Abstract
Background
Case-fatality and hospitalization rates for U.S. heart failure (HF) patients have steadily decreased over the past several decades. Diabetes mellitus (DM), a risk factor for, and frequent co-existing condition with, HF continues to increase in the general population.
Methods and Results
We used the National Inpatient Sample to estimate overall as well as age-, sex-, and race/ethnicity-specific trends in HF hospitalizations, DM prevalence and in-hospital mortality among 2.5 million discharge records from 2000–2010 with HF as primary discharge diagnosis. Multivariable logistic and Poisson regression were used to assess the impact of the above demographic characteristics on in-hospital mortality. Age-standardized hospitalizations decreased significantly in HF overall and in HF with DM . Age-standardized in-hospital mortality with HF declined from 2000 to 2010 (4.57% to 3.09%, ptrend <0.0001), while DM prevalence in HF increased (38.9% to 41.9%, ptrend < 0.0001) as did comorbidity burden. Age-standardized in-hospital mortality in HF with DM also decreased significantly (3.53% to 2.27%, ptrend <0.0001). After adjusting for year, age and comorbid burden, males remained at 17% increased risk versus females, non-Hispanics remained at 12% increased risk versus Hispanics and whites had a 30% higher mortality versus non-white minorities. Absolute mortality rates were lower in younger versus older patients although the rate of decline was attenuated in younger patients.
Conclusions
In-hospital mortality in HF patients with DM significantly decreased over the past decade despite increases in DM prevalence and co-morbid conditions. Mortality rate decreases among younger patients were significantly attenuated and mortality disparities remain among important demographic sub-groups.
Keywords: heart failure, diabetes mellitus, mortality, hospitalization
There are nearly 5 million individuals in the United States (U.S.) with a diagnosis of heart failure (HF)1 and HF is the principal diagnosis in >1 million hospitalizations annually2. Over the past decade, overall HF hospitalization and in-hospital mortality rates have declined3–6. Diabetes mellitus (DM), a disease that is increasing in prevalence7–9, is a significant risk factor for the development of cardiovascular disease and amplifies the risk for the development of HF10–12. In addition, HF itself is considered an insulin-resistant state and is associated with significant risk for the future development of DM13,14. Given these relationships, it is not surprising that DM and HF may commonly coexist.
While the “true” population-based prevalence of DM in patients with HF (ambulatory or hospitalized) is unknown, prevalences range from 20–30% in clinical trial populations15,16 to > 40% in recent registries of hospitalized patients17–19. What is clear is that the absolute number of individuals with HF will continue to increase world-wide20 as well as in the U.S.21 over the next decade while the number of individuals with DM will also continue to increase world-wide22 and in the U.S.23. Given the increasing prevalence of DM and HF and comparatively worse clinical outcomes of concomitant HF and DM in the general population18,24,25, we examined trends in hospitalizations and in-hospital mortality in patients with HF and DM from 2000–2010. We also describe the factors associated with in-hospital mortality with a focus on the impact of time, age, sex, race and ethnicity over this interval.
Methods
Data Source
The National Inpatient Sample (NIS), sponsored by the Agency for Healthcare Research and Quality (AHRQ) Healthcare Cost and Utilization Project (HCUP), is the largest all-payer inpatient database publicly available in the U.S. consisting of discharge data from over 1,000 hospitals across a majority of states and is designed to approximate a 20% stratified sample of US community hospitals26.
The NIS provides discharge-level demographic and clinical characteristics that are searchable using International Classification of Diseases, ninth revision, clinical modification (ICD-9-CM) codes. Each annual release of the NIS includes patient-level hospital discharge abstracted data for 100% of discharges from the sample of hospitals in participating states. We used NIS security files to extract administratively coded comorbid conditions of patients as established by AHRQ. The study was considered exempt from formal review by the University of New Mexico institutional review board because the NIS is a public database without personal identifiers.
Data Quality
A summary data quality report is available for review for each year of the NIS27. Individual reports for the years 2000–2010 were reviewed by one of the authors (WKL). With the exception of data for race and ethnicity (see below) edit check failure rates were consistently < 0.5% for other key data elements.
Study population
A total of 71 million hospital discharges were reported to the NIS from 2000 to 2010. We analyzed data for patients ≥ 18 years of age. HF hospital stays were defined as those with a primary discharge diagnosis of HF on the basis of the following ICD-9-CM codes: 402.01; 402.11; 402.91; 404.01; 404.03; 404.11; 404.13; 404.91; 404.93; and 428. We excluded any record containing an ICD-9-CM code for acute coronary syndrome or acute myocardial infarction in order to obviate the confounding issue of acute ischemia on in-hospital outcome. The total number of HF hospitalizations was calculated as the sum over all HF ICD-9-CM codes. We then obtained the proportion of HF discharges that occurred over the same time interval with a diagnosis of type 2 DM , identified by ICD-9-CM code 250.0 to 250.9 with a fifth digit of 0 or 2 since the majority of diagnosed cases of DM in adults are of type 2
Data Analysis
Figure 1 shows the sequence of data analysis. We recorded the number of records for each year and stratified these records by age group (< 60, 60–69, 70–79, and ≥ 80 years) sex, race and ethnicity. We also computed a measure of medical co-morbidities employed by HCUP in NIS datasets-the Elixhauser co-morbidity index28,29.
Figure 1.
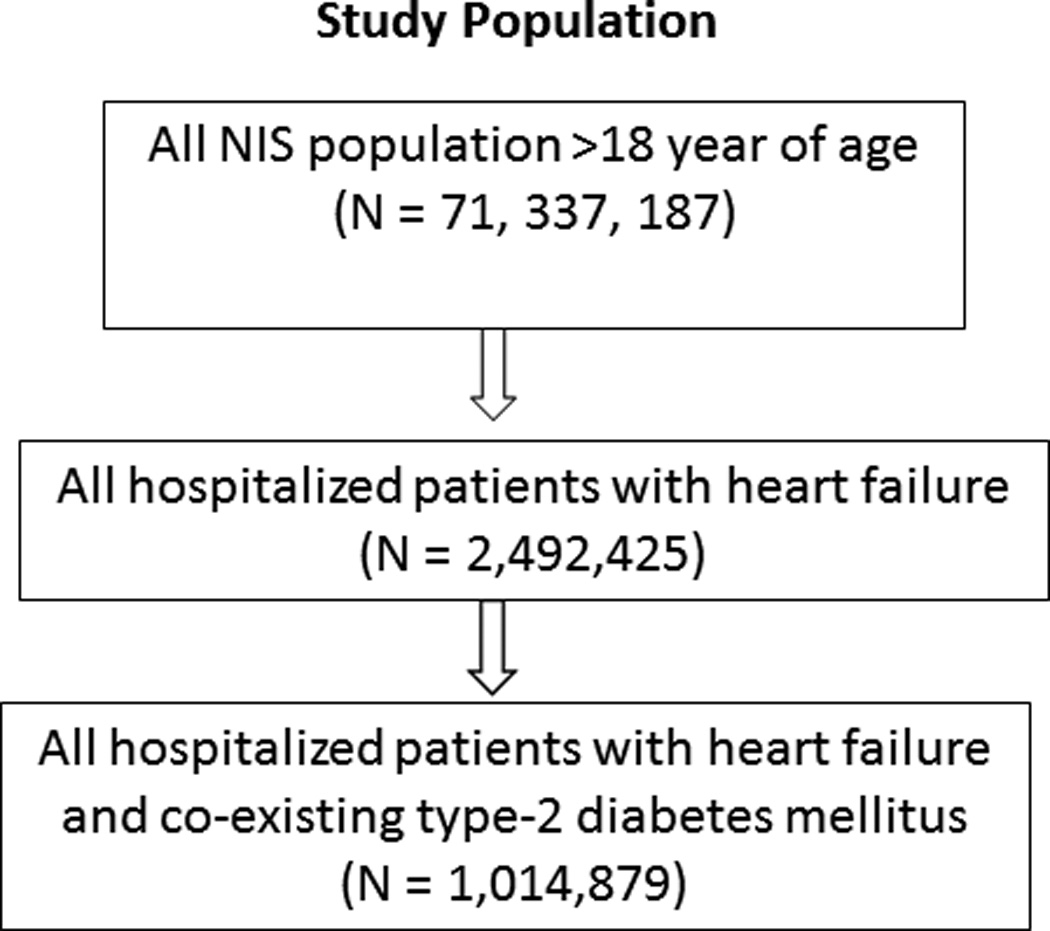
Data Analysis Sequence
Statistical sampling weights provided by the NIS allow extrapolation to estimate hospital discharge rates for the nation After weighting, this reflects approximately 95% of hospital discharges within the U.S. 30
Statistical Analysis
Hospitalizations are summarized as raw counts as well as counts per 100,000 adults (> 18 years of age) for that year obtained using the U.S. Census Bureau intercensal estimates for 2000–201031. Categorical data are summarized as percents. The outcome of interest in our analysis was in-hospital mortality. In keeping with our stated objectives, exposure variables were year, age on admission, sex, the discharge-record specified race and ethnicity and the Elixhauser co-morbidity index. Survey analysis methods were employed that used hospital-level discharge weights provided by the NIS to estimate the number of HF hospitalizations and in-hospital mortality on a national level32 Direct standardization of age was performed using the average of the 2000 and 2010 NIS data sets as the standard population. Age-standardized in-hospital mortality rates for HF with DM were calculated and reported (in percent) for the overall sample and stratified by sex. All other sub-group specific mortality rates are reported as crude mortality rates (CMR). Rates were plotted and smoothed for display using a Hamming window filter.
In order to distinguish changes in population age and/or sex composition versus age/sex-independent factors driving the observed decrease in CMR over time, the method of rate decomposition was used33. Briefly, the difference in CMR from 2000 to 2010 can be viewed as the sum of a “composition effect” (reflecting the difference in the age and/or sex composition of the sample from 2000 to 2010) and a “rate effect” (reflecting differences in the distribution of stratum-specific mortality rates from 2000 to 2010) (Δ CMR2000–2010=composition effect+rate effect). Calculations were performed for age and sex, separately and combined.
P values are based on chi-square tests for all categorical row variables or chi-square rank based group means score statistics for continuous/ordinal row variables (equivalent to Wilcoxon tests). All such tests treat the column variable as nominal. Trends in categorical variables were tested using chi-square statistics. Multivariable logistic regression that accounted for survey methodology and hospital clustering was used to estimate the magnitude of association between clinical, temporal, and demographic covariates and in-hospital mortality. Year was modeled as a continuous linear variable. An interaction term, age (group) x sex, was added to the model to test for the influence of sex on the association between age and mortality. Estimated measures of association are expressed as odds ratios (ORs) and 95% CIs. Adjusted annual rates of change in mortality were estimated from a Poisson regression model which estimated linear time trends in in-hospital mortality and included all variables used in the logistic regression model. Hospital length of stay (days) was used as the offset (“exposure”) variable in the Poisson model.
A sensitivity analysis was performed in the subgroup of hospitals with >90% completion of race/ethnicity data since missing rates of the latter frequently exceeded 10% in the overall sample. Additional sensitivity analyses examining the impact of the inclusion of ICD-9-CM codes for non-acute ischemic heart disease (ICD-9-CM 412.X, 413.X, 414.X) on the associations between age, sex, race/ethnicity, time and comorbid burden and in-hospital mortality was performed. We assessed the frequency of any acute manifestation of ischemic heart disease (ICD-9-CM codes 410.0 to 410.8) using the clinical classifications software (CCS) provided by HCUP.
The study was considered exempt from formal review by the University of New Mexico institutional review board because the NIS is a public database without personal identifiers.
A p value < 0.05 was considered statistically significant. All analyses were performed using SAS (version 9.1 or higher) or STATA (version 14)..
Results
Characteristics of HF hospitalizations from 2000 to 2010
Hospitalization with a primary diagnosis of HF steadily decreased from 227,595 in 2000 to 207, 593 in 2010 and translates to a decrease from 555/100,000 U.S. adults to 460/100,000 U.S. adults (ptrend <0.0001). (Supplemental Table 1). The overall prevalence of women was 52.5% and decreased from 55.4% in 2000 to 49.9% in 2010 (ptrend <0.0001). The mean (± SD) age of the sample was 72.6 ± 14.4 years and approximately two-thirds (65.4% in 2000 and 62.4% in 2010) were > 70 years of age. The majority were of white race (representing about 73 % in 2000 and 66 % in 2010) although non-white minority prevalence increased significantly from 27% to 34%. The prevalence of Hispanic ethnicity increased significantly from 5.3% in 2000 to 6.9% in 2010 (ptrend <0.0001). The mean (± SE) Elixhauser comorbidity index increased from 3.32±0.04 in 2000 to 5.67±0.07 in 2010 (ptrend <0.0001). The prevalence of DM increased from 38.9% to 41.9% (ptrend <0.0001) from 2000 to 2010 (Supplemental Table 1)..
Characteristics of HF with DM hospitalizations from 2000 to 2010
The study sample consisted of 1,014,879 hospitalizations with HF and co-existing DM which translates to an estimated weighted 5 million hospitalizations. As seen in Table 1, there was a statistically significant decrease in the prevalence of HF with co-existing DM hospitalizations from 217/100,000 U.S. adults in 2000 to 193/100,000 U.S. adults in 2010 (ptrend <0.0001). The prevalence of females decreased from 57% in 2000 to 50% in 2010. Although the majority of the sample was > 70 years of age a significant minority was < 60 years of age and their prevalence increased over time. The prevalence of white race decreased from 68% in 2000 to 61% in 2010 (ptrend <0.0001) whereas the cumulative prevalence of non-white minorities increased from 32% to 39% (ptrend <0.0001). The prevalence of Hispanic ethnicity increased from 7.0% in 2000 to 9.2% in 2010 (ptrend <0.0001). The annual mean Elixhauser comorbidity index significantly increased from 2.88±0.04 to 5.46±0.07 (ptrend <0.0001).
Table 1.
Characteristics among Patients with Heart Failure and Co-existing Diabetes Mellitus from 2000 to 2010
| Year | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Plinear trend | Poverall Χ2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (Sample) | 88855 | 90786 | 95278 | 99786 | 97055 | 93189 | 93909 | 89727 | 89527 | 89685 | 87184 | ||
| N (Weighted) | 435K | 452K | 460K | 479K | 469K | 456K | 459K | 444K | 438K | 454K | 436K | ||
| HF/100,000 | 217 | 222 | 224 | 230 | 223 | 214 | 213 | 204 | 199 | 204 | 193 | <0.0001 | |
| Sex (%) | <0.0001 | ||||||||||||
| Male | 43.1 | 43.6 | 44.1 | 44.9 | 46.0 | 47.5 | 47.9 | 48.4 | 49.3 | 49.9 | 50.4 | <0.0001 | |
| Female | 56.9 | 56.4 | 55.9 | 55.1 | 54.0 | 52.5 | 52.0 | 51.6 | 50.7 | 50.1 | 49.6 | <0.0001 | |
| Age (%) | <0.0001 | ||||||||||||
| <60 | 18.4 | 18.2 | 19.1 | 19.4 | 19.6 | 19.6 | 20.5 | 20.3 | 19.6 | 20.1 | 20.3 | <0.0001 | |
| 60–69 | 24.3 | 23.3 | 23.2 | 23.7 | 23.6 | 22.9 | 22.3 | 23.1 | 23.0 | 23.3 | 23.1 | <0.0001 | |
| 70–79 | 33.7 | 33.5 | 32.8 | 31.5 | 31.2 | 30.3 | 29.6 | 28.9 | 28.5 | 28.2 | 27.2 | <0.0001 | |
| ≥80 | 23.6 | 25.1 | 25.0 | 25.4 | 25.6 | 27.2 | 27.6 | 27.6 | 28.9 | 28.5 | 29.3 | <0.0001 | |
| Race (%) | <0.0001 | ||||||||||||
| White | 68.3 | 67.7 | 64.9 | 62.6 | 63.4 | 67.3 | 61.9 | 61.7 | 63.9 | 63.2 | 60.8 | <0.0001 | |
| Black | 18.7 | 18.5 | 20.7 | 20.5 | 21.6 | 17.4 | 21.2 | 22.2 | 20.6 | 20.6 | 23.6 | <0.0001 | |
| Hispanic | 9.1 | 10.2 | 9.9 | 12.6 | 10.7 | 10.9 | 12.1 | 10.6 | 9.7 | 10.2 | 10.3 | <0;0001 | |
| Asian | 1.6 | 1.6 | 1.9 | 2.0 | 1.9 | 1.7 | 1.9 | 2.1 | 2.1 | 2.1 | 2.2 | <0.0001 | |
| AI/NA | 0.4 | 0.4 | 0.4 | 0.2 | 0.5 | 0.4 | 0.8 | 0.8 | 0.8 | 0.6 | 0.8 | <0.0001 | |
| Other | 1.9 | 1.6 | 2.1 | 2.2 | 1.9 | 2.3 | 2.0 | 2.6 | 2.9 | 3.4 | 2.3 | - | |
| Ethnicity (%) | 0.0021 | ||||||||||||
| Non-Hispanic | 92.9 | 92.4 | 92.8 | 90.7 | 92.1 | 92.0 | 90.9 | 92.0 | 92.1 | 91.1 | 90.8 | <0.0001 | |
| Hispanic | 7.0 | 7.6 | 7.2 | 9.3 | 7.9 | 8.0 | 9.1 | 7.9 | 7.9 | 8.9 | 9.2 | <0.0001 | |
| Elixhauser comorbidity index | |||||||||||||
| Mean (SE) | 2.88 (0.04) |
3.03 (0.04) |
3.30 (0.06) |
4.44 (0.06) |
4.99 (0.07) |
5.53 (0.09) |
6.46 (0.10) |
0.50 (0.09) |
4.89 (0.06) |
5.25 (0.06) |
5.46 (0.07) |
<0.0001 | |
HF = Heart Failure, AI/NA = American Indian/Native Alaskan, SE = Standard error
In-hospital Mortality in HF from 2000 to 2010
There was a statistically significant decline in age-standardized in-hospital mortality from 4.57% in 2000 to 3.09% in 2010 (ptrend <0.0001) among the 2.5 million patients in the unweighted HF sample. This trend was similar for both sexes (4.71% and 3.07% for males in 2000 and 2001, respectively; 4.48% and 3.09% for females in 2000 and 2001, respectively, ptrend <0.0001). (Supplemental Figure 1). In order to better understand the driver(s) for the decrease in mortality, the method of rate decomposition (see Methods) was employed. For the entire HF population the “rate effect” was 1.4719 and the “composition effect” was 0.0006. The total, 1.4726, equals the difference in CMR from 2000 to 2010 and suggests that a change in stratum-specific risk for mortality is the main driver of the decrease in mortality and not a difference in age structure of the populations. Similar results were obtained when the analysis was limited to changes in sex distribution alone and age and sex distributions together.
In-hospital Mortality in HF with co-existing DM from 2000 to 2010
Overall and sex-specific crude and age-standardized mortality rates for HF with DM significantly decreased over this interval (Table 1, Figure 2). Rate decomposition indicated that for HF and DM the “rate effect” was 1.3511 and the “composition effect” was −0.0916. The sum of these 2 components, 1.2596, equals the difference in CMR for HF with DM from 2000 to 2010 and suggests that, as with the overall HF population, the main driver for the decrease in mortality is a change in inherent risk structure of the populations rather than a change in age structure of the populations. Similar results were obtained when the analysis was limited to changes in sex distribution alone and age and sex distributions together.
Figure 2.
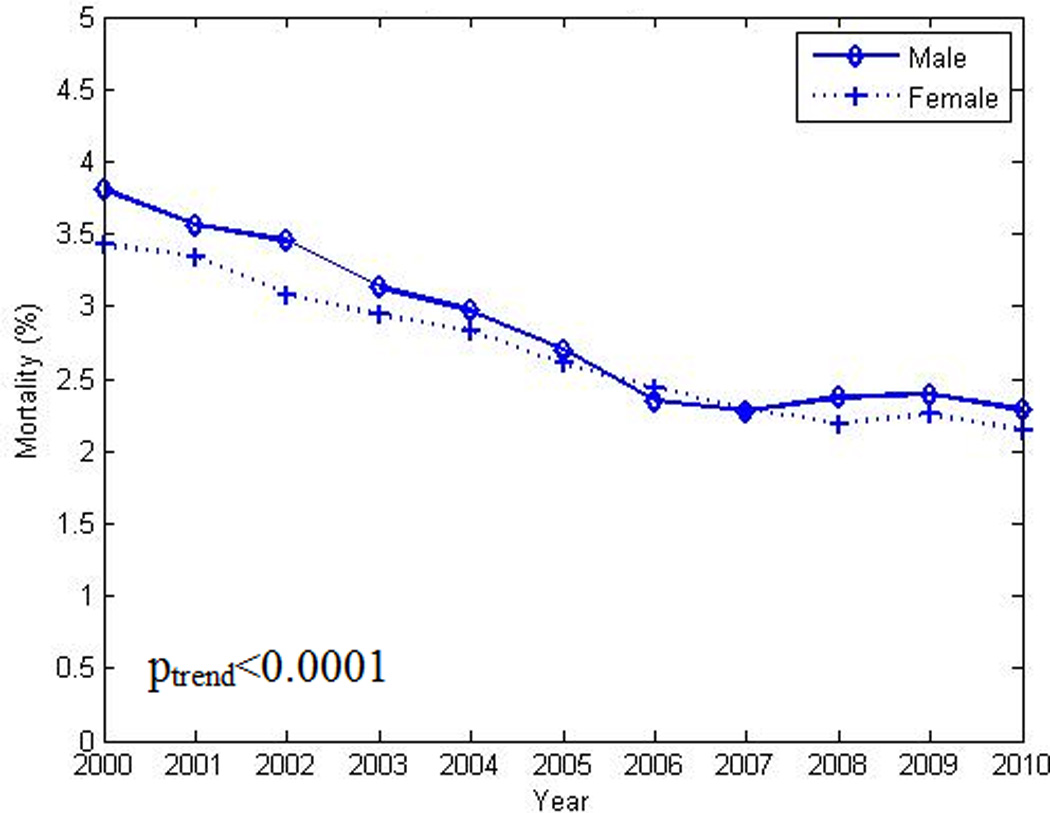
Decrease in age-standardized in-hospital mortality rate among men and women with heart failure and co-existing diabetes from 2000 to 2010. poverall χ2 <0.0001. plinear trend <0.0001
The overall decrease in in-hospital mortality was not shared equally among the selected sub-groups. As seen in Figures 3 and 4, larger decreases in case fatality rates were noted in the oldest groups when compared to their younger counterparts. Women exhibited smaller decreases in mortality over time with the largest decreases noted in the oldest group. Poisson regression analysis indicated a lower overall mortality rate and rate of decline in younger age groups (Table 2). As seen in Figure 5 and Figure 6, declines in case fatality rates in Hispanics and non-Hispanics and whites vs. non-white minorities were noted with, however, persistent absolute differences between whites and non-white minorities.
Figure 3.
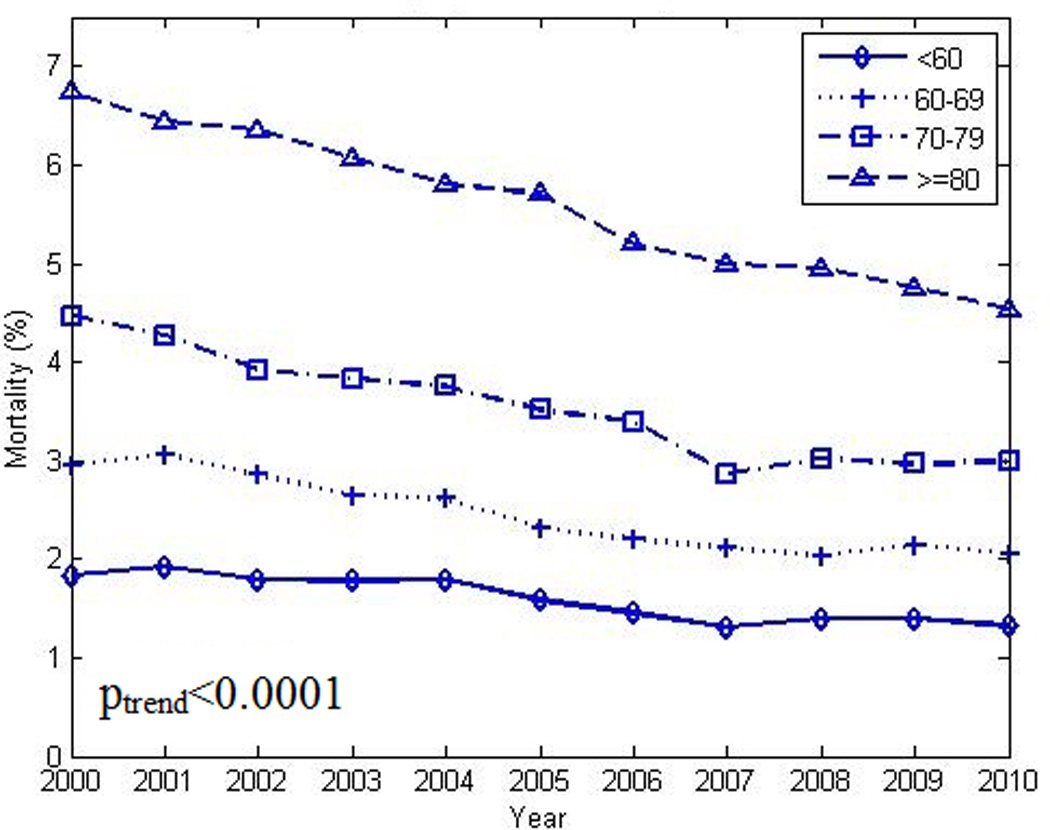
Decrease in crude in-hospital mortality rate among patients with heart failure and co-existing diabetes in different age groups (see text) from 2000 to 2010. poverall χ2 <0.0001. plinear trend <0.0001 (for each stratum)
Figure 4.
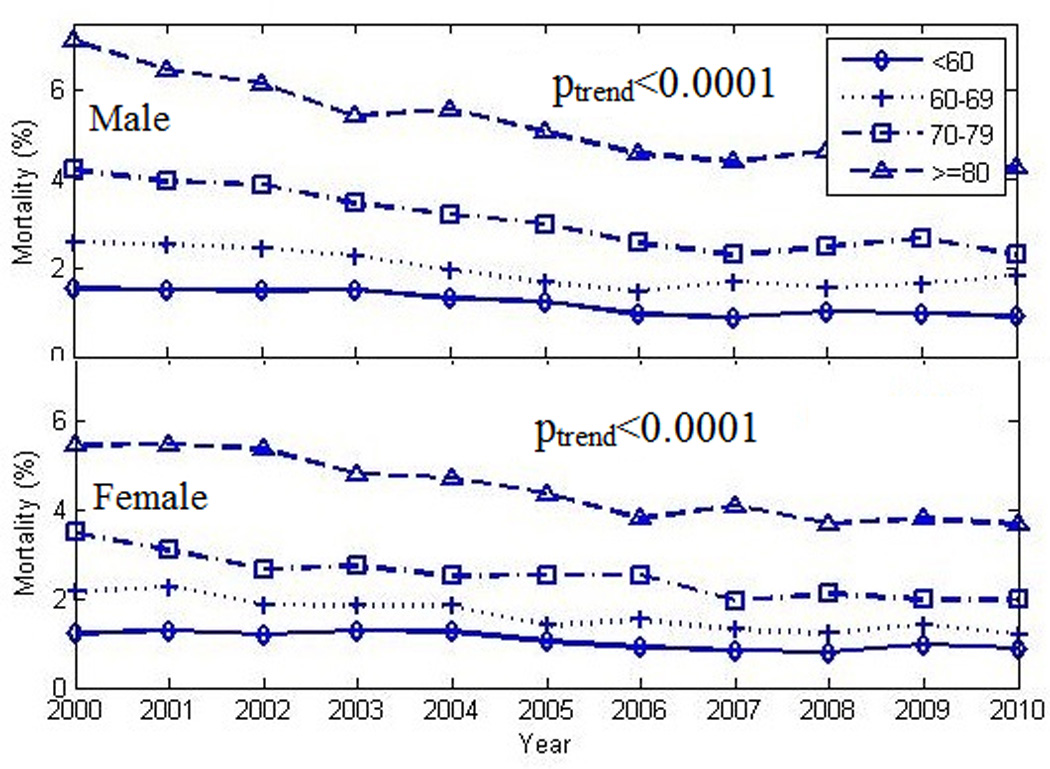
Decrease in crude in-hospital mortality rate among patients with heart failure and co-existing diabetes in different age groups (see text) stratified by sex, from 2000 to 2010. poverall χ2 <0.0001. plinear trend <0.0001 (for each stratum)
Table 2.
In-hospital mortality in patients with heart failure and co-existing diabetes mellitus. Results from Poisson regression model
| Variable | IRR | SE | p-value | 95% Confidence Interval |
|---|---|---|---|---|
| Age ≥ 80 yr | 1.00 | - | - | - |
| Age 70–79 yr | 0.58 | 0.01 | <0.0001 | 0.56–0.59 |
| Age 60–69 yr | 0.39 | 0.01 | <0.0001 | 0.38–0.40 |
| Age <60 yr | 0.27 | 0.01 | <0.0001 | 0.25–0.27 |
| Female (vs. male) | 0.81 | 0.01 | <0.0001 | 0.79–0.83 |
| Hispanic (vs. non-Hispanic) | 0.86 | 0.02 | <0.0001 | 0.82–0.89 |
| Year (per year from 2000) | 0.95 | 0.01 | <0.0001 | 0.94–0.94 |
| Elixhauser comorbidity index (per 1 unit increase) | 1.03 | 0.01 | <0.0001 | 1.03–1.04 |
Abbreviations: IRR, incidence rate ratio; SE, standard error
Figure 5.
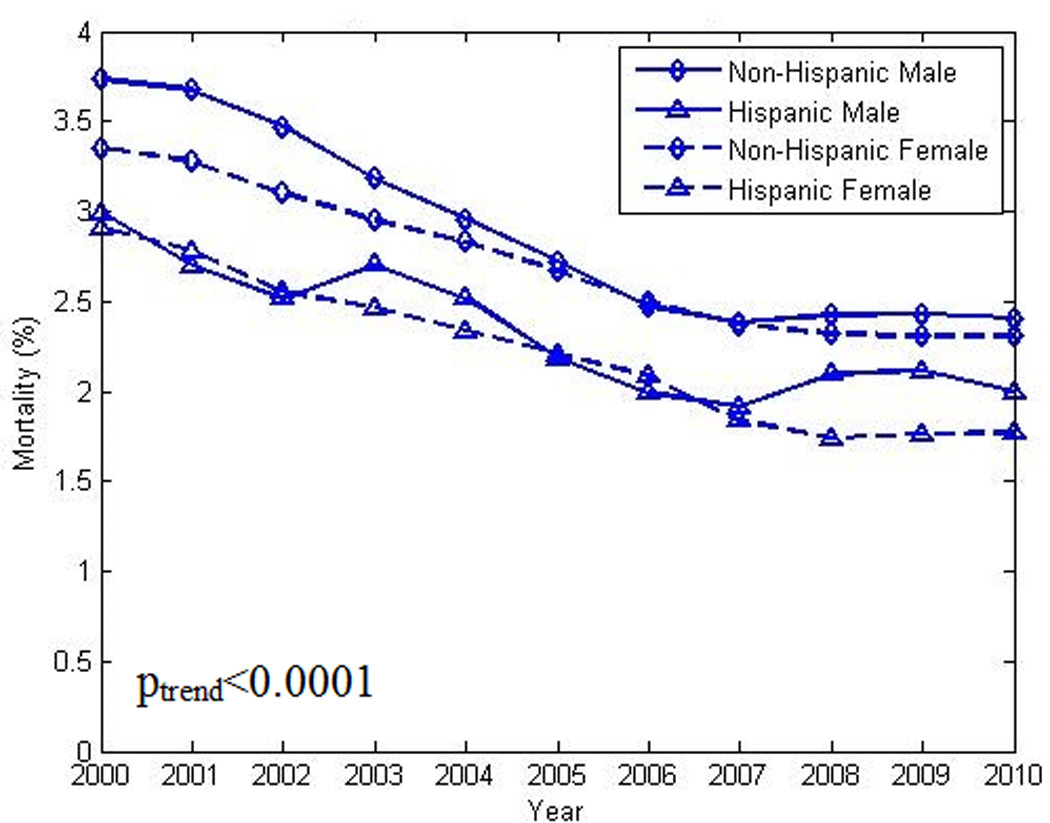
Decrease in in-hospital crude mortality rate in patients with heart failure and co-existing diabetes, by sex and ethnicity (Hispanic vs non-Hispanic), from 2000 to 2010. poverall χ2 <0.0001. plinear trend <0.0001 (for each stratum)
Figure 6.
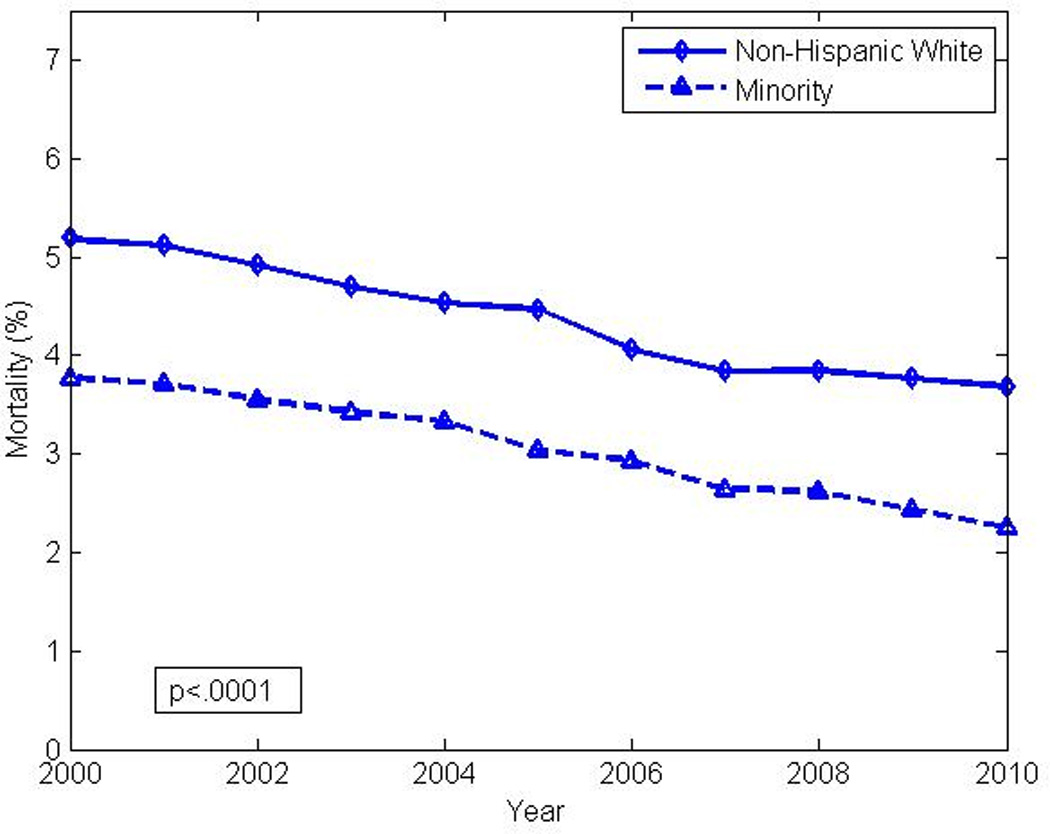
Decrease in in-hospital crude mortality rate in patients with heart failure and co-existing diabetes, by race (non-Hispanic white vs. composite non-white minority) from 2000 to 2010. poverall χ2 <0.0001. plinear trend <0.0001 (for each stratum)
Demographic, temporal and clinical factors associated with in-hospital death were assessed using multivariable regression (Table 3) with the results supporting significant disparities within and among our selected sub-groups. Males remained at 17% increased risk compared to females (OR, 1.17; 95% CI, 1.14 to 1.20); white populations remained at 30% higher risk compared to non-white minorities (OR, 1.30; 95% CI, 1.26 to 1.34); non-Hispanics remained at 12% increased risk compared to Hispanics (OR, 1.12; 95% CI, 1.06 to 1.19) and older patients (≥80 years) remained at 4 times higher risk compared to their younger counterparts <60 years (OR, 4.08; 95% CI, 3.87 to 4.29). There was no significant interaction between age and sex on the association of either with in-hospital mortality (pinteraction=0.19).
Table 3.
Factors associated with in-hospital mortality in patients with heart failure and co-existing diabetes mellitus. Results from a logistic regression model.
| Factor | ||
|---|---|---|
| Odds Ratio | Confidence Interval (95%) | |
| Sex | ||
| Male (vs. Female) | 1.17 | 1.14–1.20 |
| Age (yrs) | ||
| < 60 | 1.00 | N/A |
| 60–69 | 1.51 | 1.43–1.59 |
| 70–79 | 2.29 | 2.18–2.42 |
| ≥ 80 | 4.08 | 3.87–4.29 |
| Race | ||
| White (vs Non-White Minority) | 1.3 | 1.26–1.34 |
| Ethnicity | ||
| Non-Hispanic (vs Hispanic) | 1.12 | 1.06–1.89 |
| Year | 0.92 | 0.92–0.93 |
| Elixhauser comorbidity index | 1.06 | 1.06–1.07 |
Analysis of the above associations for only those records containing ICD-9-CM codes specific to ischemic heart disease yielded no meaningful differences in effect size (difference in magnitude of beta coefficients < 2 %) between models. Additionally, there was minimal variation in the frequency of acute coronary syndromes coded in a secondary position with an average rate for the interval of 3.1%.
Sensitivity analysis confined to those hospitals with >90% data completion for race/ethnicity confirmed the above-mentioned significant trends in prevalences over time. As well, Poisson regression utilizing data from hospitals with >90% complete records yielded similar results to those in Table 3.
Discussion
Using a nationally representative all-payer inpatient sample of U.S. hospital admissions from 2000–2010 our observations support the following conclusions. First, the total number of hospitalizations with a primary diagnosis of HF decreased over this interval. Second, the prevalence of DM as well as a measure of the burden of co-morbidities among hospitalized HF patients increased. Third, despite the increased prevalence of DM and co-morbid burden there was a 36% decrease in age-standardized in-hospital mortality among HF with DM. Fourth, in-hospital mortality rates varied by age, sex, race and ethnicity. Fifth, the decrease in in-hospital mortality rate is the result of a change in the stratum-specific risk (for mortality) rather than a change in age and/or sex structure of the 2000 and 2010 samples.
Reduction in in-hospital mortality in HF patients with co-existing DM
Several studies have now documented a decrease in hospitalization rates in the U.S for patients with HF over a time interval similar to the current study3–6. The inclusion of hospitalizations in all adults, i.e., greater than 18 years of age, distinguishes the current study from prior Medicare-derived data3,6 and the focus on the DM sub-group distinguishes the current study from prior NIS studies4,5. The inclusion of patient hospitalizations with age < 65 years allows for analysis of an important group of relatively younger patients who comprised one-fifth of all HF hospitalizations in the NIS database. .
Our study was limited to hospitalized patients. Nevertheless, secular changes in the management and characteristics of HF patients from 2000–2010 are relevant to these data. A decrease in HF-related hospitalization rates and in-hospital mortality was first reported from the Medicare and Medicaid population beginning in 19983 but reflects changes beginning prior to that date. The period from 2000–2010 was a period of increasing attention to improved management strategies for all HF patients34,35 which would likely impact hospitalization and in-hospitality mortality rates. The observed trend in the current study is consistent with the time frame for the “diffusion” of evidence-based and clinical trial data into mainstream practice36, and reflects the many changes in the timing and extent of pharmacotherapy for HF. Improved adherence to contemporary guideline-based therapies for HF and DM in conformance with national guidelines37over this time interval may also have contributed to improved in-hospital outcomes in HF and DM patients. Changes in the underlying cardiovascular risk profile of patients presenting with HF and DM7,38, whether a cohort effect or a true indicator of intensified risk factor recognition and treatment, is another possible explanation for the reduction in in-patient mortality- a finding supported by our rate decomposition analysis.
Similar39or lower40 in-hospital crude mortality rates in patients with HF and DM compared to those with HF without DM have been previously observed. Of note is the similarity of our current observations in hospitalized patients to trends in the U.S. population from 2005–201138In this latter study while hospitalizations and mortality rates declined in DM patients, including those with HF, decreases in event rates and mortality were lower or absent in those without DM. Thus, although hospitalized patients are a highly selected group from the general population, the above-noted secular trends may be powerful enough to beneficially impact this selected group of patients.
In-hospital mortality trends in HF with DM by age, sex, race and ethnicity
Our study also indicated that the reduction in in-hospitality mortality over time varied by age, sex, race and ethnicity. The current national focus on disparities in health care and health outcomes was the main driver for these additional analyses. Of particular concern is the lack of concordance in trends in-hospital mortality rates between younger and older age groups, notwithstanding higher event rates in the latter. Continuing increases in the incidence of obesity and DM, both contributors to the development of HF, in the young will likely further adversely impact these trajectories and could reverse much of the gain in survival noted to date. At the beginning of this study, females had higher rates of HF as well as HF with DM hospitalizations than males. However, the reverse trend was found by the end of the study period. These results are consistent with previous studies that suggested that the prevalence of HF in males is increasing in comparison to females2. Age standardized in-patient HF with DM mortality rates in both sexes decreased from 2000 to 2010. However the age standardized mortality in males remained higher than females throughout the study period until around 2006 and then became more comparable to females by the end of the study period.
Concordance in trends for in-hospital mortality between Hispanic and non-Hispanics was observed- findings similar to prior reports from a national HF registry41. In the adjusted logistic regression model Hispanics were at diminished risk for in-patient mortality compared to non-Hispanics. Racial differences in in-hospital mortality persist notwithstanding overall similar declines in both white and non-white groups. Lower mortality rates in the composite non-white minority group have been observed previously and remain unexplained41 but may be of relevance as the relative proportions of whites and non-whites in the U.S. changes over the next several decades42.
Limitations
The NIS remains the largest publicly available database with a statistically sound sampling design allowing for accurate identification of trends in specific diseases. However, analyses and conclusions from this large administrative database have a number of caveats. Observations reflect admissions and not unique patients. Thus, the current unit of analysis is the admission. Given the inability to account for multiple admissions for a given patient in the NIS, our observations and conclusions may be confounded by the non-trivial risk for repeat hospitalization. Thus, our reported rates may be viewed as over-estimates of a per patient admission rate. Mortality rates, however, are unlikely to be affected (a patient can only die once). Misclassification (under-or over-coding) cannot be completely ruled out without more extensive and rigorous data verification although the large number of patients in the database strongly mitigates against substantial misclassification bias43. Prior studies have shown excellent positive and negative predictive capability for ICD-9-CM codes for HF44. Our analysis could be biased by “upcoding” or “Diagnosis-related group (DRG) creep,” which may have resulted in over-reporting of comorbidities However, the impact of such would likely have been uniform across the groups, would be unlikely to bias CMRs, and would bias the results of comparisons toward the null if applied non-differentially It is even more unlikely that the highly statistically significant trend in hospitalizations and mortality from 2000–2010 is attributable to “downward” coding bias given the consistent trends across all sub-groups, the concordance with other published studies and the fact that there was no meaningful modification or revision of ICD-9-CM codes for HF or DM over the study interval. However, the possibility of bias against coding of comorbid or chronic conditions on discharge abstracts of patients who die is acknowledged45Given the statistically significant and uniform increases in the prevalence of DM and the Elixhauser index over time, the extent of systematic undercoding is admittedly unquantifiable but likely small.
Data quality assessment of the NIS is performed annually and ensures the internal validity of the data. Our data are also in agreement with a report from the National Hospital Discharge Survey, a separate and independent (from NIS) analysis of hospitalization for heart failure in the U.S. over the same time interval from the Centers for Disease Control and Prevention46.
We were only able to assess in-hospital mortality and do not have data on longer-term outcomes that may be more relevant, particularly for younger patients. Observational studies may not be able to fully adjust for residual or unmeasured confounding that might affect our estimates for the reported associations between in-hospital mortality and included covariates. Therefore, inferences based on these observational data can only be viewed as associational and hypothesis-generating and not causal in nature. Selection (survival) bias must be considered operational in all cross-sectional studies. In the absence of a prospective cohort design it is certain that hospitalized patients represent just the fraction of all HF patients, with and without DM, that survived to hospitalization. We indeed observed such a potential source of bias in our data noting that the risk ratio for mortality in HF with DM (data not shown) declined over the age spectrum (higher ratio in the younger age groups which decreases with age), consistent with a (non-testable) hypothesis in this dataset that it is the older subjects with HF and DM who survive to hospitalization40. The absence of specific data on pre- or in-hospital medical therapy for HF and DM in the NIS database precludes further analysis regarding the impact of prevalent treatment on outcomes. The absence of information on pre-hospitalization functional classification, e.g., NYHA Class and left ventricular function, precludes stratification on these important measures. However, modest differences in-hospital mortality between patients with preserved and depressed left ventricular function would not likely affect the observed trends or rates in the absence of large changes in the proportion of these entities over time47.
Finally, these observations pertain to the HF population in the U.S. and may not be generalizable to other HF populations in other countries. In a recent overview of HF hospitalization on a global scale48, it was pointed out that, at least within RCTs, there is much variability in HF hospitalization rates and that outside of the clinical trial universe, the lack of standardized, non-administrative HF-specific registries represent a major limitation to assessing and comparing HF hospitalization rates within and between countries. .Population-based and registry data from several European countries with integrated health information systems indicate a decrease in HF hospitalization rates48 consistent with the data herein, but such trends have not been seen in other European countries.
Supplementary Material
Clinical Perspective.
Given the increasing incidence and prevalence of obesity and diabetes mellitus (DM)-both being risk factors for heart failure (HF)- the impact of DM on HF outcomes warrants close study. Using a nationally representative all-payer inpatient sample of U.S. hospital admissions from 2000–2010 our observations indicate that the total number of hospitalizations with a primary diagnosis of HF decreased while the prevalence of DM as well as a measure of comorbidity burden increased. Despite the increased prevalence of DM and co-morbid burden there was a 36% decrease in age-standardized in-hospital mortality among HF with DM. The decrease in in-hospital mortality was the result of a change in the stratum-specific risk for mortality rather than a change in age and/or sex structure of the 2000 and 2010 samples. However, in-hospital mortality rates varied by age, sex, race and ethnicity and emphasize the need to survey relevant strata within an overall population as well.
Acknowledgments
Sources of Funding
WKL was supported, in part, by the Robert S. Flinn Foundation for Cardiovascular Research. HTD was supported, in part, by NIH Clinical Translational Science Award grant no. UL1TR001449 to the University of New Mexico.
Footnotes
Disclosures
None.
References
- 1.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. A American Heart Association Statistics, Committee Stroke Statistics, Subcommittee Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61:1078–1088. doi: 10.1016/j.jacc.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. doi: 10.1161/CIRCULATIONAHA.113.007787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregg EW, Williams DE, Geiss L. Changes in diabetes-related complications in the United States. N Engl J Med. 2014;371:286–287. doi: 10.1056/NEJMc1406009. [DOI] [PubMed] [Google Scholar]
- 8.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 11.Kannel WB, McGee DL. Diabetes cardiovascular disease The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 12.Nichols GA, Hillier TA, Erbey JR, Brown JB. Congestive heart failure in type 2 diabetes: prevalence, incidence, and risk factors. Diabetes care. 2001;24:1614–1619. doi: 10.2337/diacare.24.9.1614. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee D, Biggs ML, Mercer L, Mukamal K, Kaplan R, Barzilay J, Kuller L, Kizer JR, Djousse L, Tracy R, Zieman S, Lloyd-Jones D, Siscovick D, Carnethon M. Insulin resistance and risk of incident heart failure: Cardiovascular Health Study. Circ Heart Fail. 2013;6:364–370. doi: 10.1161/CIRCHEARTFAILURE.112.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M, Gheorghiade M, Fonarow GC. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015;3:136–145. doi: 10.1016/j.jchf.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ, Investigators C. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 16.Sarma S, Mentz RJ, Kwasny MJ, Fought AJ, Huffman M, Subacius H, Nodari S, Konstam M, Swedberg K, Maggioni AP, Zannad F, Bonow RO, Gheorghiade M. EVEREST investigators Association between diabetes mellitus and post-discharge outcomes in patients hospitalized with heart failure: findings from the EVEREST trial. Eur J Heart Fail. 2013;15:194–202. doi: 10.1093/eurjhf/hfs153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. ADHERE Scientific Advisory Committee Investigators Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Cavender MA, Steg PG, Smith SC, Eagle K, Ohman EM, Goto S, Kuder J, Im K, Wilson PW, Bhatt DL. Impact of Diabetes on Hospitalization for Heart Failure, Cardiovascular Events, and Death: Outcomes at 4 Years from the REACH Registry. Circulation. 2015;132:923–1031. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 19.Hsich EM, Grau-Sepulveda MV, Hernandez AF, Peterson ED, Schwamm LH, Bhatt DL, Fonarow GC. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. 2012;163:430–437. doi: 10.1016/j.ahj.2011.12.013. e3. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich P, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 24.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodcheffer RJ, Roger VL. Diabetes in heart failure: Prevalence and impact on outcome in the population. Am J Med. 2006;119:591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 26.Healthcare Cost and Utilization Project. [Accessed October 7, 2015];Overview of the Nationwide Inpatient Sample 2013. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 27.HCUP Quality Control Procedures. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality. Rockville, MD: 2013. Sep, [Accessed October 7, 2015]. Available at: http://www.hcup-us.ahrq.gov/db/quality.jsp. [Google Scholar]
- 28.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50:1109–1118. doi: 10.1097/MLR.0b013e31825f64d0. [DOI] [PubMed] [Google Scholar]
- 30.Healthcare Cost and Utilization Project. [Accessed October 7, 2015];HCUP methods series. 2010 Available at: http://www.hcup-us.ahrq.gov/reports/methods/methods_topic.jsp.
- 31.Population projections of the United States by age, sex, race, and Hispanic origin: 1995 to 2050; U.S. Bureau of the Census, current population reports. Washington (DC): US Government Printing Office; 1996. [Accessed October 7, 2015]. Available at : http://www.census.gov/prod/1/pop/p25-1130/p251130.pdf. [Google Scholar]
- 32.Healthcare Cost and Utilization Project. [Accessed October 7, 2015];Nationwide Inpatient Sample trends supplemental files. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/nistrends.Jsp.
- 33.Kitagawa EM. Components of a difference between two rates. J Am Stat Assoc. 1955;50:1168–1194. [Google Scholar]
- 34.Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O’Connor CM, Yancy CW, Young J. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): rationale and design. Am Heart J. 2004;148:43–51. doi: 10.1016/j.ahj.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Translating Research Into Practice (TRIP)-II): Fact Sheet. Agency for Healthcare Research and Quality. Rockville, MD: 2001. Mar, [Accessed on October 7, 2015]. Available at http://archive.ahrq.gov/research/findings/factsheets/translating/tripfac/trip2fac.html. [Google Scholar]
- 36.Bradley EH, Webster TR, Baker D, Schlesinger M, Inouye SK, Barth MC, Lapane KL, Lipson D, Stone R, Koren MJ. Translating research into practice: speeding the adoption of innovative health care programs. Issue Brief (Commonw Fund) 2004;(724):1–12. [PubMed] [Google Scholar]
- 37.The Joint Commission. [Accessed October 7, 2015];Comprehensive review of hospital core measures. Available at: http://www.jointcommission.org/comprehensive_review_of_hospital_core_measures.
- 38.Desai JR, Vazquez-Benitez G, Xu Z, Schroeder EB, Karter AJ, Steiner JF, Nichols GA, Reynolds K, Xu S, Newton K, Pathak RD, Waitzfelder B, Elston Lafata J, Butler MG, Kirchner HL, Thomas A, O’Connor PJ. Who Must We Target Now to Minimize Future Cardiovascular Events and Total Mortality? Lessons From the SUPREME-DM Cohort Study. Circ Cardiovasc Qual Outcomes. 2015;8:508–516. doi: 10.1161/CIRCOUTCOMES.115.001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenberg BH, Abraham WT, Albert NM, Chiswell K, Stough WG, Gheorghiade M, O’Connor CM, Sun JL, Yancy CW, Young JB, Fonarow GC. Influence of diabetes on characteristics and outcomes in patients hospitalized with heart failure: a report from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure- OPTIMIZE-HF. Am Heart J. 2007;154:277. doi: 10.1016/j.ahj.2007.05.001. e1-e8. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald MR, Jhund PS, Petrie MC, Lewsey JD, Hawkins NM, Bhagra S, Munoz N, Varyani F, Redpath A, Chalmers J, MacIntyre K, McMurray JJV. Discordant short-and long-term outcomes associated with diabetes in patients with heart failure: Importance of age and sex. Circ Heart Fail. 2008;1:234–224. doi: 10.1161/CIRCHEARTFAILURE.108.794008. [DOI] [PubMed] [Google Scholar]
- 41.Vivo RP, Krim SR, Liang L, Neely M, Hernandez AF, Eapen ZJ, Peterson ED, Bhatt DL, Heidenreich PA, Yancy CW, Fonarow GC. Short- and long-term rehospitalization and mortality for heart failure in 4 racial/ethnic populations. J Am Heart Assoc. 2014;3:e001134. doi: 10.1161/JAHA.114.001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Current population reports. Current Population Reports. Washington (DC): U.S. Government Printing Office; 1996. Population projections of the United states by age, sex, race, and hispanic origin: 1995 to 2050; U.S. Bureau of the Census. pdf. [Google Scholar]
- 43.Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, McKnight B, Ives D, Gottdiener JS, Kuller LH, Longstreth WT., Jr Study of cardiovascular health outcomes in the era of claims data. Circulation. 2016;133:156–164. doi: 10.1161/CIRCULATIONAHA.115.018610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saczynski JS, Andrade SE, Harrold LR, Tija J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):129–140. doi: 10.1002/pds.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iezzoni LI, Foley SM, Daley J, Hughes J, Fisher ES, Heeren T. Comorbidities, complications and coding bias. JAMA. 1992;267:2197–2203. doi: 10.1001/jama.267.16.2197. [DOI] [PubMed] [Google Scholar]
- 46.Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 47.Vaduganathan M, Fonarow GC. Epidemiology of hospitalized heart failure. Heart Failure Clin. 2013;9:271–276. doi: 10.1016/j.hfc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. Lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


