Abstract
The spontaneous modification of proteins, such as deamidation of asparagine residues, can significantly affect the immunogenicity of protein-based vaccines. Using a “genetically deamidated” form of recombinant protective antigen (rPA), we have previously shown that deamidation can decrease the immunogenicity of rPA, the primary component of new-generation anthrax vaccines. In this study, we investigated the biochemical and immunological mechanisms by which deamidation of rPA might decrease the immunogenicity of the protein. We found that loss of the immunogenicity of rPA vaccine was independent of the presence of adjuvant. We assessed the effect of deamidation on the immunodominant neutralizing B-cell epitopes of rPA and found that these epitopes were not significantly affected by deamidation. In order to assess the effect of deamidation on T-cell help for antibody production elicited by rPA vaccine, we examined the ability of the wild-type and genetically deamidated forms of rPA to serve as hapten carriers. We found that when wild-type and genetically deamidated rPA were modified to similar extents with 2,4-dinitrophenyl hapten (DNP) and then used to immunize mice, higher levels of anti-DNP antibodies were elicited by wild-type DNP-rPA than those elicited by the genetically deamidated DNP-rPA, indicating that wild-type rPA elicits more T-cell help than the genetically deamidated form of the protein. These results suggest that a decrease in the ability of deamidated rPA to elicit T-cell help for antibody production is a possible contributor to its lower immunogenicity.
INTRODUCTION
Inhalation anthrax is a serious, often fatal, disease caused by Bacillus anthracis. Because of the fatal nature of the disease and the ease of dispersion of B. anthracis spores, B. anthracis is one of the most feared of all bioterror weapons. Due to the low incidence of natural anthrax disease in humans, anthrax vaccines are not given routinely. However, the potential for the use of B. anthracis as a bioterror agent has prompted stockpiling of anthrax vaccines by national governments. Long-term stability is a highly desired characteristic of a stockpiled vaccine since a long shelf life significantly decreases the cost of the stockpile.
Many anthrax vaccines are based on protective antigen (PA), which is a nontoxic component of anthrax toxin. Anthrax toxin is a critical virulence factor of B. anthracis and is essential for disease symptomatology and progression (1, 2). The toxin is composed of PA, lethal factor (LF), and edema factor (EF). PA binds to cell receptors, heptamerizes, and then binds LF or EF to form lethal toxin (LT) and edema toxin (ET), respectively (3). Internalization of LT and ET leads to introduction of the enzymatically active effector proteins LF and EF into the cell cytosol where they exert their cytotoxicity (2). Vaccines based on PA or a recombinant form of PA (rPA) elicit antibodies, in particular functional toxin-neutralizing antibodies (TNAs), that have been correlated with protection against the disease (4–6).
Unfortunately, progress to develop new-generation rPA-based anthrax vaccines has been hampered by vaccine instability (7), which is believed to be due, at least in part, to spontaneous deamidation of the protein upon long-term storage (8, 9). Deamidation results in posttranslational conversion of asparagine (Asn) to aspartate (Asp) or isoaspartic acid (isoAsp). Deamidation of Asn occurs in vivo on a time scale ranging from a few seconds to centuries, depending on the local environment (10). In theory, deamidation might adversely affect the interaction of rPA with the aluminum adjuvant of the vaccine, might alter the epitope structure of the antigen, and/or might affect the ability of the protein to elicit T-cell help for antibody production which, in turn, might affect the vaccine immunogenicity (11–15).
Earlier studies have demonstrated that certain Asn residues of rPA deamidate during the purification process and upon storage (8, 16, 17). Previously, we generated a mutant form of rPA in which the six Asn residues that are the most prone to deamidation were changed to Asp residues (six-Asp mutant rPA). The residues that were changed were Asn408, Asn466, Asn537, Asn601, Asn713, and Asn719. We have used this “genetically deamidated” form of the protein as a model for the naturally deamidated form of the protein that would be expected to result upon prolonged vaccine storage (8). In that previous study, we demonstrated that six-Asp mutant rPA possesses lower immunogenicity than wild-type rPA. Recently, D'Souza et al. (9) confirmed that rPA adsorbed to Alhydrogel rapidly deamidates and further confirmed that this deamidation resulted in the loss of immunogenicity. Taken together, these results provide substantial support for the idea that the loss of stability of rPA vaccines is due, at least in part, to deamidation. However, little is known about the mechanism(s) responsible for this loss of immunogenicity. In this study, we exploited our genetically deamidated six-Asp mutant rPA in order to better understand the immunological mechanism(s) responsible for the low immunogenicity of deamidated rPA.
MATERIALS AND METHODS
Materials.
B. anthracis recombinant PA83 (NR-140), recombinant LF (NR-142), anti-rPA rabbit reference polyclonal serum (NR-3839), and murine macrophage-like J774A.1 cells (NR-28) were from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH (Bethesda, MD). Cell culture reagents were obtained from Invitrogen (Carlsbad, CA). The aluminum hydroxide adjuvant, Alhydrogel, was obtained from Brenntag Biosector (Denmark). 2,4-Dinitrobenzenesulfonic acid hydrate (DNBS), 2,4-dinitrophenyl (DNP), and Sypro Orange dye were obtained from Sigma-Aldrich (St. Louis, MO). DNP conjugated to bovine serum albumin (DNP-BSA), used for coating the enzyme-linked immunosorbent assay (ELISA) plates, was from Santa Cruz Biotechnology (Dallas, TX). The anion-exchange column (HiPrep Q HP 16/10) and the gel-filtration chromatography column (Superdex 200 10/300GL) were from GE Healthcare (Sweden).
Cloning and expression of wild-type and six-Asp mutant rPA genes.
Genes encoding wild-type and six-Asp mutant rPA were cloned in pET-22b(+) plasmid carrying an N-terminal pelB signal sequence for periplasmic localization and expressed and purified in Escherichia coli BL21(DE3) plyS essentially as previously described (18). Briefly, E. coli BL21(DE3) plyS cultures containing recombinant plasmid constructs were grown at 37°C in LB broth containing 100 μg/ml of ampicillin. Protein expression was induced with 1 mM isopropyl-β-d-thiogalactopyranoside at 30°C for 3 to 4 h. The bacterial cells were harvested and resuspended in 20 mM Tris-HCl, and 1 mM EDTA (pH 8.0) containing 20% sucrose. The suspension was stirred gently at 4°C for 10 min and centrifuged. After centrifugation, cell pellets were resuspended in 20 mM Tris-HCl (pH 8.0) containing 5 mM MgSO4 to release the periplasmic content. The suspension was again stirred gently at 4°C for 10 min, and the rPA proteins in the periplasmic content were collected after centrifugation. Wild-type and six-Asp mutant rPA proteins were then further purified using a HiPrep Q HP 16/10 anion-exchange column with a 0 to 1 M NaCl gradient. Finally, the rPA preparations were purified by size exclusion chromatography on a Superdex 200 10/300GL column. The purified rPA proteins obtained using this procedure were approximately 95 to 99% pure as determined by SDS-PAGE. We have previously shown that wild-type rPA exhibits only minor amounts of deamidation (8).
Assessment of melting temperatures.
A real-time PCR instrument (CFX96 RT-PCR; Bio-Rad, Hercules, CA) was used to monitor the protein unfolding (melting temperature) of the rPA proteins as previously described (19). Briefly, the protein unfolding was detected by binding of the dye Sypro Orange to rPA using the fluorescence resonance energy transfer (FRET) channel, which has an excitation wavelength range of 450 to 490 nm and an emission range of 560 to 580 nm. The purified protein preparations of wild-type and six-Asp mutant rPA at 0.2 mg/ml protein in 10 mM sodium phosphate buffer (pH 7.2) containing 25 mM NaCl were mixed with Sypro Orange dye at 5× concentration. A sample volume of 30 μl/well was loaded to a 48-well clear PCR plate (Bio-Rad, Hercules, CA), and the plate was sealed using optically clear PCR sealer. Samples were analyzed by holding the temperature at 25°C for 5 min followed by a temperature increase to 85°C in 1°C increments. Fluorescence measurements were collected every 1°C after the temperature was held for 1 min, and the resulting data were recorded as a plot of relative fluorescence units (RFUs) versus temperature.
Conjugation of DNP hapten to wild-type and six-Asp mutant rPA proteins.
DNP was conjugated to purified wild-type and six-Asp mutant rPA proteins as previously described (20). Briefly, 1 mg of each of the purified rPA proteins was incubated with 10 mg of DNBS in 1 ml of 0.14 M K2CO3 (pH 8.0) overnight at 25°C. After incubation, the conjugate complexes were dialyzed extensively against 10 mM sodium phosphate buffer (pH 7.2). The conjugation ratios were estimated based on the absorbance of the conjugates at 360 nm using a molar extinction coefficient of 17,500 for DNP. the DNP-conjugated wild-type (wild-type rPA-DNP) and six-Asp mutant rPA (six-Asp mutant rPA-DNP) proteins with similar conjugation ratios were used for the mouse immunization studies.
Preparation of rPA-Alhydrogel formulations and immunization studies.
All of the rPA-Alhydrogel formulations used for mouse immunization studies were prepared using either unconjugated or DNP-conjugated wild-type and six-Asp mutant rPA purified proteins at a 50 μg/ml concentration with Alhydrogel equivalent to 1.5 mg/ml of aluminum in normal saline solution (0.9% NaCl [wt/vol]). The formulation mixture was gently vortexed and allowed to stand for 1 h at room temperature (RT) for adsorption. All mouse immunizations and serum collections were carried out by Cocalico Biologicals, Inc. (Reamstown, PA) in compliance with the guidelines of its Institutional Animal Care and Use Committee. Mice were immunized once intraperitoneally with rPA-Alhydrogel formulations. Groups of 10 or 20 mice (6-week-old female CD-1 mice) were immunized with freshly prepared rPA-Alhydrogel formulations (equivalent to 10 μg of rPA/mouse). Control groups of 10 mice were simultaneously immunized with either Alhydrogel or DNP-Alhydrogel (2 μg of DNP in Alhydrogel equivalent to 1.5 mg/ml of aluminum in normal saline solution). This concentration of DNP with Alhydrogel provided 2-fold more DNP for immunization than the total DNP molecules in the DNP-labeled rPA preparations used for immunization. Mice were bled 28 days postimmunization.
Mouse immunization studies for wild-type and six-Asp mutant rPA proteins without Alhydrogel were also performed in a fashion similar to that described above. In these studies, groups of 20 mice were immunized with 15 μg of wild-type and six-Asp mutant rPA purified proteins in normal saline without any Alhydrogel. The serum samples collected from the different groups of mice were analyzed individually using the toxin-neutralizing antibody (TNA) assay or DNP ELISA, as appropriate.
TNA assays.
Sera from mouse immunization studies were analyzed by the TNA assay using J774A.1 cells essentially as described previously (21). Neutralization of LT cytotoxicity was measured by assessing the cell viability with 2-fold serial dilutions of the test serum and a reference rabbit polyclonal serum (NR-3839) as described earlier (22). A four-parameter logistic regression model was used to fit the data points generated when the optical density was plotted versus the reciprocal of the serum dilution. The inflection point, which indicates 50% neutralization, was reported as the 50% effective dose (ED50) (23).
For the homologous TNA and heterologous TNA assays, serum samples obtained from a group of mice immunized with wild-type rPA protein were tested in a TNA assay that utilized LT prepared either using wild-type rPA plus LF (homologous TNA) or six-Asp mutant rPA plus LF (heterologous TNA). Similarly, the serum samples obtained from a group of mice immunized with six-Asp mutant rPA were tested in a TNA assay that utilized LT prepared either using six-Asp mutant rPA plus LF (homologous TNA) or wild-type rPA plus LF (heterologous TNA).
DNP ELISA.
The presence of antibodies to DNP in mouse sera was measured by ELISA essentially as described previously (22). Briefly, microtiter plates were coated with DNP-BSA (100 ng/well) in phosphate-buffered saline (PBS) (pH 7.4) overnight at 4°C. Plates were washed three times with 300 μl of wash buffer (PBS [pH 7.4] with 0.05% Tween 20). Individual serum samples from different groups of immunized mice were initially diluted to 1:12.5 in 5% skim milk in PBS with 0.1% Tween 20 and then serially diluted 4-fold in a separate plate. The diluted serum samples (100 μl) were transferred to the ELISA plate and incubated for 1 h at 37°C. After incubation, the plates were washed, and 100 μl of horseradish peroxidase-labeled anti-mouse IgG(H+L) (KPL, Gaithersburg, MD) was added to each well at a 1:4,000 dilution. After 1 h of incubation at 37°C, the plates were washed, and 100 μl of SureBlue Reserve TMB (KPL, Gaithersburg, MD) substrate was added to each well and incubated for 30 min at 37°C. Color development was stopped by the addition of TMB stop solution. The absorbance at 450 nm was measured. A four-parameter logistic regression model was used to fit the data points generated when the optical density was plotted versus the reciprocal of the serum dilution. The inflection point of the curve, which indicates 50% response, was reported as the antibody titer.
Statistical analyses.
Statistical analyses were conducted using GraphPad Prism software (version 5; GraphPad Software, La Jolla, CA). For the TNA assay, all nonresponder mice were assigned an ED50 value of 18 (1/2 the limit of quantitation of the TNA assay).
RESULTS
Previously, using a genetically deamidated six-Asp mutant rPA, we demonstrated that deamidation of Asn residues to Asp residues did not affect the biological activity of the six-Asp mutant rPA vaccine but did adversely affect its immunogenicity compared to that of wild-type rPA vaccine (8). In this study, we used biochemical and immunological approaches to better understand the mechanism(s) responsible for the adverse effect of deamidation on the immunogenicity of rPA. Because deamidation results in the introduction of additional charged residues into the protein as well as changes in the amino acid sequence, we hypothesized that deamidation might alter the interaction of the protein with the vaccine adjuvant, change critical B-cell epitopes, and/or alter the T-cell response induced by the protein. In this study, we examined each of these possibilities.
Is the observed decrease in immunogenicity upon deamidation due to changes in the interaction of the protein with the adjuvant?
Because Asp residues are negatively charged and Asn residues carry no net charge, six-Asp mutant rPA has a calculated isoelectric point of 5.2, while that of wild-type rPA is 5.6 (http://isoelectric.ovh.org). Alhydrogel is positively charged at a pH below 11 and therefore will bind the negatively charged rPA proteins. Because of the differences in the pI values of the two rPA proteins, it is possible that the two proteins might bind to Alhydrogel with different strengths or even with different conformations, potentially affecting the immunogenicity. In order to test whether such potential differences might account for the lower immunogenicity that we observed for the adsorbed six-Asp mutant rPA than for the adsorbed wild-type rPA, we examined the immunogenicity of wild-type rPA and six-Asp mutant rPA that had not been adsorbed to Alhydrogel.
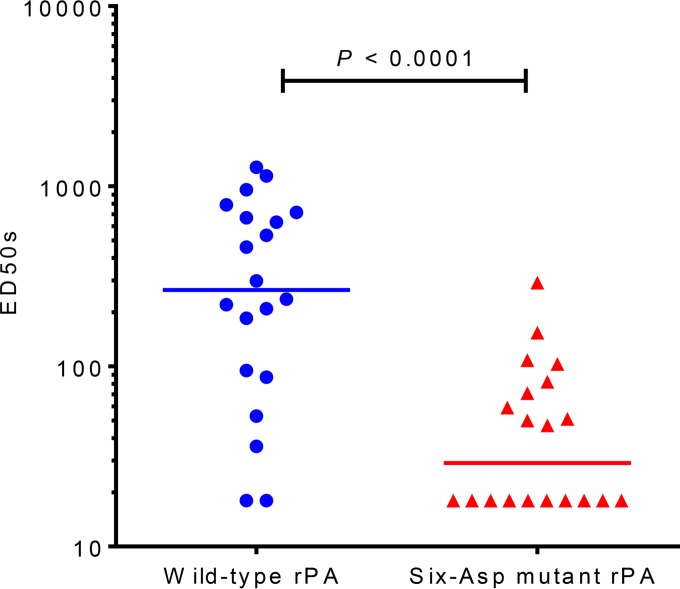
The TNA levels in sera from mice that had been immunized with either wild-type rPA or six-Asp mutant rPA without adjuvant were determined. As shown in Fig. 1, the TNA titers for both proteins were significantly lower than those previously observed with the adsorbed proteins (8), as expected since the adjuvant would increase the immunogenicity of the protein. We found that even in the absence of adsorption to the aluminum hydroxide adjuvant, differences in the immunogenicities of the two proteins were observed. Similar to the results obtained with the adsorbed proteins, the TNA titers of mice immunized with wild-type rPA protein were significantly higher (P < 0.0001; Mann-Whitney test) than those of mice immunized with the six-Asp mutant rPA protein. These data indicate that differences in the interaction of the proteins with the adjuvant would not explain the difference in immunogenicity observed between wild-type rPA and six-Asp mutant rPA.
FIG 1.
TNA titers of mice immunized with wild-type and six-Asp mutant rPA purified proteins without Alhydrogel adjuvant. Two groups of 20 mice each were immunized with either wild-type or six-Asp mutant rPA purified proteins without adjuvant (15 μg of rPA/mouse). At 28 days postimmunization, mice were bled, and serum samples were analyzed using the TNA assay. The graph shows TNA titers (expressed as ED50s) for each mouse. The horizontal lines represent the medians for the groups. A nonparametric Mann-Whitney test was performed to calculate the statistical significance using GraphPad Prism software. The P value is indicated. The median titer value for the wild-type rPA group was 234 and that for the six-Asp mutant rPA was 39. This figure is a representative of three independent immunization studies.
Does deamidation result in loss of immunodominant epitopes on rPA?
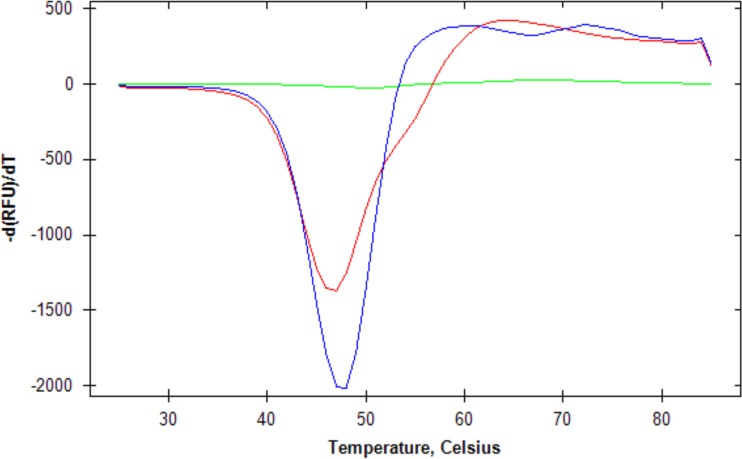
Our previous findings that wild-type rPA and six-Asp rPA have the same level of biological activity and that the far UV circular dichroism (CD) spectra of the two proteins are almost identical (8) indicate that the overall structures of the two proteins likely do not differ greatly. To further support this conclusion, we used a fluorescent dye binding assay to determine the melting temperature (i.e., the temperature at which the protein unfolds) of both wild-type rPA and six-Asp mutant rPA. We found that the melting temperatures of the two proteins are similar (48°C for wild-type rPA and 47°C for six-Asp mutant rPA) (Fig. 2). Since the thermal stability of a protein is a reflection of its structure, these results further confirm that any differences in the tertiary structures of wild-type rPA and six-Asp mutant rPA are likely to be relatively minor in nature.
FIG 2.
Melting temperatures for wild-type rPA (blue), six-Asp mutant rPA (red), and dye only (green). The melting temperatures were determined as described in Materials and Methods. The graph shows the negative first derivative of the relative fluorescence units (RFUs) as a function of temperature. The minimum of each curve represents the melting temperature.
While the tertiary structures of the two proteins appear to be similar, substitution of six Asp residues for Asn residues in wild-type rPA does change the primary structure of the protein and might result in differences in the immunodominant neutralizing B-cell epitopes between the two proteins. If that occurred, then one would expect that sera from mice immunized with wild-type rPA would neutralize LT composed of LF plus the homologous wild-type rPA better than LT composed of LF plus the heterologous six-Asp rPA since the homologous toxin would have the same immunodominant epitope structure as that of the immunogen, but the heterologous toxin would not. Likewise, sera from mice immunized with six-Asp mutant rPA would be predicted to neutralize LT composed of LF plus the homologous six-Asp mutant rPA more efficiently than LF plus the heterologous wild-type rPA. However, if critical epitopes were not altered by the substitution of the six Asn residues by Asp residues, then we would expect that sera from mice immunized with wild-type rPA would neutralize both the homologous and heterologous toxins equally well. Likewise, sera from mice immunized with the six-Asp mutant rPA would be expected to neutralize the homologous and heterologous toxins equally well. In order to test this, we conducted the study depicted schematically in Fig. 3A, in which sera from mice immunized with either the wild-type rPA-Alhydrogel or six-Asp mutant rPA-Alhydrogel formulations were tested for their abilities to neutralize both the homologous and the heterologous toxins. The results are shown in Fig. 3B. Sera from mice immunized with the wild-type rPA vaccine were able to neutralize both the homologous and the heterologous toxins equally well. Similarly, sera from mice immunized with the six-Asp mutant rPA vaccine were able to neutralize the homologous and heterologous toxins equally well. Taken together, these results suggest that alterations of deamidation-prone Asn residues to Asp residues did not result in alteration of immunodominant B-cell epitopes that are critical for the induction of toxin-neutralizing antibodies.
FIG 3.
Ability of sera from mice immunized with either wild-type rPA vaccine or six-Asp mutant rPA vaccine to neutralize the homologous and heterologous toxins. (A) A schematic diagram of the experimental design is shown. (B) Two groups of 20 mice each were immunized with either freshly prepared wild-type or six-Asp mutant rPA Alhydrogel formulations (10 μg of rPA/mouse). At 28 days postimmunization, mice were bled, and serum samples were analyzed in a TNA assay which utilized either the homologous or the heterologous toxin as described in Materials and Methods. The graph shows the TNA titers (expressed as ED50s) for each mouse. The x axis indicates the type of rPA used to prepare the LT used in the TNA assay. The horizontal lines represent the medians for the groups. A nonparametric Mann-Whitney test was performed to calculate the statistical significance using GraphPad Prism software. The P values are indicated. This figure is representative of three independent immunization studies. Abs, antibodies; rLF, recombinant lethal factor.
Does deamidation affect the ability of rPA to induce T-cell help for antibody production?
The results described above suggest that immunodominant B-cell epitopes are not significantly altered upon rPA deamidation. Another possible contributor to the lower immunogenicity observed with six-Asp rPA mutant than with wild-type rPA might be differences in the T-cell response to the antigen, possibly due to alterations in antigen processing and/or antigen presentation to T cells. Altered processing and/or presentation of T-cell epitope(s) might result in less effective T-cell help for the B-cell antibody response.
To explore this possibility, we conjugated the hapten DNP to both the wild-type and six-Asp mutant rPA proteins to assess and compare the capabilities of these rPA proteins to act as carrier proteins that provide T-cell help for the generation of anti-DNP antibodies. Haptens are small chemical molecules and cannot stimulate antibody responses by themselves. However, when coupled to a carrier protein, a hapten-specific antibody response can be achieved since the carrier protein-hapten conjugate will activate primary T cells through peptides derived from the carrier protein, thus providing the T-cell help that is needed for generation of the antibody response (24, 25). To perform these experiments, we conjugated the hapten DNP to both the wild-type and six-Asp mutant rPA proteins (wild-type rPA-DNP and six-Asp mutant rPA-DNP) such that each protein was modified to the same extent (9.5 DNP molecules/wild-type rPA molecule and 10.0 DNP molecules/six-Asp mutant rPA molecule, respectively). Groups of mice were then immunized with each of these haptenated rPA proteins that had been adsorbed to Alhydrogel. Sera from the immunized mice were then tested by ELISA for anti-DNP antibodies. As shown in Fig. 4, sera from mice immunized with wild-type rPA-DNP exhibited significantly higher anti-DNP antibody titers (P = 0.017; unpaired t test) than mice immunized with six-Asp mutant rPA-DNP. The reduced anti-DNP antibody response observed with six-Asp mutant rPA-DNP suggests that the six-Asp mutant rPA carrier protein is less effective in eliciting a primary T-cell response than the wild-type rPA carrier protein. Sera from mice immunized with DNP plus Alhydrogel did not exhibit detectable levels of anti-DNP antibodies (data not shown), as expected since the hapten alone would not be expected to elicit an antibody response.
FIG 4.
DNP ELISA titers of mice immunized with wild-type and six-Asp mutant rPA conjugated to DNP. Two groups of 20 mice each were immunized with freshly prepared wild-type rPA-DNP (9.5 DNP molecules/rPA molecule) or six-Asp mutant rPA-DNP (10.0 DNP molecules/rPA molecule) Alhydrogel formulations (10 μg of rPA conjugated to DNP/mouse). At 28 days postimmunization, mice were bled, and serum samples were analyzed by ELISA for anti-DNP antibody titers as described in Materials and Methods. The graph shows the anti-DNP antibody titer for each mouse. The horizontal lines represent the geometric mean titer (GMT) with 95% confidence interval (CI) for each group. The GMT for the wild-type rPA-DNP group was 3,475 (95% CI, 1,552 to 7,780); the GMT for the six-Asp mutant rPA-DNP group was 1,069 (95% CI, 601 to 1,896). An unpaired t test was performed to calculate the statistical significance using GraphPad Prism software. The P value is indicated. This figure is a representative of two independent immunization studies.
DISCUSSION
Over the past decade, considerable amounts of effort and resources have gone into developing new-generation anthrax vaccines and in understanding the mechanism of protection of these vaccines. Data from a number of studies suggest that PA-based anthrax vaccines can protect against disease through an antibody-mediated mechanism and that anti-PA antibodies, especially toxin-neutralizing antibodies, correlate with protection (4, 26, 27). For that reason, the antibody response generated by a PA-based vaccine is critical, and the vaccine should be able to elicit the same antibody response at the end of its dating period as that generated immediately after its manufacture.
Our previous findings (8) as well as those of others (9) suggest that deamidation of rPA upon storage of a vaccine decreases the anti-PA antibody response elicited by that vaccine and thus would be expected to decrease the protective capacity of the vaccine. In this study, we sought to better understand the mechanisms underlying this decrease in antibody response since such knowledge would inform development of strategies that will prevent or circumvent this problem. We examined three possible mechanisms by which deamidation might decrease the immunogenicity of rPA vaccines: (i) changes in the interaction of the rPA protein with the vaccine adjuvant, (ii) changes in immunodominant B-cell epitopes, and (iii) changes in the ability of the rPA to induce T-cell help to elicit an antibody response.
We first investigated whether differences in interactions of the antigen with the adjuvant could account for the lower immunogenicity of the genetically deamidated rPA protein. Previously, others have reported an inverse correlation between the strength of the interaction of an antigen with an aluminum adjuvant and its ability to elicit an antibody response (15). PA has been determined to bind to an aluminum hydroxide adjuvant primarily through electrostatic interactions (28). Deamidation of rPA increases the net negative charge of the protein and, as such, lowers the pI. Thus, the deamidated form of the protein would be expected to bind to the positively charged Alhydrogel more tightly than wild-type rPA, which might decrease its immunogenicity. In order to determine whether changes in the interactions of the antigen with the adjuvant might be the cause of the lower immunogenicity we observed with the genetically deamidated mutant, we examined the immunogenicity of wild-type and six-Asp mutant rPA that had not been adsorbed to the aluminum adjuvant. While the immunogenicity of both proteins was lower than that observed with the adjuvant, lower immunogenicity of six-Asp mutant rPA than of wild-type rPA was still observed. Thus, a difference in the adsorption of the two protein forms to the adjuvant or the strength of that interaction was not the cause of the decreased immunogenicity of the six-Asp mutant rPA.
We also investigated whether changes in B-cell epitopes that are capable of eliciting a strong neutralizing antibody response might be the cause of the lower immunogenicity of the genetically deamidated mutant. In our routine TNA assay, the abilities of antibodies elicited by rPA or rPA variants to neutralize wild-type toxin are measured. If critical B-cell epitopes were altered by the deamidation of rPA, the antibody repertoire elicited by the deamidated rPA would be expected to differ. If the epitopes that were altered were immunodominant, then the resulting antibody repertoire would likely show diminished neutralization of the wild-type toxin since the epitopes that elicited the antibody response would have different primary structures than those of the toxin that is to be neutralized. However, we found that sera from mice immunized with the wild-type rPA vaccine neutralized both the homologous (wild-type rPA plus LF) and heterologous (six-Asp mutant rPA plus LF) toxins equally well and that sera from mice immunized with the six-Asp mutant rPA vaccine neutralized its homologous (six-Asp mutant rPA plus LF) and heterologous (wild-type rPA plus LF) toxins equally well. If the immunodominant epitopes were different in the two proteins, then one would expect that the antibodies would bind to epitopes of the homologous toxin better than the heterologous toxin and therefore be more efficient in neutralizing the homologous toxin. Thus, our results suggest that deamidation does not alter B-cell epitopes to the extent that the neutralizing antibody repertoire that is elicited by the deamidated protein is substantially changed.
In contrast to our findings concerning the lack of a role of the adjuvant or the lack of effect of deamidation on the immunodominant epitopes of rPA, we did find that deamidation diminished the ability of rPA to elicit T-cell help for antibody production since six-Asp mutant rPA when coupled to DNP was less able to elicit antibodies to the hapten than was wild-type rPA when coupled to an equal amount of DNP. In the case of the rPA vaccine, T-cell help for antibody production would be elicited when the rPA is taken up by antigen-presenting cells and processed, and peptides from the processed rPA are presented to cognate T cells by major histocompatibility complex (MHC) class II molecules. Changes in the primary structure of rPA due to deamidation might affect antigen processing and presentation by several different mechanisms. First, a change from Asn to Asp or isoAsp due to deamidation, if such an amino acid were located within a critical T-cell epitope, might alter the ability of the epitope to interact with and activate T cells. Alternatively, changes in the amino acid sequence of rPA due to deamidation might affect antigen processing by altering critical target sites for the proteases involved in antigen processing. Such a phenomenon has been described for tetanus toxin fragment C (12). Those authors showed that deamidation of Asn residues interfered with processing of the protein, which affected generation of a T-cell response as assessed using an in vitro assay. Another possible mechanism for diminished T-cell help due to deamidation might be changes in the efficiency of antigen uptake by antigen-presenting cells.
The results described here provide insight into possible mechanisms by which deamidation of rPA diminishes immunogenicity of the protein. Our results suggest that while the overall structure of the protein is not substantially affected by deamidation, local changes in amino acid sequence (i.e., Asn→Asp or isoAsp) appear to affect the ability of rPA to generate T-cell help for antibody production. This knowledge should be useful in the development of strategies to minimize or circumvent the loss of immunogenicity of rPA due to spontaneous deamidation.
ACKNOWLEDGMENTS
The following reagents were obtained from the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: anthrax PA, recombinant from B. anthracis, NR-140; anthrax LF, recombinant from B. anthracis, NR-142; rabbit anti-PA reference serum pool, NR-3839; and J774A.1 monocyte/macrophage (mouse) working cell bank, NR-28.
We thank Ashley Chung for assistance in protein purification.
This work was supported by the Intramural Research Program of the Center for Biologics Evaluation and Research, Food and Drug Administration.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Moayeri M, Leppla SH. 2009. Cellular and systemic effects of anthrax lethal toxin and edema toxin. Mol Aspects Med 30:439–455. doi: 10.1016/j.mam.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young JA, Collier RJ. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem 76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 3.Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH. 1995. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun 63:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fay MP, Follmann DA, Lynn F, Schiffer JM, Stark GV, Kohberger R, Quinn CP, Nuzum EO. 2012. Anthrax vaccine-induced antibodies provide cross-species prediction of survival to aerosol challenge. Sci Transl Med 4:151ra126. doi: 10.1126/scitranslmed.3004073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitt ML, Little SF, Ivins BE, Fellows P, Barth J, Hewetson J, Gibbs P, Dertzbaugh M, Friedlander AM. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax. Vaccine 19:4768–4773. doi: 10.1016/S0264-410X(01)00234-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Schiffer JM, Dalton S, Sabourin CL, Niemuth NA, Plikaytis BD, Quinn CP. 2014. Comprehensive analysis and selection of anthrax vaccine adsorbed immune correlates of protection in rhesus macaques. Clin Vaccine Immunol 21:1512–1520. doi: 10.1128/CVI.00469-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baillie LW. 2009. Is new always better than old?: The development of human vaccines for anthrax. Hum Vaccin 5:806–816. doi: 10.4161/hv.9777. [DOI] [PubMed] [Google Scholar]
- 8.Verma A, McNichol B, Dominguez-Castillo RI, Amador-Molina JC, Arciniega JL, Reiter K, Meade BD, Ngundi MM, Stibitz S, Burns DL. 2013. Use of site-directed mutagenesis to model the effects of spontaneous deamidation on the immunogenicity of Bacillus anthracis protective antigen. Infect Immun 81:278–284. doi: 10.1128/IAI.00863-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Souza AJ, Mar KD, Huang J, Majumdar S, Ford BM, Dyas B, Ulrich RG, Sullivan VJ. 2013. Rapid deamidation of recombinant protective antigen when adsorbed on aluminum hydroxide gel correlates with reduced potency of vaccine. J Pharm Sci 102:454–461. doi: 10.1002/jps.23422. [DOI] [PubMed] [Google Scholar]
- 10.Robinson NE, Robinson AB. 2001. Molecular clocks. Proc Natl Acad Sci U S A 98:944–949. doi: 10.1073/pnas.98.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones LS, Peek LJ, Power J, Markham A, Yazzie B, Middaugh CR. 2005. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J Biol Chem 280:13406–13414. doi: 10.1074/jbc.M500687200. [DOI] [PubMed] [Google Scholar]
- 12.Moss CX, Matthews SP, Lamont DJ, Watts C. 2005. Asparagine deamidation perturbs antigen presentation on class II major histocompatibility complex molecules. J Biol Chem 280:18498–18503. doi: 10.1074/jbc.M501241200. [DOI] [PubMed] [Google Scholar]
- 13.McAdam SN, Fleckenstein B, Rasmussen IB, Schmid DG, Sandlie I, Bogen B, Viner NJ, Sollid LM. 2001. T cell recognition of the dominant I-Ak-restricted hen egg lysozyme epitope: critical role for asparagine deamidation. J Exp Med 193:1239–1246. doi: 10.1084/jem.193.11.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tleugabulova D, Falcon V, Penton E. 1998. Evidence for the denaturation of recombinant hepatitis B surface antigen on aluminium hydroxide gel. J Chromatogr B Biomed Sci Appl 720:153–163. doi: 10.1016/S0378-4347(98)00425-3. [DOI] [PubMed] [Google Scholar]
- 15.Hansen B, Sokolovska A, HogenEsch H, Hem SL. 2007. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine 25:6618–6624. doi: 10.1016/j.vaccine.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 16.Powell BS, Enama JT, Ribot WJ, Webster W, Little S, Hoover T, Adamovicz JJ, Andrews GP. 2007. Multiple asparagine deamidation of Bacillus anthracis protective antigen causes charge isoforms whose complexity correlates with reduced biological activity. Proteins 68:458–479. doi: 10.1002/prot.21432. [DOI] [PubMed] [Google Scholar]
- 17.Ribot WJ, Powell BS, Ivins BE, Little SF, Johnson WM, Hoover TA, Norris SL, Adamovicz JJ, Friedlander AM, Andrews GP. 2006. Comparative vaccine efficacy of different isoforms of recombinant protective antigen against Bacillus anthracis spore challenge in rabbits. Vaccine 24:3469–3476. doi: 10.1016/j.vaccine.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Sellman BR, Nassi S, Collier RJ. 2001. Point mutations in anthrax protective antigen that block translocation. J Biol Chem 276:8371–8376. doi: 10.1074/jbc.M008309200. [DOI] [PubMed] [Google Scholar]
- 19.Lavinder JJ, Hari SB, Sullivan BJ, Magliery TJ. 2009. High-throughput thermal scanning: a general, rapid dye-binding thermal shift screen for protein engineering. J Am Chem Soc 131:3794–3795. doi: 10.1021/ja8049063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epps JM, Phillips JO, Mestecky J. 1988. IgA-mediated clearance and tissue deposition of dinitrophenylated human serum albumin at various DNP:HSA ratios. Mol Immunol 25:731–738. doi: 10.1016/0161-5890(88)90109-5. [DOI] [PubMed] [Google Scholar]
- 21.Omland KS, Brys A, Lansky D, Clement K, Lynn F, Participating L. 2008. Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species. Clin Vaccine Immunol 15:946–953. doi: 10.1128/CVI.00003-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner L, Verma A, Meade BD, Reiter K, Narum DL, Brady RA, Little SF, Burns DL. 2012. Structural and immunological analysis of anthrax recombinant protective antigen adsorbed to aluminum hydroxide adjuvant. Clin Vaccine Immunol 19:1465–1473. doi: 10.1128/CVI.00174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngundi MM, Meade BD, Lin TL, Tang WJ, Burns DL. 2010. Comparison of three anthrax toxin neutralization assays. Clin Vaccine Immunol 17:895–903. doi: 10.1128/CVI.00513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gefen T, Vaya J, Khatib S, Rapoport I, Lupo M, Barnea E, Admon A, Heller ED, Aizenshtein E, Pitcovski J. 2015. The effect of haptens on protein-carrier immunogenicity. Immunology 144:116–126. doi: 10.1111/imm.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchison NA. 2004. T-cell-B-cell cooperation. Nat Rev Immunol 4:308–312. doi: 10.1038/nri1334. [DOI] [PubMed] [Google Scholar]
- 26.Fellows PF, Linscott MK, Ivins BE, Pitt ML, Rossi CA, Gibbs PH, Friedlander AM. 2001. Efficacy of a human anthrax vaccine in guinea pigs, rabbits, and rhesus macaques against challenge by Bacillus anthracis isolates of diverse geographical origin. Vaccine 19:3241–3247. doi: 10.1016/S0264-410X(01)00021-4. [DOI] [PubMed] [Google Scholar]
- 27.Food and Drug Administration. 2010. November 16, 2010: Vaccines and related biological products advisory committee meeting transcript. http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/ucm237045.htm.
- 28.Jendrek S, Little SF, Hem S, Mitra G, Giardina S. 2003. Evaluation of the compatibility of a second generation recombinant anthrax vaccine with aluminum-containing adjuvants. Vaccine 21:3011–3018. doi: 10.1016/S0264-410X(03)00109-9. [DOI] [PubMed] [Google Scholar]