Abstract
Controversy exists regarding the optimal use of the 23-valent pneumococcal conjugate vaccine for the protection of high-risk individuals, such as children and adults with immunocompromising conditions and the elderly. The effectiveness and immunogenicity of 23-valent pneumococcal polysaccharide vaccine (PPV23) are limited in such high-risk populations compared to the healthy, with meta-analyses failing to provide robust evidence on vaccine efficacy against invasive pneumococcal disease (IPD) or pneumonia. Moreover, several studies have demonstrated a PPV23-induced state of immune tolerance or hyporesponsiveness to subsequent vaccination, where the response to revaccination does not reach the levels achieved with primary vaccination. The clinical significance of hyporesponsiveness is not yet clarified, but attenuated humoral and cellular response could lead to reduced levels of protection and increased susceptibility to pneumococcal disease. As disease epidemiology among high-risk groups shows that we are still in need of maximum serotype coverage, the optimal use of PPV23 in the context of combined conjugate/polysaccharide vaccine schedules is an important priority. In this minireview, we discuss PPV23-induced hyporesponsiveness and its implications in designing highly effective vaccination schedules for the optimal protection for high-risk individuals.
INTRODUCTION
Streptococcus pneumoniae (the pneumococcus) is a major cause of life-threatening invasive infections accounting for considerable morbidity and mortality worldwide (1). Children and adults with certain medical conditions as well as the elderly are at increased risk for invasive pneumococcal disease (IPD) and pneumonia, with disease rates up to 20 times higher than those in the general population (2). High-risk groups consist of children and adults with chronic diseases and with primary and secondary immune deficiencies and of persons with functional or anatomical asplenia. Persons older than 65 years of age are also at increased risk for pneumococcal infection, due to attenuation of their immune response caused by advancing age, a phenomenon called immunosenescence (3). The number of patients in need for protection against IPD is continually increasing due to rising numbers of people with chronic disease or HIV infection and an aging population in many high-income countries.
Pneumococcal disease is usually more severe in such high-risk individuals than in immunocompetent subjects (4–6). While antibiotic resistance represents an additional hurdle for the successful treatment of pneumococcal infections (7, 8), optimal protection of such “high-risk” groups against S. pneumoniae infection through vaccination continues to be an important priority.
For more than 2 decades, a 23-valent pneumococcal polysaccharide vaccine (PPV23) has been recommended for the protection of immunocompromised individuals and the elderly against invasive pneumococcal disease (IPD) and pneumonia (9).
The licensure of the pneumococcal polysaccharide vaccine (PPV) was based on trials of a 6-valent PPV and a 13-valent PPV among South African gold miners (10) and a 14-valent PPV trial in Papua New Guinean highlanders (11), which showed strong vaccine efficacy against bacteremic pneumonia. In 1983, a 23-valent formulation containing a reduced amount (25 μg) of each purified capsular polysaccharide replaced the earlier polysaccharide formulations without, however, additional prelicensure trials evaluating its efficacy against bacteremic pneumonia.
Today, two types of pneumococcal vaccines are available for high-risk individuals, with different immunological characteristics and numbers of serotypes contained in each: the 23-valent pneumococcal polysaccharide vaccine, which induces a T-independent (TI) immune response with serotype-specific antibody formation but no immune memory, and a 10-valent pneumococcal conjugated vaccine (PCV10) and a 13-valent pneumococcal conjugated vaccine (PCV13), in which pneumococcal polysaccharides are coupled with a carrier protein and induce a T-dependent (TD) immune response (12). While PPV23 induces only serotype-specific antibodies, PCV13 generates the formation of both serotype-specific antibodies and B memory cells, which are associated with a longer duration of vaccine-induced immune responses.
PCV13 replaced a 7-valent formulation (PCV7) which was launched 15 years ago in the United States and was rapidly incorporated into the national immunization programs of many countries worldwide. PCV7 had to be replaced 10 years after its introduction by PCV13 due to the increased incidence of invasive pneumococcal disease (IPD) caused by non-PCV7 serotypes. PCV13 contains most emerging serotypes, e.g., serotype 19A, and is currently recommended for at-risk individuals of all ages (13–15).
In an effort to maximize protection against the pneumococcus for high-risk individuals, a combined schedule that includes PCV13 followed by PPV23 (13–15), with an additional PPV23 booster after 5 years, is recommended. PCV13/PPV23 immunization schedules have the theoretical benefit of combining establishment of immunological memory with maximum serotype coverage. However, controversy regarding PPV23 effectiveness among high-risk individuals (16), as well as evidence of PPV23-induced immunological hyporesponsiveness (17), causes skepticism about the optimal use of polysaccharide vaccines in the context of combined vaccination schedules.
In this article, we review the issue of PPV23-induced hyporesponsiveness and its implications in designing highly effective vaccination schedules for the optimal protection for immunocompromised individuals of all ages.
PNEUMOCOCCAL SEROTYPE EPIDEMIOLOGY IN HIGH-RISK INDIVIDUALS
Extended serotype coverage has long been the strongest argument in favor of PPV23 use in high-risk individuals, although the epidemiology of serotypes causing IPD in this population is not fully known.
There are over 90 different serotypes of S. pneumoniae; some are highly invasive, whereas others rarely cause disease. Among serotypes causing disease, there are significant differences in distribution between different age groups and geographical populations (18, 19).
The surveillance of pneumococcal disease after the introduction of PCV13 indicates that, similarly to what happened after the introduction of PCV7, the epidemiology is changing, with new emerging serotypes dominating carriage and disease, although it is predicted that serotype replacement following the introduction of PCV13 will not increase significantly the burden of IPD due to the low case-to-carrier ratios demonstrated for most of the replacement serotypes (20). The added value of continuing to include PPV23 in vaccination schedules for high-risk individuals in the years to come remains to be determined by monitoring whether the replacing serotypes causing IPD are covered by the 23-valent polysaccharide vaccine. Data reported from the United Kingdom since the introduction of PCV13 show that some of the emerging serotypes causing IPD in children over 5 years of age and in adults of all ages, such as serotypes 15A and 24F, are not included in PPV23 (21). Data from the United States, however, show that the proportion of IPD caused by serotypes unique to PPV23 in the elderly reached 38% in 2013 (15). Nevertheless, since the type and invasive potential of new serotypes are not easily predictable, careful monitoring of pneumococcal disease is necessary.
PPV23 EFFECTIVENESS IN HIGH-RISK INDIVIDUALS
PPV23 effectiveness against IPD and pneumonia in high-risk individuals and the elderly, the populations most in need for protection against pneumococcal disease, remains debatable despite its extensive use for over 25 years.
A number of randomized controlled trials and observational studies have been conducted among healthy adults with chronic diseases (22–25), adults with immunocompromising conditions (26–29), and the elderly (30–35), with inconclusive results regarding vaccine effectiveness against IPD and pneumonia. Differences were mainly due to study heterogeneity regarding study methodology, size, and design and population studied (16).
Moreover, such differences from individual original studies could not be resolved in several meta-analyses, which have also produced differing conclusions regarding levels of effectiveness against IPD and pneumonia in healthy and immunocompromised individuals (36–51).
The most recent Cochrane meta-analysis demonstrated effectiveness of PPV23 against IPD for healthy adults but no protection against pneumonia and all-cause mortality (52). Moreover, a subanalysis in high-risk populations of high-income countries showed no evidence of protective vaccination efficacy against IPD and pneumonia. However, the authors stressed that this could have been due to the lack of the statistical power necessary to demonstrate significant differences between the vaccinated group and the control groups (52).
The evaluation of PPV23 effectiveness with the use of immunological correlates of protection such as vaccine immunogenicity indicates that it induces antibodies in immunocompromised subjects but that antibody levels decrease substantially shortly after vaccination and that the duration of protection is relatively short-lived (53, 54). Moreover, advancing age and comorbidities have been associated with even lower antibody responses (55). Although it is not known what level of antibody should be considered protective for such high-risk individuals, it is expected that in older subjects, who are more susceptible to pneumococcal disease, PPV23 effectiveness might be diminished.
IMMUNOLOGICAL HYPORESPONSIVENESS
In an effort to protect individuals at risk, immunization with PPV23 used to be repeated every 5 years due to the exclusively humoral nature of the response induced by PPV23 and the waning of antibody levels shortly after vaccination (56).
However, the repeated use of PPV23 required for protection of high-risk individuals has been associated with hyporesponsiveness, a phenomenon where vaccine recipients are unable to mount an immune response to revaccination which represents a greater antibody response or at least a response of the same magnitude as the primary response.
Hyporesponsiveness was first described in the 1990s following vaccination with meningococcal polysaccharide vaccines and has been attributed to the immune tolerance induced by the vaccine polysaccharide antigens (57, 58). Since then, several studies demonstrated the same phenomenon following pneumococcal polysaccharide vaccination (59–68). These studies are summarized in Table 1.
TABLE 1.
Summary of trials demonstrating PPV23-related immune hyporesponsiveness
| Study(ies) | Country | Vaccination schedule | Population | No. of patients | Summary of findings |
|---|---|---|---|---|---|
| Sigurdardottir et al. (2014) (59) | Iceland | PCV9/PCV9/PCV13 | Children 7.5 yrs old | 39 | PPV23 vaccination of toddlers attenuated the subsequent response to PCV13 |
| PCV9/PPV23/PCV13 | 50 | ||||
| Papadatou et al. (2014) (60) | Greece | PCV13 | Asplenic adults with β-thalassemia | 39 | Prior history of PPV23 vaccination attenuated the response to PCV13 in a dose- and time-dependent manner |
| 1–4 PPV23 doses given prior to the study | |||||
| Clutterbuck et al. (2012) (62); Lazarus et al. (2011) (61) | United Kingdom | PPV23-PCV7-PCV7 | Adults 50–70 yrs old | 116 | Prior vaccination with PPV23 attenuated the response to subsequent vaccination with PCV7 |
| PCV7-PPV23-PCV7 | 116 | ||||
| PCV7-PCV7-PPV23 | 116 | ||||
| Russell et al. (2010) (63) | Fiji | 1–3 PCV7 doses/mPPV23a | Infants | 276 | The response to an mPPV23 challenge was lower in children who had received PPV23 at 12 mo of age |
| 1–3 PCV7 doses/PPV23/mPPV23 | 276 | ||||
| Dransfield et al. (2009) (64) | United States | PCV7 | Adults with COPD | 57 | Prior vaccination with PPV23 reduced vaccine responsiveness to either PPV23 or PCV7 |
| PPV23 | 63 | ||||
| 1–4 PPV23 doses given prior to the study | |||||
| Orthopoulos et al. (2009) (65) | Greece | PCV7/PPV23/PPV23 | Asplenic adults with β-thalassemia | 17 | Multiple PPV23 doses induced hyporesponsiveness for some serotypes |
| PCV7/PCV7/PPV23 | 18 | ||||
| 0–3 PPV23 doses given before the study | |||||
| Musher et al. (2008) (66) | United States | PCV7/PPV23 | Adults who recovered from pneumococcal pneumonia | 37 | Prior recent (>1 yr) vaccination with PPV23 attenuated the response to PPV23 and PCV7; the PPV23/PCV7 schedule resulted in lower antibody responses that the PCV7/PPV23 schedule |
| PPV23/PCV7 | 44 | ||||
| PPV23 doses given prior to the study | |||||
| de Roux et al. (2008) (67) | Germany | PCV7/PCV7 | Adults >70 yrs old | 43 | PPV23 used as the primary dose induced hyporesponsiveness |
| PCV7/PPV23 | 38 | ||||
| PPV23/PCV7 | 78 | ||||
| Mufson et al. (1991) (68) | United States | PPV14/PPV23 | Adults 56–79 yrs old | 15 | Antibody levels after revaccination were lower than after primary vaccination |
mPPV23, small dose of PPV23.
The hyporesponsiveness effect of PPV23 in the establishment of PCV-induced immunological memory has been demonstrated in both pediatric and adult populations. In a study by Russell et al., infants who had received PPV23 12 months after PCV7 priming, after challenge with a small dose (20%) of PPV23 at 17 months, had antibody titers for all PCV7 serotypes that were significantly lower than the titers seen with those who had not received PPV23 (63). A similar study by Lazarus et al. performed in adults demonstrated immunologic hyporesponsiveness to PCV7 administered after PPV23, regardless of the different vaccination schedules used for PCV7 priming; such hyporesponsiveness was not restored by additional doses of PCV7 (61).
Interestingly, the magnitudes of hyporesponsiveness seem to differ among pneumococcal serotypes (PS), possibly due to the structural diversity of different pneumococcal antigens associated with different immunological properties, e.g., T-cell dependency and immunogenicity (65).
There is also evidence that hyporesponsiveness is not an exclusively vaccine-induced phenomenon; it has also been reported after IPD or nasopharyngeal carriage in pediatric populations. A study by Borrow et al. reported hyporesponsiveness in children with IPD who were subsequently vaccinated with PCV7 and failed to reach the protective threshold of antibodies against the pathogenic serotype (69). Furthermore, Dagan et al. demonstrated that pneumococcal nasopharyngeal carriage in early infancy shortly before vaccination with PCV7 can lead to serotype-specific hyporesponsiveness to subsequent vaccination for the carried PS, implying a mechanism of B-cell unresponsiveness following colonization (70).
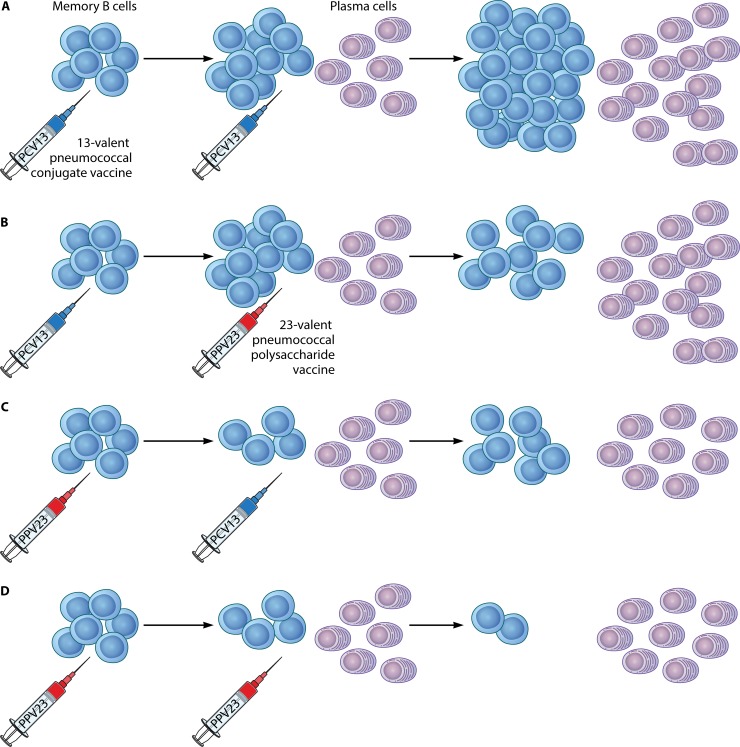
Several mechanisms, acting separately or in combination, have been suggested to be responsible for this immunological phenomenon (61–63). Polysaccharide antigens induce a T-cell-independent immune response, stimulating but not replenishing memory cells, thus resulting in overall depletion of the memory cell pool and attenuated responses on reexposure to the same PS. We have previously shown that, in contrast to vaccination with PCV13, which resulted in an increase of levels of antigen-specific memory B cells (MBCs), in splenectomized subjects with β-thalassemia, a history of previous vaccinations with PPV23 had an attenuating effect on antigen-specific MBC pool levels (60). Our findings are in accordance with recent data from studies in murine models as well as in human lymphocytes which have shown that, upon secondary challenge with a T-independent antigen, such as the antigens contained in the PPV23 vaccine, IgM and IgG memory B cells differentiate terminally toward plasma cells. In contrast, rechallenge with a TD antigen, such as the PCV13 vaccine, leads IgM memory B cells to reenter germinal center (GC) reactions and promote the enrichment of antigen-specific immunological memory (71–73). The proposed mechanisms for the effect of PPV23 on the antigen-specific memory B-cell pool and in relation to previous or subsequent vaccination with a pneumococcal conjugate vaccine are illustrated in Fig. 1.
FIG 1.
Priming and boosting with the 23-valent pneumococcal polysaccharide vaccine (PPV23) or the 13-valent pneumococcal conjugate vaccine (PCV13) and the effect on the pool of antigen-specific memory B cells (MBCs): a mechanism for immune hyporesponsiveness. (A) Priming and boosting with PCV13 result in incremental increases in the numbers of pooled MBCs. (B) Priming with PCV13 enriches the MBC pool, but a subsequent PPV23 booster depletes it. (C) Primary vaccination with PPV23 results in decreased numbers of antigen-specific MBCs. Subsequent vaccination with PCV13 enriches the depleted MBC pool. (D) Repeated immunizations with PPV23 lead to gradual depletion of the MBC pool.
It is also been suggested that the response to secondary vaccination could be attenuated by large amounts of persisting circulating antigens, which can continuously bind to naive B cells and neutralize polysaccharide-specific antibody, thus resulting in long-lasting hyporesponsiveness (74).
Finally, an immunosuppressive mechanism involving pneumococcal polysaccharide-induced interleukin-10 (IL-10) production by dendritic cells (DCs) in vitro has also been associated with downstream effects on regulatory T lymphocytes (Tregs) and antigen-specific antibody responses (75).
The magnitude of hyporesponsiveness seems to be related to the number of doses and the intervals between subsequent vaccinations. We have recently shown that multiple (>2) PPV23 doses and shorter intervals between vaccination associated with high levels of circulating antibodies have a greater impact on hyporesponsiveness (60). The negative impact of high concentrations of circulating antibodies (76) and plasma cells (77) on GC reactions has also been demonstrated in animal studies.
Interestingly, there are findings implying not only attenuated protection but possibly an increased risk for IPD in individuals with severe immunodeficiency induced by the use of polysaccharide vaccines. Increased rates of all-cause pneumonia have been demonstrated among HIV-infected PPV23 recipients in Uganda compared to unvaccinated patients (78). Although the clinical significance of hyporesponsiveness in high-risk individuals is not yet clarified, taking such concerns into account, updated Paediatric European Network for Treatment of AIDS (PENTA) guidelines do not recommend PPV23 for use in HIV-infected children (79).
DO CURRENT GUIDELINES TAKE INTO ACCOUNT PPV23-INDUCED HYPORESPONSIVENESS?
In light of the evidence of the PPV23-related immune hyporesponsiveness, guidelines for the protection of those at risk have been updated and currently PCV13 is recommended for protection of high-risk individuals of all ages. PPV23 is still in use for broader serotype coverage, but, in contrast to previous practices, repeated doses have been abandoned, and administration of a maximum of two doses is now recommended for the majority of high-risk patients (13–15).
In the currently recommended PCV13/PPV23 combined schedule, administration of the conjugate vaccine precedes administration of the polysaccharide, in order for PCV13 to establish immunological memory for the 13 serotypes that it contains and for PPV23 to subsequently induce antibody responses to the 12 additional serotypes. However, there are accumulating data indicating that PPV23 could attenuate the immunological memory induced by conjugate vaccines even when it is given soon after PCV13. In a recent study by Bjarnarson et al., where neonatal mice were immunized with a pneumococcal conjugate vaccine followed shortly by a polysaccharide booster, the booster with plain pneumococcal polysaccharide caused abrogation of conjugate-induced reactions of germinal centers and depletion of antigen-specific antibody-secreting cells that had been created in response to the conjugate primary immunization (80). Similarly, in a study by Clutterbuck et al., a decrease in the number of memory B cells was recorded when PPV23 was administered after one or two doses of PCV7 (62).
Although these data suggest that PPV23 has a depleting effect on PCV13-induced immunological memory even when given after PCV13, there is evidence that the PPV23-inflicted attenuation of immune memory might be more significant when PPV23 is given before PCV13 (81).
It is thought that hyporesponsiveness induced by the PPV23/PCV13 schedule is caused by the combined effect of two different mechanisms, i.e., (i) the direct depletion of antigen-specific MBCs by the polysaccharide antigens and (ii) the large amounts of plasma cells and antibodies that are produced in response to PPV23, which could also block the stratification of naive B cells to germinal centers (GC) in response to subsequent PCV13 by a negative-feedback mechanism (76).
Unfortunately, current guidelines do not take into consideration that hyporesponsiveness is a time-dependent phenomenon and that conjugate vaccine-induced memory is affected more significantly when PCV13 is given shortly after PPV23. This is of high importance to the majority of high-risk individuals today, as most would have already been vaccinated with at least one dose of PPV23 in the past, in accordance with previous recommendations. Short intervals between PPV23 vaccination and subsequent PCV13 vaccination for PPV23-experienced individuals are still allowed in the current guidelines; for children previously immunized with PPV23, it is recommended that a single PCV13 dose be given ≥8 weeks after the last PPV23 dose (14), whereas administration of a dose of PCV13 ≥1 year after the most recent PPV23 dose is recommended for immunocompromised adults and the elderly (81).
Such short intervals are expected to lead to suboptimal induction of immunity to vaccine antigens among the members of the populations most at risk, as the ability of PCV13 to induce a strong immunological memory is expected to be attenuated by the recent immunization with the polysaccharide vaccine. Evidence from studies performed with different intervals between PCV13 vaccination and subsequent PPV23 vaccination shows that the overall antibody response seen with a 3-to-4-year interval is superior to that seen with a 1-year interval (81). Such findings could be explained by the waning of circulating antibodies with time and the recharging of the B-cell pool by natural exposure to pneumococcal and cross-reacting antigens (60).
We have recently shown that PPV23 vaccination can affect immune responses to subsequent PCV13 vaccination in asplenic adults for as long as 6 years (82); we therefore propose that measurement of levels of anti-pneumococcus antibodies before vaccination could prove beneficial in optimizing intervals between vaccinations for such individuals. In the presence of high antibody levels, subsequent pneumococcal vaccination could be postponed, in order to maximize the potential of the immune response to future vaccination in terms of magnitude and longevity.
CONCLUSIONS
Significant progress has been achieved in recent years in incorporating accumulating research regarding PPV23-induced hyporesponsiveness and vaccine efficacy into clinical practice. The guidelines for the immunization of individuals at high risk of pneumococcus infection have significantly changed in the last 5 years, replacing the use of PPV23 alone with combined PCV13/PPV23 schedules for adults with immunocompromising conditions and introducing PCV13 into vaccination schedules of the elderly.
However, vaccination guidelines for high-risk individuals should be revised in order to maximize PCV13-induced immunological memory and long-term vaccine effectiveness. To that end, reassessment of pneumococcal vaccination policy with a special focus on the intervals between vaccinations in the context of combined conjugate/polysaccharide vaccine schedules is an important priority. Ongoing research on the mechanisms of establishment and maintenance of immunological memory is essential, and clinical trials on PPV23 effectiveness in high-risk populations are necessary in order to optimize vaccine protection against pneumococcal disease for individuals at risk.
ACKNOWLEDGMENT
We thank David Goldblatt for the constructive criticism of the manuscript.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.CDC. 2011. Active Bacterial Core surveillance report, Emerging Infections Program Network, Streptococcus pneumoniae, 2010. CDC, Atlanta, GA: http://www.cdc.gov/abcs/reports-findings/survreports/spneu10-orig.html. [Google Scholar]
- 2.Bisharat N, Omari H, Lavi I, Raz R. 2001. Risk of infection and death among post-splenectomy patients. J Infect 43:182–186. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- 3.Goronzy JJ, Weyand CM. 2013. Understanding immunosenescence to improve responses to vaccines. Nat Immunol 14:428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilks CF, Ojoo SA, Ojoo JC, Brindle RJ, Paul J, Batchelor BI, Kimari JN, Newnham R, Bwayo J, Plummer FA. 1996. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet 347:718–723. doi: 10.1016/S0140-6736(96)90076-8. [DOI] [PubMed] [Google Scholar]
- 5.Klugman KP, Madhi SA, Feldman C. 2007. HIV and pneumococcal disease. Curr Opin Infect Dis 20:11–15. doi: 10.1097/QCO.0b013e328012c5f1. [DOI] [PubMed] [Google Scholar]
- 6.Shatz DV. 2005. Vaccination considerations in the asplenic patient. Expert Rev Vaccines 4:27–34. doi: 10.1586/14760584.4.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Reacher MH, Shah A, Livermore DM, Wale MC, Graham C, Johnson AP, Heine H, Monnickendam MA, Barker KF, James D, George RC. 2000. Bacteraemia and antibiotic resistance of its pathogens reported in England and Wales between 1990 and 1998: trend analysis. BMJ 320:213–216. doi: 10.1136/bmj.320.7229.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasz A. 1995. The pneumococcus at the gates. N Engl J Med 333:514–515. doi: 10.1056/NEJM199508243330810. [DOI] [PubMed] [Google Scholar]
- 9.CDC. 2010. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPV23). MMWR Morb Mortal Wkly Rep 59:1102–1106. [PubMed] [Google Scholar]
- 10.Austrian R, Douglas RM, Schiffman G, Coetzee AM, Koornhof HJ, Hayden-Smith S, Reid RD. 1976. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians 89:184–194. [PubMed] [Google Scholar]
- 11.Riley ID, Tarr PI, Andrews M, Pfeiffer M, Howard R, Challands P, Jennison G. 1977. Immunization with polyvalent pneumococcal vaccine. Reduction of adult respiratory mortality in a New Guinea Highlands community. Lancet i:1338–1341. [DOI] [PubMed] [Google Scholar]
- 12.Stein KE. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis 165:S49–S52. doi: 10.1093/infdis/165-Supplement_1-S49. [DOI] [PubMed] [Google Scholar]
- 13.CDC. 2012. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 61:816–819. [PubMed] [Google Scholar]
- 14.CDC. 2013. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6–18 years with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 62:521–524. [PMC free article] [PubMed] [Google Scholar]
- 15.Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T; Centers for Disease Control and Prevention (CDC). 2014. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 16.Huss A, Scott P, Stuck AE, Trotter C, Egger M. 2009. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poolman J, Borrow R. 2011. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines 10:307–322. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
- 18.Fedson D, Liss C. 2004. Precise answers to the wrong question: prospective clinical trials and the meta-analysis of pneumococcal vaccine in elderly and high risk adults. Vaccine 22:927–946. doi: 10.1016/j.vaccine.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Butler J. 2004. Epidemiology of pneumococcal disease, p 148–68. In Tuomanen E, Mitchell T, Morrison D, Spratt B (ed), The pneumococcus, 1st ed, vol 10 American Society for Microbiology, Washington, DC. [Google Scholar]
- 20.Hanquet G, Kissling E, Fenoll A, George R, Lepoutre A, Lernout T, Tarragó D, Varon E, Verhaegen J. 2010. Pneumococcal serotypes in children in 4 European countries. Emerg Infect Dis 16:1428–1439. doi: 10.3201/eid1609.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. 2015. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 15:535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 22.Alfageme I, Vazquez R, Reyes N, Muñoz J, Fernández A, Hernandez M, Merino M, Perez J, Lima J. 2006. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 61:189–195. doi: 10.1136/thx.2005.043323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furumoto A, Ohkusa Y, Chen M, Kawakami K, Masaki H, Sueyasu Y, Iwanaga T, Aizawa H, Nagatake T, Oishi K. 2008. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine 26:4284–4289. doi: 10.1016/j.vaccine.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Davis AL, Aranda CP, Schiffman G, Christianson LC. 1987. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest 92:204–212. [DOI] [PubMed] [Google Scholar]
- 25.Leech JA, Gervais A, Ruben FL. 1987. Efficacy of pneumococcal vaccine in severe chronic obstructive pulmonary disease. CMAJ 136:361–365. [PMC free article] [PubMed] [Google Scholar]
- 26.Benin AL, O'Brien KL, Watt JP, Reid R, Zell ER, Katz S, Donaldson C, Parkinson A, Schuchat A, Santosham M, Whitney CG. 2003. Effectiveness of the 23-valent polysaccharide vaccine against invasive pneumococcal disease in Navajo adults. J Infect Dis 188:81–89. doi: 10.1086/375782. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, Adair RK, Clemens JD. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 325:1453–1460. doi: 10.1056/NEJM199111213252101. [DOI] [PubMed] [Google Scholar]
- 28.Simberkoff MS, Cross AP, Al-Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, Richmond AS, Smith RP, Schiffman G, Shepard DS, Van Eeckhout J. 1986. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study. N Engl J Med 315:1318–1327. doi: 10.1056/NEJM198611203152104. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro E, Clemens J. 1984. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann Intern Med 101:325–330. doi: 10.7326/0003-4819-101-3-325. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami K, Ohkusa Y, Kuroki R, Tanaka T, Koyama K, Harada Y, Iwanaga K, Yamaryo T, Oishi K. 2010. Effectiveness of pneumococcal polysaccharide vaccine against pneumonia and cost analysis for the elderly who receive seasonal influenza vaccine in Japan. Vaccine 28:7063–7069. doi: 10.1016/j.vaccine.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama T, Taguchi O, Niederman MS, Morser J, Kobayashi H, Kobayashi T, D'Alessandro-Gabazza C, Nakayama S, Nishikubo K, Noguchi T, Takei Y, Gabazza EC. 2010. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 340:c1004. doi: 10.1136/bmj.c1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Domínguez A, Izquierdo C, Salleras L, Ruiz L, Sousa D, Bayas JM, Nebot M, Varona W, Celorrio JM, Carratalà J; Working Group for the Study of Prevention of CAP in the Elderly. 2010. Effectiveness of the pneumococcal polysaccharide vaccine in preventing pneumonia in the elderly. Eur Respir J 36:608–614. doi: 10.1183/09031936.00171309. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez A, Salleras L, Fedson DS, Izquierdo C, Ruiz L, Ciruela P, Fenoll A, Casal J. 2005. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: a case control study. Clin Infect Dis 40:1250–1257. doi: 10.1086/429236. [DOI] [PubMed] [Google Scholar]
- 34.Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, Llor C; EVAN Study Group. 2006. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 43:860–868. doi: 10.1086/507340. [DOI] [PubMed] [Google Scholar]
- 35.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, Thompson WW; Vaccine Safety Datalink. 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 348:1747–1755. doi: 10.1056/NEJMoa022678. [DOI] [PubMed] [Google Scholar]
- 36.Moberley SA, Holden J, Tatham DP, Andrews RM. 2008. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008:CD000422. doi: 10.1002/14651858.CD000422.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Chang CC, Singleton RJ, Morris PS, Chang AB. 2007. Pneumococcal vaccines for children and adults with bronchiectasis. Cochrane Database Syst Rev 2007:CD006316. doi: 10.1002/14651858.CD006316.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Granger R, Walters J, Poole PJ, Lasserson TJ, Mangtani P, Cates CJ, Wood-Baker R. 2006. Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006:CD001390. doi: 10.1002/14651858.CD001390.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Chaithongwongwatthana S, Yaasmit W, Limpongsanurak S, Lumbiganon P, Desimone JA, Baxter J, Tolosa JE. 2006. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database Syst Rev 2006:CD004903. doi: 10.1002/14651858.CD004903.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Melegaro A, Edmunds WJ. 2004. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol 19:353–363. [DOI] [PubMed] [Google Scholar]
- 41.Conaty S, Watson L, Dinnes J, Waugh N. 2004. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine 22:3214–3224. doi: 10.1016/j.vaccine.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 42.Davies EG, Riddington C, Lottenberg R, Dower N. 2004. Pneumococcal vaccines for sickle cell disease. Cochrane Database Syst Rev 2004:CD003885. doi: 10.1002/14651858.CD003885.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Straetemans M, Sanders EA, Veenhoven RH, Schilder AG, Damoiseaux RA, Zielhuis GA. 2004. Pneumococcal vaccines for preventing otitis media. Cochrane Database Syst Rev 2004:CD001480. doi: 10.1002/14651858.CD001480.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Puig-Barberà J, Belenguer Varea A, Goterris Pinto M, Brines Benlliure MJ. 2002. Pneumococcal vaccine effectiveness in the elderly: systematic review and meta-analysis. Aten Primaria 30:269–281. (In Spanish.) doi: 10.1016/S0212-6567(02)79027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson L, Wilson BJ, Waugh N. 2002. Pneumococcal polysaccharide vaccine: a systematic review of clinical effectiveness in adults. Vaccine 20:2166–2173. doi: 10.1016/S0264-410X(02)00112-3. [DOI] [PubMed] [Google Scholar]
- 46.Cornu C, Yzèbe D, Léophonte P, Gaillat J, Boissel JP, Cucherat M. 2001. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomized trials. Vaccine 19:4780–4790. doi: 10.1016/S0264-410X(01)00217-1. [DOI] [PubMed] [Google Scholar]
- 47.Sheikh A, Alves B, Dhami S. 2002. Pneumococcal vaccine for asthma. Cochrane Database Syst Rev 2002:CD002165. doi: 10.1002/14651858.CD002165. [DOI] [PubMed] [Google Scholar]
- 48.Moore RA, Wiffen PJ, Lipsky BA. 2000. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Fam Pract 1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchison BG, Oxman AD, Shannon HS, Lloyd S, Altmayer CA, Thomas K. 1999. Clinical effectiveness of pneumococcal vaccine. Meta-analysis. Can Fam Physician 45:2381–2393. [PMC free article] [PubMed] [Google Scholar]
- 50.Go ES, Ballas ZK. 1996. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol 98:205–215. doi: 10.1016/S0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 51.Fine MJ, Smith MA, Carson CA, Meffe F, Sankey SS, Weissfeld LA, Detsky AS, Kapoor WN. 1994. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomized controlled trials. Arch Intern Med 154:2666–2677. [DOI] [PubMed] [Google Scholar]
- 52.Moberley S, Holden J, Tatham DP, Andrews RM. 2013. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2013:CD000422. doi: 10.1002/14651858.CD000422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, Alvarez F, Painter C, Blum MD, Silber JL. 2010. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis 201:516–524. doi: 10.1086/649839. [DOI] [PubMed] [Google Scholar]
- 54.Musher DM, Manoff SB, McFetridge RD, Liss CL, Marchese RD, Raab J, Rueda AM, Walker ML, Hoover PA. 2011. Antibody persistence ten years after first and second doses of 23-valent pneumococcal polysaccharide vaccine, and immunogenicity and safety of second and third doses in older adults. Hum Vaccin 7:919–928. doi: 10.4161/hv.7.9.15996. [DOI] [PubMed] [Google Scholar]
- 55.Romero-Steiner S, Musher DM, Cetron MS, Pais LB, Groover JE, Fiore AE, Plikaytis BD, Carlone GM. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin Infect Dis 29:281–288. doi: 10.1086/520200. [DOI] [PubMed] [Google Scholar]
- 56.Davies JM, Lewis MPN, Wimperis J, Rafi I, Ladhani S, Bolton-Maggs PHB. 2011. Review of guidelines for the prevention and treatment of infection in patients with an absent or dysfunctional spleen: prepared on behalf of the British Committee for Standards in Haematology by a Working Party of the Haemato-Oncology Task Force. Br J Haematol 155:308–317. doi: 10.1111/j.1365-2141.2011.08843.x. [DOI] [PubMed] [Google Scholar]
- 57.Granoff DM, Gupta RK, Belshe RB, Anderson EL. 1998. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis 178:870–874. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- 58.Borrow R, Goldblatt D, Andrews N, Richmond P, Southern J, Miller E. 2001. Influence of prior meningococcal C polysaccharide vaccination on the response and generation of memory after meningococcal C conjugate vaccination in young children. J Infect Dis 184:377–380. doi: 10.1086/322024. [DOI] [PubMed] [Google Scholar]
- 59.Sigurdardottir ST, Center KJ, Davidsdottir K, Arason VA, Hjalmarsson B, Elisdottir R, Ingolfsdottir G, Northington R, Scott DA, Jonsdottir I. 2014. Decreased immune response to pneumococcal conjugate vaccine after 23-valent pneumococcal polysaccharide vaccine in children. Vaccine 32:417–424. doi: 10.1016/j.vaccine.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 60.Papadatou I, Piperi C, Alexandraki K, Kattamis A, Theodoridou M, Spoulou V. 2014. Antigen-specific B-cell response to 13-valent pneumococcal conjugate vaccine in asplenic individuals with β-thalassemia previously immunized with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis 59:862–865. doi: 10.1093/cid/ciu409. [DOI] [PubMed] [Google Scholar]
- 61.Lazarus R, Clutterbuck E, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, Pollard AJ. 2011. A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin Infect Dis 52:736–742. doi: 10.1093/cid/cir003. [DOI] [PubMed] [Google Scholar]
- 62.Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, Angus B, Peto TE, Beverley PC, Mant D, Pollard AJ. 2012. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis 205:1408–1416. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AW, Tikoduadua L, Waqatakirewa L, Pryor J, Nelson J, Byrnes GB, Cheung YB, Tang ML, Mulholland EK. 2010. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial. Vaccine 28:3341–3349. doi: 10.1016/j.vaccine.2010.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, Scanlon PD, Woodruff PG, Washko GR, Connett JE, Anthonisen NR, Bailey WC; COPD Clinical Research Network. 2009. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180:499–505. doi: 10.1164/rccm.200903-0488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orthopoulos GV, Theodoridou MC, Ladis VA, Tsousis DK, Spoulou VI. 2009. The effect of 23-valent pneumococcal polysaccharide vaccine on immunological priming induced by the 7-valent conjugate vaccine in asplenic subjects with beta-thalassemia. Vaccine 27:350–354. doi: 10.1016/j.vaccine.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 66.Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. 2008. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis 198:1019–1027. doi: 10.1086/591629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Roux A, Schmöle-Thoma B, Siber GR, Hackell JG, Kuhnke A, Ahlers N, Baker SA, Razmpour A, Emini EA, Fernsten PD, Gruber WC, Lockhart S, Burkhardt O, Welte T, Lode HM. 2008. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 46:1015–1023. doi: 10.1086/529142. [DOI] [PubMed] [Google Scholar]
- 68.Mufson MA, Hughey DF, Turner CE, Schiffman G. 1991. Revaccination with pneumococcal vaccine of elderly persons 6 years after primary vaccination. Vaccine 9:403–407. doi: 10.1016/0264-410X(91)90126-Q. [DOI] [PubMed] [Google Scholar]
- 69.Borrow R, Stanford E, Waight P, Helbert M, Balmer P, Warrington R, Slack M, George R, Miller E. 2008. Serotype-specific immune unresponsiveness to pneumococcal conjugate vaccine following invasive pneumococcal disease. Infect Immun 76:5305–5309. doi: 10.1128/IAI.00796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dagan R, Givon-Lavi N, Greenberg D, Fritzell B, Siegrist CA. 2010. Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 201:1570–1579. doi: 10.1086/652006. [DOI] [PubMed] [Google Scholar]
- 71.Kugelberg E. 2015. B cell memory: making sense in humans. Nat Rev Immunol 15:133. doi: 10.1038/nri3822. [DOI] [PubMed] [Google Scholar]
- 72.Seifert M, Przekopowitz M, Taudien S, Lollies A, Ronge V, Drees B, Lindemann M, Hillen U, Engler H, Singer BB, Küppers R. 2015. Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A 112:E546–E555. doi: 10.1073/pnas.1416276112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. 2011. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Brien KL, Hochman M, Goldblatt D. 2007. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 75.Meltzer U, Goldblatt D. 2006. Pneumococcal polysaccharides interact with human dendritic cells. Infect Immun 74:1890–1895. doi: 10.1128/IAI.74.3.1890-1895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heyman B. 2000. Regulation of antibody responses via antibodies, complement and Fc receptors. Annu Rev Immunol 18:709–737. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 77.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. 2010. Plasma cells negatively regulate the follicular helper T cell program. Nat Immunol 11:1110–1118. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, Moore M, Antvelink D, Mulder D, Janoff EN, Whitworth J, Gilks CF. 2000. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355:2106–2111. doi: 10.1016/S0140-6736(00)02377-1. [DOI] [PubMed] [Google Scholar]
- 79.Menson EN, Mellado MJ, Bamford A, Castelli G, Duiculescu D, Marczyńska M, Navarro M, Scherpbier HJ, Heath PT; Vaccines Group; PENTA Steering Committee; Children's HIV Association (CHIVA). 2012. Guidance on vaccination of HIV-infected children in Europe. HIV Med 13:333–336. doi: 10.1111/j.1468-1293.2011.00982.x. [DOI] [PubMed] [Google Scholar]
- 80.Bjarnarson SP, Benonisson H, Del Giudice G, Jonsdottir I. 2013. Pneumococcal polysaccharide abrogates conjugate-induced germinal center reaction and depletes antibody secreting cell pool, causing hyporesponsiveness. PLoS One 8:e72588. doi: 10.1371/journal.pone.0072588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, Pilishvili T. 2015. Intervals between PCV13 and PPV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 64:944–947. doi: 10.15585/mmwr.mm6434a4. [DOI] [PubMed] [Google Scholar]
- 82.Papadatou I, Orthopoulos G, Theodoridou M, Spoulou V. 2015. Long-lasting hyporesponsiveness induced by the 23-valent pneumococcal polysaccharide vaccine (PPV23) in asplenic patients with β-thalassemia major. Vaccine 33:3779–3783. doi: 10.1016/j.vaccine.2015.06.100. [DOI] [PubMed] [Google Scholar]