Abstract
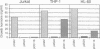
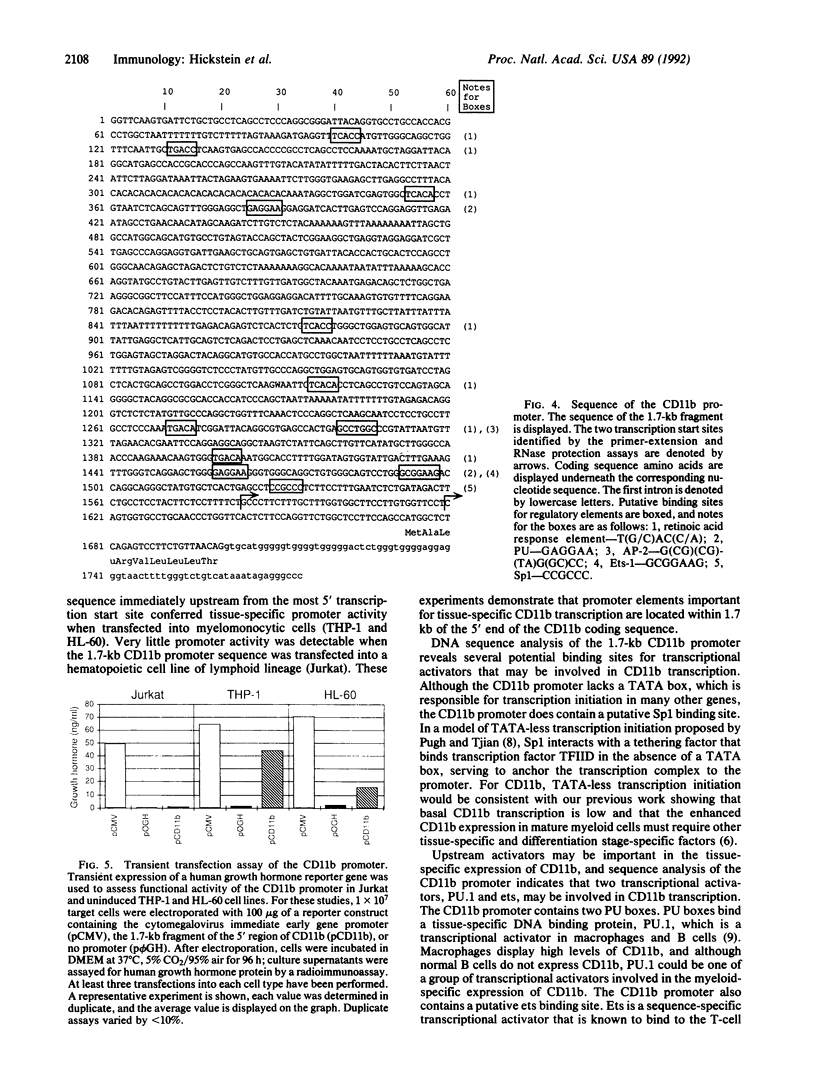
The CD11b (or macrophage-1 antigen; MAC-1) subunit of the leukocyte integrin family forms a noncovalently associated heterodimeric structure with the CD18 (beta) subunit on the surface of human granulocytes and monocyte/macrophages, where it enables these myeloid cells to participate in a variety of adherence-related activities. Expression of the CD11b subunit is restricted to cells of the myelomonocytic lineage and depends upon the stage of differentiation with the most mature myeloid cells expressing the highest levels of CD11b. To study the regulation of CD11b expression, a genomic clone corresponding to the 5' region of the CD11b gene was isolated from a human chromosome 16 library. Primer extension and RNase protection assays identified two major transcriptional start sites, located 90 base pairs and 54 base pairs upstream from the initiation methionine. DNA sequence analysis of 1.7 kilobases of the 5' flanking sequence of the CD11b gene indicated the absence of a "CAAT" or "TATA" box; however, potential binding sites for the transcription activators Sp1, PU.1, ets, and AP-2 are present, as well as retinoic acid response elements. The 1.7-kilobase CD11b promoter sequence displayed functional activity in transient transfection assays in the monocytic cell line THP-1 and the myeloid cell line HL-60. In contrast, this 1.7-kilobase promoter sequence did not display functional activity in the Jurkat T-lymphoid cell line. Detailed characterization of the CD11b promoter sequence should provide insight into the molecular events regulating the tissue-specific and developmental stage-specific expression of the CD11b molecule in myelomonocytic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Gupta S. K., Pierce M. W., Tenen D. G. Amino acid sequence of the alpha subunit of human leukocyte adhesion receptor Mo1 (complement receptor type 3). J Cell Biol. 1988 Jun;106(6):2153–2158. doi: 10.1083/jcb.106.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Corbi A. L., Kishimoto T. K., Miller L. J., Springer T. A. The human leukocyte adhesion glycoprotein Mac-1 (complement receptor type 3, CD11b) alpha subunit. Cloning, primary structure, and relation to the integrins, von Willebrand factor and factor B. J Biol Chem. 1988 Sep 5;263(25):12403–12411. [PubMed] [Google Scholar]
- Geballe A. P., Spaete R. R., Mocarski E. S. A cis-acting element within the 5' leader of a cytomegalovirus beta transcript determines kinetic class. Cell. 1986 Sep 12;46(6):865–872. doi: 10.1016/0092-8674(86)90068-1. [DOI] [PubMed] [Google Scholar]
- Hickstein D. D., Back A. L., Collins S. J. Regulation of expression of the CD11b and CD18 subunits of the neutrophil adherence receptor during human myeloid differentiation. J Biol Chem. 1989 Dec 25;264(36):21812–21817. [PubMed] [Google Scholar]
- Hickstein D. D., Hickey M. J., Ozols J., Baker D. M., Back A. L., Roth G. J. cDNA sequence for the alpha M subunit of the human neutrophil adherence receptor indicates homology to integrin alpha subunits. Proc Natl Acad Sci U S A. 1989 Jan;86(1):257–261. doi: 10.1073/pnas.86.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickstein D. D., Ozols J., Williams S. A., Baenziger J. U., Locksley R. M., Roth G. J. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J Biol Chem. 1987 Apr 25;262(12):5576–5580. [PubMed] [Google Scholar]
- Ho I. C., Bhat N. K., Gottschalk L. R., Lindsten T., Thompson C. B., Papas T. S., Leiden J. M. Sequence-specific binding of human Ets-1 to the T cell receptor alpha gene enhancer. Science. 1990 Nov 9;250(4982):814–818. doi: 10.1126/science.2237431. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Mitchell P. J., Williams T., Tjian R. Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 1989 Oct;3(10):1507–1517. doi: 10.1101/gad.3.10.1507. [DOI] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990 Jun 29;61(7):1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasios G., Mader S., Gold J. D., Leid M., Lutz Y., Gaub M. P., Chambon P., Gudas L. The late retinoic acid induction of laminin B1 gene transcription involves RAR binding to the responsive element. EMBO J. 1991 May;10(5):1149–1158. doi: 10.1002/j.1460-2075.1991.tb08055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]