Abstract
Cholera caused by Vibrio cholerae O1 confers at least 3 to 10 years of protection against subsequent disease regardless of age, despite a relatively rapid fall in antibody levels in peripheral blood, suggesting that memory B cell responses may play an important role in protection. The V. cholerae O1-specific polysaccharide (OSP) component of lipopolysaccharide (LPS) is responsible for serogroup specificity, and it is unclear if young children are capable of developing memory B cell responses against OSP, a T cell-independent antigen, following cholera. To address this, we assessed OSP-specific memory B cell responses in young children (2 to 5 years, n = 11), older children (6 to 17 years, n = 21), and adults (18 to 55 years, n = 28) with cholera caused by V. cholerae O1 in Dhaka, Bangladesh. We also assessed memory B cell responses against LPS and vibriocidal responses, and plasma antibody responses against OSP, LPS, and cholera toxin B subunit (CtxB; a T cell-dependent antigen) on days 2 and 7, as well as days 30, 90, and 180 after convalescence. In all age cohorts, vibriocidal responses and plasma OSP, LPS, and CtxB-specific responses peaked on day 7 and fell toward baseline over the follow-up period. In comparison, we were able to detect OSP memory B cell responses in all age cohorts of patients with detectable responses over baseline for 90 to 180 days. Our results suggest that OSP-specific memory B cell responses can occur following cholera, even in the youngest children, and may explain in part the age-independent induction of long-term immunity following naturally acquired disease.
INTRODUCTION
Cholera is a severe diarrheal disease that is endemic in 50 countries and associated with recurrent outbreaks and epidemics, especially in resource-limited settings (1). Vibrio cholerae can be classified into approximately 200 serogroups, and epidemic cholera can be caused by V. cholerae O1 and O139 serogroups (1, 2). V. cholerae O1 organisms can be biochemically typed into classical and El Tor biotypes. The O1 serogroup consists of Ogawa and Inaba serotypes, depending, respectively, on the presence or absence of a 2-O-methyl group in the nonreducing (upstream) terminal sugar of the O-specific polysaccharide (OSP) component of the lipopolysaccharide (LPS) (3, 4). Protection against cholera is serogroup specific, with serogroup specificity being determined by the OSP component of LPS (5–10). Previous infection with V. cholerae O1 provides no protection against cholera caused by V. cholerae O139 and vice versa (9, 11, 12). Ogawa and Inaba serotypes frequently fluctuate during cholera outbreaks, switching most commonly from Ogawa to Inaba (13). Immune responses against Inaba and Ogawa OSP cross-react, with higher immune responses targeting the homologous infecting serotype. Currently, a hybrid strain of V. cholerae O1 El Tor expressing classical cholera toxin (CT) predominates globally (14, 15).
Children under 5 years of age in regions where cholera is endemic have the highest burden of disease (16, 17), although both children and adults are vulnerable during cholera epidemics (18–20). We have previously shown that household contacts of cholera patients who are under 5 years of age have a significantly higher short-term risk of acquiring cholera infection than older household contacts in the same family (21). Unfortunately, vaccination of young children with currently available oral killed cholera vaccines results in lower protective efficacy and shorter duration of protection than those afforded by vaccination of older individuals (22, 23). Although the mechanism of protection against cholera is not well understood, epidemiological and challenge studies show that natural infection with V. cholerae O1 prompts protection against cholera that can last for at least 3 to 10 years, and protection against cholera is independent of age (24–26). The most used indirect marker of protection against cholera is the serum vibriocidal antibody response, which is a complement-dependent antibody assay in serum. However, there is no threshold vibriocidal level above which protection is ensured (27, 28). We have also previously shown that baseline plasma IgA antibody levels and circulating IgG memory B cell (MBC) responses to V. cholerae O1 LPS correlate with protection against cholera in household contacts of cholera patients (21, 29). We have recently developed the technology to isolate V. cholerae O1 OSP, and since serogroup specificity is determined by OSP, we have begun evaluating OSP responses in cholera patients (30–33). We have found that OSP serum and mucosal responses occur in patients with cholera, that the OSP response correlates with vibriocidal and LPS responses, and that the vibriocidal response can be largely adsorbed away by OSP (30–32).
We have also found that OSP serum responses are much more prominent following naturally acquired disease than following vaccination with oral killed cholera vaccine, especially in young children (30, 31). This may in part be due to the fact that OSP is a polysaccharide and as such is a T cell-independent antigen. We have also previously found that OSP memory B cell responses develop in adults following naturally acquired cholera, but whether such responses develop in young children is uncertain and of import, since young children are afforded long-term protection against cholera similarly to adults following naturally acquired disease through an unclear mechanism. As such, the aim of this current study was to characterize memory B cell responses to OSP, in young children, older children, and adults with cholera caused by V. cholerae O1 in Dhaka, Bangladesh.
MATERIALS AND METHODS
Ethics statement.
The study and all sample collections and analyses were approved by the research review and the ethical review committees of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), and the Institutional Review Board of the Massachusetts General Hospital. We obtained written informed consent from all adult participants ≥18 to 55 years of age and from parents or guardians of children 2 to 17 years of age. We also acquired assent from participants 11 to 17 years of age.
Study participants and specimen collection.
We enrolled 11 younger children (aged 2 to 5 years), 21 older children (aged 6 to 17 years), and 28 adults (aged 18 to 55 years) with cholera who were admitted to the International Centre for Diarrheal Disease Research in Dhaka, Bangladesh (ICDDR,B), with acute watery diarrhea from whom stool cultures were positive for Vibrio cholerae O1 between February 2012 and April 2014. Patients were treated with intravenous cholera saline solution and/or oral rehydration solution and azithromycin at the discretion of the attending physician (34), and all recovered. We collected venous blood samples from study participants following clinical stabilization at the acute stage of illness (day 2) and then again at convalescence on day 7, day 30, day 90, and day 180.
Isolation of PBMCs.
We isolated peripheral blood mononuclear cells (PBMCs) by centrifugation of diluted heparinized blood on Ficoll-Isopaque (Pharmacia, Piscataway, NJ) and stored plasma at −20°C for subsequent immunological analysis. We suspended freshly separated PBMCs at a concentration of 107 cells/ml in RPMI complete medium (Gibco, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT). We used the resuspended cells for a 6-day culture assay to measure antigen-specific memory B cells by using an enzyme-linked immunosorbent spot assay (ELISPOT) method (described below).
Vibriocidal antibody assay.
We assessed vibriocidal antibody responses in plasma as previously described, using guinea pig complement (Sigma-Aldrich Chemie GmbH) and V. cholerae O1 Ogawa (X-25049) as the target organism (9). We defined the vibriocidal titer as the reciprocal of the highest dilution resulting in >50% reduction of the optical density compared to that of control wells without plasma (12). We considered those individuals responders who showed a ≥4-fold increase in vibriocidal titer from baseline.
Assessing OSP-, LPS-, and CtxB-specific IgA, IgG, and IgM antibodies in plasma.
We assessed OSP-, LPS-, and CtxB-specific IgA, IgG, and IgM antibody responses in plasma by using enzyme-linked immunosorbent assay (ELISA) procedures, as previously described (9, 31, 32, 35). Briefly, we coated 96-well polystyrene plates (Nunc F; Nunc, USA) with V. cholerae O1 Ogawa OSPc-bovine serum albumin (BSA) (1 μg/ml) dissolved in carbonate buffer (pH 9.6 to 9.8), or O1 Ogawa LPS (2.5 μg/ml) dissolved in phosphate-buffered saline (PBS) (pH 7.2 to 7.4) or coated with 0.3 nmol of ganglioside GM1/ml, followed by recombinant CtxB subunit (0.5 μg/ml) (gift from A. M. Svennerholm, University of Gothenburg, Gothenburg, Sweden) (36–38). We added 100 μl of plasma (diluted 1:50 for OSP and LPS and 1:100 for CtxB in 0.1% bovine serum albumin in phosphate-buffered saline–0.05% Tween) per well and detected responses using horseradish peroxidase-conjugated secondary antibodies to human IgG, IgA, or IgM (Jackson ImmunoResearch, West Grove, PA; 1:1,000 dilution). After incubation at 37°C and washing, we developed the plates with orthophenylene diamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) and 0.012% hydrogen peroxide followed by reading the plates kinetically at 450 nm for 5 min (30, 39). We normalized the maximal rate of change in optical density in milli-absorbance units per minute across plates by calculating the ratio of the test sample to a standard of pooled convalescent-phase sera from previously infected cholera patients that was included on each plate. We considered individuals who showed a ≥2-fold increase in OSP, LPS, and CtxB responses compared to baseline values at study day 2 to be responders.
Memory B cell culture and ELISPOT.
We quantified memory B cells on days 2, 30, 90, and 180 by using an enzyme-linked immunospot assay (ELISPOT), as previously described (36, 40–42). Briefly, we placed 5 × 105 PBMCs/well in cell culture plates (BD Biosciences, San Jose, CA) containing RPMI 1640, 10% FBS, 200 U of penicillin/ml, 200 μg of streptomycin/ml, 2 mM l-glutamine, and 50 μM β-mercaptoethanol. To stimulate antigen-independent proliferation and differentiation of memory B cells into antibody-secreting cells (ASC), we added a mixture of three B cell-specific mitogens containing 6 μg/ml CpG oligonucleotide (Operon, Huntsville, AL), a 1/100,000 dilution of crude pokeweed mitogen (PWM) extract, and a 1/10,000 dilution of fixed Staphylococcus aureus Cowan (Sigma, St. Louis, MO) to all wells except those being used as negative controls, to which only medium was added. Plates were incubated at 37°C in 5% CO2 for 5 to 6 days, after which the cells were harvested and washed.
To measure antigen-specific memory B cell responses by ELISPOT, we coated nitrocellulose-bottom plates (MSHAN-4550; Millipore, Bedford, MA) with OSPc-BSA (10 μg/ml), LPS (25 μg/ml), and GM1 ganglioside (3 nmol/ml) followed by recombinant CtxB (2.5 μg/ml), 5 μg/ml affinity-purified goat anti-human immunoglobulin (Jackson Immunology Research, West Grove, PA) as a positive control, or 2.5 μg/ml keyhole limpet hemocyanin (KLH) (Pierce Biotechnology, Rockford, IL) as a negative control. After completion of blocking with RPMI 1640 containing 10% FBS, we added 20% of the cells from each culture plate well to assess total ASC in wells coated with anti-human immunoglobulin, and 80% were used to assess for antigen-specific ASC responses. Plates were incubated for 5 h at 37°C in 5% CO2. We then washed the plates and added alkaline phosphatase-conjugated goat anti-human IgG, horseradish peroxidase-conjugated goat anti-human IgA (Southern Biotech, Birmingham, AL), or horseradish peroxidase-conjugated mouse anti-human IgM (Hybridoma Reagent Laboratory, Baltimore, MD) at 1:500 dilutions. After an overnight incubation at 4°C, we developed the IgG plates with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium (NBT) (Sigma) and the IgA and IgM plates with 3-amino-9-ethylcarbazole (AEC) (Sigma). The number of memory B cell ASC per well was independently counted by two people in a sample-blinded fashion using a stereomicroscope, and the counts were averaged. We expressed the results as the percentage of antigen-specific memory B cells per total IgA, IgG, or IgM memory B cells. We used inclusion and exclusion criteria for data analysis as previously described (43, 44).
Statistical analysis.
We assessed the differences in the magnitudes of responses using the Wilcoxon signed-rank test and Mann-Whitney U test, as appropriate. We used Spearman's correlation to assess the relationship between immune responses. All reported P values were two-tailed, with a cutoff of P < 0.05 considered a threshold for statistical significance. Data analysis and figure preparation were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA) and SPSS 14 (SPSS Inc., Chicago, IL).
RESULTS
Study population.
We enrolled 11 younger children, 21 older children, and 28 adults who were admitted with cholera at the ICDDR,B. During the study period, Ogawa was the predominant serotype, and all cholera patients enrolled in this study were infected with V. cholerae O1 El Tor Ogawa. Baseline demographic and clinical characteristics of study participants are shown in Table 1.
TABLE 1.
Demographic and clinical characteristics of cholera patients in study
| Characteristic | Value for age group: |
||
|---|---|---|---|
| 2–5 yr (n = 11) | 6–17 yr (n = 21) | 18–55 yr (n = 28) | |
| Mean age, yr (range) | 3.7 (2–5) | 9.4 (6–17) | 33.9 (19–51) |
| No. (%) of female subjects | 3 (27) | 8 (38) | 10 (36) |
| No. (%) of subjects with ABO blood group | |||
| A | 2 (18) | 7 (33) | 7 (25) |
| B | 3 (27) | 7 (33) | 10 (36) |
| AB | 1 (9) | 1 (5) | 2 (7) |
| O | 5 (45) | 6 (29) | 8 (29) |
Vibriocidal responses.
We assessed Ogawa vibriocidal titers on days 2, 7, 30, 90, and 180 (Fig. 1). The baseline geometric mean (GM) reciprocal vibriocidal titer (GMT) in younger children was 31 (95% confidence interval [CI], 8 to 117); in older children, it was 24 (95% CI, 10 to 59); and in adults, it was 26 (95% CI, 16 to 42). All but one patient increased their vibriocidal titer ≥4-fold from the acute to the convalescent phase of illness. Compared to baseline, responses peaked on day 7 (younger children, GMT, 4,238; 95% CI, 2,118 to 8,479; P < 0.01; older children, GMT, 2,032; 95% CI, 1,038 to 3,978; P < 0.0001; and adults, GMT, 1,787; 95% CI, 1,218 to 2,621; P < 0.0001) and remained elevated up to day 180 in older children (GMT, 114; 95% CI, 56 to 230; P < 0.05) and adults (GMT, 109; 95% CI, 61 to 197; P < 0.001). Vibriocidal responses on day 7 were higher in young children than in adults (young children, GMT, 4,238; 95% CI, 2,118 to 8,479; adults, GMT, 1,787; 95% CI, 1,218 to 2,621; P = 0.01) but fell back to baseline by day 90.
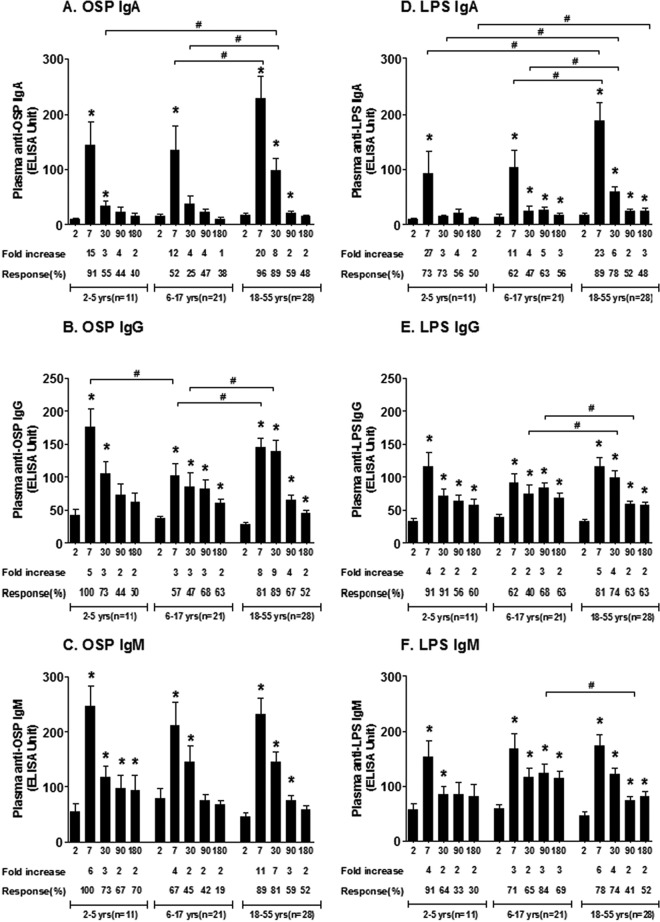
FIG 1.
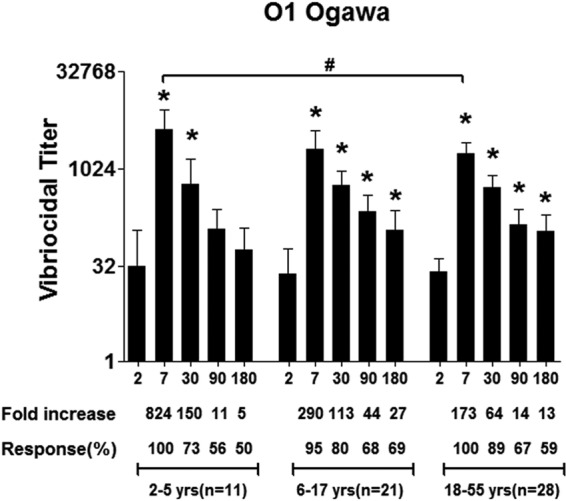
Vibriocidal antibody responses in plasma in Bangladeshi cholera patients by age group. The columns indicate geometric mean reciprocal end titers, and error bars represent 95% confidence intervals. An asterisk denotes a statistically significant difference (P < 0.05) from the baseline (day 2) titer. Mean fold changes and responder frequencies are also shown. # indicates statistically significant differences between the study groups (P < 0.05). The x axis in all figures shows day of illness or convalescence.
OSP-, LPS-, and CtxB-specific antibody responses in plasma.
We assessed Ogawa OSP- and LPS-specific IgA, IgG, and IgM antibody responses in plasma at the initial stage of infection (day 2) and at other time points during convalescence in all age groups of patients (Fig. 2). All age groups developed prominent OSP and LPS responses in all three antibody isotypes that peaked on day 7 compared to baseline levels (P < 0.01). OSP and LPS responses closely correlated with each other (Spearman r = 0.71, 0.57, and 0.56, respectively, for IgA, IgG, and IgM; all P values, <0.0001 [see Fig. S1 in the supplemental material]), and OSP responses correlated with vibriocidal responses over 6 months of follow-up (Spearman r = 0.46, 0.50, and 0.58 for IgA, IgG, and IgM, respectively; all P values, <0.0001 [see Fig. S2 in the supplemental material]). Anti-OSP and -LPS responses fell toward baseline after peaking on day 7, although the responses were more prolonged in adults and older children than in younger children.
FIG 2.
Mean plasma IgA, IgG, and IgM antibody responses to OSP and LPS by age group as measured by ELISA, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 2). Mean fold changes and responder frequencies are also shown. # indicates a statistically significant difference between the study groups (P < 0.05).
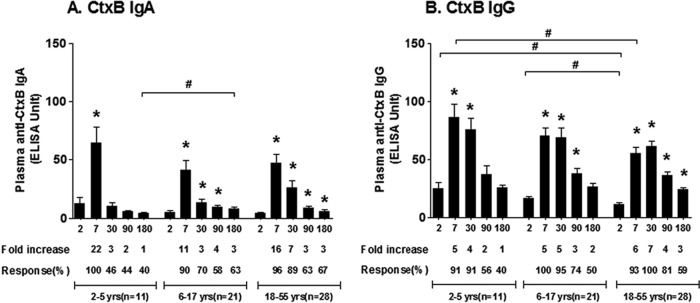
CtxB-specific IgA and IgG antibody responses similarly peaked on day 7 in all groups except in adults; in adults, CtxB-specific IgG responses peaked on day 30 compared to day 2 (Fig. 3). CtxB-specific antibody responses lasted for 6 months in adults (P < 0.05), returned to baseline level by day 180 in older children, and returned to baseline by day 30 in younger children (P < 0.02). Adults had lower baseline (day 2) CtxB-specific IgG antibody responses than did children (P < 0.01), and younger children developed higher responses on day 7 than did adults.
FIG 3.
Mean plasma IgA and IgG antibody responses to CtxB by age group as measured by ELISA, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 2). Mean fold changes and responder frequencies are also shown. # indicates a statistically significant difference between the study groups (P < 0.05).
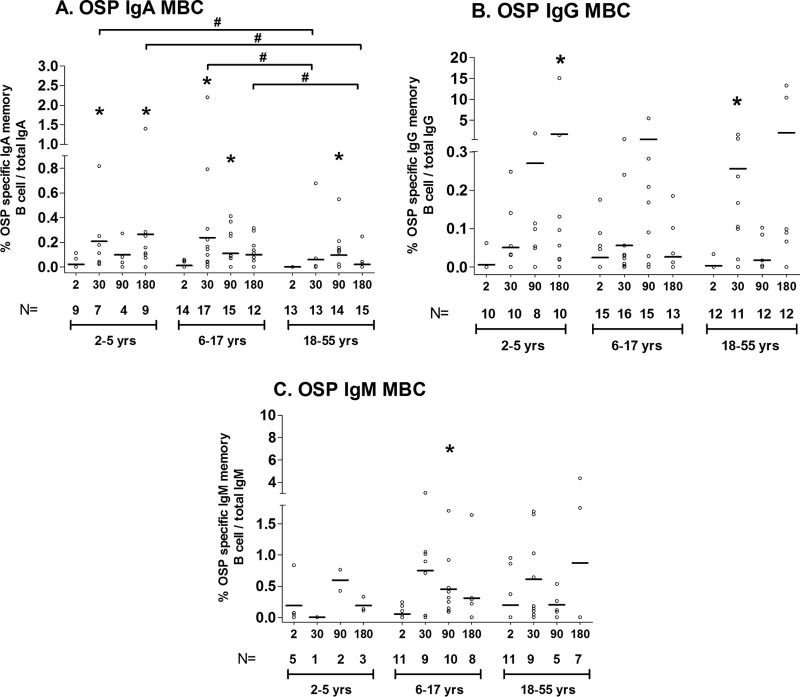
Antigen-specific memory B cell responses.
We assessed OSP- and LPS-specific memory B cell responses at four time points (days 2, 30, 90, and 180) in the three age groups of patients. Due to limited PBMC quantities, especially in the youngest children, preference was given to assessing IgG and IgA memory B cell responses over IgM responses and assessing OSP over LPS responses. Not all isotypes and antigens could be assessed in each patient. We did not assess CtxB memory responses.
Compared to baseline values, IgA OSP-specific (Fig. 4) and LPS-specific (see Fig. S3 in the supplemental material) memory B cell responses were detected in younger children, older children, and adults by day 30. OSP IgA responses were detectable through day 180 in the youngest children and day 90 in older children and adults. OSP-specific IgG and IgM memory B cell responses were also detectable in samples collected during convalescence (day 30, OSP-specific IgG in adults; day 180, OSP-specific IgG in young children; and day 90, OSP-specific IgM in older children). Interestingly, we found a negative association of the presence of an IgA memory B cell (MBC) response on day 30 and increasing age (IgA r = −0.55, P = 0.0005 [Fig. 5]), although no such association was evident for IgG or IgM OSP MBC responses (r = 0.17 and 0.07, respectively; P > 0.32). The vibriocidal peak on day 7 correlated with the development of IgA memory B cell responses on day 30 (IgA r = 0.37; P = 0.028 [see Fig. S4 in the supplemental material]) but not IgG or IgM memory responses. We were unable to demonstrate a correlation of day 7 OSP responses and the subsequent development of memory responses (see Fig. S5 in the supplemental material).
FIG 4.
Mean OSP-specific IgA, IgG, and IgM memory B cell responses by age group, as measured by ELISPOT, expressed as the percent antigen-specific responses of total isotype-specific memory B cells, with error bars representing standard errors of the means. An asterisk denotes a significant difference (P < 0.05) from the baseline (day 2). # indicates a statistically significant difference between the study groups (P < 0.05).
FIG 5.
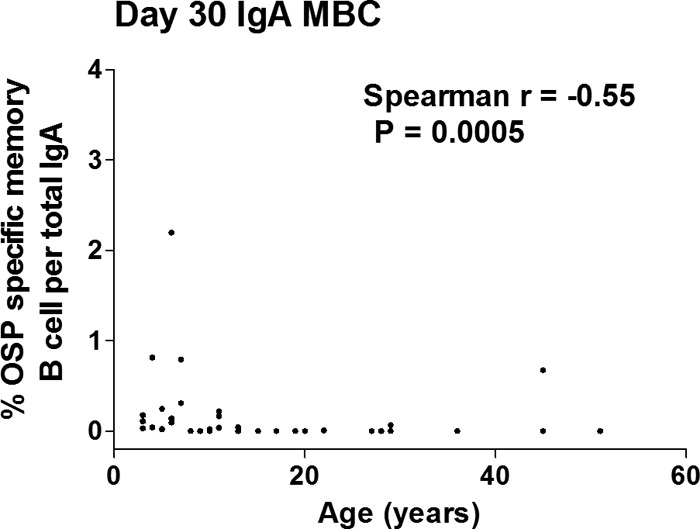
Correlation between day 30 memory B cell IgA responses and age of different age groups of cholera patients (n = 37). The Spearman correlation coefficient (r) is shown.
DISCUSSION
In this study, we found that younger children, older children, and adults develop detectable OSP memory B cell responses in peripheral blood following cholera in Bangladesh. All age groups also develop IgA, IgG, and IgM OSP-specific responses in plasma, and OSP responses correlate with LPS responses and vibriocidal responses. Although the immunologic mechanism of protection against cholera is currently unknown, there is a growing body of evidence that protection is mediated by OSP-specific antibodies. These data include the fact that protection against cholera is serogroup specific and that serogroup specificity is defined by OSP, that vibriocidal antibodies that can be adsorbed away by OSP correlate with protection (32), that OSP- and LPS-specific immune responses highly correlate with each other (33), and that LPS memory B cell and IgA plasma responses have previously been shown to correlate with protection against cholera in household contact studies, while memory B cell responses against cholera toxin (CT) are not associated with protection (21, 29). Protection against cholera can persist for at least 3 to 10 years following naturally acquired disease (24, 26), despite a return to baseline of vibriocidal and plasma antibody responses within 6 to 12 months of disease (36, 42). As such, memory responses may play a role in mediating long-term anamnestic responses upon reexposure.
In our current study, prominent vibriocidal responses developed in all our age cohorts. The highest vibriocidal response was detected in the youngest children, although it had returned to baseline by day 90 in this group, but it remained elevated in older children and adults through day 180. These prolonged responses in older children and adults may suggest prior exposure. Although the vibriocidal response was highest in the youngest children, it is of interest that OSP- and LPS-specific IgM and IgG peak responses were comparable in all age groups and that OSP- and LPS-specific IgA responses were actually highest in adults. This may suggest that mucosal IgA responses may not be fully reflected in the functionality of the antibodies assessed by the vibriocidal assay. This is of importance since the serum vibriocidal assay may be a surrogate marker of immunity at the intestinal surface that protects against V. cholerae, a noninvasive luminal pathogen.
We also detected prominent OSP-specific responses in plasma across all age groups and antibody isotypes. In general, IgG and IgM responses persisted longer into convalescence than did IgA responses, although IgA responses were detected in late convalescence in adults and older children, perhaps suggesting that IgA responses in serum may also be boosted by repetitive exposure. This was also the case for the T cell-dependent antigen CtxB.
A number of cholera vaccines are currently available or in development, with oral killed cholera vaccines currently being commercially available in a number of countries (45, 46). Oral killed cholera vaccines are safe and protective and are significant assets in global cholera control strategies. Unfortunately, protection afforded by oral killed cholera vaccines is of lower magnitude and of shorter duration in children under 5 years of age than that in older children and adults and than that afforded by naturally acquired disease in children 5 years of age and younger (22, 23, 31, 43). We have also previously shown that vaccination with an oral killed cholera vaccine (Dukoral; whole-cell oral cholera vaccine supplemented with 1 mg/dose of recombinant nontoxic cholera toxin B subunit [CtxB]; WCrBS; Crucell, Sweden) in the youngest children is associated with a proregulatory (interleukin-10 [IL-10]) response, versus a proinflammatory (IL-17, interferon gamma, and IL-13) response seen in young children with naturally acquired cholera (47), and that OSP-specific memory B cell responses following oral killed cholera vaccination of adults are markedly blunted following vaccination compared to what occurs following naturally acquired disease (30). Whether OSP-specific memory B cell responses develop following immunization with other oral cholera vaccines is currently unknown.
How memory B cell responses targeting OSP, a T cell-independent antigen, develop in young children following naturally acquired cholera is unclear. However, it should be recalled that cholera toxin is a potent immunoadjuvant in addition to being an enterotoxin (48). CT and LPS may activate mucosal innate immune responses (49), and abnormalities in innate immune factors have been repetitively associated with susceptibility to cholera (50–52). We have also recently shown that mucosal invariant intestinal T (MAIT) cells are activated during cholera and that this response correlates with the development of antibodies against T cell-independent LPS but not T cell-dependent CtxB (53). Interestingly, we found a negative association of increasing age and presence of OSP-specific IgA memory B cell responses on day 30, with the youngest children having the most prominent OSP-specific memory B cell IgA responses in peripheral blood as a percentage of total IgA cells. This is despite the observation that adults had higher day 7 and day 30 OSP-specific IgA responses in plasma than did younger children. It is possible that these observations are due to our relatively small cohort size. Alternatively, the lower percentage in adults may represent relative selection of higher-affinity B cells from previous exposure and B cell maturation. We did not find an association of age and presence of OSP-specific memory B IgG and IgM cells on day 30. In summary, we found that memory B cell responses in young children to OSP, a T cell-independent antigen, were equivalent to or more pronounced than those detected in older individuals in Dhaka, Bangladesh. We also found that the vibriocidal response correlated with the development of IgA memory B cells targeting OSP (but not IgG and IgM memory responses), perhaps again suggesting that the vibriocidal response is a marker of an as-yet-poorly defined protective mucosal immune response.
Our study has a number of limitations. Although it involved detailed immunologic analysis of 60 cholera patients in Bangladesh, our cohort size by age was relatively limited, and the volume of blood that could be drawn necessitated prioritization of analyses, especially from the youngest children. This particularly limited our ability to fully assess IgM memory responses. Our youngest children also had a mean age of 3.7 years (range, 2 to 5), and in this area of cholera endemicity, it is possible that even many of these children had previously been exposed to V. cholerae. We also assessed memory B cell responses in blood, and it is possible that long-term protection may actually be afforded by long-lived plasma cells or memory B cells residing in intestinal tissue. We also were able to assess responses only against Ogawa OSP since all of our patients were infected with this serotype. Finally, we were unable to stratify responses by other potential influences, including blood group, micronutrient deficiency, and innate haplotypes, although blood group and gender were not significantly different in our three age cohorts. Despite these limitations, our study is highly significant. We were able to detect OSP-specific memory B cell responses in cholera patients regardless of age, suggesting that long-lived immune responses that target OSP may indeed play a role in providing long-term protection against cholera, even in the youngest children.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the ICDDR,B and by the Intramural Research Program of the National Institutes of Health, NIDDK, and extramural grants from the National Institutes of Health, including the National Institute of Allergy and Infectious Diseases (R01 AI106878 [E.T.R.], U01 AI058935 [S.B.C. and E.T.R.], R03 AI063079 [F.Q.], K08 AI089721 [R.C.C.], K08AI100923 [D.T.L.]) and the Fogarty International Center, Training Grant in Vaccine Development and Public Health (TW005572 [A.A., T.U., and T.R.B.]), as well as by Swedish Sida grant INT-ICDDR,B-HN-01-AV (F.Q.), and a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (R.C.L.). ICDDR,B is thankful to the donors for their support of its research efforts. ICDDR,B is also grateful to the Government of Bangladesh, Canada, Sweden, and the United Kingdom for providing core/unrestricted support.
Funding Statement
We have also received funding from Swedish Sida grant INT-ICDDR,B-HN-01-AV for Firdausi Qadri.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00647-15.
REFERENCES
- 1.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. 2012. The global burden of cholera. Bull World Health Organ 90:209–218A. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroeher UH, Karageorgos LE, Morona R, Manning PA. 1992. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci U S A 89:2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Villeneuve S, Zhang J, Lei P, Miller CE, Lafaye P, Nato F, Szu SC, Karpas A, Bystricky S, Robbins JB, Kovac P, Fournier JM, Glaudemans CP. 1998. On the antigenic determinants of the lipopolysaccharides of Vibrio cholerae O:1, serotypes Ogawa and Inaba. J Biol Chem 273:2777–2783. doi: 10.1074/jbc.273.5.2777. [DOI] [PubMed] [Google Scholar]
- 5.Alam MM, Bufano MK, Xu P, Kalsy A, Yu Y, Freeman YW, Sultana T, Rashu MR, Desai I, Eckhoff G, Leung DT, Charles RC, LaRocque RC, Harris JB, Clements JD, Calderwood SB, Qadri F, Vann WF, Kovac P, Ryan ET. 2014. Evaluation in mice of a conjugate vaccine for cholera made from Vibrio cholerae O1 (Ogawa) O-specific polysaccharide. PLoS Negl Trop Dis 8:e2683. doi: 10.1371/journal.pntd.0002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert MJ, Alam K, Ansaruzzaman M, Qadri F, Sack RB. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 (Bengal strain) after oral immunization of rabbits with V. cholerae O1 vaccine strain CVD103-HgR. J Infect Dis 169:230–231. doi: 10.1093/infdis/169.1.230. [DOI] [PubMed] [Google Scholar]
- 7.Jonson G, Osek J, Svennerholm AM, Holmgren J. 1996. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect Immun 64:3778–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitner DR, Feichter S, Schild-Prufert K, Rechberger GN, Reidl J, Schild S. 2013. Lipopolysaccharide modifications of a cholera vaccine candidate based on outer membrane vesicles reduce endotoxicity and reveal the major protective antigen. Infect Immun 81:2379–2393. doi: 10.1128/IAI.01382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qadri F, Wenneras C, Albert MJ, Hossain J, Mannoor K, Begum YA, Mohi G, Salam MA, Sack RB, Svennerholm AM. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun 65:3571–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldor MK, Colwell R, Mekalanos JJ. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci U S A 91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert MJ, Alam K, Rahman AS, Huda S, Sack RB. 1994. Lack of cross-protection against diarrhea due to Vibrio cholerae O1 after oral immunization of rabbits with V. cholerae O139 Bengal. J Infect Dis 169:709–710. doi: 10.1093/infdis/169.3.709. [DOI] [PubMed] [Google Scholar]
- 12.Qadri F, Mohi G, Hossain J, Azim T, Khan AM, Salam MA, Sack RB, Albert MJ, Svennerholm AM. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol 2:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. 2008. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg 79:708–714. [PMC free article] [PubMed] [Google Scholar]
- 14.Nair GB, Faruque SM, Bhuiyan NA, Kamruzzaman M, Siddique AK, Sack DA. 2002. New variants of Vibrio cholerae O1 biotype El Tor with attributes of the classical biotype from hospitalized patients with acute diarrhea in Bangladesh. J Clin Microbiol 40:3296–3299. doi: 10.1128/JCM.40.9.3296-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 16.Deen JL, von Seidlein L, Sur D, Agtini M, Lucas ME, Lopez AL, Kim DR, Ali M, Clemens JD. 2008. The high burden of cholera in children: comparison of incidence from endemic areas in Asia and Africa. PLoS Negl Trop Dis 2:e173. doi: 10.1371/journal.pntd.0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sack RB, Siddique AK, Longini IM Jr, Nizam A, Yunus M, Islam MS, Morris JG Jr, Ali A, Huq A, Nair GB, Qadri F, Faruque SM, Sack DA, Colwell RR. 2003. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J Infect Dis 187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- 18.Legros D, McCormick M, Mugero C, Skinnider M, Bek'Obita DD, Okware SI. 2000. Epidemiology of cholera outbreak in Kampala, Uganda. East Afr Med J 77:347–349. [DOI] [PubMed] [Google Scholar]
- 19.Luque Fernandez MA, Mason PR, Gray H, Bauernfeind A, Fesselet JF, Maes P. 2011. Descriptive spatial analysis of the cholera epidemic 2008–2009 in Harare, Zimbabwe: a secondary data analysis. Trans R Soc Trop Med Hyg 105:38–45. doi: 10.1016/j.trstmh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki S, Suzuki H, Igarashi K, Tambatamba B, Mulenga P. 2008. Spatial analysis of risk factor of cholera outbreak for 2003–2004 in a peri-urban area of Lusaka, Zambia. Am J Trop Med Hyg 79:414–421. [PubMed] [Google Scholar]
- 21.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair D, Abba K, Zaman K, Qadri F, Graves PM. 2011. Oral vaccines for preventing cholera. Cochrane Database Syst Rev 2011(3):CD008603. doi: 10.1002/14651858.CD008603.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL. 2011. Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Negl Trop Dis 5:e1289. doi: 10.1371/journal.pntd.0001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. 2005. Refractory periods and climate forcing in cholera dynamics. Nature 436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 25.Ali M, Emch M, Park JK, Yunus M, Clemens J. 2011. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis 204:912–918. doi: 10.1093/infdis/jir416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. 1981. Duration of infection-derived immunity to cholera. J Infect Dis 143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 27.Saha D, LaRocque RC, Khan AI, Harris JB, Begum YA, Akramuzzaman SM, Faruque AS, Ryan ET, Qadri F, Calderwood SB. 2004. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis 189:2318–2322. doi: 10.1086/421275. [DOI] [PubMed] [Google Scholar]
- 28.Mosley WH, Ahmad S, Benenson AS, Ahmed A. 1968. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull World Health Organ 38:777–785. [PMC free article] [PubMed] [Google Scholar]
- 29.Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, Nazim M, LaRocque RC, Ryan ET, Calderwood SB, Qadri F, Harris JB. 2012. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol 19:842–848. doi: 10.1128/CVI.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uddin T, Aktar A, Xu P, Johnson RA, Rahman MA, Leung DT, Afrin S, Akter A, Alam MM, Rahman A, Chowdhury F, Khan AI, Bhuiyan TR, Bufano MK, Rashu R, Yu Y, Wu-Freeman Y, Harris JB, LaRocque RC, Charles RC, Kovac P, Calderwood SB, Ryan ET, Qadri F. 2014. Immune responses to O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 Ogawa in adult Bangladeshi recipients of an oral killed cholera vaccine and comparison to responses in patients with cholera. Am J Trop Med Hyg 90:873–881. doi: 10.4269/ajtmh.13-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung DT, Uddin T, Xu P, Aktar A, Johnson RA, Rahman MA, Alam MM, Bufano MK, Eckhoff G, Wu-Freeman Y, Yu Y, Sultana T, Khanam F, Saha A, Chowdhury F, Khan AI, Charles RC, Larocque RC, Harris JB, Calderwood SB, Kovac P, Qadri F, Ryan ET. 2013. Immune responses to the O-specific polysaccharide antigen in children who received a killed oral cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol 20:780–788. doi: 10.1128/CVI.00035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RA, Uddin T, Aktar A, Mohasin M, Alam MM, Chowdhury F, Harris JB, LaRocque RC, Bufano MK, Yu Y, Wu-Freeman Y, Leung DT, Sarracino D, Krastins B, Charles RC, Xu P, Kovac P, Calderwood SB, Qadri F, Ryan ET. 2012. Comparison of immune responses to the O-specific polysaccharide and lipopolysaccharide of Vibrio cholerae O1 in Bangladeshi adult patients with cholera. Clin Vaccine Immunol 19:1712–1721. doi: 10.1128/CVI.00321-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu P, Alam MM, Kalsy A, Charles RC, Calderwood SB, Qadri F, Ryan ET, Kovac P. 2011. Simple, direct conjugation of bacterial O-SP-core antigens to proteins: development of cholera conjugate vaccines. Bioconjug Chem 22:2179–2185. doi: 10.1021/bc2001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sack DA, Kauk DS, de Leeuw RA, Nelson EJ, Henning L, Pelikan J, Chisti MJ. Clinical management of cholera. Cholera Outbreak Training and Shigellosis Program. www.cotsprogram.com.
- 35.Qadri F, Ahmed F, Karim MM, Wenneras C, Begum YA, Abdus Salam M, Albert MJ, McGhee JR. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin Diagn Lab Immunol 6:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam MM, Riyadh MA, Fatema K, Rahman MA, Akhtar N, Ahmed T, Chowdhury MI, Chowdhury F, Calderwood SB, Harris JB, Ryan ET, Qadri F. 2011. Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera. Clin Vaccine Immunol 18:844–850. doi: 10.1128/CVI.00562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendall EA, Tarique AA, Hossain A, Alam MM, Arifuzzaman M, Akhtar N, Chowdhury F, Khan AI, Larocque RC, Harris JB, Ryan ET, Qadri F, Calderwood SB. 2010. Development of immunoglobulin M memory to both a T-cell-independent and a T-cell-dependent antigen following infection with Vibrio cholerae O1 in Bangladesh. Infect Immun 78:253–259. doi: 10.1128/IAI.00868-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed T, Svennerholm AM, Al Tarique A, Sultana GN, Qadri F. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433–1439. doi: 10.1016/j.vaccine.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 39.Jayasekera CR, Harris JB, Bhuiyan S, Chowdhury F, Khan AI, Faruque AS, Larocque RC, Ryan ET, Ahmed R, Qadri F, Calderwood SB. 2008. Cholera toxin-specific memory B cell responses are induced in patients with dehydrating diarrhea caused by Vibrio cholerae O1. J Infect Dis 198:1055–1061. doi: 10.1086/591500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crotty S, Aubert RD, Glidewell J, Ahmed R. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 41.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. 2003. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 42.Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, LaRocque RC, Wrammert J, Ryan ET, Qadri F, Calderwood SB, Harris JB. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun 77:3850–3856. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leung DT, Rahman MA, Mohasin M, Patel SM, Aktar A, Khanam F, Uddin T, Riyadh MA, Saha A, Alam MM, Chowdhury F, Khan AI, Charles R, LaRocque R, Harris JB, Calderwood SB, Qadri F, Ryan ET. 2012. Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh. Clin Vaccine Immunol 19:690–698. doi: 10.1128/CVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alam MM, Aktar A, Afrin S, Rahman MA, Aktar S, Uddin T, Al Mahbuba D, Chowdhury F, Khan AI, Bhuiyan TR, Begum YA, Ryan ET, Calderwood SB, Svennerholm AM, Qadri F. 2014. Antigen-specific memory B-cell responses to enterotoxigenic Escherichia coli infection in Bangladeshi adults. PLoS Negl Trop Dis 8:e2822. doi: 10.1371/journal.pntd.0002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qadri F, Ali M, Chowdhury F, Khan AI, Saha A, Khan IA, Begum YA, Bhuiyan TR, Chowdhury MI, Uddin MJ, Khan JA, Chowdhury AI, Rahman A, Siddique SA, Asaduzzaman M, Akter A, Khan A, Ae You Y, Siddik AU, Saha NC, Kabir A, Riaz BK, Biswas SK, Begum F, Unicomb L, Luby SP, Cravioto A, Clemens JD. 2015. Feasibility and effectiveness of oral cholera vaccine in an urban endemic setting in Bangladesh: a cluster randomised open-label trial. Lancet 386:1362–1371. doi: 10.1016/S0140-6736(15)61140-0. [DOI] [PubMed] [Google Scholar]
- 46.Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Nguyen TV, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD. 2009. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 47.Arifuzzaman M, Rashu R, Leung DT, Hosen MI, Bhuiyan TR, Bhuiyan MS, Rahman MA, Khanam F, Saha A, Charles RC, LaRocque RC, Weil AA, Clements JD, Holmes RK, Calderwood SB, Harris JB, Ryan ET, Qadri F. 2012. Antigen-specific memory T cell responses after vaccination with an oral killed cholera vaccine in Bangladeshi children and comparison to responses in patients with naturally acquired cholera. Clin Vaccine Immunol 19:1304–1311. doi: 10.1128/CVI.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez J, Holmgren J. 2011. Cholera toxin—a foe & a friend. Indian J Med Res 133:153–163. [PMC free article] [PubMed] [Google Scholar]
- 49.Ellis CN, LaRocque RC, Uddin T, Krastins B, Mayo-Smith LM, Sarracino D, Karlsson EK, Rahman A, Shirin T, Bhuiyan TR, Chowdhury F, Khan AI, Ryan ET, Calderwood SB, Qadri F, Harris JB. 2015. Comparative proteomic analysis reveals activation of mucosal innate immune signaling pathways during cholera. Infect Immun 83:1089–1103. doi: 10.1128/IAI.02765-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin OS, Uddin T, Citorik R, Wang JP, Della Pelle P, Kradin RL, Bingle CD, Bingle L, Camilli A, Bhuiyan TR, Shirin T, Ryan ET, Calderwood SB, Finberg RW, Qadri F, Larocque RC, Harris JB. 2011. LPLUNC1 modulates innate immune responses to Vibrio cholerae. J Infect Dis 204:1349–1357. doi: 10.1093/infdis/jir544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flach CF, Qadri F, Bhuiyan TR, Alam NH, Jennische E, Lonnroth I, Holmgren J. 2007. Broad up-regulation of innate defense factors during acute cholera. Infect Immun 75:2343–2350. doi: 10.1128/IAI.01900-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larocque RC, Sabeti P, Duggal P, Chowdhury F, Khan AI, Lebrun LM, Harris JB, Ryan ET, Qadri F, Calderwood SB. 2009. A variant in long palate, lung and nasal epithelium clone 1 is associated with cholera in a Bangladeshi population. Genes Immun 10:267–272. doi: 10.1038/gene.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung DT, Bhuiyan TR, Nishat NS, Hoq MR, Aktar A, Rahman MA, Uddin T, Khan AI, Chowdhury F, Charles RC, Harris JB, Calderwood SB, Qadri F, Ryan ET. 2014. Circulating mucosal associated invariant T cells are activated in Vibrio cholerae O1 infection and associated with lipopolysaccharide antibody responses. PLoS Negl Trop Dis 8:e3076. doi: 10.1371/journal.pntd.0003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.