Abstract
Although for many children, Autism Spectrum Disorder (ASD) is a lifelong disability, a subset of children with ASD lose their diagnosis and show typical cognitive and adaptive abilities. The ages at which this transition can occur is not known, but it sometimes occurs quite early. Participants in the current study were 207 children with an ASD at age two who were reevaluated at age four. Eighty-three percent retained an ASD diagnosis at reevaluation and 9% showed “optimal progress”: clear ASD at age two but not at age four, and average cognition, language, communication and social skills at age four. Early child-level factors predicted optimal progress: diagnosis of PDD-NOS, fewer repetitive behaviors, less severe symptomatology and stronger adaptive skills.
Keywords: ASD, optimal outcome, loss of diagnosis
Autism Spectrum Disorders (ASDs) are a group of neurodevelopmental disorders characterized by deficits in social communication accompanied by repetitive behaviors and/or restricted interests. In addition to these core deficits, individuals with ASD often experience a number of comorbid deficits including cognitive delays/intellectual disabilities, adaptive skill deficits and motor delays (Charman et al. 2011; Levy, Mandell & Schultz 2009; Lloyd, MacDonald & Lord 2013; Macdonald, Lord & Ulrich 2013; Volkmar, Lord, Bailey, Schultz & Klin 2004). The Center for Disease Control (CDC 2014) reports an overall prevalence rate for ASDs of one in 68, with boys affected at greater rates than girls (4.5:1). ASDs occur within all racial/ethnic and socioeconomic groups (CDC 2014; Fombonne 2003); however, evidence indicates that disparities in access to health care may contribute to decreased reported prevalence and later age of diagnosis for minority children and children of families with lower socioeconomic status (Fombonne 2003; Herlihy et al. 2014; Mandell, Listerud & Levy 2001).
Diagnostic Stability of ASDs
ASDs have long been considered lifelong disorders by clinicians and parents (Levy & Perry 2011; Seltzer, Shattuck, Abbeduto & Greenberg 2004). Follow-up studies of individuals diagnosed in childhood indicate that between 80 and 90% of individuals continue to meet diagnostic criteria in adolescence or adulthood (Charman et al. 2005; Seltzer et al. 2004; Woolfenden, Sarkozy, Ridley & Williams 2012). Increases in the understanding of the early behavioral profiles of individuals with ASD have allowed reliable diagnoses to be given in early childhood, often around 24 months (Chawarska, Klin, Paul, Macari & Volkmar 2009; Eaves & Ho 2004; Kleinman et al. 2008; Turner & Stone 2007). Given the increase in early diagnosis, it is of great importance that we understand the diagnostic stability of ASDs during the early years of a child’s life.
A number of studies have investigated diagnostic stability in toddlerhood. The evidence suggests that diagnostic stability is high following diagnoses given as early as 18 to 24 months. Studies have reported between 68 and 100% stability of ASD diagnoses made at approximately age two to follow-up at age three or four (Chawarska et al. 2009; Eaves & Ho 2004; Guthrie et al. 2013; Kim et al. 2015; Kleinman et al. 2008; Lord 1995; Sutera et al. 2007; Turner & Stone 2007). In a high-risk sample of later-born siblings of children with ASD, stability was found to be 93% for ASD diagnoses made at 18 months and 82% for diagnoses made at 24 months (Ozonoff et al. 2015). The stability of an ASD diagnosis is higher following a diagnosis of Autistic Disorder (AD, 68 to 100%) than a diagnosis of Pervasive Developmental Disorder- Not Otherwise Specified (PDD-NOS, 40 to 90%) (Chawarska et al. 2009; Eaves & Ho 2004; Kleinman et al. 2008; Sutera et al. 2007; Turner & Stone 2007).
A subset of children initially diagnosed with an ASD at approximately age two appear to lose their ASD diagnosis by age four. Across studies, this occurs for a range of 0 (Chawarska et al. 2009) to 37.5% of children (Turner & Stone 2007). Notably, the majority of studies investigating diagnostic stability in toddlers found that between 6 and 18% of their sample lost their diagnosis (Eaves & Ho 2004; Kim et al. 2015; Kleinman et al. 2008; Sutera et al. 2007), indicating that more extreme findings (0%, 37.5%) may be the result of specific sample characteristics. For example, Chawarska et al. (2009), who reported that no children lost their diagnosis, had a slightly earlier age at initial diagnosis and younger age at follow-up than studies that found evidence for loss of diagnosis. Additionally, Turner and Stone (2007), who reported the highest percentage of these outcomes, reported that 100% of their sample received some form of early intervention between their two diagnostic evaluations.
Predicting Diagnostic Stability and Outcome: A Brief Overview
In addition to diagnostic subtype (AD vs. PDD-NOS), a number of factors have been related to diagnostic stability in the toddler and preschool years. Children diagnosed before age three years appear to have less stable diagnoses over time as well as more positive outcomes than children diagnosed later (Woolfenden et al. 2012). In two separate studies, Turner, Stone and colleagues (2006, 2007) found that children diagnosed before age three had the least stable diagnoses and the most positive outcomes. Turner and Stone (2006) note that this does not indicate that early diagnoses are inaccurate or that clinicians should wait to diagnose until later in toddlerhood. Rather, they explain that children diagnosed early (i.e., before age three years) appear to have the greatest likelihood of benefiting from early intervention, and thus, exhibit less stable diagnoses.
Early cognitive and language abilities are associated with both diagnostic stability and later functioning more broadly. Positive outcomes including decreases in ASD symptoms and growth in social skills are predicted by stronger early language abilities (Baghdadli et al. 2012; Kim et al. 2015; Luyster et al. 2007; Sallows & Graupner 2005; Stevens et al. 2000). High verbal and nonverbal IQ at approximately age two has been shown to predict change in diagnostic status from AD to PDD-NOS or PDD-NOS to non-spectrum (Lord et al. 2006). Children who move off the ASD spectrum have also been found to show higher early visual reception and receptive language abilities on the Mullen Scales of Early Learning (Mullen, 1995) than peers who remain on the spectrum (Turner & Stone 2007). It is important to note that while some studies (e.g., Lord et al. 2006; Turner & Stone 2007) found that cognitive and language abilities helped to predict unstable versus stable diagnoses, other studies did not find such differences (e.g., Chawarska et al. 2009). Therefore, while there exists substantial support for higher cognitive and language abilities predicting more positive outcomes broadly, evidence for the predictive utility of cognitive and language abilities in terms of diagnostic stability is mixed.
Symptom severity has also been related to diagnostic stability and outcome. Lesser early symptom severity has been found to predict growth in social skills over time (Baghdadli et al. 2012). Further, diagnostic improvement or the loss of an ASD diagnosis has been predicted by lesser symptom severity at age two, particularly in the domains of social interaction (Kim et al. 2015; Turner & Stone 2007; Lord et al. 2006) and restricted, repetitive behaviors (Lord et al. 2006). There is some evidence that early adaptive skills may also help to predict diagnostic change. Stronger early daily living skills (Sallows & Graupner 2005; Sutera et al. 2007) and motor skills (Sutera et al. 2007; Turner & Stone 2007) have been found to predict movement off the ASD spectrum between ages two and four.
Outcomes Following the Loss of an ASD Diagnosis
A number of outcomes are possible for children who lose their ASD diagnosis in the toddler years or later in childhood. The majority of children who lose their ASD diagnosis are then diagnosed with another developmental disorder (60 to 100%), such as Developmental Delay or Developmental Language Disorder (Eaves & Ho 2004; Kim et al. 2015; Kleinman et al. 2008; Turner & Stone 2007). Of particular interest to the present study are the remaining children who lose their ASD diagnosis and appear to demonstrate more or less typical functioning. In the first documented report of average functioning following an ASD diagnosis, Lovaas (1987) found that 47% of his sample was functioning cognitively in the average range following intensive behavioral therapy. Importantly, however, Lovaas did not report whether individuals in his sample continued to meet criteria for an ASD following intervention. Relatively few studies have attempted to thoroughly characterize children who move off the spectrum (e.g., investigate cognitive abilities as well as remaining ASD symptoms), and therefore, it is difficult to estimate the percentage of children who move off the spectrum and are functioning in the average range in all domains. In a review of literature reporting on outcomes, Helt and colleagues (2008) determined that between 3 and 25% of children appear to lose their ASD diagnosis sometime in development and demonstrate functioning in the average range cognitively, adaptively and socially.
A few studies have characterized these children who appear to demonstrate an “optimal outcome” from an early ASD diagnosis. “Optimal outcome” has been defined as follows: the child must have previously met diagnostic criteria for an ASD following a gold standard diagnostic assessment, must no longer meet criteria for any ASD based on gold standard diagnostic assessment, must be participating in mainstream classrooms without the help of an aid, and must demonstrate a full scale IQ greater than 70 (Kelley, Naigles, & Fein 2010). Kelley and colleagues (2010) compared 13 children who attained “optimal outcome” (OO) to 14 children who demonstrated typical functioning and to 14 children who were classified as having High Functioning Autism (HFA). At a mean age of 10.5 years, children who attained OO demonstrated similar functioning to typically developing children in their adaptive skills and broad language abilities (Kelley et al. 2010).
Fein and colleagues (2013) compared a larger sample of 34 children with OO, 44 children with HFA, and 34 children with typical development at a mean age of 13 years. Criteria for OO remained largely the same as that described in Kelley et al. (2010), with the exception of stricter criteria for average social and communication functioning (i.e., scores within 1.5 SD of the mean on the Vineland Adaptive Behavior Scales (VABS) Socialization and Communication domains). In an assessment of language abilities, facial recognition abilities, socialization, communication and ASD symptoms, they found average functioning across measures for the OO group and very few differences between the OO and typically developing groups. In a more in depth analysis of language functioning in a subset of this sample, Kelley and colleagues (2006) found that the OO group demonstrated subtle residual deficits in pragmatic and semantic language when compared to typically developing peers. Overall, it appears that children with OO are functioning very similarly to their typically developing peers across domains, with very subtle deficits detectable on only the most fine-grained measures.
Predicting Highly Positive Outcomes
While a number of studies have investigated diagnostic stability, and a few studies have attempted to characterize the most optimal outcomes, relatively fewer studies have attempted to predict highly positive outcomes. In a prospective study, Sutera and colleagues (2007) found that 17.8% of children diagnosed with an ASD at age two moved off the spectrum by age four and did not exhibit cognitive impairment. This outcome was predicted by a diagnosis of PDD-NOS (vs. AD) at age two and stronger early fine motor and daily living skills.
In a large-scale, long-term prospective study, Anderson and colleagues (2014) followed a group of children diagnosed with an ASD at approximately age two to the age of about 19. In this group of young adults (with verbal IQ greater than or equal to 70), they identified a group with “very positive outcome,” with characteristics similar to children with “optimal outcome” in other studies. This “very positive outcome” at age 19 was predicted by fewer repetitive behaviors at age three years (but not at age two years), an absence of parent-reported hyperactivity at age three years, and participation in some individual intervention before age three years (Anderson et al. 2014). In a retrospective study, “optimal outcome” has been associated with milder parent-reported early social symptoms (lifetime socialization scores on the Autism Diagnostic Interview, Revised (ADI-R), lifetime scores on the Social Communication Questionnaire (SCQ)), but not by differences in parent-reported early communication symptoms or restrictive, repetitive behaviors (RRBs) (Fein et al. 2013). Additional prospective studies are needed to further clarify early predictors of highly positive outcomes from an early ASD diagnosis.
The Present Study
Given the state of the research on highly positive outcomes from ASDs in the toddler years, the current study seeks to characterize early cognitive and behavioral differences between children who demonstrate “optimal progress” (OP) and those who remain on the spectrum (ASD). The criteria for “optimal progress” used in the current study stem from criteria for “optimal outcome” (see Helt et al. 2008) with some adjustments to reflect the developmental level of preschoolers. We use the term “optimal progress” since status at age four cannot be considered an “outcome.” Optimal progress is defined as follows: a child must have met criteria for an ASD using gold standard diagnostic procedures, must no longer meet criteria for any ASD at follow up, and must demonstrate functioning in the average range (within 1.5 SD of the mean) on standardized measures of cognition, language, communication and social skills.
Specifically, we will investigate possible group differences in initial diagnosis (AD vs. PDD-NOS), cognitive abilities, language abilities, motor skills, adaptive skills and severity of ASD symptoms. Based on previous research, we hypothesize that children who demonstrate OP will be more likely to have an initial diagnosis of PDD-NOS and will show stronger early cognitive, language, and motor skills than their peers who remain on the spectrum. Additionally, we hypothesize that children who demonstrate OP will exhibit less severe ASD symptomatology at age two.
Methods
Participants
Participants include a subset of individuals participating in an ongoing study to evaluate the psychometric properties of an autism-specific screening questionnaire, the Modified Checklist for Autism in Toddlers (M-CHAT, Robins, Fein & Barton 1999) and its revision (M-CHAT-R/F; Robins, Fein & Barton 2009). Children in the current study represent a partially overlapping sample of children included in Sutera et al. (2007) (maximum overlap of approximately 40% in final sample). Children included in the current analyses (N = 207) were recruited for the study through three sources: receiving the screener at their 18 or 24 month pediatric well-child visit (n = 56), receiving the screener from an early intervention provider or psychologist (n = 134), or receiving the screener following caregiver self-referral (n = 17). Informed consent was obtained from all parents of children included in the study. This research was approved by the University of Connecticut IRB.
Following positive screening on the M-CHAT or M-CHAT-R/F, 311 children (see Figure 1) were evaluated at approximately age 26 months (Time 1), and subsequently reevaluated at an average age of 52 months (Time 2). These 311 children represent approximately 70% of all children who were evaluated at Time 1 following positive screening. Approximately 30% were lost to attrition before reevaluation, and therefore, will not be included in the current study. Within the broader study seeking to validate the M-CHAT or M-CHAT-R/F, there is evidence that individuals who did not return for reevaluation were more likely to be of non-white ethnicity and were less likely to have parents with an advanced degree (e.g., Associate’s, Bachelor’s, Master’s, etc.).
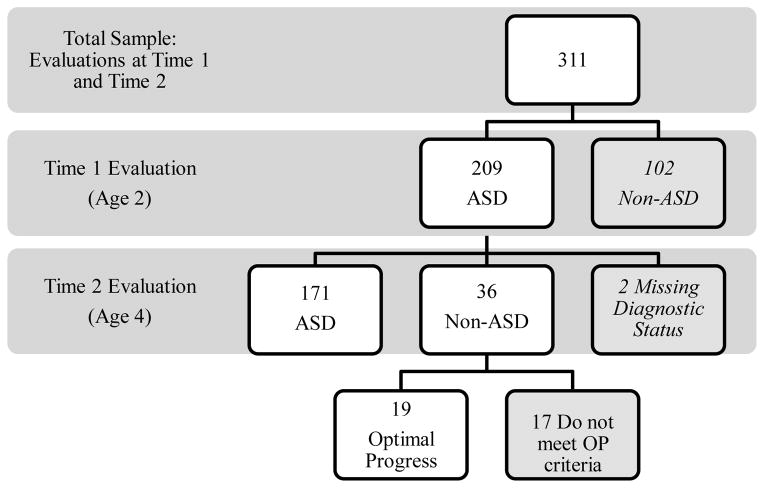
Figure 1.
Flowchart indicating diagnostic results of Time 1 and Time 2 evaluations. 102 children who received Non-ASD diagnoses at their Time 1 evaluation, and 2 children who had missing information regarding diagnostic status at Time 2 were not included in the current analyses.
Of the 311 children evaluated at both time points, 209 children were diagnosed with an ASD at their initial evaluation and were considered for inclusion in the current study. Of these 209 children, 2 were excluded due to missing data regarding diagnostic status at reevaluation (see Figure 1). See Table 1 for Time 1 and Time 2 diagnoses of these 207 children. Of the 207 children diagnosed with an ASD at Time 1, 171 (82.6%) children retained an ASD diagnosis at reevaluation (ASD). Nineteen children (9.2%) were determined to meet the previously discussed criteria for “optimal progress” (OP). The remaining 17 children moved from an ASD diagnosis at Time 1 to a different diagnosis (Developmental Delay, N = 8; Developmental Language Disorder, N = 2) or had other developmental concerns at Time 2 (i.e., did not meet the strict “optimal progress” cutoff scores on the Mullen or VABS, N = 7). As the goal of this work is to characterize children with the most highly positive outcomes from an early ASD diagnosis, these 17 children will not be considered in the subsequent analyses. Future work with a larger sample should consider the early characteristics of these children who no longer met criteria for an ASD diagnosis, but do not meet strict criteria for OP.
Table 1.
Diagnostic Stability of Time 1 ASD Diagnoses
| Time 2 Diagnosis N (%) |
|||||
|---|---|---|---|---|---|
|
| |||||
| Time 1 Diagnosis | AD | PDD-NOS | ASD-Low MA | Non-ASD | |
| AD | N = 108 | 72 (66.7) | 22 (20.3) | 0 (0.0) | 14 (13.0) |
| PDD-NOS | N = 79 | 26 (32.9) | 31 (39.2) | 0 (0.0) | 22 (27.8) |
| ASD-Low MA | N = 20 | 15 (75.0) | 1 (5.0) | 4 (20.0) | 0 (0.0) |
AD, Autistic Disorder; PDD-NOS, Pervasive Developmental Disorder - Not Otherwise Specified; ASD-Low MA, Autism Spectrum Disorder – Low Mental Age; Non-ASD, Non-Autism Spectrum Disorder (Non-ASD)
A total of 190 children, including 19 children demonstrating OP and 171 children who retained their ASD diagnosis (ASD), will be the focus of the current analyses. The sample was 82% male (n = 156) and 18% female (n = 34; see Table 2). This ratio (4.6:1) reflects the currently estimated gender ratio in the wider population of children with ASD (4.5:1) (CDC 2014). Gender did not significantly differ by group (OP vs. ASD) (X2(1) = 2.69, p = .101); however, there was a trend toward a higher percentage of girls in the OP group (see Table 2). The majority of children in the sample were white (n = 155, 81.5%), as indicated by their caregivers (see Table 2). The two groups did not differ significantly in race/ethnicity as indicated by Fisher’s Exact Test, p = .605. The two groups also did not differ significantly in maternal education (Fisher’s Exact Test, p =.719); however, information regarding maternal education was missing for a large number (n = 59) of participants.
Table 2.
Sample Demographics
| Optimal Progress | ASD | Total Sample | t or X2, p | Effect Size | |
|---|---|---|---|---|---|
|
| |||||
| N = 19 | N = 171 | N = 190 | |||
| Age in Months (M, SD) | |||||
|
| |||||
| Average age at Time 1 | 26.21 (4.81) | 26.32 (4.37) | 26.31 (4.40) | t(187) = 0.10, p = .921 | d = .02 |
| Average age at Time 2 | 51.47 (4.81) | 52.30 (9.75) | 52.22 (9.52) | t(188) = .36, p = .718, | d = .11 |
|
| |||||
| Gender (N, %) | X2(1) = 2.69, p = .101 | ϕ = .12 | |||
|
| |||||
| Male | 13 (68.4) | 143 (83.6) | 156 (82.1) | ||
| Female | 6 (31.6) | 28 (16.4) | 34 (17.9) | ||
|
| |||||
| Race/Ethnicity (N, %) | Fisher’s Exact Test = 3.85, p =.605 | ||||
|
| |||||
| White | 16 (84.2) | 139 (81.3) | 155 (81.6) | ||
| Hispanic/Latino | 1 (5.3) | 11 (6.4) | 12 (6.3) | ||
| Black or African American | 0 (0) | 7 (4.1) | 7 (3.7) | ||
| Asian or Pacific Islander | 1 (5.3) | 4 (2.3) | 5 (2.6) | ||
| Biracial | 1 (5.3) | 3 (1.8) | 4 (2.1) | ||
| Other | 0 (0) | 1 (0.6) | 1 (0.5) | ||
| Not Available | 0 (0) | 6 (3.5) | 6 (3.2) | ||
At the initial evaluation (Time 1), the OP group was on average 26.21 months (SD = 4.81) and the ASD group was 26.32 months (SD = 4.37) (See Table 2 (t(187) = .100, p = .921, d = .02). At reevaluation (Time 2), on average, the OP group was 51.47 months (SD = 7.23) and the ASD group was 52.30 (SD = 9.75) months (t(188) = .361, p = .718, d = .11).
Procedure
Children’s caregivers were provided the M-CHAT (Robins et al. 1999) (n = 176) or M-CHAT-R (Robins et al. 2009) (n = 14) screeners to complete at their pediatrician’s office during their child’s 18 or 24-month well-child visit, or at an early intervention site or psychologist’s office. The M-CHAT(-R) is a brief, autism-specific, parent-report screening measure that consists of 23 (M-CHAT) or 20 (M-CHAT-R) yes/no questions. Once the questionnaire was completed, it was sent to the University of Connecticut Early Detection laboratory to be scored. If a caregiver’s responses indicated that a child screened positive, they were contacted via telephone to complete the relevant structured Follow-Up items. If a child continued to screen positive after the Follow-Up phone interview, he or she was invited to attend a free developmental and diagnostic evaluation conducted at the University of Connecticut.
A licensed clinical psychologist or a developmental pediatrician and a graduate student in the Clinical Psychology program at the University conducted the evaluations, which consisted of measures of cognitive skills, adaptive skills, language abilities and ASD-specific measures. At the conclusion of the evaluation, caregivers were provided with feedback regarding the assessment, which included any diagnoses the child might qualify for as well as recommendations for intervention and resources. Six to eight weeks after the evaluation, caregivers received a written report detailing the results of the assessment.
A diagnosis of an ASD was assigned based on clinical judgment of experienced clinicians (licensed psychologists or developmental pediatricians) utilizing scores from all available information from direct testing and parent interviews, and in accordance with the clinicians’ best estimate diagnosis using DSM-IV-TR diagnostic criteria (APA 2000). Despite recent changes in diagnostic criteria (DSM-5, APA 2013), DSM-IV-TR diagnostic criteria were utilized throughout this longitudinal project to maintain consistency and in order to retain children with PDD-NOS diagnoses who may not have met DSM-V diagnostic criteria for ASD. ASD diagnoses included AD, PDD-NOS or Asperger’s Disorder. An additional diagnostic category, ASD – Low Mental Age (ASD-Low MA) was given to children who met DSM-IV-TR diagnostic criteria for AD or PDD-NOS and were functioning below the 12 month level across all domains on the Mullen. Clinical judgment in the assignment of ASDs has been shown to have high inter-rater reliability and is considered best practice in the field of ASDs (Klin, Lang, Cicchetti & Volkmar 2000). All children who were evaluated at approximately 24 months (Time 1) were invited for a second evaluation around their fourth birthday (approximately age 48 months, Time 2).
Measures
The following measures were utilized in the ongoing study: M-CHAT, M-CHAT-R, Autism Diagnostic Observation Schedule (ADOS), ADI(-R), Toddler Autism Symptom Interview (TASI), Mullen, VABS, and the Childhood Autism Rating Scale (CARS). These measures have been determined to have excellent psychometric properties and are widely used in the field of ASDs, with the exception of the TASI, which is currently being validated. The current study analyzes data from the measures described below, each of which was administered at Time 1 and Time 2. Please note that several measures included in the current study have been revised since the initiation of this longitudinal project. Measures were kept consistent throughout the study (except where noted below) in order to facilitate comparisons between children and across time.
Autism Diagnostic Observation Schedule - Generic (ADOS)
The ADOS (Lord et al. 2000) is a semi-structured, standardized, play-based assessment of four areas: Reciprocal Social Interaction, Communication, Stereotyped Behaviors and Restricted Interests and Play, which is intended for use with children who are suspected to have an ASD. Higher scores indicate greater severity. Modules 1 and 2 were used in the current study. Gotham and colleagues (2007, 2014) developed the ADOS Calibrated Severity Score (CSS) in order to assess symptom severity based on ADOS scores across modules. The CSS is a measure of autism severity that takes into account a child’s age and language abilities, allowing for a measure of symptom severity that is less influenced by age or verbal abilities (Gotham et al. 2007, Gotham et al. 2014). Total CSS, Social-Affect (SA) CSS and Restricted Repetitive Behavior (RRB) CSS are included in the current analyses.
Vineland Adaptive Behavior Scales – Interview Edition (Versions I and II)
The VABS (Sparrow, Balla & Cicchetti 1984) is a structured, parent-report interview measure of adaptive functioning across four domains: Communication, Daily Living Skills, Socialization and Motor Skills. Scores are determined for each domain individually, and are combined to form a total score, the Adaptive Behavior Composite (ABC). In the current study, children’s caregivers were administered the VABS (Sparrow, Balla & Cicchetti 1984) or the Vineland Adaptive Behavior Scales – Second Edition (VABS-II), an updated version which was released in 2005 (Sparrow, Cicchetti & Balla 2005). As a result of the high degree of similarly between the two versions, VABS and VABS-II scores were analyzed collectively.
Mullen Scales of Early Learning
The Mullen (Mullen 1995) assesses five domains of cognitive development. These include Visual Reception (problem solving abilities), Gross Motor, Fine Motor, Expressive Language and Receptive Language. In addition, the measure provides a summative “Early Learning Composite” (ELC) score, which is computed from the Visual Reception, Fine Motor, Expressive Language and Receptive Language domains. In the current study, the Gross Motor domain was not administered. In terms of concurrent validity, the Mullen cognitive scales and the Bayley Scales of Infant Development Mental Development Index showed correlations ranging from .53 to .59 (Mullen 1995).
Childhood Autism Rating Scale (CARS)
The CARS (Schopler 1980) is a 15-item observation-based rating scale designed to accurately differentiate children with autism from those with developmental delays without features of autism. A total score is determined by summing the ratings on all 15 items, with total CARS scores ranging from 15 to 60. Higher scores indicate greater severity. Children can be classified as being non-autistic, having mild autism or having severe autism based on established cutoff scores (Schopler et al. 1988). In order to better reflect our more current understanding of autism as a spectrum, Chlebowski and colleagues (2010) recommend a cutoff of 25.5 be used to distinguish an ASD from a non-ASD for two year olds and four year olds.
The validity of the CARS has been assessed by comparing its classification of cases to the classifications made by other frequently used measures. Saemundsen et al. (2003) found a correlation of .67 between the CARS and the ADI-R. The sensitivity and specificity of the CARS have been found to be high (.94 and .85, respectively) (Perry et al. 2005). In order to better understand domains within the CARS total score, Magyar and Pandolfi (2007) conducted a factor structure evaluation of the CARS using Principal Axis Factor Analysis (PAF) and found four factors, which accounted for 41.67% of the variance. These include Social Communication, Social Interaction, Stereotypies and Sensory Abnormalities and Emotional Regulation.
Results
Diagnostic Predictors of Optimal Progress: Time 1 Diagnoses of OP and ASD Groups
The remaining analyses include the OP (n = 19) and ASD (n = 171) groups, and do not include the 17 children who lost their ASD diagnosis but did not meet OP criteria. There was a strong trend for the OP and ASD groups to differ in Time 1 diagnosis (see Table 3). Children initially diagnosed with PDD-NOS were the most likely to meet criteria for OP at Time 2 (16.2%), followed by children initially diagnosed with AD (7.8%). No children initially diagnosed with ASD-Low MA met criteria for OP at Time 2. Notably, however, 100% of children in the ASD-Low MA group showed progress in cognitive abilities such that their mental age equivalents rose above 12 months in at least two out of four domains (Visual Reception, Fine Motor, Expressive Language, Receptive Language) and 65% showed this progress in three out of four domains.
Table 3.
Predictors of Optimal Progress: Time 1 Diagnosis
| Optimal Progress N = 19 N (%) |
ASD N = 171 N (%) |
X2 | |
|---|---|---|---|
| Diagnosis at Time 1 | X2= 5.63, p = .06, Cramer’s V = .17 | ||
|
| |||
| AD | 8 (7.8%) | 94 (92.2%) | |
| PDD-NOS | 11 (16.2%) | 57 (83.8%) | |
| ASD-Low MA | 0 (0 %) | 20 (100 %) | |
AD, Autistic Disorder; PDD-NOS, Pervasive Developmental Disorder - Not Otherwise Specified; ASD-Low MA, Autism Spectrum Disorder–Low Mental Age
Diagnostic Predictors of Optimal Progress: Time 1 Symptom Severity
Overall symptom severity at Time 1 was measured using the CARS total score. The OP group showed significantly milder symptom severity at Time 1 than the ASD group (see Table 4). In order to better understand in which specific domains OP children showed milder symptom severity, analyses were conducted for the following factors: Social Communication, Social Interaction, Stereotypies and Sensory Abnormalities, and Emotional Regulation (Magyar & Pandolfi 2007). Independent groups t-tests indicate that the OP group showed significantly milder symptom severity than the ASD group in the domains of Social Communication and Stereotypies and Sensory Abnormalities (see Table 4). There were no group differences in the Social Interaction or Emotional Regulation domains.
Table 4.
Time 1 Cognitive Abilities, Adaptive Skills and ASD Symptoms
| Measure | Optimal Progress (M, SD) | ASD (M, SD) | p value | Effect Size |
|---|---|---|---|---|
|
Mullen IQ Time 1
| ||||
| Visual Reception | 71.20 (15.19) | 66.41 (20.68) | p = .386 | d = .26 |
| Fine Motor | 80.40 (15.34) | 72.65 (22.43) | p = .195 | d = .40 |
| Expressive Language | 59.56 (21.11) | 49.56 (22.41) | p = .101 | d = .46 |
| Receptive Language | 54.92 (17.02) | 45.05 (23.21) | p = .112 | d = .49 |
|
| ||||
|
VABS Time 1
| ||||
| Total | 71.50 (7.26) | 66.20 (7.67) | p = .006 | d = .71 |
| Communication | 71.17 (9.12) | 66.82 (8.55) | p = .043 | d = .49 |
| Socialization | 73.00 (8.25) | 68.88 (8.47) | p = .051 | d = .49 |
| Daily Living | 75.00 (10.61) | 69.52 (9.23) | p = .019 | d =.55 |
| Motor | 86.72 (10.71) | 80.80 (11.68) | p = .041 | d = .53 |
|
| ||||
|
CARS Time 1
| ||||
| Total | 29.11 (5.40) | 33.02 (5.22) | p = .003 | d=.74 |
| Social Interaction | 2.18 (0.54) | 2.35 (0.57) | p = .221 | d = .31 |
| Social Communication | 2.18 (0.41) | 2.58 (0.47) | p =.001 | d =.91 |
| Stereotypies and Sens Abn | 1.86 (0.37) | 2.10 (0.45) | p = .034 | d = .58 |
| Emotion Regulation | 1.60 (0.42) | 1.73 (0.42) | p =.198 | d = .31 |
|
| ||||
|
ADOS CSS Time 1
| ||||
| Total | 5.87 (2.48) | 6.53 (2.15) | p = .262 | d = .28 |
| Social Affect | 5.81 (2.34) | 6.71 (2.09) | p = .108 | d = .41 |
| Restricted Repetitive Behaviors | 6.73 (2.55) | 6.41 (2.66) | p = .649 | d = .12 |
|
| ||||
|
DSM-IV Symptoms Time 1
| ||||
| Total | 5.19 (1.76) | 6.04 (1.75) | p = .063 | d =.48 |
| Social Interaction | 2.69 (0.95) | 2.83 (0.99) | p = .574 | d = .14 |
| Communication | 1.56 (0.73) | 1.78 (0.55) | p = .257 | d = .34 |
| RRBIs | 0.94 (0.68) | 1.43 (1.00) | p = .016 | d =.57 |
Mullen, Mullen Scales of Early Learning; VABS, Vineland Adaptive Behavior Scales; CARS, Childhood Autism Rating Scale; ADOS CSS, Autism Diagnostic Observation Schedule Calibrated Severity Score; DSM-IV RRBI, DSM-IV Restricted Repetitive Behaviors and Interests
Severity of Time 1 autism symptomatology was also measured utilizing ADOS calibrated severity scores (CSS) (total, SA, RRB) computed from participants’ scores on the ADOS, as outlined by Gotham and colleagues (2007, 2014). Independent groups t-tests indicate that the OP and ASD groups did not differ in total CSS, RRB CSS or SA CSS at Time 1 (see Table 4). Despite non-significant findings, the effect size of group differences in SA CSS was notable (d = .41), with the OP group demonstrating lesser severity than the ASD group in this domain.
Diagnostic Predictors of Optimal Progress: Time 1 DSM-IV Symptomatology
To further understand potential diagnostic differences between the OP and ASD groups, Time 1 DSM-IV symptoms were analyzed. DSM-IV total scores include symptoms in three domains: Social Interaction, Communication, Restricted Interests and Repetitive Behaviors, and reflect the total number of symptoms out of a possible 12. The OP group showed a strong trend toward fewer total DSM-IV symptoms at Time 1 than the ASD group (see Table 4). Each domain of symptomatology was then separately investigated. The OP group showed significantly fewer symptoms in the Restricted Interests and Repetitive Behaviors than the ASD group; however, the two groups did not significantly differ in number of symptoms in the Social Interaction or Communication domains (see Table 4).
Predictors of Optimal Progress: Time 1 Cognitive Abilities
Cognitive abilities were assessed using the Mullen Scales of Early Learning. Preliminary analyses indicated that the assumption of normality was violated, in that Time 1 Mullen T-scores were not normally distributed in our sample. This appeared to be due to a large number of children receiving the lowest possible T-score (20). In order to address these “floor effects,” developmental quotient scores were calculated for each domain of the Mullen for each participant. Developmental quotient scores were calculated using the following formula: mental age / chronological age × 100. In order to assure the appropriateness of using these scores in place of T-scores, developmental quotient scores were correlated with T-scores. These correlations were all found to be significant at the .01 level, ranging from .52 to .86, indicating that developmental quotient scores were highly representative of T-scores. There were no significant group differences in Time 1 developmental quotient scores for any domain of the Mullen (Visual Reception, Fine Motor, Expressive Language, Receptive Language); however, effect sizes were moderate (Fine Motor (d=.40); Expressive Language (d=.46); Receptive Language (d=.49)) (see Table 4).
Predictors of Optimal Progress: Time 1 Adaptive Skills
Adaptive skills were assessed using the VABS, version I or II. Based on the strong correlations seen between the VABS-I and VABS-II, as well as their similar overall psychometric properties (Sparrow, Cicchetti & Balla 2005), VABS-I and VABS-II scores were analyzed collectively. The OP group showed significantly stronger overall adaptive abilities at Time 1, as indicated by the VABS total score, as well as significantly stronger Time 1 skills in each domain of the VABS individually (Communication, Socialization (approaches significance, p = .051), Daily Living, Motor), with medium effect sizes for each domain (see Table 4).
Predictors of Optimal Progress: Discriminant Function Analysis
A discriminant function analysis (DFA) was conducted to determine if Time 1 variables could be utilized to significantly predict group membership at Time 2 (OP vs. ASD). Variables that significantly differed between the two groups were included (VABS Communication, VABS Socialization, VABS Daily Living Skills, VABS Motor Skills, CARS Social Communication, CARS Stereotypies and Sensory Abnormalities, DSM-IV RRBI symptoms). The resulting function was found to be significant (p = .047, Wilk’s Lambda = .914), and accurately predicted group membership for 69.5% of cases (see Table 5). Based on Time 1 scores, 69.6% of children in the ASD group and 68.4% of children in the OP group were correctly classified. Standardized canonical discriminant function coefficients indicate that variables contributed to the functioning in the following order of importance: CARS Social Communication, VABS Daily Living Skills, DSM-IV RRBI symptoms, VABS Motor Skills, VABS Communication, VABS Socialization, CARS Stereotypies and Sensory Abnormalities (see Table 6). These results indicate that within the set of variables that significantly differed between the OP and ASD groups, certain variables appear to show greater predictive value than others, with some variables (e.g., CARS Stereotypies and Sensory Abnormalities) not demonstrating substantial predictive value. Further, while the overall function was significant, the results of this DFA indicate that a larger sample size with greater power and additional variables (e.g., intervention data) may be needed to more accurately predict group membership.
Table 5.
Discriminant Function Analysis Classification Matrix
| Predicted Group Membership | |||
|---|---|---|---|
|
| |||
| Actual Group Membership | ASD | Optimal Progress | Total |
| ASD | 119 | 52 | 171 |
| Optimal Progress | 6 | 13 | 19 |
Note: 69.5% of all cases were correctly classified.
Table 6.
Standardized Canonical Discriminant Function Coefficients
| Variable | Coefficient |
|---|---|
| CARS Social Communication | 0.789 |
| VABS Daily Living Skills | −0.454 |
| DSM-IV RRBI Symptoms | 0.356 |
| VABS Motor Skills | −0.219 |
| VABS Communication | 0.203 |
| VABS Socialization | 0.173 |
| CARS Stereotypies and Sensory Ab | 0.008 |
VABS, Vineland Adaptive Behavior Scales; CARS, Childhood Autism Rating Scale; DSM-IV RRBI, DSM-IV Restricted Repetitive Behaviors and Interests
Discussion
The results of the current study support the findings of previous studies investigating diagnostic stability of ASDs in the toddler years. As in previous work, the current study found that, broadly, diagnostic stability is high, with 82.6% of children retaining an ASD diagnosis between ages two and four. Results indicate that children with severe cognitive delays (e.g., age equivalents below 12 months with chronological age of approximately 24 months) show highly stable diagnoses over time (100% in the current sample), despite cognitive improvement in the large majority of these children. This supports the early diagnosis of ASD in children who exhibit ASD symptoms accompanied by severe cognitive delays, rather than waiting to diagnosis these children until their cognitive abilities rise above the currently accepted age of diagnosis of between 18 and 24 months. Overall, our findings support continued efforts to diagnose ASDs early in development, as they appear to be stable following diagnoses made at approximately age two years.
The current study attempted to expand upon previous studies investigating predictors of highly positive outcomes from ASD in the toddler years. As hypothesized, children initially diagnosed with PDD-NOS are more likely to demonstrate OP than children initially diagnosed with AD. This is likely due to the less severe ASD symptomatology demonstrated by children with PDD-NOS, which may make them more available to participation in early intervention efforts. Children who later demonstrate OP show a strong trend toward fewer total DSM-IV symptoms at age two than their peers who remain on the spectrum. Furthermore, our results indicate that lesser early symptoms of Restricted, Repetitive Behaviors and Interests help to predict OP, but early symptoms in the Social Interaction and Communication domains do not. This is consistent with the work of Lord and colleagues (2006) who found that children with little or no repetitive behaviors during the ADOS and ADI-R were the most likely to change diagnosis from AD to PDD-NOS or from PDD-NOS to non-spectrum, as well as the work of Anderson and colleagues (2014) who found that reduction in RRBs between ages two and three predicted highly positive outcomes later in development. It is hypothesized that RRBs impede children from optimally engaging in their environment, and in turn, prevent them from fully benefitting from important learning experiences in both daily interactions and early intervention. This hypothesis was not directly tested in this work, and therefore, should be empirically investigated in future work. It is also possible that the presence of RRBs reflects more severe overall ASD symptomatology.
Our finding that the presence of fewer RRBs helps to predict OP is of particular importance given recent changes in DSM criteria, which now require individuals to demonstrate at least two symptoms in the RRB domain (APA, 2013). A recent study by Barton et al. (2013) indicates that when applying DSM-5 criteria to very young children diagnosed with an ASD using the DSM-IV, 29% of children will lose their diagnosis despite showing significant levels of impairment. It is important to note that it is largely children who would meet DSM-IV criteria for PDD-NOS who will no longer meet DSM-5 criteria for ASD. In combination, this indicates that children who may be the most likely to benefit from early intervention and to demonstrate OP (i.e., children with fewer RRBs, children with diagnoses of PDD-NOS) are the children who are most likely to no longer meet diagnostic criteria at an early age. Without a formal diagnosis of ASD, these children will be unlikely to receive adequate ASD-specific services, and in turn, may not reach the highly positive outcomes of which they are capable.
Symptom severity at age two was also investigated as a possible predictor of OP. Results of the current study indicate that, as hypothesized, children who later demonstrate OP show lesser total symptom severity at age two (as measured by the CARS, but not by the ADOS CSS) than their peers who remain on the spectrum. With less severe symptoms overall, the OP group required a lesser change in symptom severity to no longer meet criteria for ASD than their peers who demonstrated more severe symptoms. Notably, however, despite the possibility of a lesser change in symptoms between Time 1 and Time 2, the OP group demonstrated a greater magnitude of change in all domains of symptom severity on the CARS, with an average total score decrease of 10.44 points (SD=5.59) compared to an average decrease of 1.34 point (SD=5.54) for the ASD group (p<.001).
To address the discrepancy in findings between the CARS and the ADOS CSS, similarities and differences between the measures are considered here. Both measures are observation-based; however, the ADOS includes observations made during a standardized, play-based assessment, whereas the CARS includes observations made across a range of assessments (e.g., cognitive, ASD-specific) as well as clinician’s impressions based on parent report of a child’s development and symptomatology. It is possible that group differences were found on the CARS, and not the ADOS CSS, because of its broader range of symptoms assessed, the inclusion of language level in severity ratings on the CARS, the inclusion of information gleaned from parent report, and its wider range of possible scores. Further, however, a lack of differences on the ADOS CSS could also have been influenced by a lack of sufficient power, given that a moderate effect size was found for the ADOS CSS SA domain.
Analyses of individual factors within the CARS may help us to better understand which specific symptom types may predict OP. Utilizing the factors determined by Magyar and Pandolfi (2007) the current study found that children who later demonstrate OP show milder early symptom severity in the domain of Social Communication, but not in the domain of Social Interaction. Items in the Social Communication domain include imitation, verbal communication and nonverbal communication1. Items in the Social Interaction domain include a child’s general ability to relate to others and their visual response (e.g., eye contact). Therefore, it appears that children who later go on to demonstrate OP show less impaired communication skills than their ASD peers at age two, but show similar levels of impairment in the ability to relate to others. Further, the current study found that children in the OP group show similar levels of impairment in their emotional regulation abilities (e.g., emotional response, adaptation to change, and activity level) as compared to ASD peers. Our findings of similar levels of impairment in social interaction and emotional regulation should be interpreted cautiously, however, given the clinically significant effect sizes seen (.31 for both) in these analyses.
Children who later went on to demonstrate OP also showed milder early symptom severity in the domain of Stereotypies and Sensory Abnormalities on the CARS (e.g., a child’s body use, taste, smell and touch response and listening response). This finding is consistent with our finding that children in the OP group showed fewer symptoms in the RRB domain of the DSM-IV than their peers who remain on the spectrum. Results of the DFA indicated, however, that the CARS Stereotypies and Sensory Abnormalities score was not a strong independent predictor of OP. Therefore, it appears that information regarding stereotyped behavior and sensory abnormalities on the CARS should be used in concert with other similar measures with greater predictive value (e.g., DSM-IV RRBI symptoms).
Based on previous studies of both diagnostic stability and outcomes more broadly, we hypothesized that children who later demonstrate OP would show stronger early cognitive and language abilities than their peers who remain on the spectrum. Contrary to our hypothesis, we found no significant group differences in any domain of cognitive or language ability as assessed by the Mullen (Visual Reception, Receptive Language, Expressive Language). Given the small to moderate effect sizes found in the current study’s analyses (ranging from .26 to .49) it is possible that significant group differences in cognitive and/or language abilities would be found in a larger sample with greater power to detect significance. It is also possible that the Mullen may not be a sensitive enough measure to detect subtle group differences in cognitive or language abilities in two year old children, and therefore, that a more sensitive measure would be needed to characterize possible differences between the OP and ASD children.
Our results indicate that children who later demonstrate OP show stronger early adaptive skills in all domains (Communication, Socialization, Daily Living, Motor) as indicated by parent report on the VABS. Stronger social skills, in combination with stronger communication abilities, may reflect greater early social motivation, which may increase the likelihood that these children would regularly engage with peers and adults, and experience increased social learning opportunities (Chevallier, Kohls, & Troiani 2012). The OP group also demonstrated stronger motor skills at age two than their peers who remain on the spectrum. Lloyd and colleagues (2013) note that movement is a critical element of active play, which facilitates the development of social skills, understanding of the world, daily living skills and play skills. Therefore, as discussed by MacDonald and colleagues (2013), better motor skills may allow improvements in social communication skills. This, in turn, may facilitate the rapid improvements seen in social and communication abilities in these children by age four.
It is also possible that, as discussed by Mostofsky and colleagues (2007), motor and social/communication deficits are related at a more basic neurological level. Specifically, Mostofsky and colleagues (2007) argue that global deficits in procedural learning mechanisms may underlie deficits in both motor skills and social/communicative skills. Stronger early motor skills may be reflective of more typical neurological functioning, specifically, more typical patterns of white matter in the precentral cortex, which plays a role in motor functioning (Mostofsky, Burgess, & Gidley Larson 2007). Additional neuroimaging studies will be required to determine if children who show highly positive outcomes (i.e., OP) demonstrate neurological differences when compared to their peers who remain on the spectrum.
As discussed by Sutera and colleagues (2007), stronger daily living skills may reflect a number of unmeasured factors including greater independence or greater motivation to learn in these children, as well as more proactive parenting. Additionally, stronger daily living skills may be reflective of stronger motor skills, which may be important for the many reasons discussed above. Importantly, it is likely the interaction of all of these factors (e.g., motor skills, social and communication abilities) that contribute to highly positive outcomes by age four.
Limitations and Future Directions
There are several limitations to the current study. First, while a sample size of 19 is as large as may be feasible to collect given the rarity of OP, it remains a small sample with limited power. Our sample size may have limited our ability to establish a specific profile of early characteristics of children who demonstrate OP, particularly in regards to cognitive abilities. Future studies could attempt to study a larger group of children who demonstrate this type of outcome in toddlerhood in order to increase the power of analyses. Second, the age of follow up in the current study (age four) serves as both a strength and a possible limitation. Follow-up at age four allows us to demonstrate that highly positive outcomes are possible very early in development when children are diagnosed at approximately 26 months. As discussed above, children who demonstrate OP are functioning well within the average range across domains and are likely difficult to distinguish from their typically developing peers. Future studies should compare children with OP directly to typically developing peers as has been done in studies of “optimal outcome.” While the children in our OP group appear to be optimally functioning four year olds, our follow-up to age four limits our ability to assess these children’s later peer relationships and school functioning. Future studies should include longer follow-up periods to determine the extent to which these children continue on this optimal trajectory, and whether residual, subtle deficits exist for these children later in childhood. Additionally, later follow-up will allow future studies to characterize children who may not yet show this outcome at age four, but meet criteria for OP or “optimal outcome” later in development.
Third, there is little information available about the interventions received between the age two and age four evaluations. The large majority of children in our sample received early intervention (e.g., speech therapy, occupational therapy, ABA), and based on previous research (Orinstein et al. 2014; Anderson et al. 2014) it is likely that early intervention plays a large role in producing highly positive outcomes from ASD. Importantly, it is likely the interaction between child-level factors, such as those investigated in the current study, and intervention-level characteristics, that produce highly positive outcomes such as OP. Therefore, future studies should attempt to characterize these interactions so that we can best understand the mechanisms by which OP occurs. Additional factors, such as parent (e.g., mental health) and family characteristics (e.g., socioeconomic status), should also be considered.
Conclusions
The OP group represents a distinct subset of individuals with ASD who demonstrate large, clinically significant changes in symptom presentation by age four such that they no longer met criteria for any ASD, and are functioning within the average range on standardized measures of cognitive, language, social and communication abilities. The current study found that a number of early child-level factors predicted this highly positive outcome including a diagnosis of PDD-NOS, lesser early symptom severity, fewer symptoms in the domain of RRBs, and stronger early communication, social, daily living and motor skills.
Through characterizing the OP group, the current study advances our understanding of the multiple possibilities of developmental trajectories seen in children with early diagnoses of ASD. In combination with the findings of the current study, future studies should attempt to characterize the mechanisms at work in producing these outcomes, including the role of early intervention. In doing so, we can begin to promote an increase in the percentage of children attaining highly positive outcomes from ASD.
Acknowledgments
The authors thank the families who participated in the current study, the physicians who assisted by offering the screening study to their patients, and the research teams at the University of Connecticut and Georgia State University, especially Chi-Ming Chen for his assistance with data analysis. The authors also acknowledge the following funding sources: Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01HD039961, U.S. Department of Education Student-Initiated Research Grant, Maternal and Child Health Bureau Grant R40MC00270, the University of Connecticut’s Research Foundation Faculty Grant, the National Alliance of Autism Research, and a National Institute of Mental Health Predoctoral Fellowship F31MH12550.
Footnotes
Note: This domain also includes a child’s level and consistency of intellectual functioning and the clinician’s general impressions of ASD symptomatology. Given the lack of theoretical relevance of these items to the Social Communication domain, analyses were run with and without these items. No differences in results were found.
Conflict of Interest Disclosures: Deborah Fein, Marianne Barton and Diana L. Robins are co-owners of M-CHAT, LLC, which receives royalties from companies that incorporate the M-CHAT into commercial products. Data reported in the current paper is from the freely available paper version of the M-CHAT.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Publishing; 2000. text rev. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anderson DK, Liang JW, Lord C. Predicting young adult outcome among more and less cognitively able individuals with autism spectrum disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2014;55(5):485–494. doi: 10.1111/jcpp.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ML, Boorstein H, Dumont-Mathieu T, Herlihy LE, Fein D. Toddler ASD Symptom Interview (TASI) 2012. Self-published. [Google Scholar]
- Baghdadli A, Assouline B, Sonié S, Pernon E, Darrou C, Michelon C, … Pry R. Developmental trajectories of adaptive behaviors from early childhood to adolescence in a cohort of 152 children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(7):1314–25. doi: 10.1007/s10803-011-1357-z. [DOI] [PubMed] [Google Scholar]
- Barton Marianne L, Robins Diana L, Dasal Jashar, Brennan Laura, Fein D. Sensitivity and Specificity of Proposed DSM-5 Criteria for Autism Spectrum Disorder in Toddlers. Journal of Autism and Developmental Disorders. 2013;43(5):1184–1195. doi: 10.1007/s10803-013-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Manual for the Bayley Scales of Infant Development. San Antonio: The Psychological Corporation; 1969. [Google Scholar]
- Bopp KD, Mirenda P, Zumbo BD. Behavior predictors of language development over 2 years in children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research : JSLHR. 2009;52(5):1106–20. doi: 10.1044/1092-4388(2009/07-0262). [DOI] [PubMed] [Google Scholar]
- Bryson SE, Rogers SJ, Fombonne E. Autism Spectrum Disorders: Early Detection, Intervention, Education and Psychopharmacological Management. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie. 2003;48(8):506. doi: 10.1177/070674370304800802. [DOI] [PubMed] [Google Scholar]
- CDC. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. 2014;63(2):1–24. 24670961. [PubMed] [Google Scholar]
- Charman T, Jones CRG, Pickles a, Simonoff E, Baird G, Happé F. Defining the cognitive phenotype of autism. Brain Research. 2011;1380(1943):10–21. doi: 10.1016/j.brainres.2010.10.075. [DOI] [PubMed] [Google Scholar]
- Charman T, Taylor E, Drew A, Cockerill H, Brown JA, Baird G. Outcome at 7 years of children diagnosed with autism at age 2: predictive validity of assessments conducted at 2 and 3 years of age and pattern of symptom change over time. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46(5):500–13. doi: 10.1111/j.1469-7610.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(10):1235–45. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski C, Green Ja, Barton ML, Fein D. Using the childhood autism rating scale to diagnose autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(7):787–99. doi: 10.1007/s10803-009-0926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski C, Robins DL, Barton ML, Fein D. Large-scale use of the modified checklist for autism in low-risk toddlers. Pediatrics. 2013;131(4):e1121–7. doi: 10.1542/peds.2012-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves LC, Ho HH. The very early identification of autism: outcome to age 4 1/2–5. Journal of Autism and Developmental Disorders. 2004;34(4):367–78. doi: 10.1023/b:jadd.0000037414.33270.a8. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15449513. [DOI] [PubMed] [Google Scholar]
- Eldevik S, Hastings RP, Hughes JC, Jahr E, Eikeseth S, Cross S. Meta-analysis of Early Intensive Behavioral Intervention for children with autism. Journal of Clinical Child and Adolescent Psychology : The Official Journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2009;38(3):439–50. doi: 10.1080/15374410902851739. [DOI] [PubMed] [Google Scholar]
- Fein D, Barton M, Eigsti IM, Kelley E, Naigles L, Schultz RT, … Tyson K. Optimal outcome in individuals with a history of autism. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2013;54(2):195–205. doi: 10.1111/jcpp.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of Autism and Developmental Disorders. 2003;33(4):365–82. doi: 10.1023/a:1025054610557. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12959416. [DOI] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–27. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: Stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2013;54(5):582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SL, Handleman JS. Age and IQ at intake as predictors of placement for young children with autism: a four- to six-year follow-up. Journal of Autism and Developmental Disorders. 2000;30(2):137–42. doi: 10.1023/a:1005459606120. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10832778. [DOI] [PubMed] [Google Scholar]
- Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert M, Fein D. Can children with autism recover? If so, how? Neuropsychology Review. 2008;18(4):339–66. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Herlihy LE, Brooks B, Dumont-Mathieu T, Barton ML, Fein D, Chen CM, Robins DL. Standardized screening facilitates timely diagnosis of autism spectrum disorders in a diverse sample of low-risk toddlers. Journal of Developmental and Behavioral Pediatrics : JDBP. 2014;35(2):85–92. doi: 10.1097/DBP.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS Domain Scores : Separating Severity of Social Affect and Restricted and Repetitive Behaviors. 2014:2400–2412. doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013 doi: 10.1038/nature12715. advance on. Retrieved from http://dx.doi.org/10.1038/nature12715. [DOI] [PMC free article] [PubMed]
- Jónsdóttir SL, Saemundsen E, Asmundsdóttir G, Hjartardóttir S, Asgeirsdóttir BB, Smáradóttir HH, … Smári J. Follow-up of children diagnosed with pervasive developmental disorders: stability and change during the preschool years. Journal of Autism and Developmental Disorders. 2007;37(7):1361–74. doi: 10.1007/s10803-006-0282-z. [DOI] [PubMed] [Google Scholar]
- Kelley E, Naigles L, Fein D. An in-depth examination of optimal outcome children with a history of autism spectrum disorders. Research in Autism Spectrum Disorders. 2010;4(3):526–538. doi: 10.1016/j.rasd.2009.12.001. [DOI] [Google Scholar]
- Kelley E, Paul JJ, Fein D, Naigles LR. Residual language deficits in optimal outcome children with a history of autism. Journal of Autism and Developmental Disorders. 2006;36(6):807–28. doi: 10.1007/s10803-006-0111-4. [DOI] [PubMed] [Google Scholar]
- Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early Autism Spectrum Disorder: subtypes and short-term outcomes. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12448. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JM, Robins DL, Ventola PE, Pandey J, Boorstein HC, Esser EL, … Fein D. The modified checklist for autism in toddlers: a follow-up study investigating the early detection of autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(5):827–39. doi: 10.1007/s10803-007-0450-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman JM, Ventola PE, Pandey J, Verbalis AD, Barton M, Hodgson S, … Fein D. Diagnostic stability in very young children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(4):606–15. doi: 10.1007/s10803-007-0427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lang J, Cicchetti DV, Volkmar FR. Brief report: Interrater reliability of clinical diagnosis and DSM-IV criteria for autistic disorder: Results of the DSM-IV autism field trial. Journal of Autism and Developmental Disorders. 2000;30(2):163–167. doi: 10.1023/A:1005415823867. [DOI] [PubMed] [Google Scholar]
- Levy A, Perry A. Outcomes in adolescents and adults with autism: A review of the literature. Research in Autism Spectrum Disorders. 2011;5(4):1271–1282. doi: 10.1016/j.rasd.2011.01.023. [DOI] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374(9701):1627–38. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, Lord C. Motor skills of toddlers with autism spectrum disorders. Autism : The International Journal of Research and Practice. 2013;17(2):133–46. doi: 10.1177/1362361311402230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C. Follow-Up of Two-Year-Olds Referred for Possible Autism. Journal of Child Psychology and Psychiatry. 1995;36(8):1365–1382. doi: 10.1111/j.1469-7610.1995.tb01669.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EHJ, Leventhal BL, DiLavore PC, … Rutter M. The Autism Diagnostic Schedule – Generic: A standard measures of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of General Psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology. 1987;55(1):3–9. doi: 10.1037//0022-006x.55.1.3. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3571656. [DOI] [PubMed] [Google Scholar]
- Luyster R, Lopez K, Lord C. Predicting Outcomes of Children Using the MacArthur-Bates Communicative Development Inventory. 2007;50(June):667–682. doi: 10.1044/1092-4388(2007/047). [DOI] [PubMed] [Google Scholar]
- Macdonald M, Lord C, Ulrich D. Research in Autism Spectrum Disorders. The relationship of motor skills and adaptive behavior skills in young children with autism spectrum disorders. 2013;7:1383–1390. doi: 10.1016/j.rasd.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magyar CI, Pandolfi V. Factor structure evaluation of the childhood autism rating scale. Journal of Autism and Developmental Disorders. 2007;37(9):1787–94. doi: 10.1007/s10803-006-0313-9. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Listerud J, Levy SE. Race Differences in the Age at Diagnosis Among Medicaid-Eligible Children With Autism. American Academy of Child and Adolescent Psychiatry. 2001:1447–1453. doi: 10.1097/01.CHI.0000024863.60748.53. [DOI] [PubMed] [Google Scholar]
- Matson JL, Horovitz M. Stability of Autism Spectrum Disorders Symptoms over Time. Journal of Developmental and Physical Disabilities. 2010;22(4):331–342. doi: 10.1007/s10882-010-9188-y. [DOI] [Google Scholar]
- Mostofsky SH, Burgess MP, Gidley Larson JC. Increased motor cortex white matter volume predicts motor impairment in autism. Brain : A Journal of Neurology. 2007;130(Pt 8):2117–22. doi: 10.1093/brain/awm129. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services; 1995. [Google Scholar]
- Ozonoff S, Young GS, Landa RJ, Brian J, Bryson S, Charman T, … Iosif A-M. Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Condillac Ra, Freeman NL, Dunn-Geier J, Belair J. Multi-site study of the Childhood Autism Rating Scale (CARS) in five clinical groups of young children. Journal of Autism and Developmental Disorders. 2005;35(5):625–634. doi: 10.1007/s10803-005-0006-9. [DOI] [PubMed] [Google Scholar]
- Robins DL, Casagrande K, Barton M, Chen C-Ma, Dumont-Mathieu T, Fein D. Validation of the Modified Checklist for Autism in Toddlers, Revised With Follow-up (M-CHAT-R/F) Pediatrics. 2014;133(1):37–45. doi: 10.1542/peds.2013-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton M. The Modified Checklist for Autism in Toddlers (M-CHAT) 1999. Self-published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins DL, Fein D, Barton M. The Modified Checklist for Autism in Toddlers, Revised, with Follow-up (M-CHAT-R/F) 2009. Self-published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Vismara La. Evidence-based comprehensive treatments for early autism. Journal of clinical child and adolescent psychology : the official journal for the Society of Clinical Child and Adolescent Psychology, American Psychological Association, Division 53. 2008;37 doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four-year outcome and predictors. American Journal of Mental Retardation : AJMR. 2005;110(6):417–38. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Saemundsen E, Magnússon P, Smári J, Sigurdardóttir S. Autism Diagnostic Interview-Revised and the Childhood Autism Rating Scale: Convergence and discrepancy in diagnosing autism. Journal of Autism and Developmental Disorders. 2003;33(3):319–328. doi: 10.1023/A:1024410702242. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood autism rating scale (CARS) Journal of Autism and Developmental Disorders. 1980;10(1):91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler R, Renner B. The Childhood Autism Rating Scale. Los Angeles: Western Psychological Services; 1988. [Google Scholar]
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):234–47. doi: 10.1002/mrdd.20038. [DOI] [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. The Vineland Adaptive Behavior Scales. Circles Pines, MN: American Guidance Services; 1984. [Google Scholar]
- Sparrow S, Cicchetti D, Balla D. Vineland-II Adaptive Behavior Scales: Survey Forms Manual. Circle Pines, MN: AGS Publishing; 2005. [Google Scholar]
- Stevens MC, Fein Da, Dunn M, Allen D, Waterhouse LH, Feinstein C, Rapin I. Subgroups of children with autism by cluster analysis: a longitudinal examination. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(3):346–52. doi: 10.1097/00004583-200003000-00017. [DOI] [PubMed] [Google Scholar]
- Sutera S, Pandey J, Esser EL, Rosenthal Ma, Wilson LB, Barton M, … Fein D. Predictors of optimal outcome in toddlers diagnosed with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):98–107. doi: 10.1007/s10803-006-0340-6. [DOI] [PubMed] [Google Scholar]
- Turner LM, Stone WL, Pozdol SL, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism : The International Journal of Research and Practice. 2006;10(3):243–65. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- Turner LM, Stone WL. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48(8):793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(1):135–70. doi: 10.1046/j.0021-9630.2003.00317.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14959806. [DOI] [PubMed] [Google Scholar]
- Woolfenden S, Sarkozy V, Ridley G, Williams K. A systematic review of the diagnostic stability of Autism Spectrum Disorder. Research in Autism Spectrum Disorders. 2012;6(1):345–354. doi: 10.1016/j.rasd.2011.06.008. [DOI] [Google Scholar]