Abstract
It is well established that the environment contributes to health. However, few studies have evaluated environmental exposures in women that may influence future health of their offspring. Knowledge gained may inform nursing how to better advocate for patients and families; and provide individualized interventions and education. Therefore, a more comprehensive investigation of the maternal exposome to uncover mechanistic insight into complex disease in offspring is warranted. To advance understanding of biological mechanisms that contribute to high-risk birth outcomes and offspring predisposition to disease, it will be necessary to measure a range of exposures and biomarkers before and during pregnancy.
Keywords: epigenetics, maternal exposure, microbiota, pregnancy, prenatal exposure delayed effects
Background
With increasing prevalence of complex chronic disease in children such as asthma, hypertension, obesity, chronic kidney disease, and type II diabetes, there has been an emerging focus on the contributions of early life exposures that lead to chronic health outcomes. Of particular importance to this area of research is the concept of the exposome, initially proposed by Christopher Wild in 20051, defined as the totality of environmental exposures from the prenatal period across the life course. A critical consideration for the human exposome to better understand disease susceptibility is the interface with multiple exposures that occur across the lifespan, including sensitive developmental time periods. However, our current understanding of how early life exposures, including those during preconception and pregnancy, contribute to childhood disease and maladaptive development is limited and has focused primarily on single exposure health effects (e.g., relationship of alcohol to fetal alcohol syndrome). Recent reviews of health outcomes in children related to maternal preconception and pregnancy exposures have concluded that currently available data are inconsistent and inadequate to accurately identify associations with disease in humans2.
Yet, there is no question that health outcomes, and even life expectancy, are strongly influenced by the characteristics of one’s environment3. Environmental exposures comprise a significant fraction of disease risk, and longstanding evidence supports that the timing, duration, and pattern of exposures play a critical role in the profile of disease risk4. For example, chronic stress experienced by mothers during pregnancy is associated with poor birth outcomes (i.e., a sustained inflammatory response related to stress is an example of an in utero endogenous exposure)5,6. In particular, maternal stress during the fifth and sixth months of pregnancy has been shown to have an increased association with poor outcomes including preterm birth, low birth weight and small for gestational age infants5. While it is estimated that 70-90% of chronic diseases are strongly associated with a variety environmental exposures7, the extent to which preconception and pregnancy exposures contribute to poor pregnancy outcomes and predisposition to disease later in life is unclear. Major obstacles include a limited understanding of the scope of environmental heterogeneity both within and between individuals and the development of appropriate measurement strategies. This has major implications on nursing science and practice, from the provision of nursing interventions that are provided to pregnant women and mothers, to education and resources that are given (e.g., whether to advise avoiding certain foods/drinks, cosmetics, cleaning products, or other exposures before or during pregnancy). As public health advocates, nurses are well suited to advance this area of research and build the evidence required to drive health policy change aimed at reducing exposures that are linked with disease vulnerability and protecting the health of future generations.
A major challenge in this area of research is tied to one of the conceptual domains of the exposome, that is, multiple exposures with small to moderate effects likely combine to contribute to the development of complex diseases7,8. However, the exposome paradigm complements research on the molecular origins of disease that recognizes the potential for interactions between an individuals’ genetic background and exposome whereby the sensitivity to the environment is influenced by allelic variation8. For example, measuring toxicant levels in the environment [e.g., polychlorinated biphenyls (PCBs)] may not be the best proxy for associating exposures to disease outcomes, particularly when they are ubiquitous. Individuals with the same levels of exposures may not all develop disease, or individuals with lower exposures may be ill due to genetic differences. The measurement of the so-called “gene by environment interaction (GxE)” will require the collection of blood or other body fluid samples from individuals to measure toxins, or their metabolites, along with genomic biomarkers to better characterize the influence of the exposome8. Greater precision in the measurement of multiple exposures and identification of their biological modifiers will provide stronger evidence on which to base health policy and patient teaching.
Another major research challenge is the further development of the theoretical underpinning that establishes the role of environmental exposures across the lifespan on health, taking into account preconception health of the mother and pregnancy. The theory of fetal origins of adult disease can be used to guide research investigating health outcomes associated with preconception and in utero exposures9. Proposed by David Barker after observing a correlation between low birth weight and increased risk for cardiovascular disease later in life10, the theory states that early life events, especially during the critical fetal development period, predispose individuals to disease later in life. As epidemiologic evidence supporting Barker’s hypothesis continues to increase, investigations measuring the in utero environment and biological samples, similar to the aforementioned approach described by Rappaport8, are well positioned to identify causative agents and disease programming mechanisms that occur during this critical period of human development. Thus, although there are challenges in conducting exposome research, the knowledge to be gained will be instrumental for advancing personalized healthcare and precision medicine.
In this review we present an evidence-based model to describe how the preconception and pregnancy exposome can contribute to high-risk birth outcomes and increased vulnerability to disease in the offspring throughout the life course (Figure 1). We describe how the fetal system responds through biologically mediated mechanisms to maternal exposures that potentially prime the body to anticipate similar exposures after birth. While these adaptations may provide short-term gains, they may also have maladaptive consequences later in life that contribute to adult disease. Evidence is provided that biologic programming continues to be modified after birth from additional environmental exposures experienced across the life span. The discussion that follows describes the mediation of the maternal exposome to offspring through candidate biological mechanisms and highlights key relationships between the maternal preconception and pregnancy exposome and health outcomes in offspring.
Figure 1. Relationship between Maternal Exposome and Health Outcomes of Offspring.
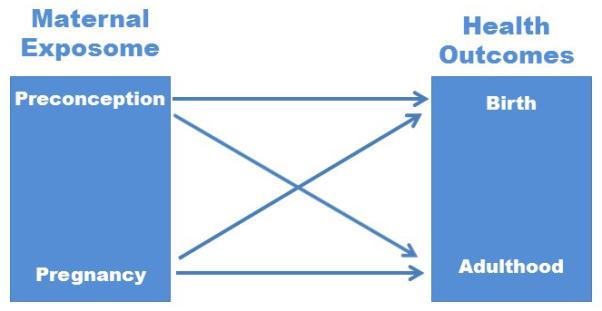
The maternal exposome contains both exogenous and endogenous exposures that can be shared with the fetus during development. Exposures are specific time points have to potential to program predisposition for specific birth and adult health outcomes.
Discussion
Investigating the impact of the maternal exposome on birth and health outcomes of offspring has traditionally been difficult due to the limited inclusion of pregnant women in clinical research. As a prime example, there remains a paucity of data concerning the consequences of in utero exposure to medications – a highly relevant topic for the 64% of pregnant women who will be prescribed a medication during pregnancy11. Of particular concern is that 90% of medications approved for use in the United States have unknown teratogenic profiles12. Similar issues exist related to chemicals present in the food supply such as methylmercury and endocrine disrupting chemicals. An extensive review of the environment and reproductive health by Caserta and colleauges13 acknowledge that practical constraints have hindered previous research in women, particularly during pregnancy. Additional issues that have limited knowledge development in this area include: lack of measurement of toxicants in maternal and fetal samples, small sample sizes, cross-sectional designs and studies that have not followed offspring to adulthood or have focused on a limited subset of the population.
Samples deposited in biobanks, such as the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) Repository (http://www.gapps.org/research/gapps_repository), provide an opportunity to investigate the relationship of the exposome and molecular biomarkers without conducting intervention studies on pregnant women that could present risk to the offspring. The GAPPS repository, for instance, contain numerous sample types from mothers (blood, urine, vaginal swabs, placental tissue) and offspring (cord blood, meconium, urine), as well as extensive questionnaire data, that can be utilized to better understand relationships between maternal exposome and offspring health. For example, Budtz-Jorgensen and colleagues14 conducted a longitudinal study questionnaire data, cord blood, and maternal hair samples previously collected at the time of birth to measure methylmercury exposure in utero. The authors concluded that methylmercury levels during fetal development were associated with neurobehavioral deficits of the infants although the effect was attenuated when polychlorinated biphenyls (PCBs) were also present14. In another example, Baron-Cohen and colleagues15 analyzed biobanked amniotic fluids from two cohort studies and found that elevated levels of specific hormones were associated with autism phenotypes in male offspring. Additional studies that incorporate the use of biobanked samples may identify associations between environment and disease that have not previously been discovered or only documented using animal models. Thus, biobanks provide a solution to the challenges of studying the exposome for the following reasons: 1) sample collection can be standardized and utilized at multiple locations to pool resources; 2) the infrastructure encourages collaboration among investigators with similar research interests; and, 3) studies can be coordinated to reduce redundancy and accelerate discovery16.
Penetrance of Exposure
Utilization of biological samples to study the exposome will encourage the discovery of alterations that represent how the exposome “gets under the skin” and contributes to health outcomes. The relationship between the maternal exposome and health outcomes of the offspring is complex and may vary depending on how and when the exposures are conferred to offspring. For example, exposures could have direct (e.g., chemical delivered directly to the fetus through the placenta) and/or indirect (e.g. the chemical causes vasoconstriction of in the placenta reducing blood flow and nutrients to the offspring) effects on the in utero environment altering fetal programming. Figure 2 illustrates the layers of “skin” that should be considering while studying how exposures may influence the health of offspring. The environment, maternal health, and fetal environment are all part of the offspring’s first environment. The effect an exposure has on offspring predisposition to disease may be eliminated or exacerbated by other maternal exposures, as the example of methylmercury and PCBs as previously described16.
Figure 2. Exposome Penetrance.
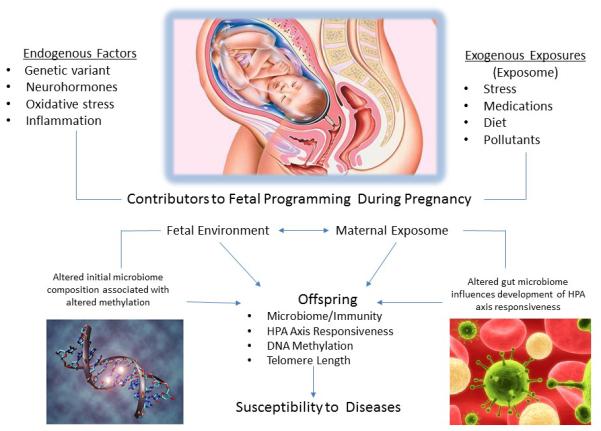
Exogenous factors in the maternal environment are also the exposome of the offspring while in utero. The exposure may impact fetal development by passing directly to the fetus or by altering maternal endogenous factors (i.e. hormones) that may alter the in utero environment. Exposures during development may alter predisposition to disease in the offspring.
Potential Mechanisms Contributing to Fetal Programming
In Figure 3, we suggest various maternal endogenous and exogenous factors that could contribute to fetal programming of later health outcomes. Several biological mechanisms resulting from maternal exposures may underlie the effects on health outcomes of the offspring – examples are listed and described in the following sections. This discussion is not intended to be all inclusive of potential programming mechanisms, instead, we highlight select mechanisms that are frequently studied and potentially function as biological mediators in fetal programming from maternal exposures, specifically DNA methylation, telomere length, the microbiome, and hypothalamic-pituitary-adrenal (HPA) axis responsiveness.
Figure 3. Variables related to programming mechanisms.
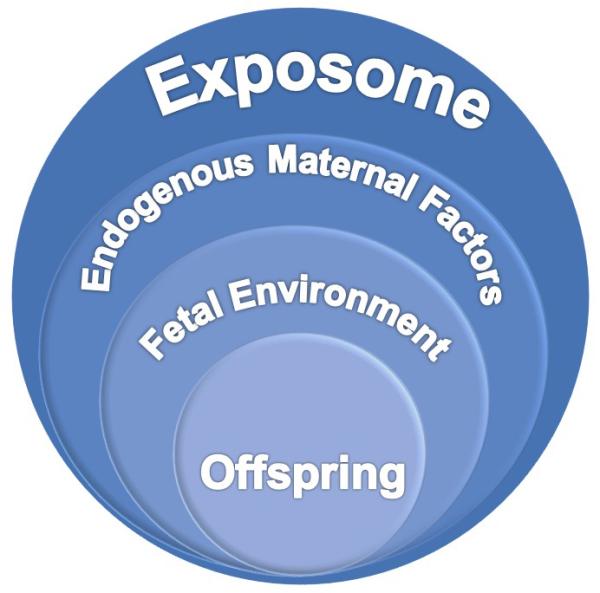
Endogenous and exogenous exposures during a woman’s life course contribute to the intrauterine environment and may influence vulnerability to disease at the level of the gamete as well as during the development of the fetus through alterations in microbiome composition, HPA axis responsiveness, DNA methylation, and telomere length. The offspring may exhibit altered health outcomes based on alterations to gametes prior to conception, exposures in utero, and throughout development. While the fetus is developing and forming gametes for the next generation, the gametes may be affected by the exposures and predispose the next generation to disease.
DNA Methylation
Epigenetic mechanisms contribute to the development of a certain phenotypes, or observable characteristics17. Epigenetic modifications can alter how the DNA sequence is packaged in cells, impacting which genes are expressed or silenced, and include DNA methylation, histone modifications, chromatin remodeling, and microRNA. DNA methylation is the most well understood epigenetic modification to date and can be modified by endogenous and exogenous environmental exposures (Table 1). Assessing preconception and early life exposures, which are likely correlated (Figure 1), to identify associations with disease processes, is novel and may be useful in identifying programming events (e.g., a change in epigenetic signature) that later result in altered gene expression when a specific secondary environmental insult occurs (e.g., diseases such as cancer). Permanent alterations in fetal DNA methylation patterns can occur in response to chemical exposures during critical periods of intrauterine development resulting in altered gene expression and health outcomes throughout the life course4,18. It is well established in animal models that variation in nutrients during pregnancy can result in DNA methylation changes that alter the phenotype of the offspring19. It was recently reported that human maternal diet at conception, falling into the preconception area of our model, can alter DNA methylation patterns in offspring although the phenotypic significance is unknown20. Furthermore, several studies have identified specific chemicals in the food supply that induce DNA methylation changes in offspring4,21, however, questions remain regarding the long-term impact on health outcomes associated with these changes.
Table 1.
Environmental Exposures that Alter DNA Methylation
| Environmental factor | Effect | Reference |
|---|---|---|
| Alcohol | ↓ DNA methylation in colonic mucosa | 22 |
| Bisphenol-A (BPA) | ↓ DNA methylation in Hoxa10 gene, altered estrogen response |
23 |
| Diethylstilbestrol (DES) | ↑ DNA methylation in Hoxa10 gene, reproductive anomalies |
21 |
| Exercise | Dose dependent ↓ DNA methylation in skeletal muscle |
24 |
| Fear | Changes in multiple genes altering memory formation |
25 |
| Hydralazine | Delayed response inhibition of DNA methylation | 26 |
| Maternal Care | ↓ DNA methylation in hippocampus with ↑ maternal care |
27 |
| Maternal Diet | ↓ protein and energy results in ↑ DNA methylation in offspring |
19, 20 |
| Microbiome | Altered initial colonization ↑ methylation in colon & lung |
28 |
| Procainamide | Inhibits DNA methylation | 26 |
| Smoking | ↑ DNA methylation in malignant lung & healthy tissue |
29 |
| Traffic pollution | ↓ DNA methylation in repetitive DNA elements | 30 |
Telomeres
Telomeres are DNA repeats present at the ends of chromosomes that are important for protecting the chromosome during cell division and for chromosome stability31. Variation in telomere length has been associated with both environmental exposures and disease32,33. For instance, psychological stress has been posited to accelerate cellular oxidative stress and lead to chronic low-grade inflammation – two synergistic mechanisms that operate to shorten telomere length through the production of reactive oxygen species that promote DNA damage33. Critically shortened telomeres provides one pathway to altered gene expression and development of disease. Because of susceptibility to the aforementioned mechanism, telomere length has potential to serve as a biomarker for studying the impact of exposure to stress in pregnant women and their offspring. A review by Zhang, Lin, Funk, and Hou32 identified a number of environmental and occupational exposures that alter telomere length, including arsenic, traffic related air pollution, and pesticides. However, it is unclear if exposures that occur while in utero have the same impact on fetal telomere length. Although telomeres tend to become shorter as humans age, a study by Eisenberg, Hayes, and Kuzawa34 found telomeres were longer in offspring born to older fathers as were the subsequent generation of offspring. The findings of Eisenberg and colleagues suggest that exposures that alter telomere length in gametes may have long-lasting health implications for future generations.
Microbiome
The microbiome of the mother and/or infant may contribute to disease by altering immunity, as well as through epigenetic modifications. The fetal intestines are theoretically sterile at birth and become colonized with approximately 100 trillion microbes by adulthood35. The intestinal microbiota modulates the maturation of the immune system and significant alterations in composition can result in immune dysfunction36. Although the precise mechanisms of how immune programming occurs between microbes and their host, a study by Olszak and colleagues28 indicates that altering the initial composition of microbes that colonize a host will alter methylation patterns of immune cells that influence cellular responses later in life. Deciphering how the microbiome contributes to health and/or as a biomarker of health status is currently underway by several research groups participating in the Human Microbiome Project37. For example, differences in microbial diversity have been identified within the gut related to obesity38 and lower diversity of bacterial species in infancy has been associated with increased risk for developing allergies in children39. A recent study investigating the gut microbiota of malnourished children found the microbiota did not mature in the same way as healthy children and the bacterial composition reverted back to the immature state even after intensive malnutrition therapy40. The long-term implications of gut dysbiosis early in life, or how long the alterations persist, are yet to be determined. Additional research in this area is needed to determine if the microbes associated with a host are merely a biomarker associated with certain conditions or if the composition of microbes independently function as an exogenous exposure to influence the health outcomes of the host.
HPA Axis
In addition to exogenous exposures that occur during preconception or in utero, there are endogenous factors of the mother and fetus that can influence health outcomes. Some of the endogenous factors listed in Figure 3 (neurohormones and inflammation) are influenced by HPA axis responsiveness. The HPA axis is involved with controlling an individual’s response to stress41. Long term effects, such as premature mortality, heightened response to stress throughout life and altered reproductive functioning, have been associated with traumatic events early in life and are thought to be the result of altered priming of the HPA axis42. Excessive cortisol levels, which may result from altered HPA axis programming, can cause damage to tissues and has been associated with the development of disease43. Furthermore, cortisol crosses the placenta and exposure to elevated levels of cortisol in utero have been associated with cognitive and affective disorders in offspring44,45. Evidence that altered levels of endogenous factors, which are modulated by the HPA axis response to environmental stress, influence health outcomes indicate HPA axis responsiveness should also be considered while examining the exposome related to health.
Interaction of mechanisms
Collectively, but perhaps not equally, the maternal exposome and fetal environment contribute to microbiome-epigenetic-HPA axis interactions (Figure 3) that result in fetal programming, the physiological parameters of adaptation. Fetal programming determines the range of molecular events and behaviors that occur in response to specific stimuli, represented by the lines in the model associating exposures in the mother to outcomes of the offspring. These mechanisms could be modifiable components of fetal programming working synergistically to influence predisposition to complex diseases. Due to the plasticity of these mechanisms, they represent potential targets for intervention. An example of the interplay between exogenous and endogenous factors that contribute to health outcomes is the crosstalk between the microbiome and the HPA axis, particularly due to the impact on other biological processes that result in measurable biomarkers. Bidirectional communication between the microbiota and central nervous system take place through multiple pathways including neuroendocrine, neuroimmune, autonomic nervous system (sympathetic and parasympathetic arms) and the enteric nervous system46. Sudo and colleagues47 provided seminal evidence of the influence of intestinal microbiota on the development of the HPA axis. Subsequent studies suggest there is a critical window during early life in which colonization must occur for normal development and appropriate regulation of the stress response in later life48.
Evidence for Trans-generational Inheritance of Exposures
Exposures of the gametes, including those that occur during the prenatal period and early development, have been found to play a large role in risk of disease over the lifespan10. Studies in animal models have documented epigenetic changes that persist from generation to generation. For example, in murine models, the methylation changes associated with DES exposure persist into the third generation suggesting in utero exposures may have a lasting impact on the health of future generations49. The same chemical clearly demonstrates a link between environment and germ-line mutations in humans as a trans-generational alteration in DNA methylation patterns of the fetus caused by exposure to DES in utero. DES is a synthetic estrogen that was administered to pregnant women to prevent spontaneous abortions prior to the mid 1970’s. DES causes hypermethylation of the homeobox protein Hox-A10 (HOXA10), a gene that controls uterine organ development, resulting in reproductive tract anomalies that persist into adulthood21. Hypermethylation of HOXA10 was specific to the fetus and did not occur in laboratory experiments using cell lines or the somatic cells of pregnant women who received DES, suggesting that this molecular alteration is unique to in utero exposure.
Investigations are ongoing in grandchildren of women given DES. Current clinical evidence suggests DES granddaughters have more menstrual irregularities, infertility, and stillbirths50; and both granddaughters and grandsons exhibit more birth defects51. Other examples of trans-generational effects of environmental exposures, or epimutations, are listed in Table 2. This accumulating evidence supports that epimutations occur from exposure during gonadal sex determination, a highly vulnerable period when the germ line DNA is demethylated and remethylated in a sex specific manner52. The chemically-induced modifications in epigenetic programming of the germ line results in differentially altered epigenomes and transcriptomes in all tissues propagated from the affected sperm or ovum, which can influence development of disease in later life53. Although data is sparse related to trans-generational effects of environmental exposures in human studies, there is a need to better inform clinicians and patients about this critically vulnerable period during, and prior to, pregnancy54-57. Confirmation of animal models of trans-generational effects will require the enrollment at least 3 generations of participants (grandparent, parent, and child).
Table 2.
Exposures demonstrating inter-generational effects on DNA methylation
| Environmental factor | Effect | Reference |
|---|---|---|
| DES | Reproductive tract anomalies, cancer | 23 |
| Dioxin | Multiple diseases, fertility & pregnancy issues | 53 |
| Famine | Cardiovascular disease, altered glucose tolerance | 56 |
| Nutrition | Heart structure, HPA responsiveness, obesity | 18,58 |
| Vinclozolin | Decreased male fertility, sperm number & viability | 56 |
Preconception Exposome related to Birth Outcomes
In light of the limited exploration related to preconception health and birth outcomes, the Centers for Disease Control and Prevention (CDC) in the United States has drafted an action plan to improve preconception health to reduce poor birth outcomes; including recommendations to improve monitoring and surveillance during the preconception period58. Several studies in animal models have documented permanent phenotypic changes in offspring that were induced by environmental exposures and alteration in the preconception diet of the mother59,60. Robledo and colleagues found that maternal preconception exposure to several different persistent organic pollutants are associated with lower birth weights of human offspring61. Additionally, multiple risk factors associated with preconception health have been associated with poor birth outcomes. For example, women with any of the following characteristics and behaviors have a higher risk of having poor birth outcomes: being underweight, obese, a smoker, excessive alcohol intake, increased levels of stress, diabetes, or poor dietary habits62-65. The biological mechanisms to explain these associations have yet to be identified
Preconception Exposome related to Health Outcomes of Offspring in Adulthood
Given the recent CDC call for action to improve preconception care and knowledge related to birth outcomes, little is known about the long term implications of preconception exposures and disease processes that present later in the life of offspring. A review by Vassoler and colleagues identifies preconception exposures to drugs associated with addictions may alter adult health of the offspring in several ways, including but not limited to: reducing fertility, altering mental health outcomes and susceptibility to drug abuse among66. Two major studies designed to enhance knowledge development in this area are: Developmental ORIgins of healthy and unhealthy AgeiNg: Human early-life exposome (HELIX)67 and the role of maternal obesity (DORIAN)68. Both studies are currently underway in Europe, to investigate the influence of pre- and postnatal exposures on the health of offspring and will provide invaluable data for guiding future fetal programming research.
Pregnancy Exposome related to Birth Outcomes
Research investigating relationships between exposures during pregnancy and birth outcomes have increased in recent years. Several meta analyses of chemical exposures during pregnancy include but are not limited to: pollution69, secondhand smoke70, efavirenz71, polychlorinated biphenyls and dichlorodiphenyldichloroethylene72, domestic violence73, antidepressant use74,75, shift work76, statins77, and maternal obesity78. Many of the exposures listed were correlated with preterm birth, altered size and weight of the offspring. The role that biologic mechanisms, possibly due to epigenetic modifications via DNA methylation, that help to explain the associations between the aforementioned exposures and altered birth outcomes, have yet to be determined.
Pregnancy Exposome related to Health Outcomes of Offspring in Adulthood
Environmental exposures during fetal development can also contribute to the future risk of disease in offspring. In particular, the in utero environment impacts fetal programming of neuroendocrine and metabolic pathways as well as immune function9. Differences in predisposition to disease have been noted in offspring based on the health status of the mother during pregnancy. For example, retrospective studies from the Dutch famine of 1944-45 have shown a significantly higher prevalence of coronary heart disease among individuals who were born at a low birth weight from women exposed to severe nutrient deprivation during pregnancy9. More recent evidence of this relationship was reported in a study that found an association between maternal farm exposure and the response of immune cells to antigens in offspring, which may potentially mediate the risk of immune-based diseases such as asthma79. Research regarding the effects of chemical exposures during human pregnancy on the development of the offspring is limited. Yet, animal studies provide evidence of developmental defects caused by over 1200 commonly used chemicals, 40 of which are considered teratogenic in humans80. Consequently, evidence for short and long-term effects of medications developed within the past 50 years and prescribed to pregnant women, is drawn from animal models81 or retrospective studies82. Furthermore, the significant differences in the human placenta from those of animal models limits the ability to accurately predict how the chemical will affect human offspring83. Of the medications that have been investigated, most initial drug safety studies were carried out in the absence of assessing for various molecular modifications, like DNA methylation, which may alter health outcomes.
Summary
It is well established that the environment influences health outcomes. With an increasing incidence of chronic adverse child health outcomes (e.g., obesity), there has been more interest in identifying environmental exposures that contribute to the mechanisms of disease via fetal programming. Accumulating evidence suggests that endogenous and exogenous environmental factors at conception and during pregnancy may influence the body’s response to future exposures. In this paper, we have argued that the exposome paradigm, including measures of biomarkers and chemicals in biological samples, provides an ideal approach toward advancing this area of research. There are many avenues for exploring the influence of the exposome on human health because environmental exposures may affect multiple systems, or be tissue specific, with additional variation based on developmental stage of the individual at the time of the exposure. The incorporation of endogenous environmental exposures including psychosocial measures (e.g., psychological stress, anxiety, depression) along with exogenous environmental exposures, such as chemicals in the air from pollution, could help to identify specific combinations that lead to adverse health outcomes. The additional consideration of preconception exposures, exposures during the fetal period, and early life when investigating potential etiological pathways of adult onset disease may be important due to the effect of timing during sensitive developmental periods. In order to address these challenges, the use of common datasets, biobanked samples, and analytical tools capable of handling large datasets hold promise to advance this field of research. Historically, nurses have studied how environmental conditions impact the wellbeing of their patients. Incorporating biological measures in the exposome-wide approach noted by Rappaport and colleauges15 has the potential to identify combinations of exposures that lead to chronic disease states and/or exacerbate and alleviate symptoms.
Assessment and critical analysis of the exposome in utero and early life may uncover mechanistic insight into complex disease states. Both exogenous exposures and endogenous factors of the mother contribute to microbiome-epigenomic interactions that occur during pregnancy, which in turn, influence fetal programming – the physiological parameters that determine the offspring’s response to the environment. Most complex diseases of our time develop from a combination of mechanisms that may be orchestrated by fetal programming, some of which are potentially reversible. Many alternations, such as DNA methylation and telomere length, have become measureable by scientists in recent decades with potential to function as surrogate measures of how the body copes with exposures throughout life. Measurement of these modifications along with chemical levels in the blood (e.g. endogenous hormones and exogenous toxicants) may improve our understanding of chronic disease etiology and symptoms. Reductionist approaches to conducting research, while useful in identifying mechanisms associated with disease states, has resulted in our inability to accurately assess multiple interactions contributing to health outcomes because many individuals have multiple concurrent comorbid conditions and exposures. Evaluation of the exposome, via questionnaires and exploratory peripheral blood samples, during pregnancy is complex mainly because of the vast number exposures encountered across the lifecourse. Characterizing the exposome of research populations will require further development of measurement instruments. Within the holistic lens of nursing science, the exposome paradigm offers a systematic method for identifying the mixture of exposures over the lifespan that contribute to disease. This review and the proposed models explicate critical endogenous and exogenous factors that should be considered when conducting investigations on the exposome and highlights plausible systems-based interactions that can ultimately influence trans-generational health and vulnerability to disease.
Acknowledgements
none.
Source of Funding: The content of this publication is solely the responsibility of the authors and grant funding was not used to produce this manuscript. AS (R01NR013932 & R01NR012667) and TY (Burroughs Wellcome Fund (1015040), R01AG037986, R01NR012667, R01AA018333, & P60MD002256) are currently receiving research grant funding from the National Institutes of Health.
Footnotes
Conflicts of Interest: The remaining author has none to declare.
Contributor Information
Michelle L. Wright, Virginia Commonwealth University School of Nursing, Richmond, VA.
Angela R. Starkweather, Department of Adult Health and Nursing Systems, Virginia Commonwealth University School of Nursing, Richmond, VA, astarkweathe@vcu.edu.
Timothy P. York, Virginia Institute for Psychiatric and Behavioral Genetics, Department of Human and Molecular Genetics, Department of Obstetrics and Gynecology, Virginia Commonwealth University School of Medicine, Richmond VA, tpyork@vcu.edu.
References
- 1.Wild CP. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC. Prenatal Exposures to Environmental Chemicals and Children’s Neurodevelopment: An Update. Saf Health Work. 2013;4(1):1–11. doi: 10.5491/SHAW.2013.4.1.1. 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon PW, Bastian B, Anderson RN, Collins JL, Jaffe HW. Potentially preventable deaths from the five leading causes of death - United States, 2008-2010. MMWR Morb Mortal Wkly Rep. 2014;63(17):369–374. [PMC free article] [PubMed] [Google Scholar]
- 4.Dahlen HG, Kennedy HP, Anderson CM, et al. The EPIIC hypothesis: Intrapartum effects on the neonatal epigenome and consequent health outcomes. Med Hypotheses. 2013;80(5):656–662. doi: 10.1016/j.mehy.2013.01.017. 10.1016/j.mehy.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Class QA, Lichtenstein P, Långström N, D’Onofrio BM. Timing of prenatal maternal exposure to severe life events and adverse pregnancy outcomes: a population study of 2.6 million pregnancies. Psychosom Med. 2011;73(3):234–241. doi: 10.1097/PSY.0b013e31820a62ce. 10.1097/PSY.0b013e31820a62ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coussons-Read ME, Lobel M, Carey JC, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26(4):650–659. doi: 10.1016/j.bbi.2012.02.009. 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41(1):24–32. doi: 10.1093/ije/dyr236. 10.1093/ije/dyr236. [DOI] [PubMed] [Google Scholar]
- 8.Rappaport S, Barupal D, Wishart D, Vineis P, Scalbert A. The blood exposome and its role in discovering causes of disease. Environ Heal …. 2014;122(8):769–774. doi: 10.1289/ehp.1308015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker DJP. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldkind S, Sahin L, Gallauresi B. Enrolling pregnant women in research—lessons from the H1N1 influenza pandemic. New Engl J …. 2010;362(24):2241–2243. doi: 10.1056/NEJMp1003462. [DOI] [PubMed] [Google Scholar]
- 12.Van Gelder MMHJ, van Rooij IALM, Miller RK, Zielhuis G a, de Jong-van den Berg LTW, Roeleveld N. Teratogenic mechanisms of medical drugs. Hum Reprod Update. 2010;16(4):378–394. doi: 10.1093/humupd/dmp052. 10.1093/humupd/dmp052. [DOI] [PubMed] [Google Scholar]
- 13.Caserta D, Mantovani A, Marci R, et al. Environment and women’s reproductive health. Hum Reprod Update. 2011;17(3):418–433. doi: 10.1093/humupd/dmq061. 10.1093/humupd/dmq061. [DOI] [PubMed] [Google Scholar]
- 14.Budtz-Jørgensen E, Debes F, Weihe P, Grandjean P. Structural equation models for meta-analysis in environmental risk assessment. Environmetrics. 2010;21(5):510–527. doi: 10.1002/env.1000. 10.1002/env.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2014 Nov;:1–8. doi: 10.1038/mp.2014.48. 2013. 10.1038/mp.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubens CE, Sadovsky Y, Muglia L, Gravett MG, Lackritz E, Gravett C. Prevention of preterm birth: Harnessing science to address the global epidemic. Sci Transl …. 2014;6(262):1–12. doi: 10.1126/scitranslmed.3009871. [DOI] [PubMed] [Google Scholar]
- 17.Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23(7):781–783. doi: 10.1101/gad.1787609. 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32(9):1373–1379. doi: 10.1038/ijo.2008.100. 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- 20.Dominguez-Salas P, Moore SE, Baker MS, et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun. 2014;5:3746. doi: 10.1038/ncomms4746. 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150(7):3376–3382. doi: 10.1210/en.2009-0071. 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi S, Stickel F, Baik H, Kim Y, Seitz H, Mason J. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J …. 1999;129(11):1945–1950. doi: 10.1093/jn/129.11.1945. [DOI] [PubMed] [Google Scholar]
- 23.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24(7):2273–2280. doi: 10.1096/fj.09-140533. 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrès R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–411. doi: 10.1016/j.cmet.2012.01.001. 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53(6):857–869. doi: 10.1016/j.neuron.2007.02.022. 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol. 1988;140(7):2197–2200. [PubMed] [Google Scholar]
- 27.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 28.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyooka S, Maruyama R, Toyooka KO, et al. Smoke exposure, histologic type and geography-related differences in the methylation profiles of non-small cell lung cancer. Int J Cancer. 2003;103(2):153–160. doi: 10.1002/ijc.10787. 10.1002/ijc.10787. [DOI] [PubMed] [Google Scholar]
- 30.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–578. doi: 10.1164/rccm.200807-1097OC. 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackburn E, Gall J. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Lin S, Funk WE, Hou L. Environmental and occupational exposure to chemicals and telomere length in human studies. Occup Environ Med. 2013;70(10):743–749. doi: 10.1136/oemed-2012-101350. 10.1136/oemed-2012-101350. [DOI] [PubMed] [Google Scholar]
- 33.Starkweather AR, Alhaeeri AA, Montpetit A, et al. An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs Res. 2014;63(1):36–50. doi: 10.1097/NNR.0000000000000009. 10.1097/NNR.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenberg DTA, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci U S A. 2012;109(26):10251–10256. doi: 10.1073/pnas.1202092109. 10.1073/pnas.1202092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Moens E, Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology. 2012;135(1):1–8. doi: 10.1111/j.1365-2567.2011.03506.x. 10.1111/j.1365-2567.2011.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson J, Garges S, Giovanni M, et al. The NIH Human Microbiome Project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):645–646. doi: 10.1016/j.jaci.2011.04.060. 10.1016/j.jaci.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 40.Subramanian S, Huq S, Yatsunenko T, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014:7. doi: 10.1038/nature13421. 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flinn MV, Nepomnaschy PA, Muehlenbein MP, Ponzi D. Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neurosci Biobehav Rev. 2011;35(7):1611–1629. doi: 10.1016/j.neubiorev.2011.01.005. 10.1016/j.neubiorev.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Manenschijn L, Schaap L, van Schoor NM, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013;98(5):2078–2083. doi: 10.1210/jc.2012-3663. 10.1210/jc.2012-3663. [DOI] [PubMed] [Google Scholar]
- 44.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012;109(20):E1312–E1319. doi: 10.1073/pnas.1201295109. 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81(1):131–148. doi: 10.1111/j.1467-8624.2009.01385.x. 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13(1):69–86. doi: 10.1111/gbb.12109. 10.1111/gbb.12109. [DOI] [PubMed] [Google Scholar]
- 47.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr J. 2009;56(1):131–139. doi: 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- 50.Titus-Ernstoff L, Troisi R, Hatch EE, et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES) Int J Epidemiol. 2006;35(4):862–868. doi: 10.1093/ije/dyl106. 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- 51.Titus-Ernstoff L, Troisi R, Hatch EE, et al. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES) Int J Androl. 2010;33(2):377–384. doi: 10.1111/j.1365-2605.2009.01010.x. 10.1111/j.1365-2605.2009.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, Zhang J, Duan J, et al. Programming and inheritance of parental DNA methylomes in mammals. Cell. 2014;157(4):979–991. doi: 10.1016/j.cell.2014.04.017. 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. doi: 10.1371/journal.pone.0055387. 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20(3):345–352. doi: 10.1016/j.reprotox.2005.04.005. 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 55.Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG, Hanson MA. Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function. J Physiol. 2008;586(8):2217–2229. doi: 10.1113/jphysiol.2007.147967. 10.1113/jphysiol.2007.147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waterland R, Dolinoy D, Lin J, Smith C. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;406(44):401–406. doi: 10.1002/dvg.20230. 10.1002/dvg. [DOI] [PubMed] [Google Scholar]
- 58.Floyd RL, Johnson KA, Owens JR, Verbiest S, Moore CA, Boyle C. A national action plan for promoting preconception health and health care in the United States (2012-2014) J Womens Health (Larchmt) 2013;22(10):797–802. doi: 10.1089/jwh.2013.4505. 10.1089/jwh.2013.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watkins AJ, Lucas ES, Wilkins A, Cagampang FR, Fleming TP. Maternal periconceptional and gestational low protein diet affects mouse offspring growth, cardiovascular and adipose phenotype at 1 year of age. PLoS One. 2011;6(12):e28745. doi: 10.1371/journal.pone.0028745. 10.1371/journal.pone.0028745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robledo C, Yeung E, Mendola P, et al. Preconception maternal and paternal exposure to persistent organic pollutants and birth size: The LIFE Study. Environ Health Perspect. 2014 Aug; doi: 10.1289/ehp.1308016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denny CH, Floyd RL, Green PP, Hayes DK. Racial and ethnic disparities in preconception risk factors and preconception care. J Womens Health (Larchmt) 2012;21(7):720–729. doi: 10.1089/jwh.2011.3259. 10.1089/jwh.2011.3259. [DOI] [PubMed] [Google Scholar]
- 63.Han Z, Mulla S, Beyene J, Liao G, McDonald SD. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. 2011;40(1):65–101. doi: 10.1093/ije/dyq195. 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- 64.Witt WP, Wisk LE, Cheng ER, Hampton JM, Hagen EW. Preconception mental health predicts pregnancy complications and adverse birth outcomes: a national population-based study. Matern Child Health J. 2012;16(7):1525–1541. doi: 10.1007/s10995-011-0916-4. 10.1007/s10995-011-0916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grieger J, Grzeskowiak L, Clifton V. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J Nutr. 2014;8:1075–1080. doi: 10.3945/jn.114.190686. 10.3945/jn.114.190686.fruit. [DOI] [PubMed] [Google Scholar]
- 66.Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2014;76(Pt B):269–275. doi: 10.1016/j.neuropharm.2013.06.016. 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vrijheid M, Slama R, Robinson O. The Human Early-Life Exposome (HELIX): project rationale and design. Env Heal …. 2014;122(6):535–544. doi: 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iozzo P, Holmes M, Schmidt MV, et al. Developmental ORIgins of Healthy and Unhealthy AgeiNg: the role of maternal obesity--introduction to DORIAN. Obes Facts. 2014;7(2):130–151. doi: 10.1159/000362656. 10.1159/000362656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–744. doi: 10.1542/peds.2011-2196. 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 71.Ford N, Mofenson L, Shubber Z, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2014;28(Suppl 2):S123–S131. doi: 10.1097/QAD.0000000000000231. 10.1097/QAD.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 72.Govarts E, Nieuwenhuijsen M, Schoeters G, et al. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European birth cohorts. Environ Health Perspect. 2012;120(2):162–170. doi: 10.1289/ehp.1103767. 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shah PS, Shah J. Maternal exposure to domestic violence and pregnancy and birth outcomes: a systematic review and meta-analyses. J Womens Health (Larchmt) 2010;19(11):2017–2031. doi: 10.1089/jwh.2010.2051. 10.1089/jwh.2010.2051. [DOI] [PubMed] [Google Scholar]
- 74.Huang H, Coleman S, Bridge JA, Yonkers K, Katon W. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. Gen Hosp Psychiatry. 2014;36(1):13–18. doi: 10.1016/j.genhosppsych.2013.08.002. 10.1016/j.genhosppsych.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA psychiatry. 2013;70(4):436–443. doi: 10.1001/jamapsychiatry.2013.684. 10.1001/jamapsychiatry.2013.684. [DOI] [PubMed] [Google Scholar]
- 76.Bonzini M, Palmer KT, Coggon D, Carugno M, Cromi A, Ferrario MM. Shift work and pregnancy outcomes: a systematic review with meta-analysis of currently available epidemiological studies. BJOG. 2011;118(12):1429–1437. doi: 10.1111/j.1471-0528.2011.03066.x. 10.1111/j.1471-0528.2011.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kusters DM, Hassani Lahsinoui H, van de Post JAM, et al. Statin use during pregnancy: a systematic review and meta-analysis. Expert Rev Cardiovasc Ther. 2012;10(3):363–378. doi: 10.1586/erc.11.196. 10.1586/erc.11.196. [DOI] [PubMed] [Google Scholar]
- 78.McDonald SD, Han Z, Mulla S, Beyene J. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. BMJ. 2010;341(jul20_1):c3428. doi: 10.1136/bmj.c3428. 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lluis A, Ballenberger N, Illi S, et al. Regulation of TH17 markers early in life through maternal farm exposure. J Allergy Clin Immunol. 2014;133(3):864–871. doi: 10.1016/j.jaci.2013.09.030. 10.1016/j.jaci.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 80.Finnell RH, Waes JG, Eudy JD, Rosenquist TH. Molecular basis of environmentally induced birth defects. Annu Rev Pharmacol Toxicol. 2002;42:181–208. doi: 10.1146/annurev.pharmtox.42.083001.110955. 10.1146/annurev.pharmtox.42.083001.110955. [DOI] [PubMed] [Google Scholar]
- 81.Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28(3):235–269. doi: 10.2165/00003088-199528030-00005. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205(1):51.e1–e8. doi: 10.1016/j.ajog.2011.02.029. 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Syme M, Paxton J, Keelan J. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet. 2004;43(8):487–514. doi: 10.2165/00003088-200443080-00001. [DOI] [PubMed] [Google Scholar]


