Abstract
A fundamental feature of the mammalian cortex is to guide movements in time. One common pattern of neural activity observed across cortical regions during temporal control of action is ramping activity. Ramping activity can be defined as consistent increases or decreases in neuronal firing rate across behaviorally relevant epochs of time. Prefrontal brain regions, including medial frontal and lateral prefrontal cortex, are critical for temporal control of action. Ramping is among the most common pattern of neural activity in these prefrontal areas during behavioral tasks. Finally, stimulating prefrontal neurons in medial frontal cortex can influence the timing of movement. These data can be helpful in approaching human diseases with impaired temporal of action, such as Parkinson's disease and schizophrenia. Cortical ramping activity might contribute to new diagnostic and therapeutic strategies for these and other debilitating human diseases.
Keywords: Climbing, dopamine, interval timing, reaction time, prefrontal cortex
Introduction
Finding food and evading threats is critical for mammalian behavior and requires the ability to guide movements in time. For humans, the temporal control of action is central to complex activities such as cooking and driving. In this review, I argue that ramping activity in the prefrontal cortex critically regulates how movements are guided in time to achieve behavioral goals. I focus on epochs of several seconds, as temporal processing at shorter or longer scales can involve distinct neural systems [1,2].
Timing has been extensively addressed by theoreticians for decades [3]. Much of this work concerns the perception of time by the brain. Perceptual timing consistently recruits subcortical networks in the cerebellum, striatum, and brainstem [4]. In the last decade, evidence has accumulated that frontal and visual cortical areas are also required for temporal control of action. Posterior cortical areas are discussed in a companion review by Shuler et al., in this issue. Here, I focus on the frontal cortex, which is chiefly concerned with motor control.
Neurophysiology facilitates investigation into how neural networks instantiate timing processes that allow movements to be coordinated in time. Most neurophysiological tasks involve some amount of temporal expectation, or the anticipation of when events or movements will occur in time. Temporal expectation can be captured mathematically via a ‘hazard’ function [5]. For instance, when an event is likely to occur within a given amount of time, if the event fails to occur at time x, then the probability that it will occur at time x+1 will increase. Organisms capitalize upon temporal information when preparing movements, as certainty regarding when events will occur will progressively increase as time unfolds. For instance, a sprinter might respond to the starting gun fastest after waiting a long time, because after a long delay they are fully prepared to respond [6]. Temporal preparation can be ‘embodied’ in movements [7]. In this sense, temporal control of action is a subset of motor control.
Furthermore, temporal control demands executive resources such as working memory and attention [8,9] albeit at an elementary level [10,11]. That is, loading executive functions such as working memory or attention can interfere with guiding movements in time [8]. These data indicate that timing shares resources with classical executive processes such as working memory, attention, and reasoning [8,10]
Ramping activity
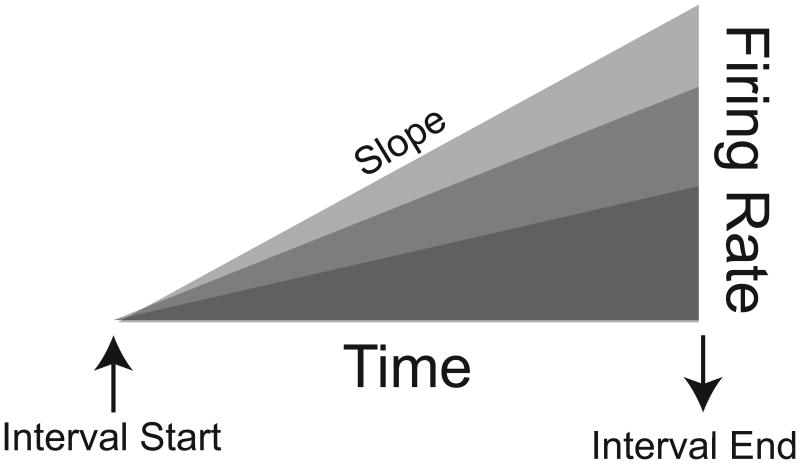
One pattern of cortical neuronal activity that robustly encodes temporal information is ramping, which can be defined as consistent increases or decreases in firing rate over time (Fig 1). Ramping is the most common pattern of activity in frontal cortex during timing tasks [12–14], and typically starts at the beginning of the interval and consistently changes until the end of the interval. This pattern of activity could readily encode the accumulation of temporal evidence; i.e., as time passes, temporal expectation increases and neural activity increases or decreases. In this sense, ramping reflects temporal integration as has been suggested by detailed modeling of timed behavior using drift-diffusion or integrative models [15,16]. Precise temporal information can be encoded in the slope and maximum activity of the ramp (Fig 1), as was first shown in recordings from inferotemporal cortex [17]. Increasing variance at longer temporal intervals might be a correlate of the scalar property of timing [3,18]. The pattern of ramping neurons need not be linear; indeed, if cortical neurons encode hazard functions they would be expected to have exponential features [16,19]. Ramping might also occur across a population of neurons via recurrent network interactions [20]. Other patterns such as persistent activity are also commonly observed that explicitly encode mnemonic information. Many cells with persistent activity can have ramping features [21]. Moreover, ramping is the integral or cumulative sum of persistent activity, indicating that ramping could encode cognitive variables in addition to timing, such as error signals or working memory [13]
Figure 1.
Ramping activity. Neural activity increases after the start of a temporal interval, as indicated by a predictive stimulus or preparatory movement. Temporal expectation anticipating movement and/or reward grows over time and neural ramps accordingly. The slope of the ramp can also encode interval duration. Ramping activity can also decrease which would be the inverse of the pattern represented here, and can also have logarithmic features.
One correlate of population-level ramping might be the Bereitschaftspotential, or ‘readiness potential’ [22]. This potential is a several microvolt-range deflection in EEG prior to voluntary movement which can begin seconds prior to movement initiation, as measured by EMG. While these potentials are largest over somatotopic motor cortex, early components of Bereitschaftspotential are distributed across frontal cortex and occur earliest over medial regions of frontal cortex [23,24]. Late components of readiness potentials also ramp, but it is as-of yet unclear how neural activity is transformed into readiness potentials.
Because of the distributed nature of temporal processing [25], it is critical to establish how cortical signals contribute to behavior. One set of criteria are that neuronal signals must be a) necessary -disruption of the signal decreases temporal control of action, b) correlated - the signal is correlated with temporal control on single trials, and c) sufficient- introducing or enhancing the temporal signal must improve how movements are guided in time. While many neuronal signals meet at least of one these criteria, even signals that appear to correlate strongly with temporally-controlled behavior can fail this test. For instance, motor cortical neurons are strongly correlated with when animals move. However, disrupting these neurons degrades specific movements but not the timing of movement initiation [26]. Similarly, ramping neurons in parietal and temporal cortex can encode highly specific aspects of temporal processing [17,18,27]; to our knowledge there is no evidence that disruption or stimulation of these areas influences timing [28]. This set of criteria can be vulnerable to conceptual flaws as the massive redundancy of neural systems makes them resistant to disruption, makes behavioral correlations omnipresent, and can make stimulation experiments difficult to interpret [29].
Prefrontal cortex ramps while animals wait to respond
Prefrontal regions include medial frontal cortex (MFC; cingulate / prelimbic cortex, BA 24/32; lead Cz from EEG) and lateral prefrontal areas in the middle frontal gyrus (BA 9/46) [30]. Several studies have shown that these areas are required for temporal processing. For example, humans with lesions of superior medial or right lateral frontal cortex have increased variability in tasks requiring temporal control [31,32]. Reversible lesions with rTMS of human right lateral frontal cortex shortened the duration that subjects pressed a spacebar when reproducing either 5 or 15 s durations[33]. Rodents lack lateral frontal regions, but rodent MFC encompasses anterior cingulate and supplementary motor areas have homologies with structures in primates [30]. Disrupting rodent MFC increases temporal errors during a time-estimation task [34]. Lesions or reversible disruptions of MFC also impair rodents' ability to move at the right time by increasing the variability of responses during interval-timing tasks, in which subjects must estimate an interval of several seconds as instructed by a stimulus [35–37]. Furthermore, inactivating MFC impairs neuronal activity related to inhibiting temporally inappropriate responses in motor cortex [38].
Temporal control in MFC depends requires dopaminergic signaling via D1 dopamine receptors (D1DR). Disrupting dopamine in mesocortical pathways impairs interval timing [14,36,39]. Focal dopamine receptor blockade in MFC implicates D1 but not D2 receptors in temporal processing [36,40]. MFC D1DR agonists or antagonists attenuates MFC ramping activity [14, 41], consistent with the role of prefrontal D1DRs in cognition [42]. Optogenetic inhibition of medial frontal neurons expressing D1DRs impairs temporal control of action on single trials [36]. These data provide convergent evidence that MFC D1DRs are necessary for temporal control of action.
MFC neurons robustly ramp [43,44]. Ramping activity predicts actions, often beginning several seconds before animals initiate their response [14,19,37]. This makes the signal unlikely to be explicitly movement-related. Ramping activity readily scales over a variety of intervals [19,37]. In premotor areas, movements are typically initiated when activity reaches a threshold [45], although it is unclear how this threshold is determined. Kim et al. found that ramping activity of MFC neurons over several intervals was best fit by logarithmic rather than linear functions [19]. These data from frontal cortex match data from macaque parietal cortex and indicate that MFC neurons might encode hazard functions of reward probabilities over time [27]. Although lateral frontal areas are not reliably activated during timing tasks in EEG and brain imaging studies [9,39], neurons in lateral frontal cortex also robustly ramp to explicitly encode time [46]. These recordings around the principal sulcus of macaques are in the same region as lesions that disrupt temporal control in humans [31].
Compared to correlative evidence, there have been far fewer studies that have stimulated cortical regions and have influenced when animals move. In parietal areas, microstimulation influences decisions, but does not reliably influence movement time [28]. Microstimulation of supplementary motor areas in primates can influence the timing of movements when significant executive control is required [47,48]. Inferences from microstimulation studies in cortical areas are challenging because many circuit elements can be stimulated. We recently addressed this issue using optogenetic stimulation of MFC D1DR-expressing neurons. We found an increase in peak responding only at the end of the interval. These data raise several questions in the context of ramping activity. First, if ramping activity represents time, optogenetically stimulating these neurons could have complex results because MFC neurons ramp both up and down [12,14]. However, MFC stimulation did not shift response-timing curves forward or backward in time. Rather, rodents responded more only at the end of the interval end, when they were most likely to get rewards. This pattern could be consistent with MFC ramping activity representing temporal rules – i.e., the rule to wait until the interval end and then respond. This idea would require some other area with explicit temporal information to provide input of temporal information to the MFC. Such areas might include the striatum [4] or the cerebellum [2]. The idea that MFC encodes temporal rules is broadly consistent with its role in exerting top-down control of action [10,38]. Still, these experiments do not explicitly manipulate ramping activity. To manipulate ramping neurons, neurons that ramp must be first identified, and then somehow specifically and dynamically manipulated. Moreover, disrupting these neurons should degrade temporal processing in areas that have well-described roles in timing, such as the striatum [9]. Finally, stimulating ramping neurons should improve timed behavior in animals with deficits in temporal control, such as animals lacking dopamine. These and related experiments likely require technical advances to specifically identify ramping neurons and dynamically stimulate them. Working towards these technical advances will help clarify the role of cortical function in elementary cognitive operations.
Clinical Implications
Timing tasks are ideal to study cortical function and dysfunction. Patients with many human diseases have reliably impaired timing (Table 1). Timing tasks can be readily adapted to patient populations with profoundly impaired cortical function. They can be deployed in the operating room or the intensive care unit, when consciousness may be impaired and experiments may be limited. Thus, timing tasks can provide a unique window on cortical function [11].
Table 1.
Diseases with impaired timing and key cortical structures and neurotransmitters involved.
For example, patients with schizophrenia have consistent impairments in their perception of time [49]. Patients with Parkinson's disease have impaired cortical function, and have executive dysfunction which can impact their ability to perform interval-timing tasks [11,39,40]. Drugs that affect dopaminergic systems reliably impair performance during timing tasks [51], and might provide a window not only into timing, but other complex processes such as emotion [52]. It is not clear how these manipulations impair ramping activity in humans; however, in rats, frontal dopamine depletion attenuates ramping [39].
Moreover, rodents and humans have strikingly similar neural correlates of timing tasks in MFC – for instance, rodents and humans have ramping activity as well as delta/theta rhythms that synchronize single neurons [53]. Delta/theta power is attenuated in both humans with PD and in rodents with MFC dopamine depletion [39], and this spectral activity can be coupled with ramping neurons in rodents [14,39]. These data lead to the hypothesis that attenuated delta/theta activity in human disease is linked to attenuated ramping activity. Future studies with intraoperative recordings in human patients might be able to directly test the idea.
In summary, this article argues that ramping activity in prefrontal cortex critically regulates the temporal control of action. Establishing precisely how the prefrontal cortex encodes and instantiates the temporal control of action might help diagnose diseases that degrade cortical function. If ramping activity is attenuated during timing tasks, this might be detectable in macro-level signals such as EEG [39]. In addition, this knowledge could help identify and optimize much-needed pharmacological and brain stimulation-based interventions for these devastating diseases.
Highlights.
Timing involves frontal areas of the cerebral cortex
Neurons in the frontal cortex ramp, or increase or decrease activity during temporal intervals
These neurons are necessary and may be sufficient for temporal control of action
These insights may be relevant for diseases with impaired timing
Acknowledgments
K08 NS078100 and R01 NS089470 from NINDS. Thank you to Krystal Parker, Eric Emmons, Ryan Kelley, Mark Laubach, and Marshall Shuler for feedback.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 2.Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann N Y Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- 4.Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Nobre A, Correa A, Coull J. The hazards of time. Curr Opin Neurobiol. 2007;17:465–470. doi: 10.1016/j.conb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Naatanen R. The diminishing time-uncertainty with the lapse of of time after the warning signal in reaction-time experiments with varying foreperiods. Acta Psychologia. 1970;1970:399–419. doi: 10.1016/0001-6918(70)90035-1. [DOI] [PubMed] [Google Scholar]
- 7.Cowen SL, McNaughton BL. Selective delay activity in the medial prefrontal cortex of the rat: Contribution of sensorimotor information and contingency. J Neurophysiol. 2007;98:303–316. doi: 10.1152/jn.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown SW. Timing and executive function: bidirectional interference between concurrent temporal production and randomization tasks. Mem Cognit. 2006;34:1464–1471. doi: 10.3758/bf03195911. [DOI] [PubMed] [Google Scholar]
- 9.Coull JT, Cheng R-K, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuster JM. The prefrontal cortex--an update: Time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 11.Parker KL, Lamichhane D, Caetano MS, Narayanan NS. Executive dysfunction in Parkinson's disease and timing deficits. Front Integr Neurosci. 2013;7:75. doi: 10.3389/fnint.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J Neurophysiol. 2009;101:2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Bekolay T, Laubach M, Eliasmith C. A spiking neural integrator model of the adaptive control of action by the medial prefrontal cortex. J Neurosci. 2014;34:1892–1902. doi: 10.1523/JNEUROSCI.2421-13.2014. *This modeling study demonstrated that elementary patterns of neuronal activity, such as ramping, could be easily identified by data-driven tools such as principal component analysis and could encode advanced aspects of timing tasks, such as memory and error signals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Narayanan NS. D1-Dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. J Neurosci. 2014;34:16774–16783. doi: 10.1523/JNEUROSCI.2772-14.2014. *In this study, authors described that focal infusion of MFC D1 agonists impaired interval timing and attenuated ramping activity by MFC neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durstewitz D. Self-organizing neural integrator predicts interval times through climbing activity. J Neurosci. 2003;23:5342–5353. doi: 10.1523/JNEUROSCI.23-12-05342.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Simen P, Balci F, de Souza L, Cohen JD, Holmes P. A model of interval timing by neural integration. J Neurosci. 2011;31:9238–9253. doi: 10.1523/JNEUROSCI.3121-10.2011. * An influential model of interval timing that incorporates drift-diffusion models highly relevant to ramping activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reutimann J, Yakovlev V, Fusi S, Senn W. Climbing neuronal activity as an event-based cortical representation of time. J Neurosci. 2004;24:3295–3303. doi: 10.1523/JNEUROSCI.4098-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38:317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- 19*.Kim J, Ghim J-W, Lee JH, Jung MW. Neural correlates of interval timing in rodent prefrontal cortex. J Neurosci. 2013;33:13834–13847. doi: 10.1523/JNEUROSCI.1443-13.2013. *Following a lesion study in rodents, this is the largest dataset of neuronal activity during a timing task and clearly shows that over of a few seconds ramping activity scales logarithmically. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong KF, Wang XJ. A recurrent network mechanism of time integration in perceptual decisions. J Neurosci. 2006;26:1314–1328. doi: 10.1523/JNEUROSCI.3733-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 22.Kornhuber HH, Deecke L. Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflügers Arch. 1965;284:1–17. [PubMed] [Google Scholar]
- 23.Yazawa S, Ikeda A, Kunieda T, Mima T, Nagamine T, Ohara S, Terada K, Taki W, Kimura J, Shibasaki H. Human supplementary motor area is active in preparation for both voluntary muscle relaxation and contraction: subdural recording of Bereitschaftspotential. Neurosci Lett. 1998;244:145–148. doi: 10.1016/s0304-3940(98)00149-9. [DOI] [PubMed] [Google Scholar]
- 24.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Karmarkar UR, Buonomano DV. Timing in the absence of clocks: Encoding time in neural network states. Neuron. 2007;53:427–438. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin JH, Ghez C. Differential impairments in reaching and grasping produced by local inactivation within the forelimb representation of the motor cortex in the cat. Exp Brain Res. 1993;94:429–443. doi: 10.1007/BF00230201. [DOI] [PubMed] [Google Scholar]
- 27.Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- 28.Hanks TD, Ditterich J, Shadlen MN. Microstimulation of macaque area LIP affects decision-making in a motion discrimination task. Nat Neurosci. 2006;9:682–689. doi: 10.1038/nn1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narayanan NS, Kimchi EY, Laubach M. Redundancy and synergy of neuronal ensembles in motor cortex. J Neurosci. 2005;25:4207–4216. doi: 10.1523/JNEUROSCI.4697-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laubach M. Neural basis of motivational and cognitive control. MIT Press; 2011. A comparative perspective on executive and motivational control by the medial prefrontal cortex. [Google Scholar]
- 31.Picton TW, Stuss DT, Shallice T, Alexander MP, Gillingham S. Keeping time: Effects of focal frontal lesions. Neuropsychologia. 2006;44:1195–1209. doi: 10.1016/j.neuropsychologia.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Koch G, Oliveri M, Carlesimo GA, Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002;59:1658–1659. doi: 10.1212/01.wnl.0000032504.45792.8f. [DOI] [PubMed] [Google Scholar]
- 33.Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60:1844–1846. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Jung AH, Byun J, Jo S, Jung MW. Inactivation of medial prefrontal cortex impairs time interval discrimination in rats. Front Behav Neurosci. 2009;3:38. doi: 10.3389/neuro.08.038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA. 2012;109:20726–20731. doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Xu M, Zhang S, Dan Y, Poo M. Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci USA. 2014;111:480–485. doi: 10.1073/pnas.1321314111. * These authors cooled and recorded from rodent frontal cortex and found strong ramping activity that scaled with how long animals were waiting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Parker KL, Chen K-H, Kingyon JR, Cavanagh JF, Naryanan NS. Medial frontal ∼4 Hz activity in humans and rodents is attenuated in PD patients and in rodents with cortical dopamine depletion. J Neurophysiol. 2015;114:1310–1320. doi: 10.1152/jn.00412.2015. *This study found that in both humans and rodents, low-frequency oscillations around the instructional cue in medial frontal cortex was decreased in PD patients without dopamine and in rodents without frontal dopamine. These oscillations could also synchronize ramping activity in rodent medial frontal cortex, implying that low-frequency activity may be dopamine-dependent and help initiate cortical ramping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parker KL, Alberico SL, Miller AD, Narayanan NS. Prefrontal D1 dopamine signaling is necessary for temporal expectation during reaction time performance. Neuroscience. 2013;255:246–254. doi: 10.1016/j.neuroscience.2013.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker KL, Ruggiero RN, Narayanan NS. Infusion of D1 dopamine receptor agonist into medial frontal cortex disrupts neural correlates of interval timing. Frontiers in Behavioral Neuroscience. 2015;9:294. doi: 10.3389/fnbeh.2015.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- 43.Niki H, Watanabe M. Cingulate unit activity and delayed response. Brain Res. 1976;110:381–386. doi: 10.1016/0006-8993(76)90412-1. [DOI] [PubMed] [Google Scholar]
- 44.Niki H, Watanabe M. Prefrontal and cingulate unit activity during timing behavior in the monkey. Brain Res. 1979;171:213–224. doi: 10.1016/0006-8993(79)90328-7. [DOI] [PubMed] [Google Scholar]
- 45.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 46.Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol. 2006;95:3281–3285. doi: 10.1152/jn.01011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuphorn V, Schall JD. Executive control of countermanding saccades by the supplementary eye field. Nat Neurosci. 2006;9:925–931. doi: 10.1038/nn1714. [DOI] [PubMed] [Google Scholar]
- 48.Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci. 2007;10:240–248. doi: 10.1038/nn1830. [DOI] [PubMed] [Google Scholar]
- 49.Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62:1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson's disease: a dopamine-related dysfunction. J Cogn Neurosci. 1998;10:316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- 51.Merchant H, Harrington DL, Meck WH. Neural basis of the perception and estimation of time. Annu Rev Neurosci. 2013;36:313–336. doi: 10.1146/annurev-neuro-062012-170349. [DOI] [PubMed] [Google Scholar]
- 52.Cheng R-K, Tipples J, Narayanan NS, Meck WH. Clock speed as a window into dopaminergic control of emotion and time perception. Timing Time Percept. in press. [Google Scholar]
- 53.Narayanan NS, Cavanagh JF, Frank MJ, Laubach M. Common medial frontal mechanisms of adaptive control in humans and rodents. Nat Neurosci. 2013;16:1888–1897. doi: 10.1038/nn.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao AK, Marder KS, Uddin J, Rakitin BC. Variability in interval production is due to timing-dependent deficits in Huntington's disease. Mov Disord. 2014;29:1516–1522. doi: 10.1002/mds.25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: Effects of duration, distraction, and stimulant medication. J Int Neuropsychol Soc. 1997;3:359–369. [PubMed] [Google Scholar]