1. Introduction
p38 kinases are members of the mitogen-activated protein kinases (MAPK) which are also referred to as stress-activated serine/threonine-specific kinases (SAPKs) with established involvement in a wide range of signaling pathways and different biological processes. The prototypic p38 MAPK, p38α MAPK, was originally identified as a tyrosine phosphorylated protein detected in LPS-stimulated macrophages with essential function for inflammatory cytokine production (Han, Lee, Bibbs, & Ulevitch, 1994; Han, Lee, Tobias, & Ulevitch, 1993) and as an upstream activating kinase of MAPK-activated protein kinase 2 (MK2) in cells stimulated with arsenite, heat shock, or interleukin-1 (Freshney et al., 1994; Rouse et al., 1994). Extensive studies have now revealed that p38 MAPKs have critical roles in many different tissues far beyond immune regulation and inflammatory responses. In this review, we will focus on the structure and molecular biology of p38 MAP kinases, and their specific roles in heart, especially regarding myocyte proliferation, apoptosis, and hypertrophic responses (Rose, Force, & Wang, 2010).
2. Molecular Structure of p38 MAPK
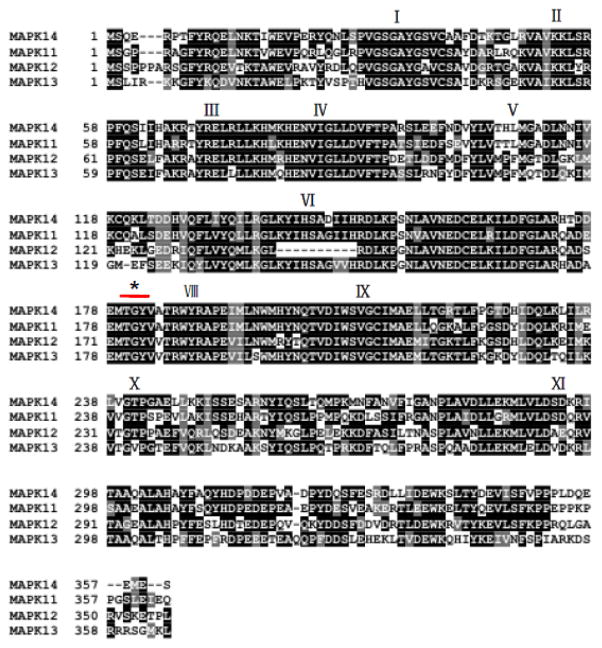
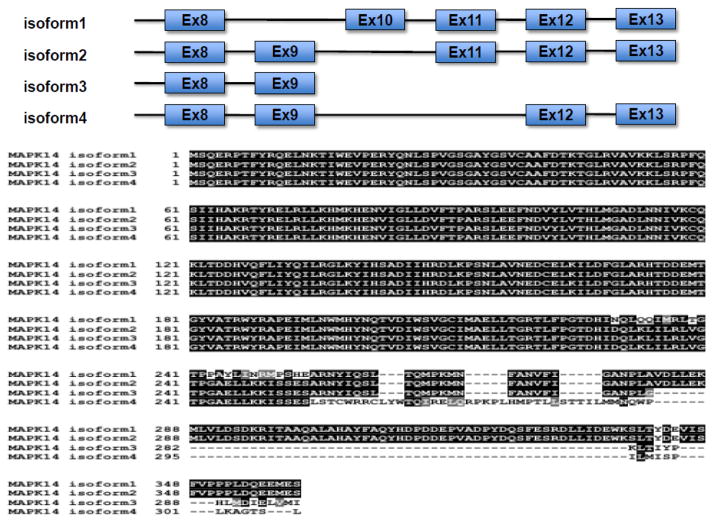
In human and mammals, there are four isoforms in the p38 MAPK sub-family including p38α , p38β , p38γ and p38δ isoforms. Human p38 MAP Kinases are encoded by MAPK14 for α (NCBI Gene ID: 1432, chromosome 6), MAPK11 for β (NCBI Gene ID: 5600, chromosome 22), MAPK12 for γ (NCBI Gene ID: 6300, chromosome 22), and MAPK13 δ (NCBI Gene ID: 5603, chromosome 6), respectively. They have high degree of sequence homologies at amino acid level (>60% identity among isoforms) (Figure 1). In addition, p38α MAPK mRNAs have 4 transcript variants due to alternative splicing (NM_001315.2, NM_139012.2, NM_139013.2, and NM_139014.2), resulting in protein variants with different sequences at the C-terminus (Figure 2). Each p38 MAPK isoform has different tissue-specific expression pattern (M. Li, Liu, & Zhang, 2011). p38α MAPK is ubiquitously expressed in many cell types, in contrast p38β MAPK is highly expressed in brain and lung, p38γ MAPK is mostly detected in skeletal muscle and nerve system, and p38δ MAPK is enriched in uterus and pancreas (Ono & Han, 2000). All isoforms possess the same conserved domains (from I to X) and a Thr-Gly-Tyr (TGY) dual phosphorylation motif that serves as activation switch for the kinases (Figure 1). As a shared mechanism among all MAP kinase families, p38 MAPKs are activated by upstream kinases (MAPKK) via targeted phosphorylation of the TGY motif, although autophosphorylation mediated activation mechanism is also reported (Han et al., 1994; Lu et al., 2006). The structures of human p38α in inactive and active states have been solved by X-ray crystallography. The phosphorylated TGY motif and the length of the activation loop are identified to be different from members of the other two MAPK branches i.e. extracellular signal-regulated kinases (ERK) and c-jun N-terminal kinases (JNK). This unique signature likely confers substrate specificity to each MAPK subfamilies (Roux & Blenis, 2004). Based on phylogenic and gene synteny analysis of MAPK 11,12,13 and 14 genes across different species, it is speculated that MAPK11/12 cluster is the gene duplication product of MAPK13/14 cluster, and MAPK12 is originated from MAPK11 also by gene duplication (M. Li et al., 2011). p38 MAPKs form functional complexes with substrates and modulators in cells. For example, MK2 is a binding partner p38α MAPK, interacting in a “head-to-head’ manner, and present active sites of both kinases with extensive intermolecular interactions that dictate substrate and intracellular localization (White, Pargellis, Studts, Werneburg, & Farmer, 2007). p38α MAPK is also reported to be a client protein for Hsp90/Cdc37 which constitutively binds and modulates its non-canonical activation by TAB1 (Ota, Zhang, Ping, Han, & Wang, 2010) Numerous inhibitors against p38 MAPKs have been reported with diverse chemical structures. Most inhibitors such as SB203580, VX745, RO3201195, and AMG548 bind competitively to the ATP binding site. On the other hand, BIRB796 inhibit p38 MAPK activity by conformational change that exclude ATP binding (J. Zhang, Shen, & Lin, 2007).
Figure 1. Primary sequence of human p38 MAPK isoforms.
Shaded blocks are conserved domains from I to XI. * indicates the TGY motif in the kinase activation loop.
Figure 2. Human p38α MAPK (MAPK14) transcription variants.
Top panel: Isoform specific exon utilization of p38α MAPK variants. Bottom panel: amino acid sequences of p38α MAPK variants.
3. Cellular function of p38 MAP Kinases
3.1. Subcellular localization of p38 MAPK
Under basal conditions, p38 MAPKs are detected in both the nucleus and the cytoplasm. However, upon activation, p38 MAPKs are trans-located into the nucleus (Zarubin & Han, 2005) and inactivated p38 MAPKs are exported to the cytoplasm. This translocation process depends on p38 MAPK phosphorylation but not its own catalytic activity, suggesting the involvement of upstream kinases (Wood, Thornton, Sabio, Davis, & Rincon, 2009). This extracellular stimulation-dependent translocation of p38 MPAKs is an essential process for its functions in various cell types. In addition to its phosphorylation, microtuble- and dynein-dependent processes are reported to be involved in p38 MAPK translocation (Gong, Ming, Deng, & Jiang, 2010). In addition, MK2 is also involved in subcellular localization of p38 MAPK s. Activated MK2 forms a complex with p38 MAPKs in the nucleus and then triggers the export p38/MK2 complex to cytoplasm, hence directing p38 MAPK activities to cytosolic downstream targets (Ben-Levy, Hooper, Wilson, Paterson, & Marshall, 1998). Different p38 MAPK isoforms also have distinct subcellular localization patterns. p38-regulated/activated protein kinase (PARK) interacts with p38α MAPK and localizes in the nucleus, whereas p38β-PARK complex localizes in the cytosol. This differential localization is determined by specific motifs between amino acid 145 and 156 in both p38 MAPK isoforms (Q. Li et al., 2008). In addition to the translocation between nucleus and cytosol, p38 MAPK also translocates to mitochondria. p38 MAPK is known to activate p53 and then promote apoptosis by inducting expression and translocation of Bax in mitochondria (S. J. Kim, Hwang, Shin, Kang, & Chun, 2002; Mayr, Hu, Hainaut, & Xu, 2002). In neuronal cell, p38 MAPK was shown to translocate to mitochondria in response to nerve growth factor withdrawal, resulting in phosphorylation of Bcl-2 and inactivation of its anti-apoptotic effects (Torcia et al., 2001).
3.2. Activation/deactivation of p38 MAPK
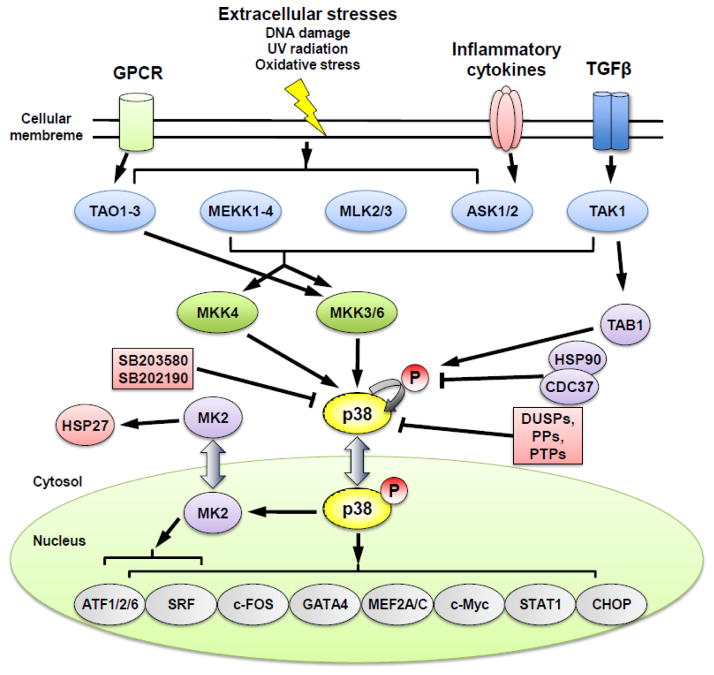
Activation of p38 MAPK pathway has been implicated in a variety of stress response in addition to inflammation, including osmotic shock, heat, and oxidative stress (Johnson & Lapadat, 2002; Kyriakis & Avruch, 2001; Ono & Han, 2000). The canonical pathway for p38 MAPK activation involves cascades of MAP3Ks and MAP2Ks as upstream kinases, including MAP kinase kinase kinases (MEKK1-4), TGFβ-activated kinase (TAK1), thousand-and-one amino acid 1-3 (TAO1-3), mixed-lineage kinase 2/3 (MLK2/3), and apoptosis signal-regulating kinase 1/2 (ASK1/2) at the MAP3K level, and MKK 3, 6 and 4 at the MAP2K level (Figure 3). Phosphorylation of p38 MAPKs at the threonie/tyrosine residues (TGY) in the kinase activation loop resulted in a conformational change which induces the kinase catalytic activity and binding to substrates (Cuadrado & Nebreda, 2010). Other than MKK dependent activation, TAK1-binding protein 1 (TAB1), ZAP70 and HSP90/Cdc37 have been reported to regulate non-canonical activation of p38 MAPK via autophosphorylation (Ge et al., 2002; Ota et al., 2010; Salvador et al., 2005; Tanno et al., 2003). Uniquely, ZAP70 phosphorylates p38α on Tyr323 before it is further activated by autophoshorylation (Salvador et al., 2005). Furthermore, acetylation of p38 at lys-53 in the ATP-binding pocket also enhances p38 MAPK activity during cellular stress (Pillai et al., 2011). Therefore, p38 MAPKs can be induced by varies stresses via different mechanisms.
Figure 3.
Signaling pathways and molecular network of p38 MAPK activation and downstream targets.
Inactivation of p38 MAPK is primarily carried out through the dephosphorylation of the TGY motif. Several phosphatases from protein phosphatase (PP) family, protein tyrosine phosphatase (PTP) family, and dual-specificity phosphatase (DUSP) family are implicated in this process. For example, ser/thr phosphatases PP2Cα /β suppress activity of p38 MAPKs through direct interaction as well as suppression of MKKs/TAK1 in mammalian cell (Hanada et al., 1998; Takekawa, Maeda, & Saito, 1998). PTPs, such as hematopoietic PTP (HePTP) and striatal-enriched phosphatase (STEP), are known to bind to MAPKs through a kinase-interaction motif (KIM) and inactivate p38 MAPK s by dephosphorylating the phosphotyrosine residue in their activation loop (Pulido, Zuniga, & Ullrich, 1998; Saxena, Williams, Brockdorff, Gilman, & Mustelin, 1999; Saxena, Williams, Gilman, & Mustelin, 1998). DUSPs, which have a docking domain to MAPKs and dual-specific phosphatase activity, bind to p38 MAPK s and dephosphorylate both phosphotyrosine and phosphothreonine residues in the TXY motif (reviewed in (Cuadrado & Nebreda, 2010)). In addition to these phosphatases, molecular chaperones such as Hsp90-Cdc37 complex can also modulate p38 MAPK autophosphorylation activity and non-canonical activation (Ota et al., 2010).
3.3. Downstream targets of p38 MAPK
Diverse functions of p38 MAPKs are mediated through a large variety of downstream substrates. As reviewed by Young et al, p38 MAPK target molecules are first characterized by using p38 MAPK specific inhibitors, SB203580 and SB202190, and more recently in gene deletion mouse models. Inhibition/deficiency of p38 MAPKs causes changes in cell survival/apoptosis, proliferation, differentiation, migration, mRNA stability, and inflammatory response in different cell types (Young, 2013). Although a vast majority of the literature focus on p38α MAPK isofrom function, a significant number of studies have been conducted to demonstrate the different functions of the distinct isoforms of p38 MAPK, For instance, p38β MAPK contributes to regulate Store Operated Calcium Entry (SOCE) and permeability responses in endothelial cell and bladder cancer cell migration through STIM1 and Hsp27, respectively (Sundivakkam, Natarajan, Malik, & Tiruppathi, 2013; Yu et al., 2014). Although distinct roles of p38γ and δ MAPK isoforms are not well-known, several evidences have been provided that they have critical roles in biological processes such as tumorigenesis (Del Reino et al., 2014). p38γ regulates G2-M transition in mitotic processs via stabilizing chromosome and an energetic signaling in skeletal muscle contraction (Brault, Pizzimenti, Dentel, & Wiseman, 2013; Kukkonen-Macchi et al., 2011). p38δ MAPK plays a role of differentiating monocyte to bone-forming monoosteophils in bone repair process and insulin secretion and survival of pancreastic β cells (Sumara et al., 2009; Z. Zhang & Shively, 2013). From the p38 MAPK isoform knockouts study, deficiency of p38α MAPK isoform in mouse leads to embryonic death as a result of defective placental development, whereas that of other isofoms shows subtle phentypes (Allen et al., 2000; Beardmore et al., 2005; Mudgett et al., 2000; Sabio et al., 2005; Tamura et al., 2000). Considering the severity of phenotypes in isoform specific knockout mice, p38α MAPK seems to be the major isofom of the family at least during development and inflammatory responses, although p38β MAPK, p38γ MAPK, and p38δ MAPK have also pivotal roles under other conditions. (Aouadi, Binetruy, Caron, Le Marchand-Brustel, & Bost, 2006; Xing, Bachstetter, & Van Eldik, 2013).
Many kinases are activated by p38 MAPKs such as MK2/3, PARK, MAPK interacting protein kinases 1/2 (MNK1/2), mitogen and stress activated protein kinase 1/2 (MSK1/2), and eukaryotic elongation factor 2 kinase (eEF2k) (Cuadrado & Nebreda, 2010; Gallo & Johnson, 2002). MK2 is one of the well-studied downstream targets of p38 MAPKs and its activity in turn can regulate cell survival/apoptosis, proliferation, differentiation, mRNA stability and inflammatory response. In addtion, MK2/p38 complex stabilizes each other (Gaestel, 2006; Kotlyarov et al., 2002; Sudo, Kawai, Matsuzaki, & Osada, 2005). p38/MK2-dependent cellular processes are regulated by numerous downstream substrates such as small heat shock protein 27 (HSP27), lymphocyte-specific protein1 (LSP1), cAMP response element-binding protein (CREB), cyclooxygenase 2 (COX2), activating transcription factor 1 (ATF1), serum response factor (SRF), and mRNA-binding protein tristetraprolin (TTP) (Rose et al., 2010; Zarubin & Han, 2005). CREB and TTP can regulate mRNA stability and transcriptional factor activity in a MK2-dependent manner (Rolli, Kotlyarov, Sakamoto, Gaestel, & Neininger, 1999; Stoecklin et al., 2004). MK3 is highly homologous to MK2 and these two kinases have similar functions (Cargnello & Roux, 2011). Another well-studied downstream target of p38 MAPKs is MSK1/2, which is thought to regulate the subcellular localization of p38 MAPK and ERK1/2 (Cargnello & Roux, 2011). These kinases are involved in the transcriptional regulation by CREB, STAT3, NFkB-dependent transcription and chromatin remodeling (Drobic, Perez-Cadahia, Yu, Kung, & Davie, 2010; Pierrat, Correia, Mary, Tomas-Zuber, & Lesslauer, 1998; Vermeulen, De Wilde, Van Damme, Vanden Berghe, & Haegeman, 2003; Wierenga, Vogelzang, Eggen, & Vellenga, 2003).
In addition to protein kinases such as MK2/3 and MSK1/2, many transcription factors are direct downstream targets of p38 MAPKs, including ATF1/2/6, c-MYC, c-FOS, GATA4, MEF2A/C, SRF, STAT1, and CHOP. ATF2 isa member of the ATF/cAMP response element-binding protein family and a regulator of tumorgenesis. It can be activated by p38 MAPKs as well as JNK through phosphorylation on threonine 69 and 71 (Raingeaud et al., 1995; Vlahopoulos et al., 2008). ATF2 regulates other transcription factors in extracellular stresses; genes related with growth and tumorgenesis; and genes associated with homeostasis (Bhoumik, Lopez-Bergami, & Ronai, 2007). Furthermore, ATF2 has a negative feedback mechanism for p38 MAPK regulation through DUSPs (Breitwieser et al., 2007). In short, upstream and downstream signaling molecules constitute the signaling network of p38 MAPKs as activators, modulators and effectors (Figure 3).
4. Function of p38 MAPK in Cardiovascular System
4.1. Regulation of cardiomyocyte proliferation
Whereas fetal and neonatal cardiomyocytes have a high mitotic activity, terminally differentiated cardiomyocytes in adult heart have diminished capacity to proliferate. Myocyte proliferation is dynamically regulated during cardiac maturation in postnatal heart and is an important area of investigation for cardiac regeneration after injury. Recently, several lines of evidence have indicated that cell cycle reentry and progression can be induced in adult myocytes while cell proliferation can be enhanced in neonatal and progenitor cardiomyocyte by inhibition of p38 MAPK activities. Engel et al. first showed that binucleation of cardiomyocyte is regulated by p38 MAPK which accumulates at the mid-body during myocyte cytokinesis and disrupts anillin localization (Engel, Schebesta, & Keating, 2006). They also demonstrated that p38 MAPK inhibition in serum-treated cardiomyocyte up-regulated core components of the central spindle and resulted in the mid-body formation. Combination FGF1 stimulation and p38 MAPK inhibitor up-regulates cell cycle regulating genes including cyclin A2, cdc2a, and cyclin B, resulting in induction of mitosis in both adult and fetal cardiomyocyte (Engel, Hsieh, Lee, & Keating, 2006; Engel et al., 2005). Therefore, p38 MAPK is associated with cell-cycle arrest in mammalian cardiomyocytes and its inhibition may represent a strategy to promote cardiac regeneration in response to injury.
4.2. Apoptotic roles in the heart
Under stressed conditions such as ischemia and oxidative injury, cardiomyocytes suffer from apoptotic death in heart. Myocardial ischemia is a potent inducer of p38 MAPK activation. Using both in vivo and ex-vivo cardiac ischemia/reperfusion (I/R) injury models, p38 MAPK inhibition has been demonstrated to blunt apoptosis in I/R injured hearts. Similar cardioprotection is also observed in a transgenic heart expressing a dominant negative mutant of p38α MAPK (Ma et al., 1999; Ren, Zhang, Kovacs, Wang, & Muslin, 2005). Consistent with these studies, overexpression of p38α MAPK reduced anti-apoptotic protein Bcl-xl expression in cultured neonatal cardiomyocyes and p38 inhibition reduced stress-induced apoptosis in cultured cardiomyocyes (Kaiser et al., 2004; Sharov et al., 2003). It is reported that p38 MAPK dependent apoptosis in heart is mediated via downstream events mediated by STAT1, CHOP, FAK, SMAD, cytochrome c, NF-kB, PTEN, and p53 (Eiras et al., 2006; Fiordaliso et al., 2001; Ghosh, Das, Manna, & Sil, 2009; Qian et al., 2012; Schroder, Heger, Piper, & Euler, 2006; Stephanou et al., 2001; Zhao et al., 2010). However, some reports demonstrated that p38 MAPK also involves in anti-apoptotic effect via phosphorylation of α β-Crystallin or induction of Pim-3 during early response to oxidative stress or anoxic preconditioning respectively (Aggeli, Beis, & Gaitanaki, 2008; D. Liu et al., 2009; Mitra, Ray, Datta, Sengupta, & Sarkar, 2014). More interestingly, p38α MAPK and p38β MAPK appear to have an opposite role in apoptosis (Wang et al., 1998). Whereas p38α MAPK has a pro-apoptotic role via p53 activation, p38β MAPK has a pro-survival role via inhibition of ROS formation (J. K. Kim, Pedram, Razandi, & Levin, 2006; H. Liu, Pedram, & Kim, 2011). Interestingly, a recent report suggests that the protective effect of estrogen against oxidative stress via manganese superoxide dismutase induction is mediated by active mitochondrial localized p38β MAPK in cardiomyocytes (H. Liu, Yanamandala, Lee, & Kim, 2014). Therefore distinct function of p38α MAPK and p38β MAPK in cardiac apoptosis regulation is potentially contributed by their distinct intracellular localization, . Under ischemic condition, chronic insulin exposure and metabolic stresses induce p38 MAPK activation and promote insulin receptor substrate 1/2 (IRS1/2) degradation, resulting in AKT inactivation and subsequent myocyte apoptosis and heart failure (Qi et al., 2013). In general, chronic activation of p38 MAPK activity is viewed as pathological and pro-apoptotic, and inhibition of p38 MAPK activity is in clinical evaluation as a potential therapy to mitigate acute injury in ischemic heart failure (Marber, Rose, & Wang, 2011).
4.3. Cardiac Hypertrophy Regulation
Cardiac hypertrophy is a significant component of pathological remodeling in the diseased hearts and a major risk factor for heart failure and advert outcome. In past decade, numerous reports have demonstrated that p38 MAPK inhibition using pharmacological inhibitors or dominant negative p38 MAPK mutant expression can attenuate cardiomyocyte growth in response to hypertrophic stimuli in vitro (Liang & Molkentin, 2003; Nemoto, Sheng, & Lin, 1998; Wang et al., 1998; Zechner, Thuerauf, Hanford, McDonough, & Glembotski, 1997). In addition to inhibitory studies, chronic activation of p38 MAPK pathway by overexpression upstream kinases has indicated that p38 MAPK activation is sufficient to induce hypertrophic response in cultured cardiomyocytes (Nemoto et al., 1998; Wang et al., 1998; Zechner et al., 1997). Adenoviral infection of constitutive active mutant of MKK3 or MKK6 in neonatal rat ventricular myocytes (NRVMs) result in characteristic hypertrophic responses, including an increase in cell size, enhanced sarcomeric organization, and elevated atrial natriuretic factor expression. Furthermore, the hypertrophic response by MKK3/6 is enhanced by co-infection of an adenoviral vector expressing p38β MAPK, and was suppressed by the p38β MAPK dominant negative mutant (Wang et al., 1998). In addition, adenoviral infection of p38α MAPK in NRVMs upregulated fibrosis related gene expression, whereas that of p38β MAPK upregulated BNP gene at transcriptional level (Koivisto et al., 2011). As mentioned above, p38α MAPK enhanced MKK3 activation-induced apoptotic activity. Taken together, these observations indicate that p38α MAPK and p38β MAPK have distinct roles in cardiac hypertrophy and pathological remodeling in vitro.
Despite the evident in vitro impacts of p38 MAPK activation on hypertrophic response, the in vivo impact is far from uniformed. Cardiac-specific overexpression of active MKK3 and MKK6 mice do not show the significant increase in cardiomyocyte size, although they show marked cardiac interstitial fibrosis, ventricular wall thinning, left ventricular dysfunction, immature death, and expression of fetal marker genes characteristic of cardiac failure (Liao et al., 2001). In addition, cardiac-specific overexpression of dominant negative p38α MAPK in mice promotes cardiomyocyte growth (Braz et al., 2003). In contrast with these reports, acute activation of endogenous p38 MAPK in adult heart using cardiac-specific and inducible expression of a constitutively active MKK3 results in cardiac hypertrophy and fibrosis (Streicher, Ren, Herschman, & Wang, 2010). This discrepancy might be caused by developmental timing and the duration of p38 MAPK activation. Because our unpublished data and other report suggest p38 MAPK involves in post-natal cardiac maturation and proliferation (Engel et al., 2005), In addition, cardiac-specific p38 MAPK dominant negative transgenic mice and cardiac-specific p38α knockout mouse showed an elevated cardiac hypertrophy in response to pressure overload (Nishida et al., 2004; S. Zhang et al., 2003). Recent studies have reported that p38 MAPK also plays critical roles in the development of physiological hypertrophy. Using ASK1 knockout and cardiac – specific p38α MAPK knockout mice, Taniike et al demonstrate that loss of p38α MAPK results in enhanced physiological hypertrophy through an increase in AKT activity in response to swimming exercise (Taniike et al., 2008). On the other hand, the transgenic mice expressing p38α MAPK dominant negative mutant do not show the enhancement in physiological hypertrophy in response to swimming exercise (Watanabe et al., 2007). Taken together with pressure overload and swimming exercise studies, loss of function through cardiac-specific knockout and transgenic dominant negative p38α MAPK expression yield distinct outcome in response to hypertrophic stimuli. Therefore, although p38 MAPK activation is related to cardiac hypertrophy, its role in vivo is controversial and remains to be further elucidated. More mechanistic studies regarding isoform specific function and stress-specific response are needed before a targeted therapy can be developed for heart diseases.
Acknowledgments
This work is supported in part by grants from NHLBI (HL070079, HL108186, HL103205) to YW. TY is supported by Construction of Labo-exchange Type Health & Biomedical Science Research Consortium. This review and the corresponding Gene Wiki article are written as part of the Cardiac Gene Wiki Review series--a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative, and the BD2K initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (GM089820 and GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org/wiki/MAPK14
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggeli IK, Beis I, Gaitanaki C. Oxidative stress and calpain inhibition induce alpha B-crystallin phosphorylation via p38-MAPK and calcium signalling pathways in H9c2 cells. Cell Signal. 2008;20(7):1292–1302. doi: 10.1016/j.cellsig.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Allen M, Svensson L, Roach M, Hambor J, McNeish J, Gabel CA. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med. 2000;191(5):859–870. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88(9):1091–1098. doi: 10.1016/j.biochi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Beardmore VA, Hinton HJ, Eftychi C, Apostolaki M, Armaka M, Darragh J, … Arthur JS. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol Cell Biol. 2005;25(23):10454–10464. doi: 10.1128/MCB.25.23.10454-10464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ. Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol. 1998;8(19):1049–1057. doi: 10.1016/s0960-9822(98)70442-7. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, Lopez-Bergami P, Ronai Z. ATF2 on the double - activating transcription factor and DNA damage response protein. Pigment Cell Res. 2007;20(6):498–506. doi: 10.1111/j.1600-0749.2007.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault JJ, Pizzimenti NM, Dentel JN, Wiseman RW. Selective inhibition of ATPase activity during contraction alters the activation of p38 MAP kinase isoforms in skeletal muscle. J Cell Biochem. 2013;114(6):1445–1455. doi: 10.1002/jcb.24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, … Molkentin JD. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003;111(10):1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser W, Lyons S, Flenniken AM, Ashton G, Bruder G, Willington M, … Jones N. Feedback regulation of p38 activity via ATF2 is essential for survival of embryonic liver cells. Genes Dev. 2007;21(16):2069–2082. doi: 10.1101/gad.430207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- Del Reino P, Alsina-Beauchamp D, Escos A, Cerezo-Guisado MI, Risco A, Aparicio N, … Cuenda A. Pro-oncogenic role of alternative p38 mitogen-activated protein kinases p38gamma and p38delta, linking inflammation and cancer in colitis-associated colon cancer. Cancer Res. 2014;74(21):6150–6160. doi: 10.1158/0008-5472.CAN-14-0870. [DOI] [PubMed] [Google Scholar]
- Drobic B, Perez-Cadahia B, Yu J, Kung SK, Davie JR. Promoter chromatin remodeling of immediate-early genes is mediated through H3 phosphorylation at either serine 28 or 10 by the MSK1 multi-protein complex. Nucleic Acids Res. 2010;38(10):3196–3208. doi: 10.1093/nar/gkq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiras S, Fernandez P, Pineiro R, Iglesias MJ, Gonzalez-Juanatey JR, Lago F. Doxazosin induces activation of GADD153 and cleavage of focal adhesion kinase in cardiomyocytes en route to apoptosis. Cardiovasc Res. 2006;71(1):118–128. doi: 10.1016/j.cardiores.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Engel FB, Hsieh PC, Lee RT, Keating MT. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc Natl Acad Sci U S A. 2006;103(42):15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, … Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19(10):1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Keating MT. Anillin localization defect in cardiomyocyte binucleation. J Mol Cell Cardiol. 2006;41(4):601–612. doi: 10.1016/j.yjmcc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, … Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50(10):2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78(6):1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Gaestel M. MAPKAP kinases - MKs - two's company, three's a crowd. Nat Rev Mol Cell Biol. 2006;7(2):120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3(9):663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, … Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295(5558):1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Das J, Manna P, Sil PC. Taurine prevents arsenic-induced cardiac oxidative stress and apoptotic damage: role of NF-kappa B, p38 and JNK MAPK pathway. Toxicol Appl Pharmacol. 2009;240(1):73–87. doi: 10.1016/j.taap.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Gong X, Ming X, Deng P, Jiang Y. Mechanisms regulating the nuclear translocation of p38 MAP kinase. J Cell Biochem. 2010;110(6):1420–1429. doi: 10.1002/jcb.22675. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265(5173):808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Tobias PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268(33):25009–25014. [PubMed] [Google Scholar]
- Hanada M, Kobayashi T, Ohnishi M, Ikeda S, Wang H, Katsura K, … Tamura S. Selective suppression of stress-activated protein kinase pathway by protein phosphatase 2C in mammalian cells. FEBS Lett. 1998;437(3):172–176. doi: 10.1016/s0014-5793(98)01229-0. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Bueno OF, Lips DJ, Doevendans PA, Jones F, Kimball TF, Molkentin JD. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J Biol Chem. 2004;279(15):15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]
- Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281(10):6760–6767. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFkappa B-dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277(36):33501–33508. doi: 10.1074/jbc.M202862200. [DOI] [PubMed] [Google Scholar]
- Koivisto E, Kaikkonen L, Tokola H, Pikkarainen S, Aro J, Pennanen H, … Ruskoaho H. Distinct regulation of B-type natriuretic peptide transcription by p38 MAPK isoforms. Mol Cell Endocrinol. 2011;338(1–2):18–27. doi: 10.1016/j.mce.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Kotlyarov A, Yannoni Y, Fritz S, Laass K, Telliez JB, Pitman D, … Gaestel M. Distinct cellular functions of MK2. Mol Cell Biol. 2002;22(13):4827–4835. doi: 10.1128/MCB.22.13.4827-4835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen-Macchi A, Sicora O, Kaczynska K, Oetken-Lindholm C, Pouwels J, Laine L, Kallio MJ. Loss of p38gamma MAPK induces pleiotropic mitotic defects and massive cell death. J Cell Sci. 2011;124(Pt 2):216–227. doi: 10.1242/jcs.068254. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81(2):807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Li M, Liu J, Zhang C. Evolutionary history of the vertebrate mitogen activated protein kinases family. PLoS One. 2011;6(10):e26999. doi: 10.1371/journal.pone.0026999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang N, Zhang D, Wang Y, Lin T, Wang Y, … Han J. Determinants that control the distinct subcellular localization of p38alpha-PRAK and p38beta-PRAK complexes. J Biol Chem. 2008;283(16):11014–11023. doi: 10.1074/jbc.M709682200. [DOI] [PubMed] [Google Scholar]
- Liang Q, Molkentin JD. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J Mol Cell Cardiol. 2003;35(12):1385–1394. doi: 10.1016/j.yjmcc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, … Wang Y. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci U S A. 2001;98(21):12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, He M, Yi B, Guo WH, Que AL, Zhang JX. Pim-3 protects against cardiomyocyte apoptosis in anoxia/reoxygenation injury via p38-mediated signal pathway. Int J Biochem Cell Biol. 2009;41(11):2315–2322. doi: 10.1016/j.biocel.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Liu H, Pedram A, Kim JK. Oestrogen prevents cardiomyocyte apoptosis by suppressing p38alpha-mediated activation of p53 and by down-regulating p53 inhibition on p38beta. Cardiovasc Res. 2011;89(1):119–128. doi: 10.1093/cvr/cvq265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yanamandala M, Lee TC, Kim JK. Mitochondrial p38beta and manganese superoxide dismutase interaction mediated by estrogen in cardiomyocytes. PLoS One. 2014;9(1):e85272. doi: 10.1371/journal.pone.0085272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Kang YJ, Han J, Herschman HR, Stefani E, Wang Y. TAB-1 modulates intracellular localization of p38 MAP kinase and downstream signaling. J Biol Chem. 2006;281(9):6087–6095. doi: 10.1074/jbc.M507610200. [DOI] [PubMed] [Google Scholar]
- Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, … Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99(13):1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- Marber MS, Rose B, Wang Y. The p38 mitogen-activated protein kinase pathway--a potential target for intervention in infarction, hypertrophy, and heart failure. J Mol Cell Cardiol. 2011;51(4):485–490. doi: 10.1016/j.yjmcc.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr M, Hu Y, Hainaut H, Xu Q. Mechanical stress-induced DNA damage and rac-p38MAPK signal pathways mediate p53-dependent apoptosis in vascular smooth muscle cells. FASEB J. 2002;16(11):1423–1425. doi: 10.1096/fj.02-0042fje. [DOI] [PubMed] [Google Scholar]
- Mitra A, Ray A, Datta R, Sengupta S, Sarkar S. Cardioprotective role of P38 MAPK during myocardial infarction via parallel activation of alpha-crystallin B and Nrf2. J Cell Physiol. 2014;229(9):1272–1282. doi: 10.1002/jcp.24565. [DOI] [PubMed] [Google Scholar]
- Mudgett JS, Ding J, Guh-Siesel L, Chartrain NA, Yang L, Gopal S, Shen MM. Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc Natl Acad Sci U S A. 2000;97(19):10454–10459. doi: 10.1073/pnas.180316397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Sheng Z, Lin A. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol Cell Biol. 1998;18(6):3518–3526. doi: 10.1128/mcb.18.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, … Otsu K. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24(24):10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12(1):1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Ota A, Zhang J, Ping P, Han J, Wang Y. Specific regulation of noncanonical p38alpha activation by Hsp90-Cdc37 chaperone complex in cardiomyocyte. Circ Res. 2010;106(8):1404–1412. doi: 10.1161/CIRCRESAHA.109.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrat B, Correia JS, Mary JL, Tomas-Zuber M, Lesslauer W. RSK-B, a novel ribosomal S6 kinase family member, is a CREB kinase under dominant control of p38alpha mitogen-activated protein kinase (p38alphaMAPK) J Biol Chem. 1998;273(45):29661–29671. doi: 10.1074/jbc.273.45.29661. [DOI] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Samant SA, Wolfgeher D, Trivedi CM, Gupta MP. Acetylation of a conserved lysine residue in the ATP binding pocket of p38 augments its kinase activity during hypertrophy of cardiomyocytes. Mol Cell Biol. 2011;31(11):2349–2363. doi: 10.1128/MCB.01205-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17(24):7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, … Guo S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38alpha MAPK during insulin resistance. Diabetes. 2013;62(11):3887–3900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Ling S, Castillo AC, Long B, Birnbaum Y, Ye Y. Regulation of phosphatase and tensin homolog on chromosome 10 in response to hypoxia. Am J Physiol Heart Circ Physiol. 2012;302(9):H1806–1817. doi: 10.1152/ajpheart.00929.2011. [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270(13):7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Ren J, Zhang S, Kovacs A, Wang Y, Muslin AJ. Role of p38alpha MAPK in cardiac apoptosis and remodeling after myocardial infarction. J Mol Cell Cardiol. 2005;38(4):617–623. doi: 10.1016/j.yjmcc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Rolli M, Kotlyarov A, Sakamoto KM, Gaestel M, Neininger A. Stress-induced stimulation of early growth response gene-1 by p38/stress-activated protein kinase 2 is mediated by a cAMP-responsive promoter element in a MAPKAP kinase 2-independent manner. J Biol Chem. 1999;274(28):19559–19564. doi: 10.1074/jbc.274.28.19559. [DOI] [PubMed] [Google Scholar]
- Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, … Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78(6):1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G, Arthur JS, Kuma Y, Peggie M, Carr J, Murray-Tait V, … Cuenda A. p38gamma regulates the localisation of SAP97 in the cytoskeleton by modulating its interaction with GKAP. EMBO J. 2005;24(6):1134–1145. doi: 10.1038/sj.emboj.7600578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador JM, Mittelstadt PR, Guszczynski T, Copeland TD, Yamaguchi H, Appella E, … Ashwell JD. Alternative p38 activation pathway mediated by T cell receptor-proximal tyrosine kinases. Nat Immunol. 2005;6(4):390–395. doi: 10.1038/ni1177. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Brockdorff J, Gilman J, Mustelin T. Inhibition of T cell signaling by mitogen-activated protein kinase-targeted hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1999;274(17):11693–11700. doi: 10.1074/jbc.274.17.11693. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Gilman J, Mustelin T. Negative regulation of T cell antigen receptor signal transduction by hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1998;273(25):15340–15344. doi: 10.1074/jbc.273.25.15340. [DOI] [PubMed] [Google Scholar]
- Schroder D, Heger J, Piper HM, Euler G. Angiotensin II stimulates apoptosis via TGF-beta1 signaling in ventricular cardiomyocytes of rat. J Mol Med (Berl) 2006;84(11):975–983. doi: 10.1007/s00109-006-0090-0. [DOI] [PubMed] [Google Scholar]
- Sharov VG, Todor A, Suzuki G, Morita H, Tanhehco EJ, Sabbah HN. Hypoxia, angiotensin-II, and norepinephrine mediated apoptosis is stimulus specific in canine failed cardiomyocytes: a role for p38 MAPK, Fas-L and cyclin D1. Eur J Heart Fail. 2003;5(2):121–129. doi: 10.1016/s1388-9842(02)00254-4. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Scarabelli TM, Brar BK, Nakanishi Y, Matsumura M, Knight RA, Latchman DS. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J Biol Chem. 2001;276(30):28340–28347. doi: 10.1074/jbc.M101177200. [DOI] [PubMed] [Google Scholar]
- Stoecklin G, Stubbs T, Kedersha N, Wax S, Rigby WF, Blackwell TK, Anderson P. MK2-induced tristetraprolin:14-3-3 complexes prevent stress granule association and ARE-mRNA decay. EMBO J. 2004;23(6):1313–1324. doi: 10.1038/sj.emboj.7600163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher JM, Ren S, Herschman H, Wang Y. MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ Res. 2010;106(8):1434–1443. doi: 10.1161/CIRCRESAHA.109.213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Kawai K, Matsuzaki H, Osada H. p38 mitogen-activated protein kinase plays a key role in regulating MAPKAPK2 expression. Biochem Biophys Res Commun. 2005;337(2):415–421. doi: 10.1016/j.bbrc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, … Ricci R. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136(2):235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundivakkam PC, Natarajan V, Malik AB, Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38beta mitogen-activated protein kinase. J Biol Chem. 2013;288(23):17030–17041. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M, Maeda T, Saito H. Protein phosphatase 2Calpha inhibits the human stress-responsive p38 and JNK MAPK pathways. EMBO J. 1998;17(16):4744–4752. doi: 10.1093/emboj/17.16.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102(2):221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, … Otsu K. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117(4):545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, … Marber MS. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res. 2003;93(3):254–261. doi: 10.1161/01.RES.0000083490.43943.85. [DOI] [PubMed] [Google Scholar]
- Torcia M, De Chiara G, Nencioni L, Ammendola S, Labardi D, Lucibello M, … Cozzolino F. Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release. J Biol Chem. 2001;276(42):39027–39036. doi: 10.1074/jbc.M102970200. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22(6):1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahopoulos SA, Logotheti S, Mikas D, Giarika A, Gorgoulis V, Zoumpourlis V. The role of ATF-2 in oncogenesis. Bioessays. 2008;30(4):314–327. doi: 10.1002/bies.20734. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273(4):2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ma M, Hirabayashi K, Gurusamy N, Veeraveedu PT, Prakash P, … Aizawa Y. Swimming stress in DN 14-3-3 mice triggers maladaptive cardiac remodeling: role of p38 MAPK. Am J Physiol Heart Circ Physiol. 2007;292(3):H1269–1277. doi: 10.1152/ajpheart.00550.2006. [DOI] [PubMed] [Google Scholar]
- White A, Pargellis CA, Studts JM, Werneburg BG, Farmer BT., 2nd Molecular basis of MAPK-activated protein kinase 2:p38 assembly. Proc Natl Acad Sci U S A. 2007;104(15):6353–6358. doi: 10.1073/pnas.0701679104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga AT, Vogelzang I, Eggen BJ, Vellenga E. Erythropoietin-induced serine 727 phosphorylation of STAT3 in erythroid cells is mediated by a MEK-, ERK-, and MSK1-dependent pathway. Exp Hematol. 2003;31(5):398–405. doi: 10.1016/s0301-472x(03)00045-6. [DOI] [PubMed] [Google Scholar]
- Wood CD, Thornton TM, Sabio G, Davis RA, Rincon M. Nuclear localization of p38 MAPK in response to DNA damage. Int J Biol Sci. 2009;5(5):428–437. doi: 10.7150/ijbs.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing B, Bachstetter AD, Van Eldik LJ. Deficiency in p38beta MAPK fails to inhibit cytokine production or protect neurons against inflammatory insult in in vitro and in vivo mouse models. PLoS One. 2013;8(2):e56852. doi: 10.1371/journal.pone.0056852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PR. Perspective on the discovery and scientific impact of p38 MAP kinase. J Biomol Screen. 2013;18(10):1156–1163. doi: 10.1177/1087057113497401. [DOI] [PubMed] [Google Scholar]
- Yu L, Yuan X, Wang D, Barakat B, Williams ED, Hannigan GE. Selective regulation of p38beta protein and signaling by integrin-linked kinase mediates bladder cancer cell migration. Oncogene. 2014;33(6):690–701. doi: 10.1038/onc.2013.20. [DOI] [PubMed] [Google Scholar]
- Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15(1):11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139(1):115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shen B, Lin A. Novel strategies for inhibition of the p38 MAPK pathway. Trends Pharmacol Sci. 2007;28(6):286–295. doi: 10.1016/j.tips.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Zhang S, Weinheimer C, Courtois M, Kovacs A, Zhang CE, Cheng AM, … Muslin AJ. The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J Clin Invest. 2003;111(6):833–841. doi: 10.1172/JCI16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Shively JE. Acceleration of bone repair in NOD/SCID mice by human monoosteophils, novel LL-37-activated monocytes. PLoS One. 2013;8(7):e67649. doi: 10.1371/journal.pone.0067649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Chu WF, Wu L, Li J, Liu QM, Lu YJ, … Yang BF. PAF exerts a direct apoptotic effect on the rat H9c2 cardiomyocytes in Ca2+-dependent manner. Int J Cardiol. 2010;143(1):86–93. doi: 10.1016/j.ijcard.2009.01.068. [DOI] [PubMed] [Google Scholar]