, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17(4):1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in neurosciences. 2007;30(8):399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. REVIEW: Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction biology. 2010;15(2):169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addiction biology. 2009;14(1):108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RG. Epigenetic effects of stress and corticosteroids in the brain. Frontiers in cellular neuroscience. 2012;6 doi: 10.3389/fncel.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, Blank S, Backhaus J. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol and Alcoholism. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, Wiedemann K. Alcohol self-administration, craving and HPA-axis activity: an intriguing relationship. Psychopharmacology. 2002;164:239–240. doi: 10.1007/s00213-002-1255-3. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain research. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Spagnolo PA, Schwandt ML, Thorsell A, George DT, Momenan R, Rio DE, Huestis M, Anizan S, Concheiro M, Sinha R, Heilig M. The Corticotropin Releasing Hormone-1 (CRH1) Receptor Antagonist Pexacerfont in Alcohol Dependence: A Randomized Controlled Experimental Medicine Study. Neuropsychopharmacology. 2015;40(5):1053–1063. doi: 10.1038/npp.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison J. Brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2014;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N, Tranel D, Bechara A. Visceral and decision-making functions of the ventromedial prefrontal cortex. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. 2006. pp. 325–353. [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. Endocrine reviews. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology. 2013;39(3):670–680. doi: 10.1038/npp.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2008;34(5):1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addiction biology. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KIA, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress-and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of general psychiatry. 2011;68(9):942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Behavioral Neurobiology of Alcohol Addiction. Springer; Berlin Heidelberg: 2013. Modeling relapse situations in the human laboratory; pp. 379–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: animal studies and clinical significance. Trends in neurosciences. 2014;37(4):219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Buller KM, Day TA. Medial prefrontal cortex control of the paraventricular hypothalamic nucleus response to psychological stress: possible role of the bed nucleus of the stria terminalis. J Comp Neurol. 2005;481(4):363–376. doi: 10.1002/cne.20376. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. The neurobiology of alcohol craving and relapse. Alcohol and the Nervous System: Handbook of Clinical Neurology. 2014;125:355. doi: 10.1016/B978-0-444-62619-6.00021-5. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews. 2012;36(2):747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Buijs RM. Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Progress in brain research. 2000;126:49–62. doi: 10.1016/S0079-6123(00)26006-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences. 2011;108(37):15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart showing sample recruitment for the current independent analysis from AD patients who participated in previously reported studies. Inclusion required participation in each of the following procedures and assessments during the same abstinence and treatment episode: stress neuroendocrine laboratory experiment, brain imaging experiment, and prospective longitudinal 90-day follow-up assessment after discharge, in addition to complete, statistically normal data for all measurements.


