Abstract
CT screening for lung cancer is now being implemented in the US and China on a widespread national scale but not in Europe so far. The review gives a status for the implementation process and the hurdles to overcome in the future. It also describes the guidelines and requirements for the structure and components of high quality CT screening programs. These are essential in order to achieve a successful program with the fewest possible harms and a possible mortality benefit like that documented in the American National Lung Screening Trial (NLST). In addition the importance of continued research in CT screening methods is described and discussed with focus on the great potential to further improve this method in the future for the benefit of patients and society.
Keywords: Lung cancer, CT screening, organization, implementation
Introduction
Lung cancer is the leading cause of cancer death world-wide both in the US and Europe the overall survival is still between 10–18% (1,2) in spite of improvements in treatment over the last decades (3). Early diagnosis by CT screening has in the US lead to a significant reduction in lung cancer mortality of 20% in the large randomized clinical trial, National Lung Screening Trial (NLST) (4,5). As a consequence lung cancer CT screening is now being implemented on a wide population based scale both in the US (6-12) and in China (13,14). In Europe most national health authorities are awaiting results from the Dutch-Belgian NELSON screening trial (15,16), expected in 2016 before making decisions regarding implementation (17-19). However it seems probable that eventually lung cancer screening will be part of the health care scenario also in Europe, irrespective of whether it is privately or publically funded. The way a lung cancer screening program is organized and structured will have a profound influence on the results and costs generated by the program, and mismanagement of the screening process may jeopardize the mortality benefit which is the overall goal of screening. Already in 2011 the International Association for the Study of Lung Cancer (IASLC) emphasized the need to develop well-structured guidelines and recommendations for subsequent screening programs (20), and several guidelines have been published mainly in the US (6-12).
A lung cancer screening program with low dose CT screening is a complex endeavor with the purpose of identifying persons without symptoms with lung cancer in an early stage allowing curative treatment, avoiding causing harm to the persons that do not have the disease. To achieve this during large scale implementation requires that the screening program is performed according to a systematic, structured, standardized and validated protocol, and that the quality of the performance is monitored continuously.
This review deals with essential requirements and components for organizing and running a CT screening program for lung cancer. These need to be taken into consideration when planning or implementing CT screening.
International collaboration
Globally there may be huge differences in the actual screening environment and conditions, therefore the screening program will have to be adapted to local conditions accordingly while still maintaining a high quality performance. The first to implement this was the ELCAP (Early Lung Cancer Action Program) which in 1992 was established in New York by Dr. Henschke et al. (21). In 1999 this was extended into an international alliance of multiple screening centers across the globe, with one supervising and coordinating center in the US: the International Early Lung Cancer Action Program (IELCAP) (22). High quality and excellent results of the screening process was achieved, by the commitment and supervision by the founders, and numerous publications from IELCAP have expanded our knowledge of CT screening (23). IELCAP demonstrated that a common screening protocol and techniques may be applied in a global network with high quality results (22) (see website: http://www.ielcap.org). To facilitate international collaboration on this issue another organization, the IASLC, hosted international workshops on LC screening at the IASLC World conferences on Lung Cancer in 2011, 2013 and 2015 (see website www.iaslc.org), and published a statement on LC screening in 2011 (20).
Implementation and organization in the US
In the US implementation of CT screening is based on the results obtained in the NLST trial (4,5) which also is reflected in the recommendations/requirements set up by Medicare (8), the United States Preventive services Task Force (USPTF) (7) and many other organizations (9-12).
In compliance with these requirements, an organization has been established by the Radiological Society of North America (RSNA) and American College of Radiology (ACR) to support implementation of CT screening in the US. This organization will comprise all aspects of the screening process, designated as “the 10 pillars of Lung Cancer Screening” (Figure 1) (24-26). This also includes the information (informed consent) and eligibility process prior to inclusion in CT screening (Figure 2), which also is covered in a chapter in this issue. The core activities however are:
Figure 1.
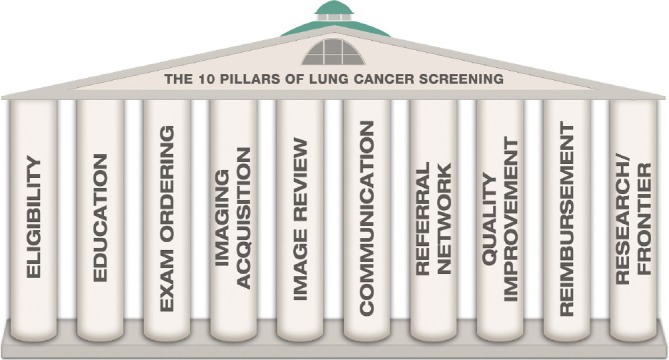
The 10 pillars of lung cancer screening. Published in: Fintelmann et al. The 10 pillars of lung cancer screening: rationale and logistics of a lung cancer screening program. Radiographics 2015;35:1893-1908.
Figure 2.
Example of Informed consent form used in CT screening program in the US. Published in: Fintelmann et al. The 10 pillars of lung cancer screening: rationale and logistics of a lung cancer screening program. Radiographics 2015;35:1893-1908.
The ACR Lung Cancer Screening Registry, is at present the only registry approved by the Centers for Medicare & Medicaid Services (CMS) for reimbursement and the Physician Quality Reporting System (PQRS) participation, including audit measures and peer comparisons;
Lung Cancer Screening Education, in which to learn how to implement a comprehensive, multidisciplinary program, receive Continuing Medical Education (CME) credits and comply with ACR requirements for lung cancer screening interactive eLearning activities;
ACR Designated Lung Cancer Screening Center. The ACR Designated Lung Cancer Screening Center status demonstrates that the center provides safe and effective care;
Lung-RADS. A standardized lung cancer screening CT reporting and management recommendations with the ACR Lung Imaging Reporting and Data System (Lung-RADS). This has been implemented by several groups and been shown to reduce the rate of false positive test results in CT screening (25,26).
In the US Lung Cancer Alliance advocacy groups have taken initiative to establish a Lung Cancer Screening framework in order to encourage institutions providing screening services to use “best practice” screening and treatment measures, including minimally invasive surgical techniques, so that the screening program has a high quality (18). In US funding of CT screening is covered mostly by private health care insurance, or Medicare (8). This approach may create bias in achieving equal access to screening also for the underserved, low income, heavy smoker, who may be hard to reach and come to a screening clinic (27).
Implementation outside the US
In Europe CT screening is so far only recommended in a white paper by European Society of Radiology (ESR) and European Respiratory Society (ERS) (28) and in a statement from the Swiss University Hospitals (29). No European national funding bodies have yet decided to support implementation of CT screening even though the most important CT screening trial outside the US is performed in the Netherlands-Belgium (NELSON trial) (15,16). This is because the final results of the trial was expected in 2015, but have not yet been published (17). The general consensus in Europe is to await the final results of the NELSON CT screening trial, before making decisions regarding implementation of LC screening (17,27). If screening is decided it will presumably follow traditions from other screenings programs as for example breast and colorectal cancer which are already implemented in many European countries (27). This implies that CT screening for lung cancer would be implemented in a form with complete public financing and public screening centers with population based recruitment (27). However there is considerable variability between the European countries. In the UK plans will probably follow the methodology applied in the United Kingdom Lung Screening trial (UKLS), with risk stratification of participants selecting a high risk cohort with minimum 5% risk of getting lung cancer within the next 5 years (30). This is in order to increase the cost effectiveness of the screening program, which is expected to be in higher focus compared to the US (29). In Germany extrapolation of the NLST results, and assuming 50% recruitment rate, indicates that 1.3 million persons would have to undergo annual CT screening (31). In many countries it seems probable that public radiology services may be at a shortage and it is expected that some countries may have to integrate private operators and perhaps financing in this process (29).
In China, a demonstration project of a prospective, multi-center, population-based lung cancer screening was initiated in 2010 to evaluate the feasibility of conducting population-based LDCT lung cancer screening (32). Therefore guidelines recommending implementation of CT screening have now been published (13,14), and it is expected to be given high priority due to the very high rates of lung cancer in both men and women in China (13).
How should screening be done?
Guidelines published by the ACR, “the 10 pillars of lung cancer screening”, illustrates the elements of a screening program (24), which are:
Eligibility
Who should be screened is determined by the criteria specified in the screening protocol (age, smoking history, duration of ex smoking, family history etc.) (see separate chapter in this issue) (9), so that both inclusion and exclusion criteria are clearly stated (28). So far there are no good risk predictors and indication for CT screening of non-smokers (7-9,28).
Education
Participants and staff should be educated in benefits and harms of screening and information material should inform on both benefits and possible harms (7-10,28). An example of such an information and informed consent form from the ACR is shown in Figure 2 (24). In the statement from Switzerland the adoption of an ethical code regarding recruitment for screening was suggested. “Institutions performing lung cancer screening, should adopt an ethical chart stating that they do not recruit patients through direct or indirect advertisement, use fear of cancer to promote lung cancer screening, let patients believe that the cancer risk can be eliminated by screening, or offer screening at reduced costs with the aim of generating profit from additional diagnostic and therapeutic procedures.” (29). The generalizability of such a code is at present unknown and it may only be applicable in some countries in Europe.
A smoking cessation program should be an integrated part of the screening program (see Chapter on smoking cessation and lung cancer screening) (8-10,13,28,29). Education also involves staff training and certification as specified in the ACR program (24).
Imaging acquisition
Low dose CT should be performed in a standardized manner according to technical specifications in protocols, as for example described by ACR (24) and NLST (4,5). Approximately one cancer death may have been caused by radiation from CT per 2,500 persons screened; thus, the benefit in preventing lung cancer death using the NLST was greater than the radiation risk. Multidetector LDCT with at least 16 detector rows provide isotropic high spatial resolution (slice thickness of 1 mm with an increment of 0.7 mm) (28). A CT dose index (CTDIvol) of 2–3 mGy was used as target in NLST (5,33) and in NELSON (15). The resulting effective dose is 1–1.3 mSv for a CTDIvol of 2.5 mGy. Technical improvements in CT scanners and settings will lead to lower doses in the future and a further decrease in radiation exposure to a level of approximately 0.2 mSV may be possible (34,35). Data should be collected to ensure that the actual radiation dose is in accordance with recommendations (24). The radiation exposure was generated by follow up scans and derived diagnostic evaluations may be significant and should be included in the monitoring process (31).
Image review
A complete flow chart for management of nodules with a care pathway should be developed, including criteria for when to initiate invasive diagnostic procedures (see section on Workup and surgery). Management of screen detected nodules should involve clinicians and radiologist with expertise in the management of lung nodules and treatment of lung cancer and planning done at multi-disciplinary treatment (MDT) conferences (20,27).
Criteria for lung nodule identification, and for size, character and growth of nodules to define test as positive, indeterminate or negative should be described (15,23,27). The use of volumetric nodule measurements for the assessment of growth (tumor volume doubling time) has primarily been used in Europe by NELSON (15), Danish Lung Cancer Screening trial (DLCST) (36,37), UKLS (27,30). Volumetric evaluation is a more sensitive and accurate way to measure growth than linear measurements (38,39) and may reduce number of false positive tests (28).
Data should be collected on location, number, size and character of all lung nodules detected and registered and reported. A structured reporting system, such as the Lung-RADS (25,26), or an equivalent should be used. Adherence to the screening process should also be monitored.
Positron emission tomography (PET) scanning
The use of PET in the diagnostic evaluation of screen detected nodules has been an important tool in several studies (40-42). The combination of PET and volumetric measurements increased the diagnostic accuracy and reduced the rate of false positive test results in DLCST (42).
Screening interval
The time interval between the CT scans in the screening program, has a great impact on the costs but also on the cumulative radiation dose the participants are exposed to (28,33). An increase in the time interval may however reduce the diagnostic sensitivity of the screening test. The recommended time interval, based on the NLST data, at present is annual screening (7-9,28). In the NELSON trial a screening interval of 1, 2 and 2.5 years is being evaluated (43). The results so far show that a 2-year interval after a baseline screening and one annual repeat scan did not impair the diagnostic sensitivity, however during a 2.5-year interval the frequency of interval cancer increased significantly (43). In the future perhaps individually tailored screening intervals based on baseline CT scan characteristics and individual risk profile may be possible and hopefully reduce both the number of CT scans and radiation exposure (44).
Communication
Results of the screening test should be communicated and explained to the participant in both writing and direct oral communication in case of a positive or indeterminate result. A negative (normal) result may often be communicated in writing. All test results have an impact on the persons receiving them, and this should be taken into consideration when organizing the screening protocol (45).
Quality improvement and research
Continuous research and audits is essential to ensure persistent high quality and performance in the screening program. Important Research areas in lung cancer screening at present include:
Biomarkers, including gene methylation, micro-ribonucleic acid, and autoantibodies to be used for potential screening; but most of these need prospective population based validation.
Chemoprevention studies within screening programs (46);
Methods to recruit the “hard to reach population (27,47,48);
Optimal screening intervals in CT screening. Annual vs. biannual screening (49) in addition to more individually tailored programs based on individual risk profile;
Further development of minimal invasive treatment options in early lung cancer (50).
Minimum requirements to a lung cancer CT screening center
The present guidelines for CT screening from Medicare (8), USPSTF (7), NCCN (9), ALS (10), AATS (11), ACS (12) and IASLC (20) state that screening should only be done in centers with multidisciplinary capabilities and organization, and this has been endorsed by many others (18,24,28,29,31).
The following MDT board certified capabilities should be available: pulmonology, pathology, radiology, thoracic surgery and oncology (20,24,28,29).
The center should be certified, authorized and accredited to do lung cancer screening (if such a national authorization exists) (20,24,28).
CT scanner capabilities (min, 16 slices) with lung nodule volumetric software, and reporting system (fex. Lung-RADS), radiation quality control (24,28,29).
Radiologist or pulmonologists with CT guided biopsy expertise or other minimal invasive technology for biopsy of small lung nodules (<10 mm) (20,24,28,29).
Invasive pulmonology service (Bronchoscopy, EBUS, EUS, ENB) (20,24,28,29).
Minimal Invasive VATS surgery program allowing a full spectrum of surgical options (wedge resection, anatomical segmental resections, lobectomy, lymph node dissection etc.) (See also chapter on Work up and surgery) (20,24,29).
PET or PET-CT scanner capabilities for diagnostic evaluation of suspicious nodules and preoperative staging (29).
Data registration and Research capabilities (20,24,28).
Reporting to a national Lung Cancer CT screening register (20,24,28).
Conclusions
Lung cancer CT screening is at present being implemented in the US and China. It is expected that many European countries also will start an implementation process within the next few years. Successful screening requires a multidisciplinary organization with focus on both delivering accurate and curative diagnosis and treatment to participants identified with lung cancer, but also focus on minimizing harms to the vast majority of screens without the disease. Well organized and validated screening programs as outlined above are the optimal way to achieve this.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095-128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551-64. 10.1136/thoraxjnl-2012-202297 [DOI] [PubMed] [Google Scholar]
- 3.Vallières E, Peters S, Van Houtte P, et al. Therapeutic advances in non-small cell lung cancer. Thorax 2012;67:1097-101. 10.1136/thoraxjnl-2011-201043 [DOI] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team , Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team , Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology 2011;258:243-53. 10.1148/radiol.10091808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyer VA, U.S. Preventive Services Task Force . Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [DOI] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force. Final recommendation statement, screening for lung cancer: US preventive services. Available online: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening
- 8.Description of Medicare coverage regarding lung cancer screening available at Medicare web site (Jan 2016). Available online: https://www.medicare.gov/coverage/lung-cancer-screening.html
- 9.Wood DE. National Comprehensive Cancer Network (NCCN) clinical practice guidelines for lung cancer screening. Thorac Surg Clin 2015;25:185-97. 10.1016/j.thorsurg.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 10.American Lung Association. Providing guidance on lung cancer screening to patients and physicians. Available online: http://www.lung.org/assets/documents/lung-cancer/lung-cancer-screening-report.pdf
- 11.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33-8. 10.1016/j.jtcvs.2012.05.060 [DOI] [PubMed] [Google Scholar]
- 12.Wender R, Fontham ET, Barrera E, Jr, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:107-17. 10.3322/caac.21172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou QH, Fan YG, Bu H, et al. China national lung cancer screening guideline with low-dose computed tomography (2015 version). Thorac Cancer 2015;6:812-8. 10.1111/1759-7714.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao SJ, Wu N. Early detection of lung cancer: Low-dose computed tomography screening in China. Thorac Cancer 2015;6:385-9. 10.1111/1759-7714.12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. 10.1056/NEJMoa0906085 [DOI] [PubMed] [Google Scholar]
- 16.Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med 2013;187:848-54. 10.1164/rccm.201209-1651OC [DOI] [PubMed] [Google Scholar]
- 17.Heuvelmans MA, Vliegenthart R, Oudkerk M. Contributions of the European trials (European randomized screening group) in computed tomography lung cancer screening. J Thorac Imaging 2015;30:101-7. 10.1097/RTI.0000000000000135 [DOI] [PubMed] [Google Scholar]
- 18.Mulshine JL, D'Amico TA. Issues with implementing a high-quality lung cancer screening program. CA Cancer J Clin 2014;64:352-63. 10.3322/caac.21239 [DOI] [PubMed] [Google Scholar]
- 19.Field JK, van Klaveren R, Pedersen JH, et al. European randomized lung cancer screening trials: Post NLST. J Surg Oncol 2013;108:280-6. 10.1002/jso.23383 [DOI] [PubMed] [Google Scholar]
- 20.Field JK, Smith RA, Aberle DR, et al. International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol 2012;7:10-9. 10.1097/JTO.0b013e31823c58ab [DOI] [PubMed] [Google Scholar]
- 21.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. 10.1016/S0140-6736(99)06093-6 [DOI] [PubMed] [Google Scholar]
- 22.International Early Lung Cancer Action Program Investigators , Henschke CI, Yankelevitz DF, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763-71. 10.1056/NEJMoa060476 [DOI] [PubMed] [Google Scholar]
- 23.Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246-52. 10.7326/0003-4819-158-4-201302190-00004 [DOI] [PubMed] [Google Scholar]
- 24.Fintelmann FJ, Bernheim A, Digumarthy SR, et al. The 10 pillars of lung cancer screening: rationale and logistics of a lung cancer screening program. Radiographics 2015;35:1893-908. 10.1148/rg.2015150079 [DOI] [PubMed] [Google Scholar]
- 25.McKee BJ, Regis SM, McKee AB, et al. Performance of ACR Lung-RADS in a clinical CT lung screening program. J Am Coll Radiol 2015;12:273-6. 10.1016/j.jacr.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 26.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med 2015;162:485-91. 10.7326/M14-2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field JK, Devaraj A, Duffy SW, et al. CT screening for lung cancer: is the evidence strong enough? Lung Cancer 2016;91:29-35. 10.1016/j.lungcan.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 28.Kauczor HU, Bonomo L, Gaga M, et al. ESR/ERS white paper on lung cancer screening. Eur Radiol 2015;25:2519-31. 10.1007/s00330-015-3697-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frauenfelder T, Puhan MA, Lazor R, et al. Early detection of lung cancer: a statement from an expert panel of the Swiss university hospitals on lung cancer screening. Respiration 2014;87:254-64. 10.1159/000357049 [DOI] [PubMed] [Google Scholar]
- 30.Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. 10.1136/thoraxjnl-2015-207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stang A, Schuler M, Kowall B, et al. Lung cancer screening using low dose CT scanning in Germany. Extrapolation of results from the National Lung Screening Trial. Dtsch Arztebl Int 2015;112:637-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Q, Fan Y, Wu N, et al. Demonstration program of population-based lung cancer screening in China: rationale and study design. Thorac Cancer 2014;5:197-203. 10.1111/1759-7714.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol 2011;197:1165-9. 10.2214/AJR.11.6533 [DOI] [PubMed] [Google Scholar]
- 34.Katsura M, Matsuda I, Akahane M, et al. Model-based iterative reconstruction technique for ultralow-dose chest CT: comparison of pulmonary nodule detectability with the adaptive statistical iterative reconstruction technique. Invest Radiol 2013;48:206-12. [DOI] [PubMed] [Google Scholar]
- 35.Huber A, Landau J, Ebner L, et al. Performance of ultralow-dose CT with iterative reconstruction in lung cancer screening: limiting radiation exposure to the equivalent of conventional chest X-ray imaging. Eur Radiol 2016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen JH, Ashraf H, Dirksen A, et al. The Danish randomized lung cancer CT screening trial--overall design and results of the prevalence round. J Thorac Oncol 2009;4:608-14. 10.1097/JTO.0b013e3181a0d98f [DOI] [PubMed] [Google Scholar]
- 37.Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax 2012;67:296-301. 10.1136/thoraxjnl-2011-200736 [DOI] [PubMed] [Google Scholar]
- 38.Field JK, Oudkerk M, Pedersen JH, et al. Prospects for population screening and diagnosis of lung cancer. Lancet 2013;382:732-41. 10.1016/S0140-6736(13)61614-1 [DOI] [PubMed] [Google Scholar]
- 39.Xie X, Zhao Y, Snijder RA, et al. Sensitivity and accuracy of volumetry of pulmonary nodules on low-dose 16- and 64-row multi-detector CT: an anthropomorphic phantom study. Eur Radiol 2013;23:139-47. 10.1007/s00330-012-2570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastorino U, Bellomi M, Landoni C, et al. Early lung-cancer detection with spiral CT and positron emission tomography in heavy smokers: 2-year results. Lancet 2003;362:593-7. 10.1016/S0140-6736(03)14188-8 [DOI] [PubMed] [Google Scholar]
- 41.Veronesi G, Travaini LL, Maisonneuve P, et al. Positron emission tomography in the diagnostic work-up of screening-detected lung nodules. Eur Respir J 2015;45:501-10. 10.1183/09031936.00066514 [DOI] [PubMed] [Google Scholar]
- 42.Ashraf H, Dirksen A, Loft A, et al. Combined use of positron emission tomography and volume doubling time in lung cancer screening with low-dose CT scanning. Thorax 2011;66:315-9. 10.1136/thx.2010.136747 [DOI] [PubMed] [Google Scholar]
- 43.Horeweg N, Scholten ET, de Jong PA, et al. Detection of lung cancer through low-dose CT screening (NELSON): a prespecified analysis of screening test performance and interval cancers. Lancet Oncol 2014;15:1342-50. 10.1016/S1470-2045(14)70387-0 [DOI] [PubMed] [Google Scholar]
- 44.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. 10.1056/NEJMoa1214726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasmussen JF, Siersma V, Pedersen JH, et al. Psychosocial consequences in the Danish randomised controlled lung cancer screening trial (DLCST). Lung Cancer 2015;87:65-72. 10.1016/j.lungcan.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 46.Veronesi G, Guerrieri-Gonzaga A, Infante M, et al. Chemoprevention studies within lung cancer screening programmes. Ecancermedicalscience 2015;9:597. 10.3332/ecancer.2015.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali N, Lifford KJ, Carter B, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015;5:e008254. 10.1136/bmjopen-2015-008254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanner NT, Gebregziabher M, Hughes Halbert C, et al. Racial differences in outcomes within the national lung screening trial. Implications for Widespread Implementation. Am J Respir Crit Care Med 2015;192:200-8. 10.1164/rccm.201502-0259OC [DOI] [PubMed] [Google Scholar]
- 49.Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. 10.1097/CEJ.0b013e328351e1b6 [DOI] [PubMed] [Google Scholar]
- 50.Petersen RH, Hansen HJ, Dirksen A, et al. Lung cancer screening and video-assisted thoracic surgery. J Thorac Oncol 2012;7:1026-31. 10.1097/JTO.0b013e31824fe942 [DOI] [PubMed] [Google Scholar]



