Abstract
Background
The NHANES study identified several pollens and cat dander among the most common allergens that induce allergic sensitization and allergic diseases. We recently reported that ragweed pollen extract (RWPE) requires TLR4 to stimulate CXCL-mediated innate neutrophilic inflammation that facilitates allergic sensitization and airway inflammation. Myeloid differentiation protein-2 (MD2) is a TLR4 coreceptor, but its role in pollen and cat dander-induced innate and allergic inflammation has not been critically evaluated.
Objective
To elucidate the role of MD2 in inducing pollen and cat dander-induced innate and allergic airway inflammation.
Methods
TCMNull (TLR4Null, CD14Null, MD2Null), TLR4Hi, TCMHi cells and human bronchial epithelial cells with siRNA-induced downregulation of MD2 were stimulated with RWPE, other pollen allergic extracts, or cat dander extract (CDE), and activation of NF-κB and/or secretion of the NF-κB-dependent CXCL8 were quantified. Wild type (WT) mice or mice with siRNA knockdown of lung MD2 were challenged intranasally with RWPE or CDE, and innate and allergic inflammation were quantified.
Results
RWPE stimulated MD2-dependent NF-κB activation and CXCL secretion. Likewise, Bermuda, rye, timothy, pigweed, Russian thistle, cottonwood, walnut and CDE stimulated MD2-dependent CXCL secretion. RWPE and CDE challenge induced MD2-dependent, CD14-independent innate neutrophil recruitment. RWPE induced MD2-dependent allergic sensitization and airway inflammation.
Conclusions
MD2 plays an important role in induction of allergic sensitization to cat dander and common pollens relevant to human allergic diseases.
Keywords: Allergic inflammation, Antigen, Cat dander, Pollen, MD2, Neutrophil, NF-κB, Ragweed, TLR4
Introduction
Pollens and cat dander are major causes of allergic airway disorders like rhinitis and asthma.1-3 The NHANES study identified several pollens and cat dander among the most common allergens that induce allergic sensitization and allergic diseases.2 The role of adaptive immune responses in induction of allergic diseases by allergens has been extensively studied. However, relatively little is known about innate immune receptors that contribute to allergic sensitization. Recent studies have identified a role of innate responses mediated by Toll-like receptor 4 (TLR4) in pollen-induced allergic inflammation.4-6 One study reported that short ragweed pollen induces allergic conjunctivitis by stimulating TLR4-dependent TSLP (Thymic stromal lymphopoietin) secretion in sensitized mice.4 Another study reported that the adaptive allergic immune responses to birch pollen extract was reduced in Tlr4 KO mice, thus implying a role of TLR4 in induction of allergic immune responses.5 We recently reported that ragweed pollen extract (RWPE) challenge induces CXCR2 and TLR4-dependent innate recruitment of activated neutrophils to the lungs.6 We reported that deletion of TLR4 abrogated RWPE-induced allergic sensitization and allergic inflammation.6 We further demonstrated that passive transfer of neutrophils to Tlr4KO recipient mice reconstitutes allergic sensitization and allergic airway inflammation in Tlr4KO mice.6
Myeloid differentiation protein-2 (MD2) is a 160 amino acids glycoprotein.7 MD2 directly binds lipopolysaccharide (LPS) presented by CD14,8, 9 and stimulates TLR4 homodimerization induced canonical inflammatory signaling.10 MD2 belongs to the ML (MD-2-related lipid-recognition) domain superfamily that also includes mite allergens Der p 2 and Der f 2 in addition to MD-1, GM2 activator protein, Niemann-Pick C2 protein (Npc2), and phosphatidylinositol phosphatidylglycerol transfer protein (PG/PI-TP). The structural and functional mimicry of MD2 by Der p 2 stimulates TLR4.11 However the role of MD2 in pollen-induced innate and allergic airway inflammatory responses has not been reported. We hypothesized that because RWPE requires TLR4 to induce innate inflammation-mediated allergic sensitization,6 it might also require MD2 to mediate these effects. We further hypothesized that this pathway is shared by other common allergens relevant to human allergic diseases. 2, 3
Materials and Methods
Mice
Eight- to 12-wk-old male wild type (WT) C57BL/10SNJ mice, Tlr4 knock out (KO) mice (C57BL/10ScNJ), WT mice (C57BL/6J), and Cd14KO mice (B6.129S-Cd14tm1Frm/J) were purchased from (Jackson Laboratory, Bar Harbor, ME) for these studies. The mice were maintained in a pathogen-free environment at the University of Texas Medical Branch (Galveston, TX). Animal experiments were performed according to the NIH Guide and were approved by the UTMB Animal Care and Use Committee.
Allergenic extracts
We have previously reported that lyophilized RWPE containing very low amount of endotoxin (< 0.1pg LPS /1μg allergen protein, Greer Laboratories, NC) induces a TLR4-dependent innate neutrophilic inflammation and allergic sensitization.6 For the present study we purchased lyophilized RWPE, Bermuda grass, timothy grass, rye grass, firebush, pigweed, Russian thistle, black walnut, eastern cottonwood, mountain cedar, and cat dander extract (CDE) from Greer Labs (Lenoir, NC). Like our previous study,6 all tested allergenic extracts had very low (< 0.1pg LPS /1μg allergen protein) using LAL chromogenic endotoxin quantitation kit (Thermo Scientific, Hudson, NH).
Protocols used for animal studies
Mice were sedated with low dose intraperitoneal xylazine-ketamine anesthetic mixture for intranasal sensitization, or challenge and sacrificed by lethal anesthetic mixture overdose.12
• Single-challenge model (Fig. S1A)
WT mice were intranasally challenged with a single dose of RWPE or CDE (100 μg/60μl), and sacrificed after 16 hours. In additional experiments, one hour before RWPE challenge, WT mice were treated with or without intranasal administration of an NF-κB inhibitor that selectively irreversibly blocks IκBα phosphorylation BAY 11-7082 (Calbiochem; 10mg/kg body weight) 13, or NEMO-Binding Domain Binding Peptide (Calbiochem; 25μg/mouse).14 These treated mice were challenged with RWPE and sacrificed as described above.
• Single-challenge model after single siRNA treatment (Fig. S1B)
Two HPLC-purified predesigned siRNA against myeloid differentiation protein-2 (Md2) (catalog no: s69441; Ambion Silencer) and Tlr4 (catalog no: s75206; Ambion Silencer), and control nonspecific siRNA oligos (catalog no: 12935-100; stealth RNAi Negative Control duplexes; Ambion) were diluted in 5% glucose mixed with in vivo JET-PEI (Polyplus -transfection, New York, NY). We selected intravenous route of siRNA administration because it has been shown suppress specific gene expression by 80% in airway epithelial cells, and has minimal toxicity unlike intranasal administration.15 40μg of each siRNA was administered in WT mice on day 0. The mice were challenged intranasally with 100 μg of RWPE or CDE on day 2, and sacrificed 16 hours after challenge.
• Repeated-challenge allergy model after repeated siRNA treatment (Fig. S1C)
WT mice were administred control siRNA oligos or siRNA against MD2 as described above on day -2, 1, and 9. These mice were administered 5 intranasal doses of RWPE (100 μg/60μl) on days 0, 1, 2, 3, 4, and 11 to mimic chronic exposure of humans to RWPE6, 16 and sacrificed on day 14 as described above.
Processing of mouse fluid and tissue samples
Total and differential bronchoalveolar lavage fluid (BALF) cells counts were performed.16 Lungs were perfused and fixed with Zn fixative (BD Biosciences, San Jose, CA), and sections stained with periodic acid-Schiff (PAS) for mucus staining.
qRT-PCR of mouse lung mRNA
RNA from mouse lung tissue was reverse-transcribed, and amplified using real-time PCR in ABI 7000 (Applied Biosystems, Foster City, CA). The primer sequences of MD2 were as follows: forward, 5'-AGCTCTGCAAAAAGAATAGTCATC-3', reverse, 5'-ATAAGACTGAGGGGAACCAATG-3'; this primer were obtained from Integrated DNA Technologies (Coralville, IA).
Mucin production
Mucin production was assessed by two investigators who were blinded to the treatment groups using a modification of a method reported6, 16 on a subjective scale of 0, 1, 2, 3, and 4 corresponding to none, mild, moderate, marked, or severe mucin deposition, respectively. The data were expressed as mean of score recorded by two blinded investigators.6, 16
Measurement of IL-5, IL-13, IL-33, and TSLP in BALF
The BALF from the WT mice in repeated-challenge allergy model were assayed for IL-5, IL-13, IL-33, and TSLP using a DuoSet ELISA development kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Measurement of RWPE-specific serum IgE
RWPE-specific IgE was measured using a previously described method.6, 16 Briefly, 96-well plates were coated with 100ug/ml of RWPE protein overnight. After thrice washing, the plates were blocked with Sea Block blocking buffer (Pierce Biotechnology, Inc, Rockford, IL). Diluted sera from the mice were added to the plates and incubated overnight. After washing, the plates were incubated with biotin-conjugated rat IgE (clone R35-72; BD Biosciences, San Jose, CA) for 2 hours at room temperature, washed and incubated with avidin-conjugated alkaline phosphatase for 45 minutes at 4°C. After washing, fluorometric values for each well were measured after addition of AttoPhos substrate solution (Promega, Madison, WI).
Studies involving HEK 293 cell lines and HBEC
Three HEK 293 cell lines TCMNull (TLR4Null, CD14Null, MD2Null), TLR4Hi (TLR4Hi, CD14Null, MD2Null) and TCMHi (TLR4Hi, CD14Hi, MD2Hi) (InvivoGen, San Diego, CA) were used. In some experiments, hTERT immortalized normal human bronchial epithelial cells (HBEC) were used as previously described.17
• NF-κB dual luciferase reporter assays
TCMNull, TLR4Hi, and TCMHi cells were transiently transfected with pGL4.32[luc2P/NF-κB-RE/Hygro] (Promega, Madison, WI) vector containing multiple NF-κB response elements (NF-κB-RE) that drives transcription of the luciferase reporter gene luc2P (Photinus pyralis) and pRL-SV40 Renilla Luciferase Reporter Vector (Promega, Madison, WI) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). 24 hours after transfection, the cells were stimulated with RWPE or PBS for 6 hours, and cell lysates were assessed with the Dual-Luciferase™ Reporter Assay System (Promega, Madison, WI).
• Transfection with MD2, or sham transfection
In some experiments, TLR4Hi cells were transfected with 2μg of plasmid encoding MD2 (TLR4Hi-MD2) (plasmid 13028, Addgene, Cambridge, MA) using Lipofectamine 2000, or sham transfected with Lipofectamine 2000 (TLR4Hi-ST). 24 hours post transfection, the cells were stimulated with pollen allergenic extracts or CDE for 18 hours, and CXCL8 in cell supernatant was quantified.
• Transfection with siRNA against MD2
HBEC were transfected with siRNA against human MD2 siRNA (Life Technologies, Carlsbad, CA) with Lipofectamine 2000 or sham transfected with Lipofectamine 2000.
• Measurement of CXCL8 in cell supernatants
TCMNull, TLR4Hi, TCMHi, TLR4Hi-ST, or TLR4Hi-MD2 cells were stimulated with RWPE, Bermuda grass, rye grass, pigweed, mountain cedar, cat dander, LPS, or Amb a1 at 100μg/ml for 18 hours. In some experiments, HBEC, HBEC transfected with siRNA against MD2, or HBEC sham transfected were starved for 24 hours, and stimulated with RWPE, Bermuda grass, rye grass, timothy grass, pigweed, Russian thistle, cottonwood, walnut, and cat dander allergenic extracts. Cell supernatants were assayed for CXCL8.
• Determination of LPS binding analysis by FACS
In some experiments TLR4Hi, TLR4Hi-MD2, TLR4Hi-ST or TCMHi cells were incubated with 1 μg/ml LPS-Alexa-568 for 30 min, and cell-bound LPS-Alexa-568 was detected by flow cytometry (BD Biosciences, San Jose, CA).
• BODIPY-RWPE conjugate preparation and flow cytometry
RWPE was labeled with BODIPY® FL STP Ester (B10006, Life technologies) and excess unbound dye was removed by column purification. TCMNull, TLR4Hi, TCMHi, TLR4Hi-ST, and TLR4Hi-MD2 cells were incubated with 100μg /ml BODIPY-RWPE conjugates for 30 mins on ice, washed, then subjected to flow cytometric analysis.
Statistical Analysis
Results of the study are presented as means ± SEM. Difference between two groups were analyzed by unpaired t-test. Multiple comparisons were analyzed by ANOVA. The software package GraphPad Prism 6 (GraphPad Software, San Diego, CA) was used for all data analyses and preparation of graphs. All statistical analyses considered data significant at p < 0.05. In all figures, data is expressed as means ± SEM. The p values are designated with the following asterisk: * = P < .05, ** = P < .01, *** = P < .001, **** = P < .0001.
Results
RWPE activates NF-κB and stimulates secretion of CXCL8 in TCMHi cells
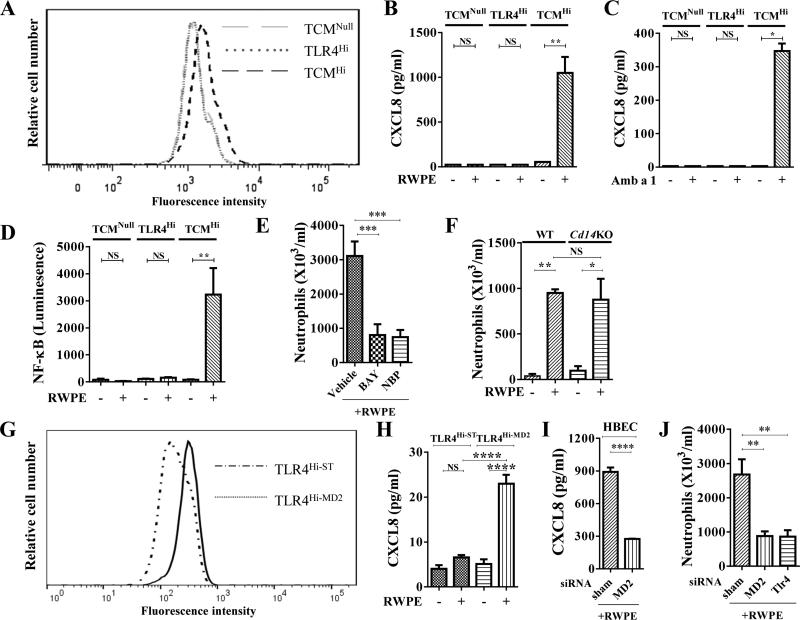
We have recently shown that a single RWPE challenge in naïve mice induces TLR4 and CXCL-mediated neutrophil recruitment, and this neutrophil recruitment is critical for induction of allergic sensitization and allergic airway inflammation.6 Here we hypothesized that RWPE may require CD14 and/or MD2 in addition to TLR4 to stimulate cells. To test this hypothesis, we initially selected HEK 293 cells for our studies because their inherent property of lacking TLR4, CD14 or MD2 has been extensively utilized in the literature to define TLR4 innate responses.18, 19 Three types of HEK 293 cells, TCMNull, TLR4Hi, and TCMHi were cultured with BODIPY-labeled RWPE for 30 mins and subjected to FACs analysis. Compared to TCMNull and TLR4Hi cells (<3%), TCMHi cells demonstrated higher BODIPY-RWPE staining (12.9%) (Fig. 1A). We have recently shown that RWPE challenge induces TLR4-dependent CXCL chemokine secretion.6 Building on this published observation, taken together with our observation in the current study that TCMHi cells demonstrate higher BODIPY-RWPE staining, we hypothesized that RWPE should increase CXCL chemokines only in TCMHi cells. Consistent with our hypothesis, RWPE induced CXCL8 secretion from TCMHi but not from TCMNull cells or TLR4Hi cells (Fig. 1B), validating our hypothesis. These results also suggest that RWPE requires CD14 and/or MD2 in addition to TLR4 to induce CXCL chemokine secretion. Amb a 1 is a major allergen in RWPE; like RWPE, it also induced CXCL8 secretion only in TCMHi cells (Fig. 1C), indicating that a single protein in RWPE can also stimulate this pathway. Since TLR4 signaling stimulates NF-κB activation, next we examined whether RWPE requires TLR4 along with either CD14 and/or MD2 to activate NF-κB. TCMNull, TLR4Hi cells, and TCMHi, were thus transfected with an NF-κB luciferase construct. Stimulation with RWPE induced NF-κB in TCMHi but not TCMNull or TLR4Hi cells (Fig. 1D). These studies suggest that RWPE requires CD14 and/or MD2 in addition to TLR4 to stimulate NF-κB and induce CXCL chemokine secretion. To elicit the in vivo role of NF-κB activation in mounting RWPE-induced innate inflammatory response in the lungs, IκB kinase inhibitor, BAY 11-7082 or NEMO-binding domain binding peptide were administered to mice prior to intranasal RWPE instillation (Fig. S1A). Both NF-κB inhibitors decreased RWPE-challenge induced neutrophil recruitment into the airways (Fig. 1E), indicating a critical role of the NF-κB pathway in RWPE-induced innate inflammatory responses.
Fig. 1. RWPE requires MD2 to induce NF-κB activation, CXCL8 secretion, and recruitment of neutrophils.
(A) FACS of HEK cells cultured with BODIPY-RWPE. (B, C) RWPE (B) and Amb a 1 (C) induce CXCL8 secretion from TCMHi cells. (D) RWPE activates NF-κB in TCMHi cells. (E, F) Effect of NF-κB inhibition (E) and CD14 (F) on RWPE- innate inflammation. (G) FACS of HEK cells cultured with BODIPY-RWPE. (H) RWPE induces CXCL8 secretion from TLR4Hi-MD2 cells. (I) Md2 siRNA suppresses RWPE-induced CXCL8 secretion from HBEC. (J) Tlr4 or Md2 siRNAs suppress RWPE- innate inflammation.
MD2 is the critical co-receptor of TLR4 that induces RWPE-mediated innate inflammatory responses
To elicit the role of CD14 in RWPE-induced innate inflammation, a single-challenge model with RWPE was performed in WT and Cd14KO mice, and mice were sacrificed 16 hours later. RWPE challenge induced the same level of neutrophil recruitment in the BALF from Cd14KO as WT mice (Fig. 1F), indicating that CD14 is not an essential co-receptor for TLR4 to induce RWPE-induced innate response in the lungs. To validate the role of MD2 in pollen-induced CXCL chemokine synthesis, TLR4Hi cells were sham–transfected (TLR4Hi-ST) or transfected with a plasmid to overexpress MD2 (TLR4Hi-MD2). FACs analysis of TLR4Hi-ST and TLR4Hi-MD2 cultured with BODIPY-labeled RWPE demonstrated higher BODIPY-RWPE staining in TLR4Hi-MD2 than TLR4Hi-ST cells (Fig. 1G). Stimulating TLR4Hi cells (data not shown) and TLR4Hi-ST cells with RWPE failed to induce CXCL8 secretion (Fig. 1H). By contrast, stimulating TLR4Hi-MD2 cells with RWPE induced CXCL8 secretion (Fig. 1H). Next we sought to determine the relevance of these data from HEK 293 cells to human bronchial epithelial cells, HBEC.17 Stimulation of HBEC with RWPE induced CXCL8 secretion (Fig.1I). Suppression of Md2 in HBEC by siRNA inhibited RWPE-induced CXCL8 secretion (Fig.1I). To test the in vivo relevance of MD2 in stimulating RWPE-induced innate immune response, we first tested the efficacy of siRNA against Md2 or Tlr4 in suppressing lung expression of these genes. The siRNA against Md2 or Tlr4 (Fig. S1B) suppressed Md2 or Tlr4 mRNA expression in the lungs by 60% or 71%, respectively (data not shown). Similar to our previously reported observations in Tlr4KO mice6, suppression of Tlr4 mRNA by siRNA administration inhibited RWPE-induced neutrophil recruitment (Fig. 1J), validating this strategy as a tool to test the in vivo role of MD2. Consistent with our studies in HEK cells, in vivo suppression of Md2 by siRNA administration (Fig. S1B) reduced RWPE-induced neutrophil recruitment (Fig. 1J). These studies indicate an important role of MD2 in mediating the innate immune responses to RWPE.
Pollen extracts from diverse plant families utilize MD2 as a critical co-receptor of TLR4 to stimulate CXCL chemokine secretion
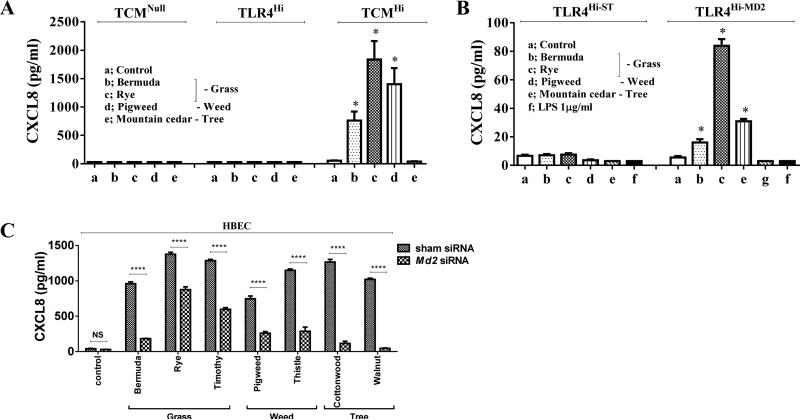
Next we examined the broader role TLR4, CD14 and MD2 in stimulating CXCL chemokine in response to culture with allergenic pollen extracts from diverse plant families. Pollen allergenic extracts belonging to diverse families – grasses (Bermuda and rye) and weeds (pigweed) – induced CXCL8 secretion from TCMHi but not TCMNull cells or TLR4Hi cells (Fig. 2A). By contrast, ten replicates stimulated with tree pollen extract (mountain cedar) failed to induce CXCL8 secretion, demonstrating the specificity of innate immune recognition of allergenic extracts. Like RWPE, stimulating TLR4Hi cells (data not shown) and TLR4Hi-ST cells with these pollen allergenic extracts failed to induce CXCL8 secretion (Fig. 2B). By contrast, stimulating TLR4Hi-MD2 cells with Bermuda, rye, firebush, and pigweed, but not mountain cedar, induced CXCL8 secretion (Fig. 2B). This pattern of CXCL8 secretion was similar to TCMHi cells (Fig. 2A), demonstrating reproducibility and specificity of innate recognition of diverse allergens. By contrast, very high concentration of LPS (1μg/ml) failed to bind (Fig.S2) or induce CXCL8 secretion (Fig. 2B) from TLR4Hi-MD2 cells. Together, these observations indicate that diverse pollen allergenic extracts require MD2 in addition to TLR4 to mount innate immune responses characterized by secretion of CXCL chemokines, and utilize a mechanism that is distinct from LPS to induce this secretion. Next we sought to validate our results by using human bronchial epithelial cells, HBEC.17 Stimulation with diverse pollen allergenic extracts like Bermuda, rye, timothy, pigweed, Russian thistle, eastern cottonwood, and black walnut induced CXCL8 secretion (Fig. 2C). Suppression of Md2 by siRNA inhibited pollen allergenic extract-induced CXCL8 secretion (Fig. 2C). Firebush and mountain cedar did not induce CXCL8 secretion from HBEC (data not shown), indicating specificity of innate immune recognition of these pollen extracts.
Fig. 2. Diverse pollen allergens requires MD2 in addition to TLR4 to induce CXCL8 secretion.
(A) Specific pollen extracts stimulate CXCL8 secretion from TCMHi cells. (B) Specific pollen extracts stimulate CXCL8 secretion from TLR4Hi-MD2 cells. (C) Md2 siRNA suppresses pollen extract-induced CXCL8 secretion from HBEC cells.
Cat dander extract requires MD2 in addition to TLR4 to induce CXCL8 secretion and neutrophil recruitment
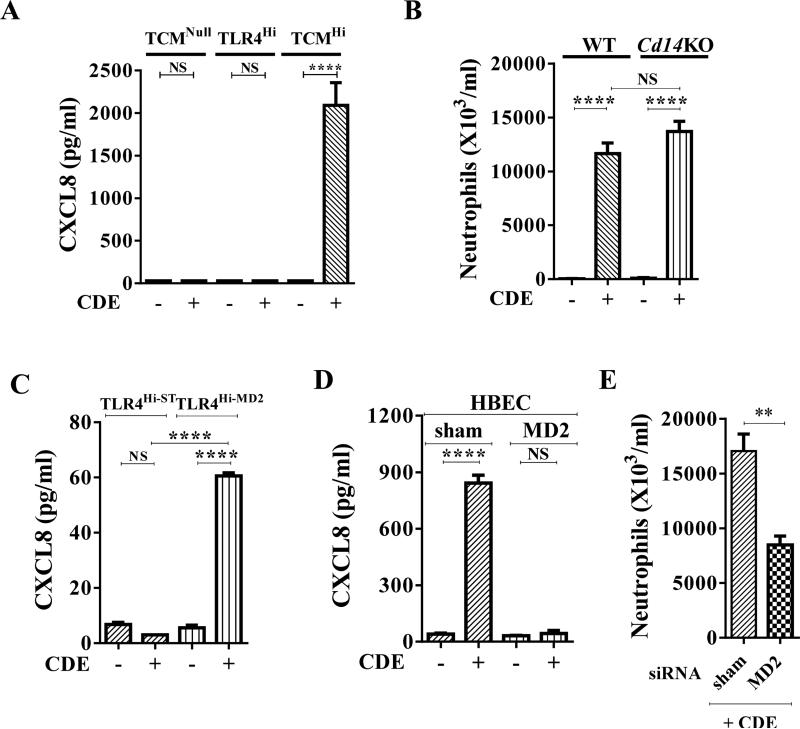
Next we examined whether CDE, an allergenic extract completely unrelated to pollen extracts, stimulates an MD2-dependent innate immune response. Similar to RWPE and other pollen allergens, CDE induced CXCL8 secretion in TCMHi but not TCMNull cells or TLR4Hi cells (Fig. 3A). Intranasal challenge with CDE induced the same level of neutrophil recruitment in the BALF from Cd14KO as WT mice (Fig. 3B). These results indicated that CD14 is not an essential co-receptor for TLR4 for CDE to induce innate recruitment of neutrophils. Stimulating TLR4Hi-ST cells with CDE failed to induce CXCL8 secretion (Fig. 3C). By contrast, stimulating TLR4Hi-MD2 cells with CDE induced CXCL8 secretion (Fig. 3C). Likewise, stimulation of HBEC cells with CDE induced CXCL8 secretion (Fig. 3D), and suppression of MD2 by siRNA inhibited the CDE-induced CXCL8 secretion from these cells (Fig. 3D). siRNA suppression of lung Md2 prior to intranasal single-challenge model with CDE (Fig. S1B) inhibited CDE-induced neutrophil recruitment (Fig. 3E). Together these studies indicate that CDE requires MD2 in addition TLR4 to induce CXCL chemokine secretion and induce recruitment of neutrophils to the airways.
Fig. 3. Requirement of MD2 for cat dander extract-induced recruitment of neutrophils.
(A) CDE requires CD14 and/or MD2 in addition to TLR4 to induce CXCL8 secretion. (B) A CDE single-challenge induces similar neutrophil recruitment in WT and Cd14KO mice. (C) CDE induces CXCL8 secretion from TLR4Hi-MD2 cells. (D) Effect of MD2 siRNA suppression on CDE induced-CXCL8 secretion from HBEC. (E) Effect of siRNA knockdown of MD2 on CDE-induced innate inflammation.
MD2 facilitates RWPE-induced allergic sensitization and allergic airway inflammation
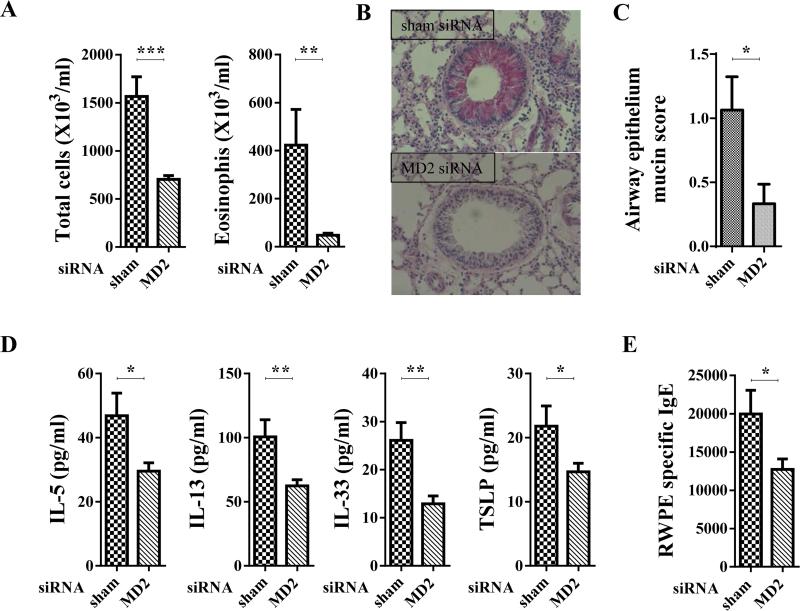
Building on our observation that MD2-mediates pollen and CDE-induced CXCL chemokine secretion and neutrophil recruitment, taken together with our recent report that RWPE challenge-induced TLR4 and CXCL-mediated neutrophil recruitment is critical for induction of allergic sensitization and allergic airway inflammation,6 we hypothesized that MD2 is important for inducing allergic sensitization and allergic inflammation to pollen and cat dander. To test this hypothesis, we utilized RWPE as a model system. An siRNA against Md2 was administered to WT mice before and during RWPE instillations in the repeated-challenge model (Fig. S1C). Compared to administration of control siRNA, administration of siRNA against Md2 strongly attenuated RWPE-induced allergic sensitization and allergic airway inflammation. This inhibition consisted of a decrease in recruited eosinophils and total inflammatory cells (Fig. 4A), a decrease in the accumulation of mucin in epithelial cells (Fig. 4B and C), a decrease in IL-5, IL-13, IL-33, and TSLP in BALF (Fig. 4D), and a decrease in RWPE-specific serum IgE (Fig. 4E). These observations indicate that MD2 facilitates RWPE-induced allergic sensitization and allergic airway inflammation.
Fig. 4. Effect of disrupting MD2 on RWPE-induced allergic inflammation.
(A-E) Effect of suppression of MD2 by administration of siRNA on allergic inflammation induced in RWPE repeated-challenge model. (A) Number of total inflammatory cells and eosinophils in BALF, (B,C) mucin secretion in airway epithelial cells. (B) Original magnifications, X400. (D) BALF level of IL-5, IL-13, IL-33, and TSLP. (E) Serum RWPE-specific IgE.
Discussion
Neutrophils have long been viewed as terminally differentiated cells that clear extracellular pathogens. However, a growing body of literature indicates that neutrophils have numerous additional effects that regulate innate and adaptive immune responses.20, 21 Neutrophil recruitment is a hall mark of innate immune responses,22 and innate recruitment of neutrophil to the skin through leukotriene B4 (LTB4) is critical for induction of subsequent allergic skin inflammation.22, 23 We recently reported that RWPE challenge induces CXCR2 and TLR4-dependent innate recruitment of activated neutrophils to the airways, and these recruited neutrophil are critical for induction of allergic sensitization and allergic airway inflammation.6 In the present study, we extend our earlier observations by demonstrating a broad role of MD2 in induction of innate and allergic airway inflammation and allergic sensitization by CDE, RWPE and extracts of pollens from grasses, weeds and trees.6
Similar to the present study, several earlier studies reported neutrophil influx in response to allergen challenge in human asthma subjects.24-26 One interpretation of neutrophil recruitment after allergen exposure is that endotoxin contamination of allergenic extracts or stimulation of TLR4/MD2 signaling by allergen-LPS complex could explain neutrophil recruitment after pulmonary allergen challenge in those studies and in the present study.27, 28 However several lines of evidence strongly indicate that endotoxin contamination/signaling cannot explain the observations in the present study. First, in the present study all allergenic extracts had very low (< 0.1pg LPS /1μg allergen protein) endotoxin levels, well below the no-azide low-endotoxin neutralizing antibodies used in numerous cell culture and in vivo studies (BD Biosciences, San Jose, CA). Second, we demonstrate in this manuscript that RWPE and CDE utilize a CD14-independent pathway to induce an innate neutrophilic airway inflammation, distinguishing it from LPS-induced inflammatory response. Third, using TLR4Hi-MD2 cells that do not bind or respond to very high concentrations of LPS, we show that RWPE, CDE and other allergenic extracts induce CXCL chemokine secretion. Finally, we have reported that repeated RWPE challenges together with passive transfer of neutrophils from donor mice to Tlr4 KO recipient mice (that lack the major receptor to respond to LPS) overcomes the blockade of RWPE-induced allergic sensitization and airway inflammation observed in TLR4 null mice.6 Together these data indicate that allergenic extracts directly induce CD14-independent, MD2 and TLR4-dependent innate immune responses in the lungs, possibly using a mechanism similar to HIV-1 Tat that binds TLR4-MD2 and stimulates an innate cytokine response independent of CD14.29 This MD2/TLR4-induced innate neutrophil recruitment facilitates allergic sensitization and allergic airway inflammation.6, 23
In the present study, even though the pattern of stimulation of CXCL8 by pollen extracts in TCMHi cells was similar to TLR4Hi-MD2, the levels of CXCL8 chemokine was 20-50-fold higher in TCMHi cells. This striking difference in pollen extract-induced CXCL8 secretion between two cell types may reflect cell damage to TLR4Hi-MD2 cells during transient transfection with plasmid DNA and lipofection vs. no damage in stably transfected TCMHi cells. Alternatively, transient transfection of TLR4Hi-MD2 may have induced relatively low levels of MD2 expression compared to long-term stably transfected and selected TCMHi cells.
We have recently reported that BAL neutrophil numbers distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1.30 Likewise, the dominance of neutrophils has been reported in the airways in severe asthma 31 and sudden-onset fatal asthma.32 Future studies will have to investigate whether stimulation of MD2-TLR4 signaling by allergens, as elucidated in the present study, provides a molecular mechanism basis of neutrophilic inflammation in the earlier studies. If proven correct, additional carefully conducted mechanistic studies will be required to determine whether these recruited neutrophils contribute to severe asthma and sudden-onset fatal asthma by stimulating allergic sensitization and allergic inflammation.6, 23
Our data indicate that diverse allergenic extracts require both MD2 and TLR4 to induce innate and allergic inflammation,6 distinguishing pollen extracts and CDE from several TLR4 ligands such as lipid A,33 taxol,34 nickel and cobalt that stimulate TLR4 without MD2.35 Der p2, the major component of house dust mite (HDM), shows structural homology with MD2 and enhances allergic inflammation by facilitating TLR4 signaling.11, 36 Since Amb a1 that has no structural similarity with MD2 but can stimulate MD2-dependent CXCL chemokine secretion, it utilizes a mechanism that is distinct from the structural mimicry of MD2 used by HDM.11
MD2 has been reported as one of seven SNPs in six genes associated with asthma.37 Our data suggest that future studies should investigate whether MD2 inhibitor or antagonists, like curcumin,38 prenylated flavonoids,39 rifampin,40 and Eritoran41 can inhibit allergic disorders, particularly those induced by ragweed, Bermuda, rye, timothy, pigweed, Russian thistle, cottonwood, and cat dander extracts. Future studies should investigate whether use of specific inhibitors of MD2 pathway could be a strategy to prevent neutrophil-dominant forms of asthma, such as severe asthma.31
Supplementary Material
Clinical Implications.
Blocking MD2 may be a novel strategy of inhibiting allergic sensitization and allergic inflammation induced by common allergenic pollens and cat dander.
Capsule summary.
Specific pollens and cat dander require MD2 in addition to TLR4 to stimulate innate inflammation, allergic sensitization and allergic airway inflammation.
Acknowledgements
We thank Dr. David Konkel's (Institute for Translational Sciences, UTMB) for his scientific input and for critical editing of the manuscript.
A declaration of all sources of funding for the research reported in the manuscript.
NAID P01 AI062885-06, NIEHS RO1 ES18948, NHLBI Proteomic Center, N01HV00245 NIEHS T32 ES007254, Leon Bromberg Professorship at UTMB
Abbreviations used
- BALF
bronchoalveolar lavage fluid
- CDE
cat dander extract
- DCs
dendritic cells
- HDM
House dust mite
- LPS
lipopolysaccharide
- LTB4
Leukotriene B4
- MD2
myeloid differentiation protein-2
- RWPE
Ragweed pollen extract
- TCMHi
HEK 293 cells that stably overexpress TLR4, CD14 and MD2
- TCMNull
HEK 293 cells that do not express TLR4, CD14 or MD2 cells
- TLR4
Toll-like receptor 4
- TLR4Hi
HEK 293 cells that overexpress TLR4, but not CD14 and MD2
- TLR4Hi-MD2
TLR4Hi cells were transfected with plasmid encoding MD2.
- TLR4Hi-ST
TLR4Hi cells were sham transfected.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts-Mills TA. Mite, cat, and cockroach exposure, allergen sensitisation, and asthma in children: a case-control study of three schools. Thorax. 1999;54:675–80. doi: 10.1136/thx.54.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salo PM, Calatroni A, Gergen PJ, Hoppin JA, Sever ML, Jaramillo R, et al. Allergy-related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2011;127:1226–35. e7. doi: 10.1016/j.jaci.2010.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salo PM, Arbes SJ, Jr., Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li DQ, Zhang L, Pflugfelder SC, De Paiva CS, Zhang X, Zhao G, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol. 2011;128:1318–25. e2. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shalaby KH, Allard-Coutu A, O'Sullivan MJ, Nakada E, Qureshi ST, Day BJ, et al. Inhaled birch pollen extract induces airway hyperresponsiveness via oxidative stress but independently of pollen-intrinsic NADPH oxidase activity, or the TLR4-TRIF pathway. J Immunol. 2013;191:922–33. doi: 10.4049/jimmunol.1103644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosoki K, Aguilera-Aguirre L, Brasier AR, Kurosky A, Boldogh I, Sur S. Pollen-induced Innate Recruitment of Neutrophils Facilitates Induction of Allergic Sensitization and Airway Inflammation. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2015-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viriyakosol S, Tobias PS, Kitchens RL, Kirkland TN. MD-2 binds to bacterial lipopolysaccharide. J Biol Chem. 2001;276:38044–51. doi: 10.1074/jbc.M105228200. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–35. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 10.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–72. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 11.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, et al. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–79. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med. 2007;175:805–15. doi: 10.1164/rccm.200608-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haeberle HA, Casola A, Gatalica Z, Petronella S, Dieterich HJ, Ernst PB, et al. IkappaB kinase is a critical regulator of chemokine expression and lung inflammation in respiratory syncytial virus infection. J Virol. 2004;78:2232–41. doi: 10.1128/JVI.78.5.2232-2241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaskill J, Singhania R, Burgess M, Allavena R, Wu S, Blumenthal A, et al. Efficient Biodistribution and Gene Silencing in the Lung epithelium via Intravenous Liposomal Delivery of siRNA. Mol Ther Nucleic Acids. 2013;2:e96. doi: 10.1038/mtna.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, et al. IFN-gamma-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–10. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 17.Rada B, Boudreau HE, Park JJ, Leto TL. Histamine stimulates hydrogen peroxide production by bronchial epithelial cells via histamine H1 receptor and dual oxidase. Am J Respir Cell Mol Biol. 2014;50:125–34. doi: 10.1165/rcmb.2013-0254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem. 2008;283:24748–59. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junker Y, Zeissig S, Kim SJ, Barisani D, Wieser H, Leffler DA, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 21.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–5. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyoshi MK, He R, Li Y, Mondal S, Yoon J, Afshar R, et al. Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation. Immunity. 2012;37:747–58. doi: 10.1016/j.immuni.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lommatzsch M, Julius P, Kuepper M, Garn H, Bratke K, Irmscher S, et al. The course of allergen-induced leukocyte infiltration in human and experimental asthma. J Allergy Clin Immunol. 2006;118:91–7. doi: 10.1016/j.jaci.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Hoskins A, Reiss S, Wu P, Chen N, Han W, Do RH, et al. Asthmatic airway neutrophilia after allergen challenge is associated with the glutathione S-transferase M1 genotype. Am J Respir Crit Care Med. 2013;187:34–41. doi: 10.1164/rccm.201204-0786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzger WJ, Richerson HB, Worden K, Monick M, Hunninghake GW. Bronchoalveolar lavage of allergic asthmatic patients following allergen bronchoprovocation. Chest. 1986;89:477–83. doi: 10.1378/chest.89.4.477. [DOI] [PubMed] [Google Scholar]
- 27.Hunt LW, Gleich GJ, Ohnishi T, Weiler DA, Mansfield ES, Kita H, et al. Endotoxin contamination causes neutrophilia following pulmonary allergen challenge. Am J Respir Crit Care Med. 1994;149:1471–5. doi: 10.1164/ajrccm.149.6.8004300. [DOI] [PubMed] [Google Scholar]
- 28.Herre J, Gronlund H, Brooks H, Hopkins L, Waggoner L, Murton B, et al. Allergens as immunomodulatory proteins: the cat dander protein Fel d 1 enhances TLR activation by lipid ligands. J Immunol. 2013;191:1529–35. doi: 10.4049/jimmunol.1300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben Haij N, Leghmari K, Planes R, Thieblemont N, Bahraoui E. HIV-1 Tat protein binds to TLR4-MD2 and signals to induce TNF-alpha and IL-10. Retrovirology;2013;10:123. doi: 10.1186/1742-4690-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosoki K, Ying S, Corrigan C, Qi H, Kurosky A, Jennings K, et al. Analysis of a Panel of 48 Cytokines in BAL Fluids Specifically Identifies IL-8 Levels as the Only Cytokine that Distinguishes Controlled Asthma from Uncontrolled Asthma, and Correlates Inversely with FEV1. PLoS ONE. 2015;10:e0126035. doi: 10.1371/journal.pone.0126035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med. 1999;160:1532–9. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 32.Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148:713–9. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 33.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–9. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 35.Raghavan B, Martin SF, Esser PR, Goebeler M, Schmidt M. Metal allergens nickel and cobalt facilitate TLR4 homodimerization independently of MD2. EMBO Rep. 2012;13:1109–15. doi: 10.1038/embor.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Poon A, Himes BE, Lasky-Su J, Sordillo JE, Belanger K, et al. Association of variants in innate immune genes with asthma and eczema. Pediatr Allergy Immunol. 2012;23:315–23. doi: 10.1111/j.1399-3038.2011.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z, Chen G, Chen L, Liu X, Fu W, Zhang Y, et al. Insights into the binding mode of curcumin to MD-2: studies from molecular docking, molecular dynamics simulations and experimental assessments. Mol Biosyst. 2015;11:1933–8. doi: 10.1039/c5mb00085h. [DOI] [PubMed] [Google Scholar]
- 39.Peluso MR, Miranda CL, Hobbs DJ, Proteau RR, Stevens JF. Xanthohumol and related prenylated flavonoids inhibit inflammatory cytokine production in LPS-activated THP-1 monocytes: structure-activity relationships and in silico binding to myeloid differentiation protein-2 (MD-2). Planta Med. 2010;76:1536–43. doi: 10.1055/s-0029-1241013. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Grace PM, Pham MN, Cheng K, Strand KA, Smith C, et al. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 2013;27:2713–22. doi: 10.1096/fj.12-222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.