Abstract
Light can be used to consolidate sleep in individuals with Alzheimer’s disease and related dementias, but the light delivery method is one of the greatest challenges for successful treatment. Based on our field observations, it was hypothesised that a self-luminous light table would be a practical way to deliver light because persons with Alzheimer’s disease typically spend a significant amount of time sitting at tables. Compared to a baseline week, sleep percent and efficiency significantly increased and agitation and depression scores significantly decreased during the four intervention weeks. The self-luminous light table was an effective and practical method to deliver circadian-effective light to persons with Alzheimer’s disease.
1. Background
Irregular and unconsolidated sleep is common among individuals with Alzheimer’s disease and related dementias (ADRD). In addition to being problematic for the person with ADRD, these sleep problems, together with associated agitation and depression, place a significant burden on caregivers. Consequently, these behaviours are frequently the basis for moving individuals with ADRD from private residences to long-term care facilities, and from assisted living settings to nursing homes. The psychological and financial costs of these transitions to more controlled environments can be high.
Presumably these adverse sleep effects are caused, or at least exacerbated by irregular or inadequate 24-hour light–dark exposure patterns that disrupt circadian regulation. Consistent with this idea, several research studies have demonstrated that daytime bright light exposure can consolidate sleep at night and increase nighttime sleep efficiency, whilst increasing daytime wakefulness and reducing evening agitation in persons with ADRD.1–4 Although these studies show positive effects of daytime bright light treatment on sleep, cognition and behaviour, others have failed to show any effect.5 A recent Cochrane review, for example, states that that there is not enough evidence to conclude that bright light treatment during the day is effective at improving sleep in persons with ADRD.6
After visiting several nursing homes, we observed that residents with ADRD spend most of their waking hours in their rooms alone or, more commonly, they are brought together into a common area because nurses find it easier to provide personalised care. We also observed that these common areas tend to be dimly illuminated. We hypothesised that the negative results reported in the Cochrane review may simply reflect the use of ineffective bright light delivery methods during the day that would be required for circadian system regulation. With this in mind, we also observed that residents with ADRD in common areas often sat together around a table for many hours. We hypothesised that a self-luminous light table designed to provide high circadian light stimulation during daytime hours might be practical and effective at regulating circadian rhythms and thereby improve sleep outcomes for these residents. The goal of the present study was to test this hypothesis in an actual clinical setting.
2. Method
2.1 Participant selection
Six participants with ADRD living in a nursing home were recruited for the study (four females; mean age was 84 ± 8.6 years). The mean Brief Interview for Mental Status (BIMS) score of the participants was 5.3 ± 1.9. Scores between 0 and 7 suggest that residents are severely impaired and scores between 8 and 12 suggest that residents are moderately impaired. All study materials and procedures were reviewed and approved by the Institutional Review Board at Rensselaer Polytechnic Institute. Informed consent was obtained from participants’ family members after full explanation of the procedures, in accordance with the Declaration of Helsinki.7
2.2 Experimental protocol
The experiment comprised a baseline week, four intervention weeks with an energised self-luminous light table, and four post-intervention weeks after the light table was removed from the facility. After the baseline week, the self-luminous light table was installed in a common area and remained there for four weeks. The common area did not have direct access to daylight and was lit by 60 × 120 cm fluorescent light fixtures, delivering less than 50 lx at eye level. The light table was placed on a timer and operated during daytime from 7:00 a.m. to 6:00 p.m. Upon waking, caregivers directed the participants to the light table for breakfast. Although they were allowed to get up and walk around the facility during the day, our observations were that participants remained sitting at the light table for most of the day. Lunch and dinner were also served at the light table. Participants were taken back to their rooms after dinner, where they stayed until the following morning.
A device that continuously measured light and activity was placed on the nondominant wrist at the start of the baseline week, immediately after the participants were selected and signed consent documents had been collected. Light and activity data were collected for seven consecutive days during the baseline, intervention and post-intervention periods. All caregivers were instructed to keep the device on the participants for the entire day and night, except when showering. They were also asked to ensure that blankets and long-sleeve sweaters and shirts did not cover the device.
The primary, nighttime caregivers completed standardised questionnaires related to observed sleep quality, depression and agitation at the end of the baseline week, after the four intervention weeks and after the four post-intervention weeks. The nightshift caregivers who completed the standardised questionnaires were blind to the purpose and operation of the light table intervention.
2.3 Light intervention
Custom self-luminous tables were built for the study from 178 cm (70-in) LED edge-lit televisions (Figure 1). The flat panel display was incorporated into a t-slotted aluminum frame (MiniTec Framing Systems, Victor, NY) that allowed it to be used as a light table. A protective clear acrylic sheet (0.64-cm thick) was attached to the top of the frame.
Figure 1.
The self-luminous light table. An LED edge-lit television was inserted in a customised table frame to deliver light to persons with ADRD at a long-term care facility.
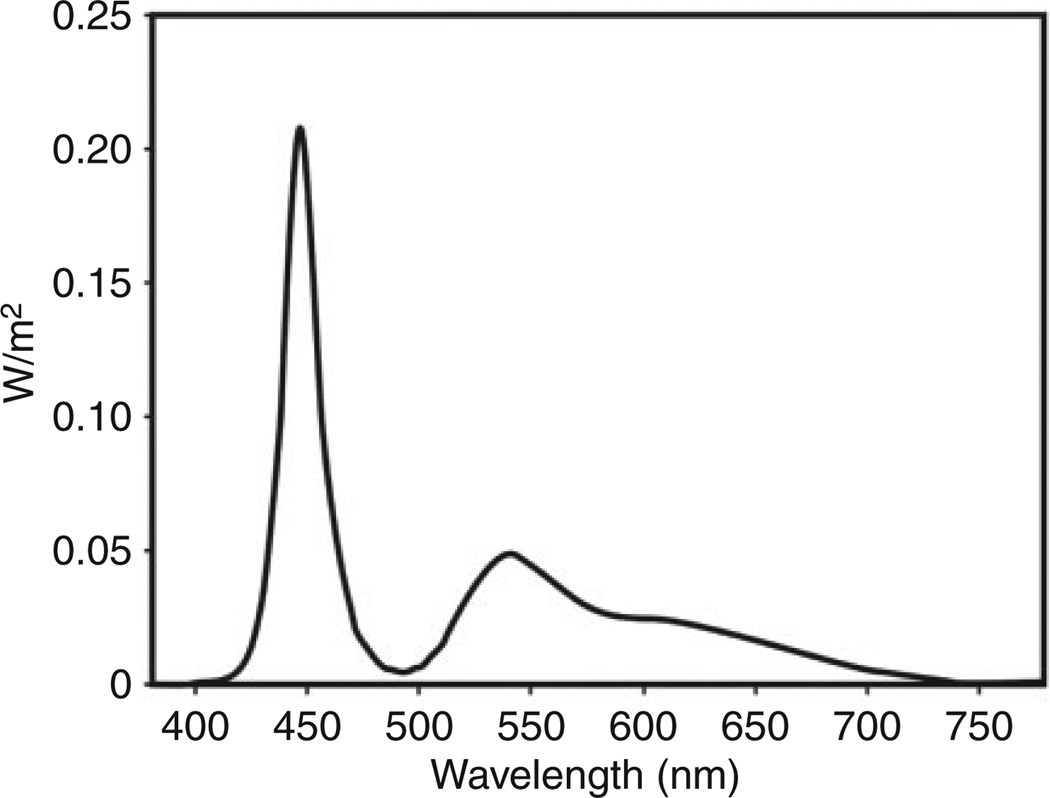
Spectroradiometric measurements were performed in the laboratory. Measurements at 12 different points on the surface of the table (4 × 3 grid) were taken with a spectroradiometer (Photo Research SpectraScan PR 740) placed at eye level at 46 cm from each of the points on the table. Figure 2 shows the average spectral irradiance distribution of the measurements at the 12 points. The measured photopic illuminance at 46 cm was 1190 lx when the sensor was placed perpendicular to the plane of the table surface and 2780 lx when it was oriented towards the centre of the table.
Figure 2.
Average measured spectral irradiance distribution at 46 cm from the table.
The model of human circadian phototransduction proposed by Rea et al.8,9 was used to estimate the circadian-effective light potentially available to participants during daytime operation of the self-luminous light table. At 2780 lx, the light table would provide a circadian stimulus (CS) value of 0.5. A CS value of 0.7 is the highest possible, indicating that the light table could deliver very high levels of circadian-effective light.10 CS is directly proportional to nocturnal melatonin suppression after one-hour exposure (0–70%).10 A value of 0.7 is comparable to morning daylight exposure.
2.4 Outcome measures
2.4.1 Personal light exposure and activity levels
The Daysimeter is a small device that continuously records light–dark patterns (using red, green and blue [RGB] sensors) and is calibrated in units of photopic illuminance (lux), circadian illuminance (CLA) and CS, which characterises both the spectral and absolute sensitivities of the human circadian system.11 Rest–activity patterns are also recorded from a three-axis, monolithic solid-state accelerometer calibrated in g-force (1 g force = 9.8 m/s2) with an upper frequency limit of 6.25 Hz. An activity index (AI) is determined using the rest–activity data.11
2.4.2 Sleep analyses
The Daysimeter activity data were used to estimate percent sleep time and sleep efficiency. Percent sleep time was defined as the sum of epochs scored as sleep multiplied by the total epoch length and then divided by the assumed sleep time as determined by the actigraphy data. Sleep efficiency was defined as total sleep time divided by the total amount of time in bed. Sleep start was defined as the first 10-minute period with one or less epochs scored as mobile. Similarly sleep end was defined as the last 10-minute period with one or less epochs scored as mobile. Due to low compliance rates, the activity data were not evaluated for the post-intervention period and are not discussed.
2.4.3 Standardised questionnaires
The following questionnaires were administered to assess subjective sleep quality, depression and agitation: (1) Pittsburgh Sleep Quality Index (PSQI):12 PSQI is a tool that can be used to measure sleep quality in clinical populations, composed of 19 items that generate seven component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication and daytime dysfunction). The sum of the seven component scores yields one global score. A person with a global score above 6 is considered to have sleep disturbances; (2) Cornell Scale for Depression in Dementia (CSDD):13 CSDD is a 19-item tool designed to rate symptoms of depression in persons with dementia. This tool evaluates the presence and extent of mood-related signs (anxiety, sadness, irritability), behavioural disturbances (agitation, loss of interest), physical signs (loss of appetite, weight loss), cyclic functions (mood variation, sleep quality) and ideational disturbances (suicidal thoughts, poor self-esteem); (3) Cohen-Mansfield Agitation Inventory (CMAI).14 The purpose of the CMAI is to assess the frequency of manifestations of agitated behaviours in elderly persons. The CMAI is a caregivers’ rating questionnaire consisting of 29 agitated behaviours, each rated on a 7-point scale.
3. Results
3.1 Daysimeter data
Complete data were available from four participants during baseline and intervention periods and these data are reported here. Two-tailed Student’s t-tests were performed comparing data obtained during the baseline week and during the four intervention weeks.
3.1.1 Light exposure
The intervention increased the CS values measured by the Daysimeter, but this increase was not statistically significant (p = 0.15). The mean and standard deviation CS of participants at baseline and during the intervention period were 0.04 ± 0.005 and 0.08 ± 0.02. More importantly, the absolute values were too low to be representative of corneal light exposures, most likely because the wrist-worn device was below the self-luminous light table (Figure 1) and did not record the light at the cornea.
3.1.2 Actigraphy
Percent sleep significantly (t = 3.9; p = 0.02) increased during the intervention weeks. Table 1 provides the data for the individual participants obtained during baseline and intervention periods. Sleep efficiency increased during intervention weeks, but this difference did not reach statistical significance.
Table 1.
Mean ± standard deviation (SD) percent sleep and sleep efficiency from the actigraph data for the individual participants obtained during baseline and intervention periods.
| Subject | Baseline | Intervention | ||
|---|---|---|---|---|
| Percent sleep (%) |
Sleep efficiency (%) |
Percent sleep (%) |
Sleep efficiency (%) |
|
| 1 | 75 | 45 | 86 | 65 |
| 2 | 84 | 75 | 91 | 86 |
| 3 | 83 | 67 | 87 | 73 |
| 4 | 82 | 51 | 86 | 49 |
| Mean | 81.1 | 59.4 | 87.4 | 68.2 |
| SD | 4.3 | 13.6 | 2.2 | 15.6 |
3.2 Questionnaire data
Complete data for all six participants were obtained. Compared to baseline, light exposure significantly reduced depression scores from the CSDD (t = 5.2; p = 0.003) and agitation scores from the CMAI (t = 2.6; p = 0.046). Both depression and agitation scores remained significantly lower after post-intervention compared to baseline (t = 3.9; p = 0.01 and t = 3.6; p = 0.01, respectively), suggesting a carryover effect of the light intervention. Compared to baseline, the PSQI scores decreased during the intervention, suggesting an improvement in sleep quality, consistent with the sleep analyses, but this difference did not reach statistical significance (t = 1.8; p = 0.13). Table 2 provides the questionnaire data for the individual participants obtained during baseline, intervention and post-intervention periods.
Table 2.
Mean ± standard deviation (SD) data for the individual participants obtained during baseline, intervention and postintervention periods.
| Subject | Baseline | Intervention | Post Intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PSQI | CSDD | CMAI | PSQI | CSDD | CMAI | PSQI | CSDD | CMAI | |
| 1 | 12 | 12 | 53 | 5 | 1 | 39 | 9 | 9 | 43 |
| 2 | 9 | 17 | 73 | 2 | 3 | 29 | 7 | 3 | 31 |
| 3 | 11 | 10 | 52 | 9 | 0 | 29 | 7 | 3 | 38 |
| 4 | 4 | 5 | 32 | 8 | 1 | 30 | 2 | 6 | 37 |
| 5 | 14 | 8 | 40 | 6 | 3 | 29 | 3 | 8 | 33 |
| 6 | 8 | 14 | 39 | 7 | 8 | 34 | 4 | 12 | 35 |
| Mean | 9.7 | 11.0 | 48.2 | 6.2 | 2.7 | 31.7 | 4.6 | 6.8 | 36.2 |
| SD | 3.5 | 4.3 | 14.6 | 2.5 | 2.9 | 4.1 | 2.7 | 3.5 | 4.2 |
Note: PSQI: Pittsburgh Sleep Quality Index; CSDD: Cornell Scale for Depression in Dementia; CMAI: Cohen-Mansfield Agitation Inventory.
4. Discussion
Consistent with previous reports,1,15–17 the present results add to the literature showing that bright, daytime light exposures promote circadian regulation in persons with ADRD, helping to consolidate nighttime sleep, reduce depression and reduce agitation. From a practical perspective, it should be noted that, consistent with several other studies15,17 these data showed significant carryover effects from the light treatment period. Depression and agitation scores and sleep quality were reduced well after the light treatment was removed. This suggests that the light treatment might not have to be delivered every day, enabling caregivers to vary the daily routines of those in their care, as well as their own, without fear of sudden relapse.
The increase in sleep percent and sleep efficiency observed in this study as a result of the lighting intervention may be clinically important because recent studies suggest a relationship between sleep efficiency and deposition of fibrillar amyloid β (Aβ) in the brain, which is one of the hallmarks of Alzheimer’s disease.18–20
Although the sample size was small for this study and replication in a larger population is warranted, it is important to note that, as shown in Tables 1 and 2, most, if not all of the participants exhibit a change in the outcome measures consistent with the hypothesised direction. In fact, the statistically significant effects of the light treatment based upon the small sample size should be considered an asset for generalisation of the findings that daily, bright light treatment can be an effective intervention to improve the lives of individuals with ADRD and those of their caregivers.
Finally, it should be noted that the questionnaire data obtained in the present study need to be considered with caution because the caregivers that provided responses may not have been completely blinded to the intervention. Although the nighttime caregivers were not likely to know whether the light table had been on during the day, they may have noticed whether or not the participants were wearing the Daysimeter devices and answered accordingly. However, this seems unlikely because the caregivers did not know the purpose of the study and were unfamiliar with the instruments prior to the questionnaires. A second important caution stems from the use of proxy data. Proxy data were used, rather than self-reports from the participants themselves, because all participants were moderately to severely demented. It is important to note, however, that the objective measures of sleep (efficiency and sleep percent) from the participants were consistent with the proxy data obtained from the caregivers. Future studies should also include a placebo control condition to minimise potential bias in the subjective data from caregivers.
5. Conclusion
The present study demonstrated that a self-luminous light table was an effective and practical method to deliver circadian light to improve sleep, mood and behaviour in individuals with ADRD. Two related features of the light intervention probably led to the successful outcomes: (1) the self-luminous light table has the potential to deliver an amount and a spectrum that can strongly stimulate the human circadian system (CS values above 0.5) and (2) the self-luminous light table can be very effective at delivering light to individuals with ADRD because they were commonly observed sitting together around a table with their heads tilted down. Recognising that the light delivery method is one of the biggest challenges for successful light treatment, the self-luminous light table tested here is probably a highly effective and practical way to improve sleep and reduce mood disorders for individuals with ADRD living in controlled environments or at home.
Acknowledgments
Sharon Lesage, Anna Murphy, Martin Overington, Geoffrey Jones, Rebekah Mullaney and Dennis Guyon of the Lighting Research Center are acknowledged for their technical and editorial assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the National Institute on Aging (R01AG034157). Products were donated by Sharp Corporation.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Mishima K, Hishikawa Y, Okawa M. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer’s type. Chronobiology International. 1998;15:647–654. doi: 10.3109/07420529808993200. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos C, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. International Journal of Geriatric Psychiatry. 1999;14:520–525. [PubMed] [Google Scholar]
- 3.Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep–wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry and Clinical Neurosciences. 2000;54:352–353. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Someren EJW, Kessler A, Mirmirann M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biological Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 5.Colenda CC, Cohen W, McCall WV, Rosenquist PB. Phototherapy for patients with Alzheimer disease with disturbed sleep patterns: results of a community-based pilot study. Alzheimer Disease and Associated Disorders. 1997;11:175–178. doi: 10.1097/00002093-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. The Cochrane Database of Systematic Reviews. 2014;2:CD003946. doi: 10.1002/14651858.CD003946.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Medical Association. Declaration of Helsinki. JAMA. 2000;284:3043–3045. [PubMed] [Google Scholar]
- 8.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Research Reviews. 2005;50:213–228. doi: 10.1016/j.brainresrev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Rea MS, Figueiro MG, Bierman A, Hamner R. Modeling the spectral sensitivity of the human circadian system. Lighting Research and Technology. 2012;44:386–396. [Google Scholar]
- 10.Rea MS, Figueiro MG, Bierman A, Bullough JD. Circadian light. Journal of Circadian Rhythms. 2010;8:2. doi: 10.1186/1740-3391-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Lighting Research and Technology. 2013;45:421–434. doi: 10.1177/1477153512450453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Alexopolous GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biological Psychiatry. 1988;23:271. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. The Journals of Gerontology. 1989;44:M77–M84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 15.Figueiro MG, Plitnick B, Lok A, Jones G, Higgins P, Hornick T, Rea MS. Tailored lighting intervention improves measures of sleep, depression and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Journal of Clinical Interventions in Aging. 2014;9:1527–1537. doi: 10.2147/CIA.S68557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Someren EJW, Hagebeuk EE, Lijzenga C, Scheltens P, de Rooij SE, Jonker C, Pot AM, Mirmiran M, Swaab DF. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biological Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 17.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 18.Kang J-E, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, Zhou Y, Wong DF, Ferrucci L, Resnick SM. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spira AP, Yager C, Brandt J, Smith GS, Zhou Y, Mathur A, Kumar A, Brašić JR, Wong DF, Wu MN. Objectively measured sleep and β-amyloid burden in older adults: A pilot study. SAGE Open Medicine. 2014;2:1–6. doi: 10.1177/2050312114546520. [DOI] [PMC free article] [PubMed] [Google Scholar]