Abstract Abstract
Riociguat is a soluble guanylate cyclase stimulator approved for the treatment of pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH). This randomized, double-blind, placebo-controlled study investigated the pharmacokinetics of riociguat and its metabolite M1 in young (18–45 years) and elderly (64.5–80 years) healthy volunteers of both sexes to assist planning of the dose regimens for clinical trials. The data were also used to draw comparisons with the effects of age and sex on riociguat pharmacokinetics in patients with PAH and CTEPH from the riociguat phase 3 trials, PATENT and CHEST. Volunteers received an oral dose of either riociguat 2.5 mg or placebo, and the concentrations of riociguat and M1 in blood and urine samples were determined using mass spectrometry. In elderly healthy volunteers, overall riociguat and M1 exposure tended to be higher than in young healthy volunteers (P > 0.05), partly because of reduced renal clearance (approximately 28% reduction) and differences in body weight. Although the mean maximum concentrations of riociguat and M1 were significantly higher in women than in men (35% and 50% higher, respectively), total exposure was similar. Despite differences in riociguat and M1 pharmacokinetics, riociguat was well tolerated with a comparable safety profile across all subgroups, suggesting that differences in drug exposure due to age or sex were not sufficient to warrant a dose adjustment in clinical trials. Furthermore, similar pharmacokinetics were observed in patients with PAH and CTEPH. However, particular care should be exercised during individual dose titration of riociguat in elderly patients.
Keywords: pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, drug exposure
Pulmonary hypertension (PH) is a progressive disease of the pulmonary vasculature characterized by vasoconstriction, vascular remodeling, and pathologically increased pulmonary arterial pressure.1 The workload of the right heart is therefore increased, leading to right ventricular failure, progressive impairment of exercise capacity, and eventually death. PH is a devastating condition with a poor prognosis and high unmet therapeutic need.2,3 Despite modern therapies, the mortality of patients with pulmonary arterial hypertension (PAH) remains high: 32% at 3 years in a large US registry.4
In the healthy lung, vascular pressure is regulated by numerous mediators, including the vasodilator agent nitric oxide (NO).5 NO acts on soluble guanylate cyclase (sGC), catalyzing the generation of cyclic guanosine monophosphate (cGMP), which regulates vascular tone, proliferation, fibrosis, and inflammation.6-8 In patients with PH, endothelial dysfunction impairs the NO-sGC-cGMP pathway, leading to vascular injury, vasoconstriction, and pulmonary vascular remodeling.7,8
Riociguat (BAY 63-2521) is an sGC stimulator with a dual mode of action on the NO-sGC-cGMP pathway; it stimulates sGC directly via an NO-independent mechanism and sensitizes sGC to NO, thus enhancing the effect of low concentrations of NO.9 Riociguat is the first drug to be approved for the treatment of 2 separate PH indications: PAH and chronic thromboembolic pulmonary hypertension (CTEPH). The safety and efficacy of riociguat was demonstrated in the phase 3 trials PATENT-1 (ClinicalTrials.gov identifier: NCT00810693), in patients with symptomatic PAH,10 and CHEST-1 (NCT00855465), in patients with symptomatic CTEPH.11 In both studies, riociguat significantly improved exercise capacity and a range of secondary end points, including hemodynamic parameters, World Health Organization (WHO) functional class (FC), and levels of N-terminal prohormone of brain natriuretic peptide.10,11 In the new European Society of Cardiology/European Respiratory Society guidelines for the diagnosis and treatment of PH, riociguat has a class I recommendation for patients with PAH (WHO FC II and III) or CTEPH that is recurrent or persistent after surgical treatment or is judged inoperable.12
This clinical pharmacology study in healthy volunteers was performed to assist the development of the dose regimens of riociguat for the phase 3 clinical trials. There is clear evidence that the pharmacokinetic and pharmacodynamic properties of drugs can be altered in the elderly population, owing partly to reduced renal clearance, decreased liver perfusion, and a reduction in body weight.13,14 Sex can also influence the disposition and pharmacokinetics of drugs.15,16 This study was therefore designed to determine and compare the pharmacokinetics of riociguat and its metabolite M1 (BAY 60-4552) in young (18–45 years) and elderly (64.5–80 years) healthy volunteers of both sexes and to examine the effects of age and sex on the pharmacokinetics of both compounds. In this study, we also evaluated and compared safety and tolerability of riociguat across age and sex groups. In addition, the effects of age and sex on the pharmacokinetics of riociguat in healthy volunteers were compared with these effects in patients with PAH and CTEPH, using population pharmacokinetic data from the phase 3 trials.
Methods
Healthy volunteer study
The healthy volunteer study was a placebo-controlled, double-blind, parallel-group study conducted at a single center (ProMedica Clinical Research Center, Boston), in accordance with the Declaration of Helsinki and the good clinical practice guidelines of the International Conference on Harmonisation and with approval from the local ethics committee. All subjects gave written informed consent before the start of any study-specific procedures.
Study population
Healthy male and female volunteers were eligible for inclusion in the study if they were aged 18–45 or 64.5–80 years and had a body mass index between 20 and 32 inclusive. In addition, subjects had to test negative for drug abuse and hepatitis B and C at screening and could not be taking any long-term medication, except specified medications for elderly participants. Women in the young group had to have been sterilized, as documented by medical records. If such documentation was not available, negative serum human chorionic gonadotropin and urine pregnancy tests were required. Exclusion criteria included clinically significant and relevant past medical history or current medical illness; history of a gastrointestinal disorder that could result in incomplete absorption of the study drug; significant history of alcoholism; smoking ≥15 cigarettes/day or equivalent; or any medical or psychiatric disorder or condition or treatment with any drug that could (in the opinion of the investigator or the sponsor) impair the individual’s ability to complete the trial.
Study design
Subjects were randomized to receive a single oral tablet of riociguat 2.5 mg or placebo following an overnight fasting period within the following 4 subgroups: young (aged 18–45 years) male (YM), elderly (aged 64.5–80 years) male (EM), young female (YF), and elderly female (EF). In each subgroup, 9 volunteers received riociguat and 3 volunteers received placebo, with the exception of the YF group, in which 2 volunteers received placebo.
Sample and data collection
Blood samples for pharmacokinetics were collected before the riociguat or placebo dose and then at specified time points from 0.25 to 72 hours after dosing. Urine samples for pharmacokinetics were collected at the time of dosing and at specified intervals up to 72 hours after dosing. Samples were stored below −15°C and were analyzed within 9 months after sampling. A DINAMAP ProCare Model 100 monitor (GE Medical Systems Information Technologies, Milwaukee) was used for all blood pressure and pulse rate recordings.
Pharmacokinetic and safety evaluations
Concentrations of riociguat and M1 in plasma and urine samples were determined by high-performance liquid chromatography coupled with mass spectrometry, using [methoxycarbonyl (2H3)]riociguat and [2H3]M1 as internal standards. For both analytes, the calibration ranges were 0.5–100 μg/L for plasma and 2–200 μg/L for urine.
For levels of both analytes in plasma, quality control (QC) samples in the concentration range of 1.5–80.0 μg/L were determined for riociguat with an accuracy of 92.0%–97.1% and a precision of 6.88%–15.10% and for M1 with an accuracy of 97.7%–99.1% and a precision of 6.00%–6.53%. For levels of both analytes in urine, QC samples in the concentration range of 6.0–160.0 μg/L were determined for riociguat with an accuracy of 95.0%–102.0% and a precision of 2.88%–3.67% and for M1 with an accuracy of 95.7%–101.0% and a precision of 2.89%–5.14%.
Pharmacokinetic parameters were calculated using noncompartmental methods in WinNonlin (ver. 4.1a; Pharsight, Cary, NC), and the logarithms of pharmacokinetic variables—with the exception of time to maximum concentration in plasma—were analyzed using a 2-way analysis of variance with terms for age, sex, and age × sex. Ratios of geometric means (90% confidence intervals) were used to conduct the following pairwise comparisons of study subgroups: YM with EM, YF with EF, YM with YF, and EM with EF. In addition, the total elderly population was compared with the total young population, and all men as a group were compared with all women as a group. The placebo arm enabled a safety and tolerability comparison to be made with the active treatment arms; therefore, participants receiving placebo were not included in the above analyses.
Safety and tolerability were assessed by monitoring adverse events, vital signs, clinical laboratory parameters, and electrocardiogram recordings and by physical examination. All participants received a follow-up assessment by telephone approximately 7 days after dosing.
Phase 3 trials
PATENT-110 and CHEST-111 were multicenter, randomized, double-blind, placebo-controlled trials of riociguat at doses up to 2.5 mg 3 times daily for 12 weeks in PATENT-1 and 16 weeks in CHEST-1. In both studies, men and women aged 18–75 years were enrolled. PATENT-2 (NCT00863681) and CHEST-2 (NCT00910429) were long-term, single-arm extensions to PATENT-1 and CHEST-1, respectively, in which all patients (including those previously randomized to placebo) received open-label riociguat.17-20 Detailed methodology and trial designs have been published previously. The institutional review board at each participating center approved the trial protocols.
Sample and data collection
During the double-blind studies, protocol-specified blood samples were taken from all patients at the first visit (day 0), at each subsequent biweekly study visit, and at the termination visit. During the long-term extension studies, samples were collected on day 1 and again after 8 weeks. Samples were collected approximately 1 hour before administration of study drug in the main and extension phases of the PATENT and CHEST studies. In PATENT-1, samples were also taken 2–3 hours after the first and second doses of study drug at the first visit. All samples were stored at −15°C and analyzed within 18 months after sampling.
Pharmacokinetic and safety evaluations
Plasma concentrations of riociguat and M1 were measured using a validated high-pressure liquid chromatography/tandem mass spectrometry method. QC and calibration samples were analyzed concurrently with study samples. Previous studies had established that the calibration range of the procedure was from 2 μg/L (the lower limit of quantification) to 500 μg/L for both riociguat and M1. QC samples in the concentration range of 6–400 μg/L indicated an accuracy of 96.8%–104.0% and a precision of 5.02%–8.49% for riociguat and an accuracy of 96.9%–100.0% and a precision of 3.35%–7.56% for M1.
All pharmacokinetic analyses were performed using a population pharmacokinetic modeling approach with a first-order conditional estimation method (NONMEM software, ver. 7.2.0). A pharmacokinetic model was derived that accounted for random effects, interindividual and interoccasion variability, and patient covariates. A visual predictive check was carried out to determine the validity of the model.
Results
Healthy volunteer study
Study population
In total, 47 participants were randomized, received either riociguat or placebo, and were eligible for pharmacokinetic and safety analyses. Thirty-six participants (9 in each study subgroup) received a single oral dose of riociguat 2.5 mg, and 11 participants (3 in each of the YM, EM, and EF subgroups and 2 in the YF subgroup) received a single dose of placebo.
Generally, the placebo and riociguat groups were well matched for age and body mass index (Table 1). The age and sex subgroups were also generally well matched (Table S1). Each subgroup was predominantly white, except for the YF riociguat subgroup (in which 33% of participants were white, 56% black, and 11% Hispanic) and the YM riociguat group (in which 44% were white or black and 11% Hispanic).
Table 1.
Demographic characteristics of the healthy volunteer study population
| Characteristic | Placebo (n = 11) | Riociguat 2.5 mg (n = 36) |
|---|---|---|
| Race, no. (%) | ||
| White | 9 (82) | 23 (64) |
| Black | 2 (18) | 10 (28) |
| Asian | 0 | 1 (3) |
| Hispanic | 0 | 2 (5) |
| Age at enrollment, years | 56.7 ± 14.3 | 54.1 ± 16.2 |
| Body mass index | 27.5 ± 3.1 | 27.1 ± 3.4 |
Unless otherwise stated, data are expressed as mean ± standard deviation.
Pharmacokinetic evaluations
Effect of age on pharmacokinetic characteristics. The mean maximum concentration in plasma (Cmax) of riociguat did not vary widely between age groups (Tables 2, 3; Fig. 1). Mean renal clearance of riociguat was reduced in elderly individuals (male and female) by approximately 28% compared with young participants (Table 2). The mean terminal elimination half-life (t1/2) of riociguat was significantly prolonged by approximately 40% in elderly compared with young participants (Table 3). Overall exposure to riociguat—measured as area under the plasma concentration–time curve (AUC)—was approximately 40% higher in elderly than in young participants, but this finding was not statistically significant (P > 0.05). When exposure was normalized relative to body weight, the difference between elderly and young participants was approximately 28% (Tables 2, 3). The differences between elderly and young subjects for M1 were similar to those for riociguat but were generally less pronounced (Tables 2, 3, S2; Fig. 2), although the difference in t1/2 of M1 between the age groups was statistically significant. Pharmacokinetic results for the pooled young and elderly populations and for pooled male and female participants are shown in Table S2.
Table 2.
Pharmacokinetic parameters of riociguat and M1 by age and sex following a single oral dose of riociguat 2.5 mg in the healthy volunteer study
| Female | Male | |||
|---|---|---|---|---|
| Parameter | Young (n = 9) | Elderly (n = 9) | Young (n = 9) | Elderly (n = 9) |
| Riociguat | ||||
| AUC, μg h/L | 803 (55) | 1,145 (36) | 750 (58) | 1,036 (48) |
| Cmax, μg/L | 113.0 (22) | 125.0 (35) | 80.4 (25) | 96.7 (32) |
| AUCnorm, g h/L | 24.8 (57) | 32.6 (45) | 26.5 (55) | 32.8 (50) |
| Cmax,norm, g/L | 3.48 (20) | 3.56 (36) | 2.84 (23) | 3.06 (22) |
| t1/2, hours | 8.97 (51) | 11.80 (31) | 8.10 (39) | 12.20 (62) |
| tmax, hours, median (range) | 1.5 (1.0–2.0) | 2.0 (1.0–2.5) | 1.5 (1.0–3.0) | 2.0 (1.0–3.0) |
| CLR, L/ha | 0.320 (28.1) | 0.223 (33.3) | 0.389 (36.6) | 0.287 (28.1) |
| Aeur(0–72), %a | 11.1 (45.6) | 11.1 (59.1) | 12.0 (44.0) | 12.8 (49.7) |
| M1 | ||||
| AUC, μg h/L | 640 (26) | 651 (38) | 476 (20) | 687 (44) |
| Cmax, μg/L | 28.1 (53) | 22.4 (52) | 14.9 (44) | 18.8 (71) |
| AUCnorm, g h/L | 20.5 (26) | 19.2 (30) | 17.4 (28) | 22.5 (35) |
| Cmax,norm, g/L | 0.90 (51) | 0.661 (42) | 0.544 (51) | 0.615 (65) |
| t1/2, hours | 16.0 (34) | 16.7 (21) | 14.9 (28) | 21.3 (33) |
| tmax, hours, median (range) | 3.0 (2.5–10) | 8.0 (2.5–16) | 10.0 (2.5–24) | 10.0 (8.0–24) |
| CLR, L/ha | 0.824 (31.8) | 0.487 (36.4) | 0.718 (39.2) | 0.573 (44.0) |
| Aeur(0–72), %a | 22.4 (41) | 14.1 (51) | 14.5 (44) | 17.8 (75) |
Unless otherwise stated, data are expressed as geometric means (percentage coefficient of variation). Aeur(0–72): proportion of dose excreted into urine from time 0 to 72 hours; AUC: area under the plasma concentration–time curve; AUCnorm: AUC divided by dose per kilogram of body weight; CLR: renal clearance; Cmax: maximum concentration in plasma; Cmax,norm: Cmax divided by dose per kilogram of body weight; t1/2: terminal elimination half-life; tmax: time to Cmax.
Arithmetic mean (percentage coefficient of variation).
Table 3.
Comparison of selected pharmacokinetic parameters for riociguat and M1 between different subgroups in the healthy volunteer study
| Parameter | Elderly vs. young (n = 18 per group) | Female vs. male (n = 18 per group) |
|---|---|---|
| Riociguat | ||
| AUC | 1.40 (1.06–1.86) | 1.09 (0.82–1.44) |
| Cmax | 1.16 (0.98–1.36) | 1.35 (1.14–1.59)a |
| AUCnorm | 1.28 (0.95–1.71) | 0.97 (0.72–1.30) |
| Cmax,norm | 1.05 (0.91–1.22) | 1.20 (1.03–1.38)a |
| t1/2 | 1.41 (1.08–1.84)a | 1.04 (0.79–1.35) |
| M1 | ||
| AUC | 1.21 (1.00–1.46) | 1.13 (0.94–1.36) |
| Cmax | 1.00 (0.73–1.38) | 1.50 (1.09–2.05)a |
| AUCnorm | 1.10 (0.93–1.30) | 1.00 (0.85–1.19) |
| Cmax,norm | 0.91 (0.68–1.23) | 1.33 (0.99–1.79) |
| t1/2 | 1.22 (1.04–1.44)a | 0.92 (0.78–1.08) |
Values are shown as ratios (90% confidence limits). AUC: area under the plasma concentration–time curve; AUCnorm: AUC divided by dose per kilogram of body weight; Cmax: maximum concentration in plasma; Cmax,norm: Cmax divided by dose per kilogram of body weight; t1/2: terminal elimination half-life.
P < 0.05 for ratio = 1.
Figure 1.
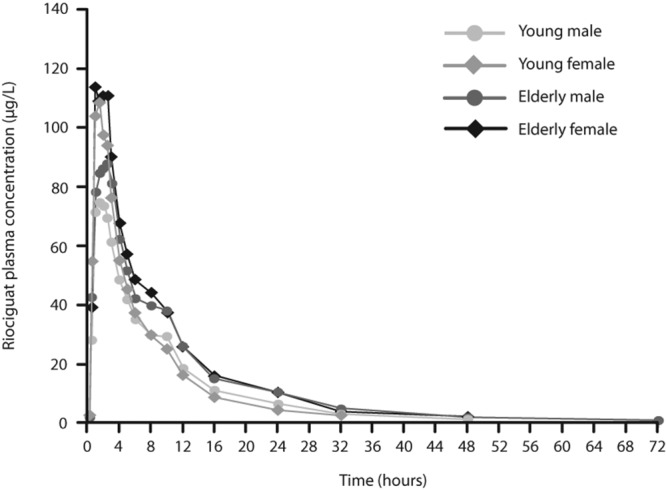
Geometric mean riociguat plasma concentrations in age and sex subgroups following administration of a single oral dose of riociguat 2.5 mg in the healthy volunteer study. Means were calculated when at least two-thirds of the data were above the lower limit of quantification of 0.5 μg/L. For calculation of mean values, concentrations below the lower limit of quantification were replaced by 0.25 μg/L.
Figure 2.
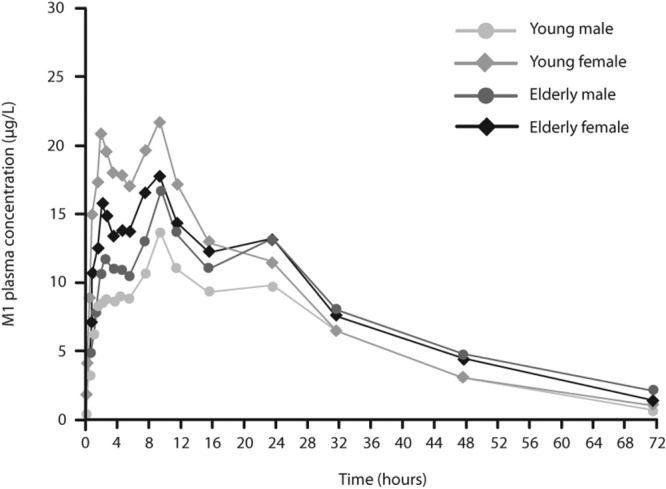
Geometric mean M1 plasma concentrations in age and sex subgroups following administration of a single oral dose of riociguat 2.5 mg. Means were calculated when at least two-thirds of the data were above the lower limit of quantification of 0.5 μg/L. For calculation of mean values, concentrations below the lower limit of quantification were replaced by 0.25 μg/L.
Effect of sex on pharmacokinetic characteristics. The mean Cmax of riociguat was approximately 35% higher in women than in men (P < 0.05). This difference was smaller (approximately 20%) when normalized for body weight (Cmax,norm) but remained statistically significant (P < 0.05; Table 3). Despite this difference, riociguat exposure (AUC) and t1/2 were similar in women and in men (Table 3).
The greatest difference between sexes was observed for Cmax values of M1, which were significantly higher in women than in men (50%, P < 0.05; Table 3). However, after normalization for body weight, the difference between sexes was reduced and no longer statistically significant (Cmax,norm 33% higher in women than in men, P > 0.05). Similar to the observed pharmacokinetics of riociguat, despite the significant difference in M1 Cmax values, there was little difference in exposure (AUC) to M1 between the sex groups.
Safety and tolerability
Riociguat was generally well tolerated. Three (8%) participants receiving riociguat reported study-drug-related adverse events. Mild nausea and vomiting were each reported by 1 EM and 1 YF participant. One EF participant experienced moderate dizziness and severe hypotension, the latter of which was considered serious. Both events resolved following intravenous administration of normal saline. There were no discontinuations due to adverse events, all of which had resolved by the conclusion of the study. No adverse events were reported by participants in the placebo subgroups, no drug-induced clinically relevant changes in laboratory parameters were detected, and electrocardiogram recordings showed no unexpected safety signals.
Patients receiving riociguat experienced a reduction in blood pressure. This change in blood pressure reached a mean maximum of −10.8 ± 16.4 mmHg for sitting systolic blood pressure and −8.5 ± 9.0 mmHg for sitting diastolic blood pressure 2 hours after dosing. At the same time point, sitting heart rate increased by +5.9 ± 6.0 beats/minute. The corresponding changes in the placebo group were +3.5 ± 11.1 mmHg, +0.4 ± 6.2 mmHg, and −1.9 ± 4.2 beats/minute. These effects waned substantially 16 hours after dosing, and blood pressure values had almost returned to baseline levels 24 hours after receiving riociguat.
Phase 3 trials
Study populations
Baseline demographic characteristics of the population of patients from PATENT-1/2 and CHEST-1/2 who were valid for the population pharmacokinetic analysis are shown in Table 4. Patients in the PATENT studies tended to be younger and weigh less than those in the CHEST studies. There was a higher percentage of women in the PATENT studies than in the CHEST studies.
Table 4.
Baseline demographic characteristics of the patients in the PATENT-1/2 and CHEST-1/2 studies
| Characteristic | PATENT-1/2 | CHEST-1/2 |
|---|---|---|
| Patients, no. | 438 | 260 |
| Age, years | 50.6 ± 16.4 | 59.3 ± 13.5 |
| Female, no. (%) | 347 (79) | 171 (66) |
| Weight, kg | 69.1 ± 17.8 | 74.7 ± 17.7 |
| Race, no. (%) | ||
| White | 269 (61) | 184 (71) |
| Black | 6 (1) | 8 (3) |
| Asian | 139 (32) | 57 (22) |
| Hispanic | 22 (5.0) | 10 (4) |
| Unknown | 2 (<1) | 1 (<1) |
Unless otherwise stated, data are expressed as mean ± standard deviation.
Pharmacokinetic evaluations: effect of age and sex on pharmacokinetic characteristics
A total of 642 patients had pharmacokinetic data available for analysis, providing more than 5,000 samples for riociguat and M1 (Table S3). Data from samples collected on the last day of the main study (day 84 of PATENT-1 and day 112 of CHEST-1) showed that riociguat exposure was age dependent, modestly increasing with age (Fig. 3, left). The AUC for riociguat was approximately 10% higher in women than in men (Fig. 3, right). Figure 4 shows the effects of the presence versus the absence of PH as well as age, weight, and sex on exposure to riociguat in the pooled studies.21 In a population pharmacokinetic analysis of the phase 3 trials of riociguat, covering an age range from 18 to 80 years (median: 56 years; n = 698), age was not a significant covariate for riociguat clearance but was strongly correlated with creatinine clearance, which was a significant covariate in the final pharmacokinetic model.
Figure 3.
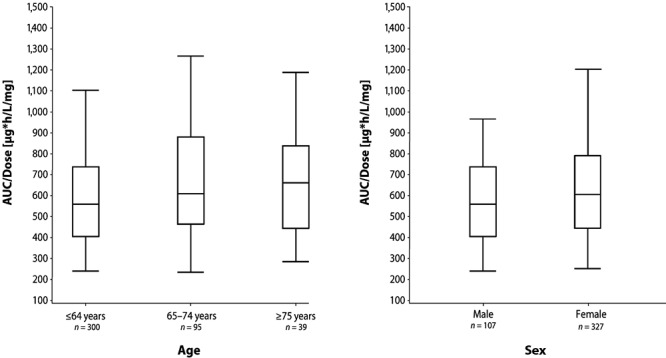
Riociguat dose-normalized area under the plasma concentration–time curve (AUC) at steady state on last day of main study (day 84 of PATENT-1; day 112 of CHEST-1) in patients aged ≤64, 65–74, and ≥75 years (left) and in men and women (right). Data show box-and-whisker plots with fifth, 25th, 50th, 75th, and 95th percentiles.
Figure 4.
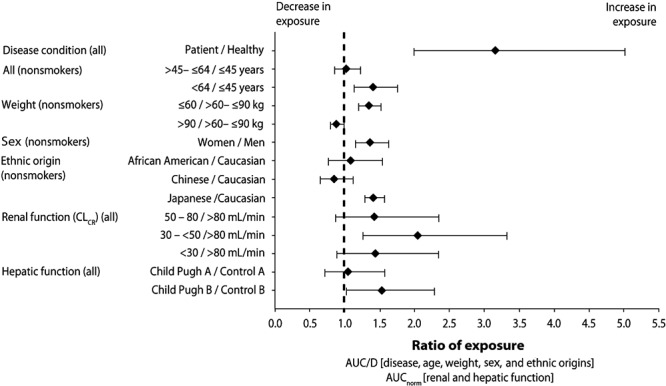
Impact of pulmonary hypertension, age, body weight, and sex on riociguat exposure (area under the plasma concentration–time curve/dose [AUC/D]), normalized for renal and hepatic function (AUCnorm), in subjects included in the pharmacokinetic analysis.21 CLCR: creatinine clearance.
Safety and tolerability
Riociguat was generally well tolerated in both the PATENT and CHEST studies, and safety and tolerability results for all 4 studies have been published previously.10,11,17,18 In summary, the most common adverse events were headache, dyspepsia, peripheral edema, nausea, dizziness, diarrhea, nasopharyngitis, and vomiting. Drug-related serious adverse events were reported in 2.4% of patients receiving riociguat 2.5 mg 3 times daily in PATENT-1 and in 3.5% of patients receiving riociguat in CHEST-1. Discontinuation of study drug because of adverse events was reported in 3.1% and 2.9% of patients, respectively. Riociguat was associated with significant reductions in mean arterial pressure of 9 ± 11 mmHg in PATENT-1 and 9 ± 12 mmHg in CHEST-1, and hypotension was reported as an adverse event in 10% and 9% of patients, respectively. Pooled safety data from the PATENT and CHEST trials revealed no significant effect of age, sex, or ethnicity on the tolerability of riociguat.21
Discussion
As described above, age and sex can influence the pharmacokinetics of many drugs. These effects may be relevant to the management of PAH and CTEPH because they can occur in both sexes and across a broad age range. Therefore, in the healthy volunteer study described here, we sought to identify any differences in the safety and pharmacokinetic properties of riociguat between young (18–45 years) and elderly (64.5–80 years) men and women. The results showed higher exposure to riociguat and its metabolite M1 in elderly than in young subjects: following a single oral dose (2.5 mg), the AUCs for riociguat and M1 were 40% and 21% greater in elderly than in young participants, respectively. The study also revealed a mean Cmax approximately 35% higher for riociguat and 50% higher for M1 in women than in men but no significant differences in AUC for either compound. Riociguat was associated with decreases in systolic and diastolic blood pressure and an increase in heart rate and was generally well tolerated.
The pharmacokinetic differences between subgroups were smaller when corrected for body weight. This observation suggests that the differences were partly related to the lower weight of elderly compared with young subjects and of women compared with men. The lower renal clearance in the elderly compared with the young subjects most likely also made a contribution to the greater drug exposure in the former group.
The reductions in blood pressure with riociguat in healthy volunteers were an expected consequence of its vasodilating action, and the increase in heart rate may have been a reflex response to this. Both effects have been seen previously with riociguat in healthy volunteers.22 The one subject with a serious adverse event was an elderly woman and thus belonged to the group with the highest AUC, highest Cmax, and lowest clearance of riociguat; however, it is not clear to what extent increased drug exposure contributed to the event. While hypovolemia is more common in the elderly and increases their vulnerability to hypotension, the fall in blood pressure in this subject occurred when she stood up, suggesting that an orthostatic mechanism may have been involved.
Overall exposure to riociguat among the patients in the phase 3 trials was significantly higher than in the healthy volunteers. This difference results principally from a longer elimination phase in patients compared with healthy subjects. Some intrinsic factors, such as older age, may contribute to increased riociguat exposure because of age-related declines in renal and hepatobiliary clearance processes. Moreover, PH per se also alters renal and/or hepatobiliary elimination of riociguat because of factors such as reduced cardiac output and worsening renal function. The effect of PH on riociguat exposure is greater than the effects of age or sex.
Despite the differences in total exposure, the effects of demographic characteristics on the population pharmacokinetics of riociguat in patients with PAH and CTEPH were consistent with those seen in healthy volunteers. Thus, older age and female sex were associated with a modestly increased AUC of riociguat; treatment was generally well tolerated, and riociguat was associated with reductions in mean arterial pressure and reports of hypotension. Pharmacokinetic analyses of phase 2 trials indicated that the increased exposure in older patients results from an age-dependent decline in renal clearance of riociguat. The lower riociguat exposure in men than in women may have been partly a consequence of the greater body weight of the men.
However, age and sex appear to have little effect on the clinical efficacy of riociguat. In PATENT-110 and CHEST-1,11 there was no statistically significant interaction between age (<65 years vs. ≥65 years) or sex and the change from baseline in 6-minute walking distance (the primary efficacy end point).
Of the total number of subjects in clinical studies of riociguat, 23% were aged 65 and over, and 6% were aged 75 and over. No overall differences in safety or effectiveness were observed between these and young subjects, but in view of their higher exposure to riociguat, greater sensitivity of some older individuals cannot be ruled out. Particular care should be exercised during individual dose titration of riociguat in elderly patients (65 years or older).23,24
In practice, women might experience adverse events with riociguat or require dose reduction more often than men because of their higher drug exposure. This could be especially important in PAH because this condition is more common in women than in men,25-27 as observed in PATENT-1, in which 79% of patients were women.10
Registries have reported an average patient age of approximately 50 years for PAH25 and 63 years for CTEPH.28 Consequently, a substantial proportion of patients, particularly with CTEPH, will be elderly, and therefore riociguat exposure may be higher. As a result, they may experience pharmacological effects at low doses, and the optimal dose may be below the maximum of 2.5 mg 3 times daily. Indeed, this forms part of the rationale behind the individual dose adjustment regimen that is recommended for treatment with riociguat.23,24
The studies described here have some limitations. By definition, the healthy volunteer study excluded subjects with diseases or medications that could influence pharmacokinetics, while in practice, many patients may have comorbidities or may be receiving medications that could have an influence. Most of the participants in all studies of riociguat were white. The responses to some drugs vary between ethnic groups,29 but the observation that ethnicity had only a minor effect on the efficacy or tolerability of riociguat suggests that inclusion of more nonwhite subjects would not have substantially altered the conclusions of the current study.
In conclusion, the results of the healthy volunteer study indicated that the effects of age and sex on the pharmacokinetics of riociguat were not sufficient to warrant a dose adjustment for these characteristics in subsequent clinical trials beyond the individual dose adjustment scheme employed for all studies. This dose scheme is indicated for all patients receiving riociguat treatment.23,24 This enables the optimal dose to be selected for the individual patient across variations in riociguat exposure resulting from differences in age, sex, or other characteristics.
Acknowledgments
We thank John Lettieri (Clinical Pharmacology, Bayer HealthCare Pharmaceuticals, Montville, NJ) and Andrea Nadel (Clinical Statistics, Bayer HealthCare Pharmaceuticals, Montville, NJ) for their contributions to the study and data analysis. We thank Miguel Zinny, Maryellen Fitzgerald, Arthur LaFluer, and Carolyn Maloney of ProMedica Clinical Research Center for their contributions to the conduct of the study.
Appendix. Supplementary tables
Table S1.
Demographic characteristics of age and sex subgroups of the healthy volunteer study
| Young male | Young female | Elderly male | Elderly female | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Placebo (n = 3) | Riociguat (n = 9) | Placebo (n = 2) | Riociguat (n = 9) | Placebo (n = 3) | Riociguat (n = 9) | Placebo (n = 3) | Riociguat (n = 9) |
| Race, no. (%) | ||||||||
| White | 2 (67) | 4 (44) | 1 (50) | 3 (33) | 3 (100) | 8 (89) | 3 (100) | 8 (89) |
| Black | 1 (33) | 4 (44) | 1 (50) | 5 (56) | 0 | 1 (11) | 0 | 0 |
| Asian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (11) |
| Hispanic | 0 | 1 (11) | 0 | 1 (11) | 0 | 0 | 0 | 0 |
| Age, years | 42.9 ± 0.6 | 36.9 ± 7.2 | 41.3 ± 5.2 | 40.8 ± 4.1 | 67.6 ± 2.9 | 69.8 ± 4.8 | 69.9 ± 5.5 | 68.8 ± 2.9 |
| Body mass index | 28.2 ± 0.5 | 28.2 ± 2.5 | 29.4 ± 3.1 | 28.1 ± 2.9 | 27.9 ± 3.1 | 25.6 ± 3.4 | 25.2 ± 4.6 | 26.4 ± 4.2 |
Unless otherwise stated, data are expressed as mean ± standard deviation.
Table S2.
Pharmacokinetic parameters of riociguat and M1 following a single oral dose of riociguat 2.5 mg: pooled results for young, elderly, male, and female subjects
| Parameter | Young (n = 18) | Elderly (n = 18) | Male (n = 18) | Female (n = 18) |
|---|---|---|---|---|
| Riociguat | ||||
| AUC, μg h/L | 775 | 1,089 | 881 | 958 |
| Cmax, μg/L | 95.1 | 110.0 | 88.2 | 118.6 |
| AUCnorm, g h/L | 25.6 | 32.7 | 29.4 | 28.4 |
| Cmax,norm, g/L | 3.14 | 3.30 | 2.94 | 3.52 |
| t1/2, hours | 8.52 | 12.01 | 9.93 | 10.30 |
| M1 | ||||
| AUC, μg h/L | 552 | 669 | 572 | 645 |
| Cmax, μg/L | 20.5 | 20.5 | 16.7 | 25.1 |
| AUCnorm, g h/L | 18.9 | 20.8 | 19.8 | 19.8 |
| Cmax,norm, g/L | 0.700 | 0.638 | 0.579 | 0.771 |
| t1/2, hours | 15.44 | 18.84 | 17.80 | 16.34 |
Data are expressed as geometric least squares means of the samples in each group. AUC: area under the plasma concentration–time curve; AUCnorm: AUC divided by dose per kilogram of body weight; Cmax: maximum concentration in plasma; Cmax,norm: Cmax divided by dose per kilogram of body weight; t1/2: terminal elimination half-life.
Table S3.
Sample sizes (n) for pharmacokinetic data in the PATENT-1/2 and CHEST-1/2 studies
| PATENT-1 | CHEST-1 | PATENT-2 | CHEST-2 | Total | |
|---|---|---|---|---|---|
| Patients with pharmacokinetic data | 314 | 172 | 355 | 192 | 642 |
| Plasma samples for riociguat | 3,131 | 1,131 | 640 | 343 | 5,245 |
| Plasma samples for M1 | 3,131 | 1,131 | 640 | 344 | 5,246 |
Source of Support: The study was funded by a research grant from Bayer Pharma. Medical writing support was provided by Adelphi Communications and was funded by Bayer Pharma.
Conflict of Interest: All authors are employees of Bayer Pharma.
Supplement
Appendix: Supplementary tablesPulmCirc-006-S58.s001.pdf (414.9KB, pdf)
References
- 1.Rosenkranz S. Pulmonary hypertension: current diagnosis and treatment. Clin Res Cardiol 2007;96(8):527–541. [DOI] [PubMed]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 3.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012;39(4):945–955. [DOI] [PubMed]
- 4.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from REVEAL. Chest 2012;142(2):448–456. [DOI] [PubMed]
- 5.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006;5(9):755–768. [DOI] [PMC free article] [PubMed]
- 6.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA 1977;74(8):3203–3207. [DOI] [PMC free article] [PubMed]
- 7.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 2009;191:277–308. [DOI] [PubMed]
- 8.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123(20):2263–2273. [DOI] [PMC free article] [PubMed]
- 9.Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Muller D, Schluter KD, Dingendorf A, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J 2008;32(4):881–891. [DOI] [PubMed]
- 10.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 11.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369(4):319–329. [DOI] [PubMed]
- 12.Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37(1):67–119. [DOI] [PubMed]
- 13.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 2009;41(2):67–76. [DOI] [PubMed]
- 14.Turnheim K. When drug therapy gets old: pharmacokinetics and pharmacodynamics in the elderly. Exp Gerontol 2003;38(8):843–853. [DOI] [PubMed]
- 15.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet 2003;42(2):107–121. [DOI] [PubMed]
- 16.Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol 2011;2011:187103. [DOI] [PMC free article] [PubMed]
- 17.Rubin LJ, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh A, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J 2015;45(5):1303–1313. [DOI] [PubMed]
- 18.Simonneau G, D’Armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J 2015;45(5):1293–1302. [DOI] [PubMed]
- 19.Rubin LJ, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): 2-year results from the PATENT-2 long-term extension. Eur Respir J 2014;44(suppl. 58):1803. [DOI] [PubMed]
- 20.Simonneau G, D’Armini AM, Ghofrani HA, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH): 2-year results from the CHEST-2 long-term extension. Eur Respir J 2014;44(suppl. 58):1802. [DOI] [PubMed]
- 21.Bayer HealthCare Pharmaceuticals. Briefing document for Cardiovascular and Renal Drugs Advisory Committee: riociguat (BAY 63-2521). Published August 6, 2013. Accessed August 20, 2015. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm363543.pdf.
- 22.Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol 2008;48(8):926–934. [DOI] [PubMed]
- 23.Bayer HealthCare Pharmaceuticals. Prescribing information [Adempas (riociguat) tablets]. Accessed June 27, 2014. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf.
- 24.European Medicines Agency. Annex I: summary of product characteristics [Adempas (riociguat tablets)]. Accessed November 25, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf.
- 25.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137(2):376–387. [DOI] [PubMed]
- 26.Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grünig E, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2012;168(2):871–880. [DOI] [PubMed]
- 27.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Circulation 2009;119(16):2250–2294. [DOI] [PubMed]
- 28.Pepke-Zaba J, Delcroix M, Lang I, Mayer E, Jansa P, Ambroz D, Treacy C, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation 2011;124(18):1973–1981. [DOI] [PubMed]
- 29.Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 2008;84(3):417–423. [DOI] [PubMed]


