Abstract
Background
The combination of topotecan and cyclophosphamide is active in relapsed Ewing sarcoma family of tumors (ESFT). The feasibility of adding these agents combined with vincristine (VTC) to standard 5-drug chemotherapy with vincristine, doxorubicin, cyclophosphamide (VDC) and ifosfamide, etoposide (IE) administered in an interval compressed (2 week instead of 3 week intervals) schedule was investigated.
Procedure
Newly diagnosed patients with localized ESFT < 31 years, with good performance status and adequate organ function were eligible. Seventeen alternating cycles of chemotherapy with VTC, VDC and IE were to be administered at 2-week intervals. Local control of the primary tumor (LC) occurred following 6 cycles. Primary endpoints were the ability to deliver chemotherapy in an interval compressed schedule and, the rate of grade 3 or greater non-hematologic toxicity and grade 4 hematologic toxicity that delayed chemotherapy by ≥ 2 weeks. Secondary endpoints were event free survival (EFS) and survival (OS).
Results
35 patients with a median age of 11 years enrolled. The mean time to last dose of chemotherapy prior to LC was 12.6 +/− 1.4 weeks and 45.5% of patients received intended chemotherapy without any delay prior to LC. There were no toxic deaths or unexpected toxicities. Five -year event free survival was 79.6% (95% CI 61.8–89.7%) and five year overall survival was 88% (95% CI 71.4–95.3%).
Conclusions
The addition of VTC to standard therapy was tolerable with sufficient interval compression compared to historical standard three week cycles.
Keywords: Ewing Sarcoma, PNET, interval compression, dose dense, topotecan, cyclophosphamide
Introduction
Successful treatment of patients suffering from Ewing Sarcoma Family of Tumors (ESFT) requires the use of systemic multi-agent chemotherapy and surgery, and/or radiation therapy for local tumor control1. Previously conducted Ewing Sarcoma clinical trials have established the importance of doxorubicin in the treatment of ESFT and demonstrated that a regimen of vincristine-doxorubicin-cyclophosphamide (VDC) alternating with ifosfamide-etoposide (IE) was superior to VDC and dactinomycin2–3. Thus VDC alternating with IE became the North American standard chemotherapy regimen for patients with ESFT. Subsequently, over the next decade and a half, the Pediatric Oncology Group (POG)/Children’s Cancer Group (CCG) and the Children’s Oncology Group (COG) conducted two randomized phase 3 clinical trials INT-0154 and AEWS0031 in ESFT to investigate the role of increasing the dose intensity of chemotherapy. INT-0154 randomized patients between 17 cycles of standard 5-drug chemotherapy given every three weeks and 11 cycles of 5-drug chemotherapy with escalated doses of the alkylating agents. There was no difference in survival between the two regimens4. AEWS0031 compared the outcomes of 14 cycles of standard 5-drug chemotherapy given once every 3 weeks to 14 cycles of an experimental regimen of the same 5-drugs given every two weeks. The interval compressed experimental arm proved to be superior to the standard arm with a 5-year event free survival of 73% compared to 65% (P 0.048)5. Thus VDC alternating with IE with chemotherapy cycles administered every 2 weeks has become the new North American standard for the treatment of patients with localized ESFT.
Given the successful strategy in the past of adding the active agents IE to standard chemotherapy to improve outcomes for patients with ESFT, adding other active agents to standard therapy has the potential to further improve outcomes in this group of patients. One such active combination is topotecan and cyclophosphamide, which when administered together has shown activity in patients with relapsed and metastatic ESFT. This combination was recognized as the first new combination that had activity following the identification of IE with response rates of 57% and 33–41% in metastatic and relapsed patients respectively6–7. Based on the standard application of vincristine in the treatment of ESFT, its synergy with camptothecins demonstrated in preclinical sarcoma models8 and in clinical trials for metastatic and relapsed rhabdomyosarcoma9,10, it was also hypothesized that the addition of vincristine to topotecan and cyclophosphamide (VTC) would also benefit patients with ESFT. However, this combination had never been studied in an interval compressed schedule. Therefore we conducted a clinical trial to investigate the feasibility of adding VTC to standard interval compressed 5-drug therapy in ESFT before embarking on a randomized Phase 3 clinical trial.
Methods
Eligibility
Patients eligible for AEWS07P1 had newly diagnosed, localized, previously untreated, biopsy proven osseous or extra-osseous ESFT and were < 31 years of age with an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2. Adequate organ function was required as defined by a creatinine clearance or radioisotope glomerular filtration rate (GFR) of ≥ 70 ml/min/1.73 m2, or threshold age and gender based serum creatinine values derived from the Schwarz formula for estimating GFR11; serum bilirubin ≤ 1.5 times upper limit of normal (ULN) for age; serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) < 2.5 times ULN; left ventricular shortening fraction of > 27% by echocardiogram or ejection fraction of > 50% by gated radionuclide scan. A negative pregnancy test was required for all post-menarchal female patients. Sexually active patients who did not agree to use effective contraception, lactating females who wished to continue breast feeding and those with intra-dural primaries were excluded. Written informed consent prior to starting protocol therapy was required from all participants and/or their parents/legal guardians after all institutional, US Food and Drug Administration, and National Cancer Institute (NCI) requirements for human studies were met.
Clinical Trial Design
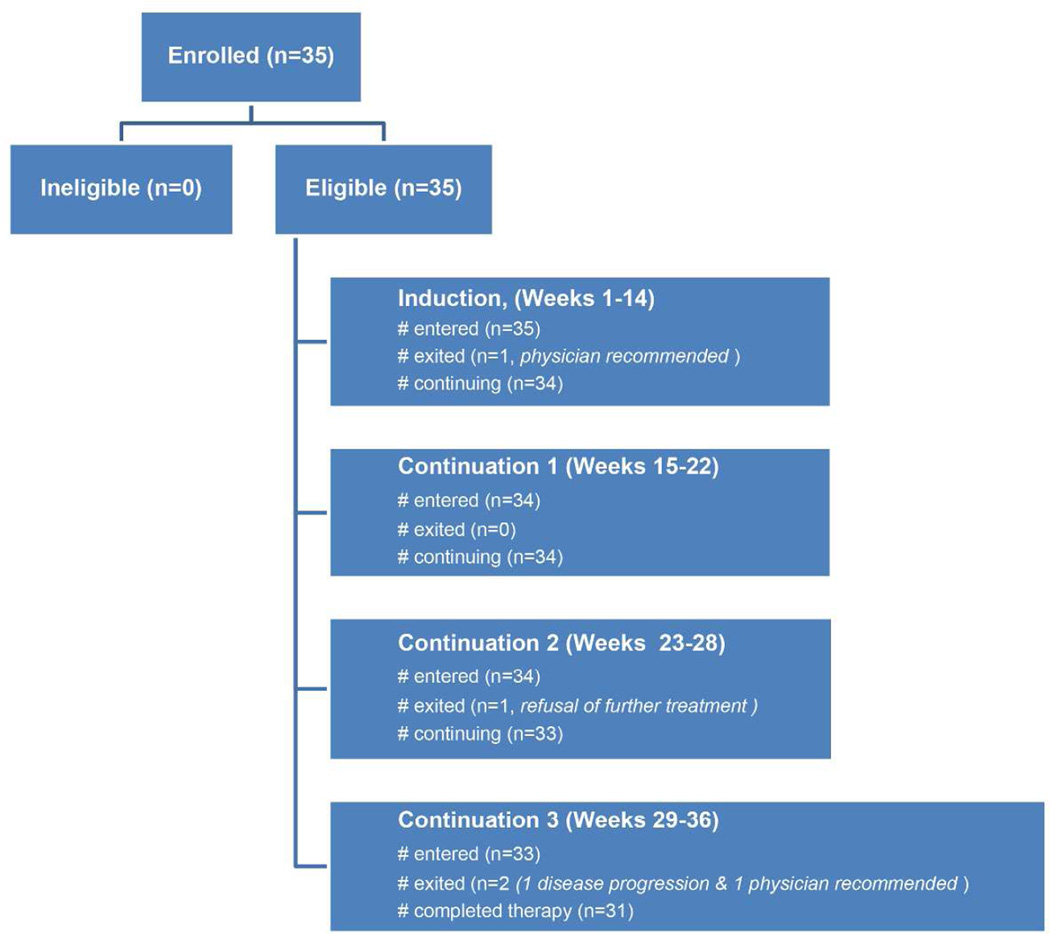
The CONSORT diagram for AEWS07P1 trial is shown in Figure 1 and included induction (cycles 1–6), local control and continuation therapy for a total of 17 cycles of chemotherapy. It was divided into 4 reporting periods (RP); RP 1, weeks 1–14 (cycles 1–6), RP 2, weeks 15–22 (cycles 7–10), RP 3 week 23–28 (cycles 11–13) and RP 4, week 29–36 (cycles 14–17). The treatment schema including schedule, route of administration and doses of VTC, VDC and IE chemotherapy are depicted in Table I. Patients who did not experience progressive disease during induction therapy underwent surgery alone if the lesion could be resected with negative surgical margins and with a reasonable functional result. Continuation chemotherapy was initiated following recovery from surgery with adequate wound healing. If radiation therapy (total dose 55.8 Gray) was used as the primary modality for local control, it began concomitantly with cycle 7 of chemotherapy at chronological week 13. If the surgical margins were involved microscopically radiation was administered at a lower dose (50.4 Gray) as soon as adequate wound healing was achieved following surgical resection concomitantly with continuation chemotherapy. Dexrazoxane was administered as a short infusion at 10 times the dose of doxorubicin immediately prior to doxorubicin infusion for the last two VDC cycles. Granulocyte colony stimulating factor (G-CSF) was administered 24–36 hours after the last dose of myelosuppressive chemotherapy with each cycle and continued for a minimum of 7 days and until the absolute neutrophil count (ANC) was greater than 750/mm3 following the nadir. The subsequent cycle of chemotherapy was to be initiated as soon as the platelet count was self-sustained ≥ 75,000/mm3 and a minimum time of 24 hours had elapsed after the last dose of myeloid growth factor administration. The use of granulocyte macrophage colony stimulating factor (GM-CSF) was not permitted since its use did not facilitate interval compression12.
Figure 1.
AEWS07P1 CONSORT diagram
TABLE I.
Treatment Regimen for AEWS07PI
| Week | Chemotherapy |
|---|---|
| 1 | VTc |
| 2 | V |
| 3 | IE |
| 4 | |
| 5 | VDC |
| 6 | V |
| 7 | IE |
| 8 | |
| 9 | VTc |
| 10 | V |
| 11 | VDC |
| 12 | V |
| 13* 14 |
Surgical Local Control |
| 15* | VTc |
| 16 | V |
| 17 | IE |
| 18 | |
| 19 | IE |
| 20 | |
| 21 | VTc |
| 22 | V |
| 23 | IE |
| 24 | V |
| 25 | IE |
| 26 | |
| 27# | VDC |
| 28 | V |
| 29 | VTc |
| 30 | V |
| 31 | IE |
| 32 | |
| 33# | VDC |
| 34 | V |
| 35 | IE |
| 36 |
V = Vincristine 1.5 mg/m2 (Maximum dose 2 mg)
T = Topotecan 0.75 mg/m2
c = Cyclophosphamide 250 mg/m2/day × 5 days
C= Cyclophosphamide 1200 mg/m2
I = Ifosfamide 1800 mg/m2
E = Etoposide 100 mg/m2/day × 5 days
Patients receiving radiation therapy alone for primary tumor local control began week 15 chemotherapy at chronological week 13
Dexrazoxane 375 mg/m2 (Pre-Doxorubicin)
Note: All drugs administered intravenously
Staging studies at diagnosis included magnetic resonance imaging and/or computed tomography (CT) of primary site, CT of the chest and whole body bone scintigraphy as well as bilateral bone marrow biopsies. The imaging studies were repeated prior to LC and at the end of therapy. Response at the time of LC was assessed using 3-dimensional volume measurement. Patients with greater than 40% increase in volume at the time of LC were considered to have progressive disease and were taken of protocol therapy.
Statistical Analysis
This pilot clinical trial was designed to assess the feasibility of administering the treatment described considering the following primary endpoints: i) incidence of death due to toxicity, ii) incidence rate of dose limiting toxicity (DLT) and iii) the time from the start of therapy to the initiation of LC at week 13. Any patient who received at least one cycle of protocol therapy, and who died as a result of complications of therapy prior to completing therapy or within one month of stopping protocol therapy, and whose death is considered by the institutional investigator to be possibly, probably or definitely related to treatment was considered to be an “on treatment death”. If 2 or more “on treatment deaths” were observed, the study was to be suspended and considered for early termination or modification. Each patient was to be evaluated for toxicity during each RP. Toxicity was assessed using National Cancer Institute Common Toxicity Criteria ® version 3.0 for adverse event reporting. DLT was defined as a Grade 3 or greater non-hematological adverse event that was possibly, probably or definitely related to therapy with the specific exceptions of Grade 3 or greater nausea or vomiting controlled by standard supportive care measures and, Grade 3 infection; or Grade 4 hematological AE that delayed the administration of chemotherapy more than two weeks. If the reporting period was associated with a true incidence of DLT of 30%, the therapy would be identified as excessively toxic with probability 0.92. The rule rate was designed to identify the need for formal review of toxicity by the study committee and Data and Safety Monitoring committee of COG. DLTs were reported as assessed by treating physician and centrally reviewed by the principal investigator. Any patient who completed the first reporting period and was evaluated for local control was evaluated for the third primary endpoint. The average number of weeks from enrollment to the end of the last week of chemotherapy prior to local control was the sample characteristic used to evaluate the feasibility of the accelerated regimen. This average was to be compared with 12 weeks using a one-sided t-test of size 0.05. The hypothesis that the median time was 18 weeks from enrollment to the start of first local control method was tested using the one-sided Wilcoxon signed rank test of a median of 18 weeks and included any patient who was not removed from protocol therapy prior to the initiation of local control measures13.
A maximum of 30 patients evaluable for the interval between start of therapy to the initiation of local control measures was required to address the goals of the study. The secondary end points were event free survival (EFS) and overall survival (OS). EFS was defined from study enrollment until disease progression, occurrence of a second malignant neoplasm, death or last contact, whichever occurred first. Patients who experienced a relapse, SMN or died were considered to have experienced an analytic event; otherwise, the patient was considered censored at the date of last follow-up. OS was defined as the time from enrollment on the study until death or last contact, whichever came first. Patients who died were considered to have experienced an OS event; otherwise, the patient was considered censored at the date of last follow-up. EFS and OS were characterized by the method of Kaplan and Meier14. No formal statistical testing was considered for these two outcome measures.
Results
Patient Characteristics
AEWS07P1 enrolled 35 patients between March and October 2008 (Figure 1). Data current to June 30, 2015 were used for this analysis. Characteristics of patients are given in Table II. One patient was taken off protocol therapy by the treating physician prior to LC to pursue a different treatment plan. No patients received preoperative radiotherapy during LC. The majority of patients received surgery only for LC as expected with only 3 patients receiving surgery followed by adjuvant radiation for microscopic residual disease (Table II).
Table II.
Patient Characteristics (n=35)
| Age (median) | 11 years (0–22) |
| Gender (M/F) | 19/16 |
| Primary site: | |
| Osseous: | 26 |
| Extremity: | 16 |
| Rib: | 6 |
| Pelvis: | 3 |
| Spine: | 1 |
| Extra osseous: | 9 |
| Local Control* | |
| Surgery only: | 20 |
| Radiation only: | 11 |
| Surgery & Radiation: | 3 |
1 patient was taken off protocol therapy prior to local control
Toxicity
Thirty five patients were evaluable for toxicity in RP1, 34 in RP2 and RP3, and 33 in RP4. The reasons for patient attrition are shown in Figure 1. The incidence of DLTs as assessed by treating physician for RP1, RP2, RP3 and RP 4 were 31%, 29%, 21% and 36% compared with central review rates of 34%, 26%, 26% and 39% for the same time periods respectively. There were no deaths related to treatment toxicity and no unexpected toxicities. Table III illustrates the toxicities where the grade was grade 3 or higher in at least 10% of patients during any RP. The incidence of any grade 3 or higher hematological toxicity ranged from 44.1–62.9%, while febrile neutropenia was reported in 18.2–28.6% of patients. Documented infections requiring hospitalization were noted in 18.2–32.4% of patients. Non hematologic toxicity was generally uncommon other than hypokalemia in RP1 and elevated alanine aminotransferase levels in RP4. Hematologic DLT occurred only in RP2, RP3 and RP4 and was exclusive to those patients exposed to radiation therapy. The incidences were 8.8%, 8.8% and 12.1% respectively.
Table III.
Grade 3 or greater toxicities occurring in 10% or more patients in any reporting period
| Chemotherapy Cycles |
1–6 (n=35) |
7–10 (n=34) |
11–13 (n=34) |
14–17 (n=33) |
|---|---|---|---|---|
| Anemia | 62.9% | 35.3% | 44.1% | 45.5% |
| Low ANC | 65.7% | 58.8% | 55.9% | 57.6% |
| Low Platelets | 51.4% | 44.1% | 61.8% | 69.7% |
| Febrile neutropenia | 28.6% | 23.5% | 26.5% | 18.2%` |
| Infection | 25.7% | 32.4% | 26.5% | 18.2% |
| Hypokalemia | 14.3% | 5.9% | 5.9% | 9.1% |
| Elevated ALT | 5.7% | 0 | 2.9% | 12.1% |
ANC- Absolute neutrophil count, ALT – Alanine aminotransferase
Interval Compression
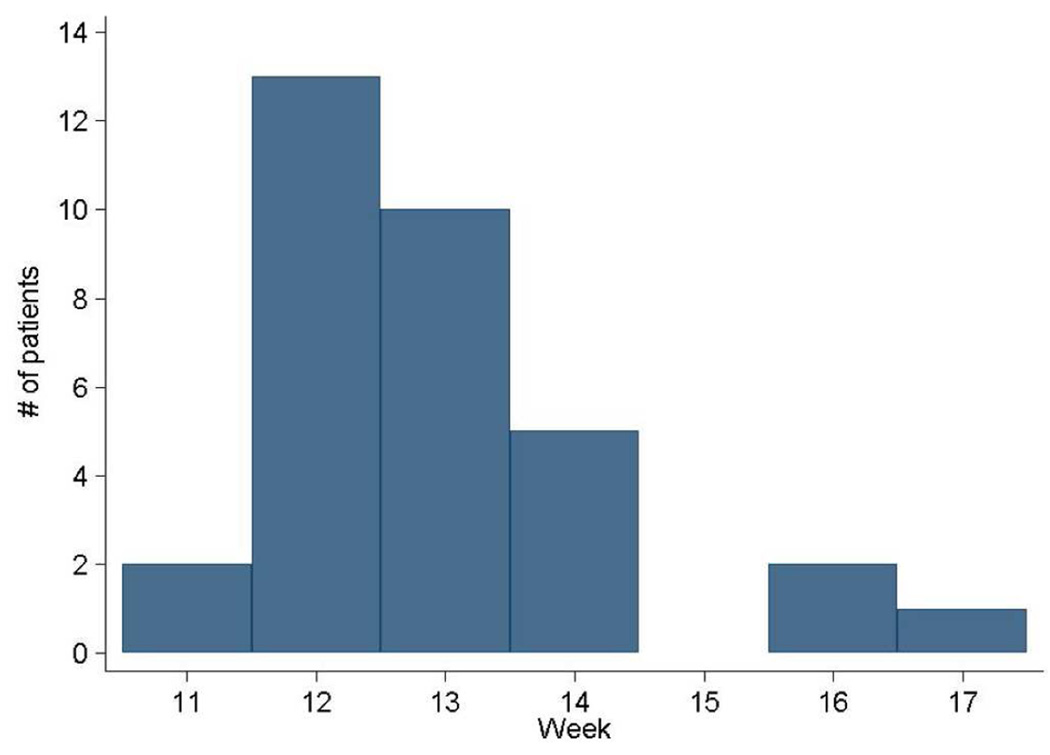
The mean time to LC from the start of treatment was 15.9 weeks (standard deviation 1.2 weeks). This was significantly different from the expected time of 12 weeks by protocol calendar (p< 0.0001). Time to LC ranged from 11.9–20.6 weeks. The mean time from enrollment to last dose of chemotherapy administered prior to local control was 12.6 weeks (standard deviation 1.4 weeks). This was significantly more than the expected protocol calendar of 12 weeks (p< 0.0001). Fifteen of 33 (45.5%) patients received all intended induction chemotherapy by week 12 (Figure 2) and 90% by week 14. Delays were mainly due to scheduling LC surgery rather than due to toxicity (data not shown). The median time from enrollment to the start of the first local control was 14.9 weeks. This was significantly less than a theoretical median of 18 weeks of 6 cycles of chemotherapy administered every 3 weeks (p <0.0001)
Figure 2.
Histogram depicting number completing induction chemotherapy in weeks from starting therapy.
Outcome
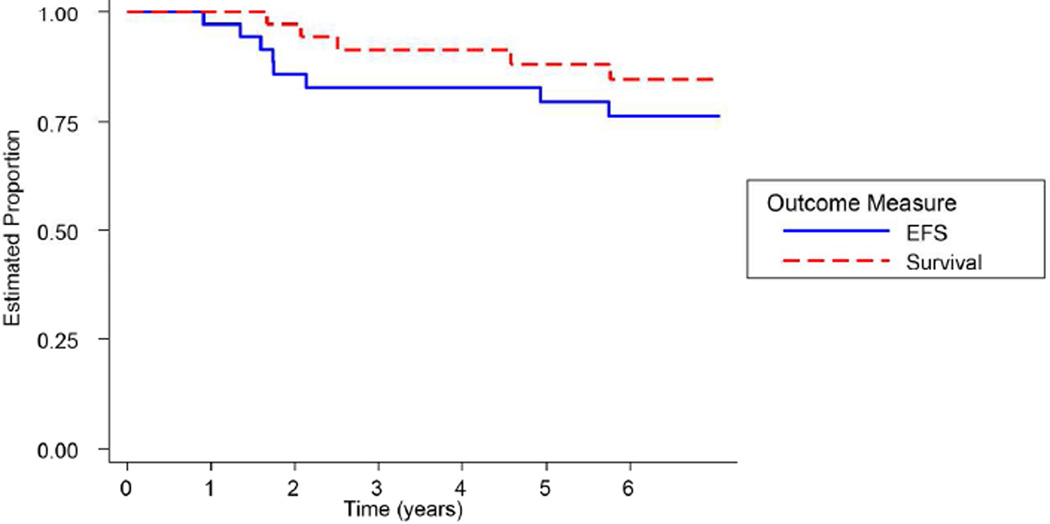
All 35 patients were followed for a median of five years for EFS and OS. Figure 3 illustrates the Kaplan Meier survival plots for the entire cohort of patients. The five-year EFS was 79.6% (95% confidence intervals 61.8 %–89.7%). Six patients relapsed and two patients developed second malignant neoplasms (1 acute myelogenous leukemia, 1 undifferentiated bone sarcoma). The undifferentiated bone sarcoma occurred in the radiation field. Four patients died, three due to relapsed ESFT and 1 due to acute myelogenous leukemia. The five-year OS was 88% (95% confidence interval 71.04–95.3%).
Figure 3.
Probability of event-free survival and survival (n=35).
Discussion
In order to investigate the combination of VTC with interval compressed standard 5-drug frontline therapy for patients with ESFT, it was imperative to test its feasibility. This clinical trial was designed based on the preliminary results of AEWS0031 that confirmed superior EFS for interval compression compared to chemotherapy cycles being administered at 3 week intervals. There were no treatment related deaths on this clinical trial and there were no unexpected toxicities. The DLT rate exceeded 30% in RP1 and RP4. However, the 30% DLT rate rule set in the statistical plan was not designed to identify the RP as excessively toxic, but rather to identify the need for formal review of toxicity by the study committee and Data and Safety Monitoring committee of COG. This was accomplished and the adverse event profile was considered to be acceptable. Infection related complications on AEWS0031 were reported in up to 16% of patients and selected other non-hematological toxicities at less than 5%5. However, toxicity on AEWS0031 was reported using a different toxicity assessment schedule (CTCAE Version 2.0) than that used for AEWS07P1 (CTCAE Version 4.0). Further, toxicities collected on AEWS0031 were not evaluated by the dose-limiting criteria utilized in AEWS07P1. The EURO E.W.I.N.G. 99 clinical trial reported 79–84% grade 4 or greater hematologic toxicity and 50–53% grade 3 or greater non hematologic toxicity in newly diagnosed ESFT patients treated with vincristine, ifosfamide, doxorubicin, etoposide (VIDE) and vincristine, dactinomycin, cyclophosphamide (VAC) or vincristine, dactinomycin, ifosfamide (VAI)15,16. The incidence of grade 3 or greater toxicities exceeded 80% in patients treated on Intergroup Rhabdomyosarcoma Study (IRS) IV where patients received similar chemotherapy with VAC, VAI or vincristine, ifosfamide, etoposide (VIE) administered at three week intervals18. Considering this, the acute toxicity rate noted on our pilot trial appears to be acceptable.
Time to local control was significantly longer than the a priori 12 week criterion set by our pilot trial. The median time to local control was 14.9 weeks (range 11.9–20.6 weeks). Unfortunately, the study design did not take into account delays due to patient preference, the availability of surgical expertise and operating room time, scheduling convenience of the surgeon, lack of elective services on weekend days and the delays incurred by some patients needing general anesthesia for radiation planning and delivery. These are some of the reasons that could have potentially contributed to delays and are more reflective of what happens with patients with ESFT in clinical circumstances. While the true measure of interval compression during induction would be the time point when the patient was clinically ready for local control (platelet count recovery to >/= 75,000/mm3 following week 12 of chemotherapy), this information was not collected. Instead, we used the surrogate data point of when the last dose of chemotherapy was administered prior to LC. The mean of this characteristic was 12.6 weeks from the start of treatment. AEWS0031 did not measure length of time to local control, but rather the number of days from the start of one chemotherapy cycle to the start of the next. While the median time for the standard arm on this trial was 21 days in all groups, the median cycle time for the experimental arm ranged from 15–19 days and was influenced by time point of protocol therapy and whether or not the patient received radiation7. The median time to LC of 14.9 weeks on AEWS07P1 was significantly less than 18 weeks, the best projected time taken to deliver the same 6 cycles of chemotherapy every 21 days. Given that both arms of AEWS0031 had the same 5 active drugs, we inferred that it was the dose dense, interval compressed schedule of chemotherapy administration that improved the EFS of patients with localized ESFT. Though this pilot clinical trial was not designed to assess improvement of EFS and OS, the survival rates appear to be similar to those reported on AEWS00317. This study was also not designed to assess the response rate of newly diagnosed localized ESFT to VTC but rather the feasibility of inclusion of VTC with standard 5-drug chemotherapy. While a phase 2 window design could have provided this information, pre-existing data in metastatic and recurrent patients and the difficulty of accurately measuring response in patients with bone primaries made this question less compelling. Based on the above information, the bone sarcoma committee of COG concluded that the addition of VTC to standard 5-drug chemotherapy was feasible with acceptable toxicity and sufficient interval compression. This regimen is now being investigated in a COG randomized Phase 3 clinical trial (AEWS1031) where it is being compared to standard 5-drug therapy administered in an interval compressed schedule.
Acknowledgments
The National Cancer Institute (NCI) Grant Numbers U10CA180886, U10CA180999, U10CA98543, and U10CA98413 provided infrastructure support for conducting this multi-institutional study through the Children’s Oncology Group (COG). The authors thank Ms. Sanas Javadian for her careful review and formatting of the manuscript.
Footnotes
Conflict of Interest Statement
None declared.
References
- 1.Jürgens H, Dirksen U. Ewing sarcoma treatment. Eur J Cancer. 2011;47(Suppl 3):S366–S367. doi: 10.1016/S0959-8049(11)70206-4. [DOI] [PubMed] [Google Scholar]
- 2.Nesbit ME, Jr, Perez CA, Tefft M, Burgert EO, Jr, Vietti TJ, Kissane J, Pritchard DJ, Gehan EA. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: an Intergroup Study. Natl Cancer Inst Monogr. 1981;(56):255–262. [PubMed] [Google Scholar]
- 3.Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJH, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 4.Granowetter L, Womer R, Devidas M, Krailo M, Wang C, Bernstein M, Marina N, Leavey P, Gebhardt M, Healey J, Shamberger RC, Goorin A, Miser J, Meyer J, Arndt CAS, Sailer S, Marcus K, Perlman E, Dickman P, Grier HE. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children’s Oncology Group Study. J Clin Oncol. 2009;27(15):2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Womer RB, West DC, Krailo MD, Dickman PS, Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP, Weiss AR. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(33):4148–4154. doi: 10.1200/JCO.2011.41.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein ML, Devidas M, Lafreniere D, Souid A-K, Meyers PA, Gebhardt M, Stine K, Nicholas R, Perlman EJ, Dubowy R, Wainer IW, Dickman PS, Link MP, Goorin A, Grier HE Pediatric Oncology Group, Children’s Cancer Group Phase II Study 9457, Children’s Oncology Group. Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children’s Cancer Group Phase II Study 9457--a report from the Children’s Oncology Group. J Clin Oncol. 2006;24(1):152–159. doi: 10.1200/JCO.2005.02.1717. [DOI] [PubMed] [Google Scholar]
- 7.Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, Jürgens H. Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer. 2006;47(6):795–800. doi: 10.1002/pbc.20719. [DOI] [PubMed] [Google Scholar]
- 8.Thompson J, George EO, Poquette CA, Cheshire PJ, Richmond LB, de Graaf SS, Ma M, Stewart CF, Houghton PJ. Synergy of topotecan in combination with vincristine for treatment of pediatric solid tumor xenografts. Clin Cancer Res. 1999;5(11):3617–3631. [PubMed] [Google Scholar]
- 9.Pappo AS, Lyden E, Breneman J, Wiener E, Teot L, Meza J, Crist W, Vietti T. Upfront window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: an intergroup rhabdomyosarcoma study. J Clin Oncol. 2001;19(1):213–219. doi: 10.1200/JCO.2001.19.1.213. [DOI] [PubMed] [Google Scholar]
- 10.Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Lager JJ, Lyden ER, Anderson JR, Pappo AS, Meyer WH, Breitfeld PP. Pooled analysis of phase II window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol. 2006;24(21):3415–3422. doi: 10.1200/JCO.2005.01.9497. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106(3):522–526. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 12.Wexler LH, Weaver-McClure L, Steinberg SM, Jacobson J, Jarosinski P, Avila N, Pizzo PA, Horowitz ME. Randomized trial of recombinant human granulocyte-macrophage colony-stimulating factor in pediatric patients receiving intensive myelosuppressive chemotherapy. J Clin Oncol. 1996;14(3):901–910. doi: 10.1200/JCO.1996.14.3.901. [DOI] [PubMed] [Google Scholar]
- 13.Hajek J, Sidak Z. The Theory of Rank Tests. New York: Academic Press; 1967. [Google Scholar]
- 14.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 15.Le Deley M-C, Paulussen M, Lewis I, Brennan B, Ranft A, Whelan J, Le Teuff G, Michon J, Ladenstein R, Marec-Bérard P, van den Berg H, Hjorth L, Wheatley K, Judson I, Juergens H, Craft A, Oberlin O, Dirksen U. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: results of the randomized noninferiority Euro-EWING99-R1 trial. J Clin Oncol. 2014;32(23):2440–2448. doi: 10.1200/JCO.2013.54.4833. [DOI] [PubMed] [Google Scholar]
- 16.Juergens C, Weston C, Lewis I, Whelan J, Paulussen M, Oberlin O, Michon J, Zoubek A, Juergens H, Craft A. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006;47(1):22–29. doi: 10.1002/pbc.20820. [DOI] [PubMed] [Google Scholar]
- 17.Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, Breneman J, Qualman SJ, Wiener E, Wharam M, Lobe T, Webber B, Maurer HM, Donaldson SS. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]