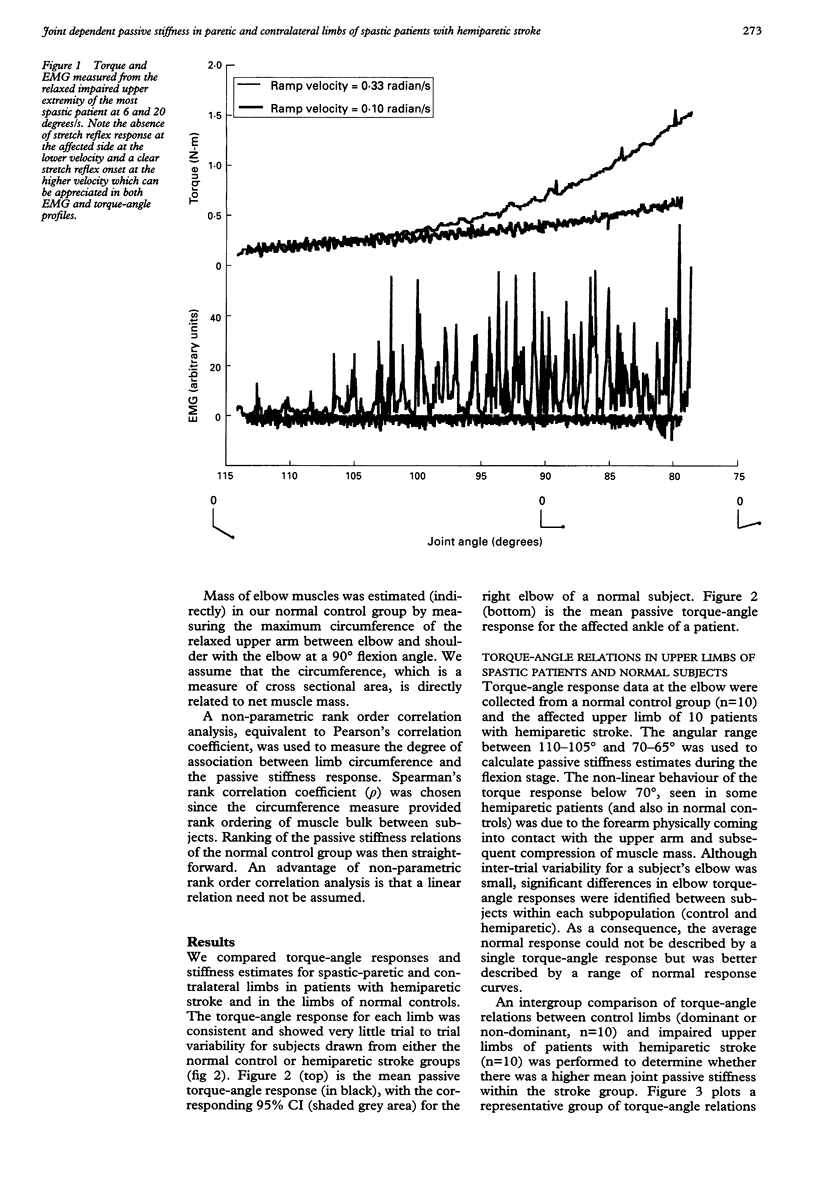
Abstract
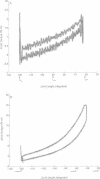
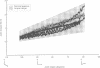
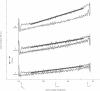
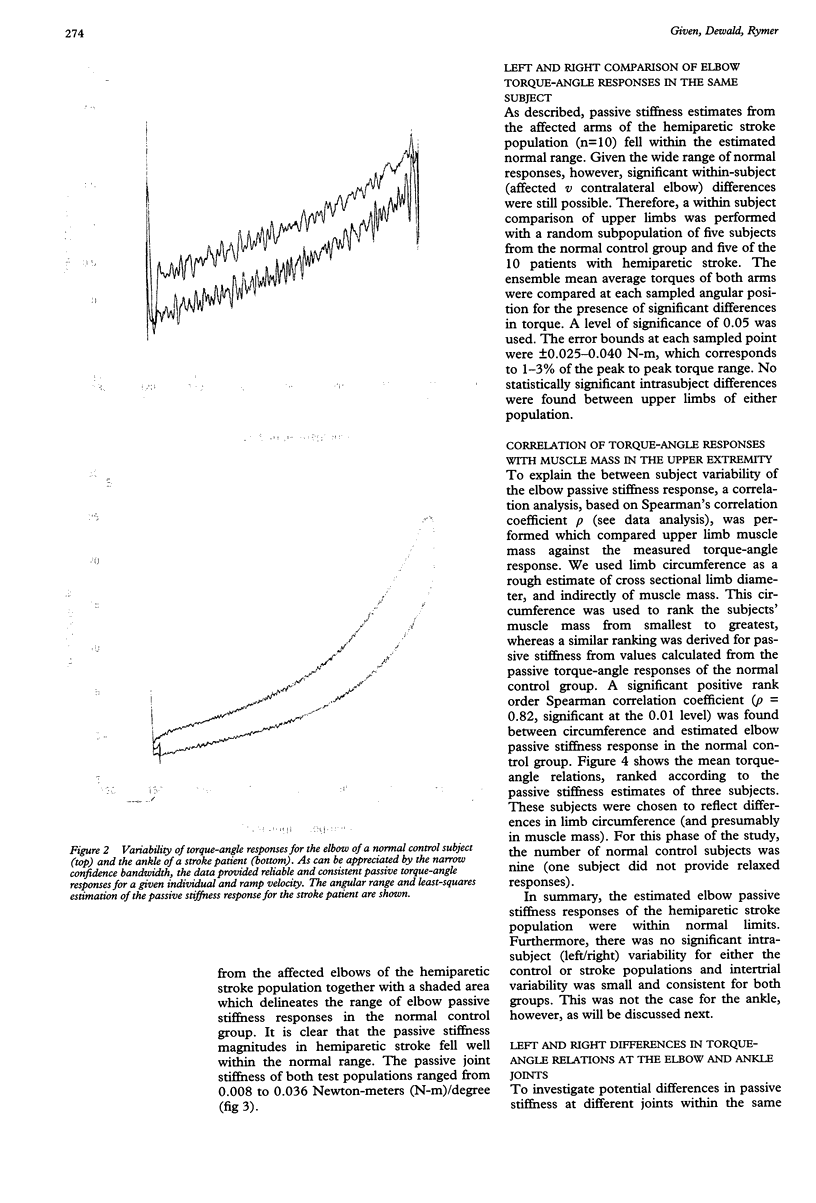
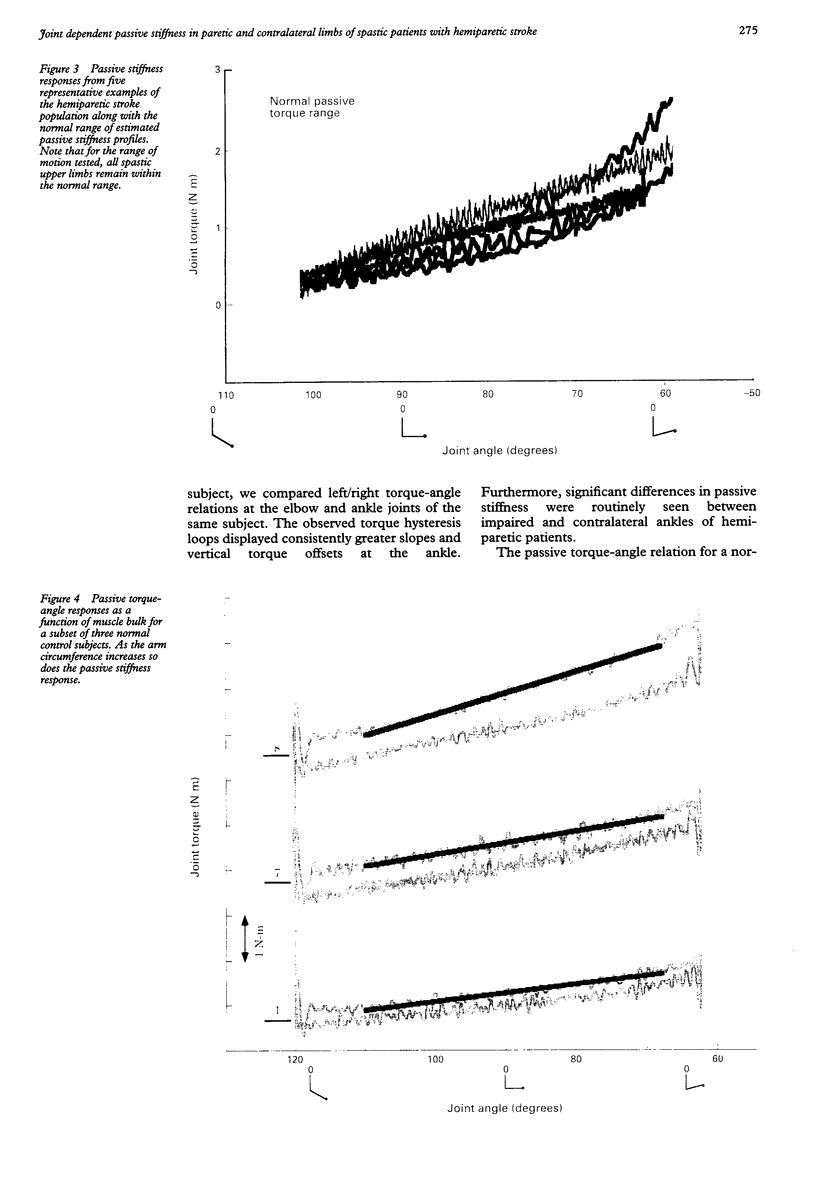
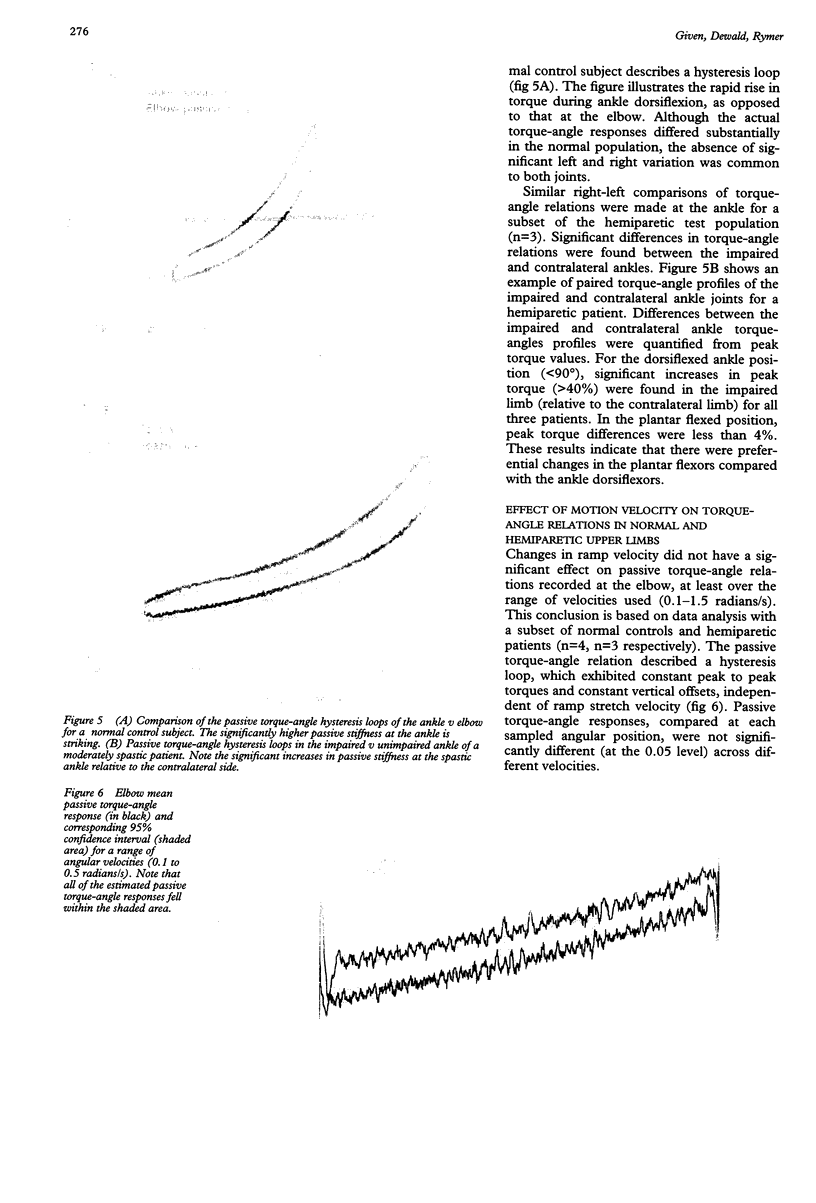
Torque-angle relations at the elbow and ankle joints of relaxed normal controls and patients with hemiparetic stroke were compared. Low velocity flexion/hold/extension angular perturbations were applied to the joint under examination. The resulting torque-angle profiles described a hysteresis loop with similar slopes during the extension and flexion stages but separated by a vertical torque offset. Torque-angle responses obtained in the absence of significant muscle activation, as recorded by surface electromyographic activity, were designated as passive. Elbow passive stiffness estimates were calculated from the slope of the torque-angle response during the flexion stage of the perturbation. The elbow torque-angle plots exhibited linear passive stiffness with magnitude significantly lower than the passive stiffness of the ankle in both normal subjects and spastic patients. Changing ramp velocity had no significant effect on the passive torque-angle hysteresis loop at the elbow. A comparison of the torque-angle relations between hemiparetic spastic and normal control arms showed no significant differences in passive stiffness. Furthermore, no significant differences were found between paretic and contralateral upper limbs of a given hemiparetic subject. By contrast, significant differences in the torque-angle hysteresis loop were present between the paretic and contralateral ankles in all hemiparetic patients tested. These differences were more significant during dorsiflexion, and therefore seem to be related to preferential changes in mechanical properties of plantar flexor muscles. It is hypothesised that the differences in the torque-angle hysteresis loop between elbow and angle joints are related primarily to the larger amount of connective tissue in the calf muscles, as well as to a larger total physiological cross sectional area of calf muscles compared with elbow muscles. It is further hypothesized that the preferential increases in passive stiffness at the ankle in spastic legs result from immobilisation induced changes in muscle connective tissue, which are most prominent in muscles with predominantly slow-twitch fibres (such as soleus). Connective tissue surrounding such slow twitch muscle fibres have been shown to be more sensitive to immobilisation than those in fast twitch muscle. The functional, pathophysiological, and clinical implications of our findings are reviewed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH B. PRELIMINARY TRIAL OF CARISOPRODOL IN MULTIPLE SCLEROSIS. Practitioner. 1964 Apr;192:540–542. [PubMed] [Google Scholar]
- An K. N., Kwak B. M., Chao E. Y., Morrey B. F. Determination of muscle and joint forces: a new technique to solve the indeterminate problem. J Biomech Eng. 1984 Nov;106(4):364–367. doi: 10.1115/1.3138507. [DOI] [PubMed] [Google Scholar]
- Broberg C., Grimby G. Measurement of torque during passive and active ankle movements in patients with muscle hypertonia. A methodological study. Scand J Rehabil Med Suppl. 1983;9:108–117. [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981 Sep;104(3):431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Friederich J. A., Brand R. A. Muscle fiber architecture in the human lower limb. J Biomech. 1990;23(1):91–95. doi: 10.1016/0021-9290(90)90373-b. [DOI] [PubMed] [Google Scholar]
- HARKNESS M. L., HARKNESS R. D. Changes in the physical properties of the uterine cervix of the rat during pregnancy. J Physiol. 1959 Oct;148:524–547. doi: 10.1113/jphysiol.1959.sp006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerkens Y. F., Woittiez R. D., Kiela J., Huijing P. A., Huson A., van Ingen Schenau G. J., Rozendal R. H. Mechanical properties of passive rat muscle during sinusoidal stretching. Pflugers Arch. 1987 Aug;409(4-5):438–447. doi: 10.1007/BF00583799. [DOI] [PubMed] [Google Scholar]
- Hufschmidt A., Mauritz K. H. Chronic transformation of muscle in spasticity: a peripheral contribution to increased tone. J Neurol Neurosurg Psychiatry. 1985 Jul;48(7):676–685. doi: 10.1136/jnnp.48.7.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozsa L., Thöring J., Järvinen M., Kannus P., Lehto M., Kvist M. Quantitative alterations in intramuscular connective tissue following immobilization: an experimental study in the rat calf muscles. Exp Mol Pathol. 1988 Oct;49(2):267–278. doi: 10.1016/0014-4800(88)90039-1. [DOI] [PubMed] [Google Scholar]
- Józsa L., Kannus P., Thöring J., Reffy A., Järvinen M., Kvist M. The effect of tenotomy and immobilisation on intramuscular connective tissue. A morphometric and microscopic study in rat calf muscles. J Bone Joint Surg Br. 1990 Mar;72(2):293–297. doi: 10.1302/0301-620X.72B2.2312572. [DOI] [PubMed] [Google Scholar]
- Kovanen V., Suominen H., Heikkinen E. Collagen of slow twitch and fast twitch muscle fibres in different types of rat skeletal muscle. Eur J Appl Physiol Occup Physiol. 1984;52(2):235–242. doi: 10.1007/BF00433399. [DOI] [PubMed] [Google Scholar]
- Kovanen V., Suominen H., Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech. 1984;17(10):725–735. doi: 10.1016/0021-9290(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Kovanen V., Suominen H., Peltonen L. Effects of aging and life-long physical training on collagen in slow and fast skeletal muscle in rats. A morphometric and immuno-histochemical study. Cell Tissue Res. 1987 May;248(2):247–255. doi: 10.1007/BF00218191. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Bates P. C., Millward D. J. Turnover of muscle protein in the fowl. Collagen content and turnover in cardiac and skeletal muscles of the adult fowl and the changes during stretch-induced growth. Biochem J. 1978 Nov 15;176(2):419–427. doi: 10.1042/bj1760419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T., Toft E., Larsen K., Andreassen S., Hansen H. J. Non-reflex and reflex mediated ankle joint stiffness in multiple sclerosis patients with spasticity. Muscle Nerve. 1993 Jan;16(1):69–76. doi: 10.1002/mus.880160112. [DOI] [PubMed] [Google Scholar]
- Tardieu C., Lespargot A., Tabary C., Bret M. D. Toe-walking in children with cerebral palsy: contributions of contracture and excessive contraction of triceps surae muscle. Phys Ther. 1989 Aug;69(8):656–662. doi: 10.1093/ptj/69.8.656. [DOI] [PubMed] [Google Scholar]
- Thilmann A. F., Fellows S. J., Ross H. F. Biomechanical changes at the ankle joint after stroke. J Neurol Neurosurg Psychiatry. 1991 Feb;54(2):134–139. doi: 10.1136/jnnp.54.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viidik A. Functional properties of collagenous tissues. Int Rev Connect Tissue Res. 1973;6:127–215. doi: 10.1016/b978-0-12-363706-2.50010-6. [DOI] [PubMed] [Google Scholar]
- Watts R. L., Wiegner A. W., Young R. R. Elastic properties of muscles measured at the elbow in man: II. Patients with parkinsonian rigidity. J Neurol Neurosurg Psychiatry. 1986 Oct;49(10):1177–1181. doi: 10.1136/jnnp.49.10.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickiewicz T. L., Roy R. R., Powell P. L., Edgerton V. R. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983 Oct;(179):275–283. [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J Anat. 1978 Dec;127(Pt 3):459–468. [PMC free article] [PubMed] [Google Scholar]
- Williams P. E., Goldspink G. Connective tissue changes in immobilised muscle. J Anat. 1984 Mar;138(Pt 2):343–350. [PMC free article] [PubMed] [Google Scholar]