Abstract Abstract
Renal impairment is a common comborbidity in patients with pulmonary hypertension. The breakdown of riociguat, an oral soluble guanylate cyclase stimulator used to treat pulmonary hypertension, may be affected by smoking because polycyclic aromatic hydrocarbons in tobacco smoke induce expression of one of the metabolizing enzymes, CYP1A1. Two nonrandomized, nonblinded studies were therefore performed to investigate the pharmacokinetics and safety of a single oral dose of riociguat 1.0 mg in individuals with mild, moderate, or severe renal impairment compared with age-, weight-, and sex-matched healthy controls, including either smokers and nonsmokers (study I) or nonsmokers alone (study II). Pharmacokinetic analyses focused on the integrated per-protocol data set of both studies (N = 63). In patients with renal impairment, the renal clearance of riociguat was reduced and its terminal half-life prolonged compared with those in healthy controls. There was a monotonic relationship between creatinine clearance on treatment day and riociguat renal clearance (R2 = 0.62). However, increased riociguat exposure with decreasing renal function was not strictly proportional. Riociguat exposure appeared to be greater in nonsmokers than in the combined population of smokers and nonsmokers, irrespective of renal function. Adverse events were mild to moderate and in line with the mode of action of riociguat. No serious adverse events occurred. In conclusion, renal impairment was associated with reduced riociguat clearance compared with that in controls; however, riociguat exposure in patients with renal impairment was highly variable, and ranges overlapped with those observed in healthy controls.
Keywords: soluble guanylate cyclase, CYP1A1, pulmonary hypertension, clinical pharmacology, renal function
Pulmonary hypertension (PH) is a debilitating disease associated with a poor prognosis and high mortality. Vascular remodeling and pulmonary vasoconstriction lead to increased vascular resistance and ultimately to right heart failure.1,2 After diagnosis, median survival of patients with untreated primary PH has been estimated as 2.8 years.3 Although recent advances in treatment have improved outcomes, the 3-year survival rates for patients with PH of various etiologies remain low: 68% for pulmonary arterial hypertension (PAH), 71% for chronic thromboembolic PH (CTEPH), and 44% for PH associated with lung diseases.4
The vasodilatory agent nitric oxide (NO) modulates the pulmonary vasculature via stimulation of soluble guanylate cyclase (sGC), which leads to increased intracellular levels of the secondary messenger cyclic guanosine monophosphate (cGMP). The NO-sGC-cGMP vasodilatory pathway is disrupted in patients with PH,5,6 with the lung tissues of patients with primary PH having less NO than those of healthy individuals. In addition, patients with PH exhibit lower levels of exhaled NO, and severity of PH correlates inversely with levels of NO reaction products in the lung.7,8 Riociguat (BAY 63-2521) targets the NO-sGC-cGMP vasodilatory pathway via a dual mode of action: the drug stimulates sGC directly by an NO-independent but heme-dependent mechanism, and it increases the sensitivity of sGC to endogenous NO, thereby restoring cGMP levels.9,10 Riociguat is the first oral sGC stimulator developed for the treatment of PH,10,11 and it is approved in more than 40 countries, including the United States and countries of the European Union.
In a study of 58 healthy male volunteers, a single dose of riociguat (0.25–5 mg) was well tolerated, had a favorable safety profile, and exhibited dose-proportional pharmacokinetics.12 Phase 2 studies in patients with PAH, CTEPH, or PH associated with interstitial lung disease demonstrated suitable efficacy, tolerability, and safety of riociguat to warrant progression to phase 3 trials.13-15 Results from the placebo-controlled phase 3 Pulmonary Arterial Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 (PATENT-1) showed promising efficacy and safety profiles with a 12-week course of riociguat (0.5–2.5 mg 3 times daily) in treatment-naïve patients with PAH and those previously exposed to endothelin receptor antagonists or nonintravenous prostanoids.16 A similar study in patients with inoperable CTEPH, the Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase–Stimulator Trial 1 (CHEST-1), reported significant improvements in exercise capacity and hemodynamics after 16 weeks of riociguat (0.5–2.5 mg 3 times daily) compared with placebo.17,18
N-demethylation of the parent drug to the pharmacologically active metabolite M1 (BAY 60-4552), catalyzed primarily by the cytochrome P450 (CYP) mono-oxygenases CYP1A1, CYP3A4, CYP2C8, and CYP2J2, is the major biotransformation pathway for riociguat in humans.19 CYP1A1, which is induced by tobacco smoke,20 is thought to be the most important enzyme involved in the metabolic clearance of riociguat; therefore, smoking is considered to be the major source of variability in clearance of the drug.
Mass-balance data indicate that the kidney contributes substantially to the total body clearance of riociguat, with 33%–45% of the administered parent drug ([14C]-radiolabeled riociguat 1.0 mg, oral solution) excreted via the renal-urinary route as unchanged riociguat or M1.19 Renal impairment may thus affect the renal clearance of riociguat and consequently the pharmacokinetics, safety, and tolerability of the drug.
The clinical pharmacology studies described here were undertaken primarily to assess the pharmacokinetics and safety of a single oral dose of riociguat in individuals with mild, moderate, or severe renal impairment compared with those in healthy individuals. In addition, the effects of smoking on riociguat pharmacokinetics were evaluated.
Methods
Study design and treatments
Two nonrandomized, nonblinded studies with group stratification were conducted at a single center in Germany (study I from October 2008 to February 2009, study II from February 2010 to March 2011). After completion of study I, it became evident from the results of preclinical studies that riociguat is metabolized to the primary metabolite M1 by CYP1A1,19 a CYP enzyme known to be highly inducible by agents in tobacco smoke.20 Thus, active smokers are likely to have an elevated rate of metabolism of riociguat to M1 compared with nonsmokers. To reduce the effect of smoking as a confounding variable on the results of the pharmacokinetic analysis, a second study was conducted, with the same design but restricted to nonsmoking individuals (study II).
All study participants were stratified into one of four groups according to their creatinine clearance (CLCR): group 1 had CLCR > 80 mL/min (normal renal function; age-, weight- and sex-matched healthy controls); group 2 had CLCR = 50–80 mL/min (mild renal impairment); group 3 had CLCR = 30–49 mL/min (moderate renal impairment); and group 4 had CLCR < 30 mL/min (severe renal impairment; not receiving dialysis from 4 weeks before enrollment to study conclusion). CLCR was calculated by serum creatinine concentration and excretion of creatinine collected in urine samples over 24 hours.
In study I, because the exposure and tolerability of riociguat in individuals with severe renal impairment was previously unknown, participants with CLCR < 30 mL/min received a single oral immediate-release tablet of riociguat 0.5 mg. All other participants in both study I and study II received a 1.0-mg dose of riociguat consisting of 2 oral immediate-release 0.5-mg tablets. Participants remained at the study center for 3 days and underwent a final medical examination within the 2-week period after riociguat dosing.
Both studies were conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guidelines, and the German drug law (Arzneimittelgesetz). Both studies observed the US Food and Drug Administration and European Medicines Agency guidelines for industry and were approved by the Ethics Committee of the Schleswig-Holstein Medical Council, Bad Segeberg, Germany.21,22 All individuals gave written informed consent to participate in the studies.
Study population
Men and women aged 18–75 years (study I) or 18–79 years (study II) were eligible to participate in the studies if they had a body mass index (BMI) in the range of 18–34, a resting heart rate of 45–90 beats per minute, and a negative drug screen. A negative screen for hepatitis B, hepatitis C, and human immunodeficiency virus was also required. Study participants were permitted a daily consumption of not more than 40 g (5 units) of alcohol or 1 L of xanthine-containing beverages. In study I, participants were allowed a regular daily consumption of up to 25 cigarettes; only nonsmoking individuals who had not smoked for at least 3 months were eligible to participate in study II.
Renal impairment was indicated by a CLCR of no more than 80 mL/min, determined 2–14 days before drug administration. Individuals with renal impairment and stable renal disease (defined as <20% variation between serum creatinine values measured at the prestudy visit and those determined at least 3–6 months before the prestudy visit) were eligible to participate in the study. A systolic blood pressure (SBP) of no more than 180 mmHg and a diastolic blood pressure (DBP) of no more than 100 mmHg were required. Healthy individuals who had a CLCR of >80 mL/min, an SBP in the range of 100–145 mmHg, and a DBP of no more than 95 mmHg were enrolled as controls. Mean age and body weight of healthy controls did not vary from those of the renally impaired groups by more than 10 years and 10 kg, respectively. Exclusion criteria applicable to both studies are detailed in Table 1. Use of medicinal products other than study drugs was not permitted during the study without consulting the investigator. Individuals with renal impairment, however, were allowed to continue permitted medications (for details, see below and Table 1). Any concomitant medication was documented. The administration of concomitant medication was withheld until 4 hours after riociguat intake to avoid possible confounding effects of concomitant medications on the absorption of riociguat; for example, the use of oral iron supplements; antacids such as magnesium, aluminum, or calcium compounds; or drugs for the treatment of hyperphosphatemia such as calcium compounds, sevelamer, or lanthanum was not permitted from 16 hours before until 8 hours after riociguat administration. For the same reason, drugs for acid-related disorders, such as H2-receptor antagonists and proton-pump inhibitors, were not permitted from 48 hours before until 8 hours after riociguat administration.
Table 1.
Main exclusion criteria applicable to both studies
| Criteria |
|---|
| Applicable to all individuals |
| History of medical disorders or conditions that could impair an individual’s ability to participate in or complete the study |
| Febrile illness in the week before the study |
| History of severe allergies and nonallergic drug reactions |
| Pathological changes in the electrocardiogram, such as a second- or third-degree atrioventricular block, prolongation of the QRS complex to more than 120 ms, or prolongation of the QTc interval to more than 450 ms |
| Participation in another clinical study in the previous 3 months |
| Donation of more than 100 mL blood in the preceding 4 weeks or 500 mL in the preceding 3 months |
| Pregnant women and women of childbearing age not using double-barrier contraception |
| Applicable to patients with renal impairment |
| Concurrent diagnosis of severe cerebrovascular or cardiac disorders, acute renal failure, acute nephritis, or failure of any organ system other than the kidney |
| Diagnosis of malignancy within the previous 5 years |
| Severe infection or any clinically significant illness in the 4 weeks before drug administration |
| Any organ transplant |
| Percutaneous transluminal coronary angioplasty or coronary artery bypass graft <6 months before riociguat administration |
| Concomitant thrombotic disorders |
| Platelet count less than 100 × 109/L |
| Diabetes mellitus with a fasting blood glucose level of >220 mg/dL or glycosylated hemoglobin levels of >10% |
| History of bleeding in the past 3 months |
| Concomitant use of potent CYP3A4 inhibitors, phosphodiesterase-5 inhibitors, endothelin receptor antagonists, prostacyclins, or treatment with any medication (except those necessary for the treatment of kidney disease or related complications) |
| Change in chronic medication less than 4 weeks before dosing |
Safety and tolerability
Safety and tolerability were evaluated by monitoring standard vital signs, electrocardiography, and laboratory investigations, including hematology, urinalysis, and biochemical tests. Adverse events (AEs) were identified by questioning of participants and through spontaneous self-reporting. All AEs were classified according to their degree of severity (mild, moderate, or severe) and whether or not they were serious.
Pharmacokinetic evaluation
For analysis of riociguat and M1 pharmacokinetic parameters, plasma samples were taken at the time of riociguat administration (0 hours) and at 15, 30, and 45 minutes and 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 24, 36, 48, 60, and 72 hours after dosing. Urine samples were obtained at 0–4, 4–8, 8–12, 12–24, 24–48, and 48–72 hours. Samples were stored below −15°C until analysis and analyzed within 6 months.
In study I, total (bound and unbound) riociguat and M1 concentrations in plasma and urine were determined by high-performance liquid chromatography coupled with mass spectrometry (HPLC/MS). Internal standards were [2H3]riociguat and [2H3]M1, with working ranges from 0.500 μg/L (the lower limit of quantification) to 100 μg/L in plasma and from 10.0 to 1,000 μg/L in urine. Quality control (QC) samples in the plasma concentration range 1.50–80.0 μg/L were determined with an accuracy of 104%–113% and a precision of 3.95%–9.81% for riociguat and with an accuracy of 100%–112% and a precision of 5.24%–7.47% for M1. QC samples in the urine concentration range 30.0–800 μg/L were determined with an accuracy of 95.3%–101% and a precision of 3.80%–6.50% for riociguat and with an accuracy of 98.7%–101% and a precision of 4.56%–7.93% for M1.
In study II, total riociguat and M1 concentrations in plasma and urine were determined by HPLC/MS, with [2H3]riociguat and [2H3]M1 as the internal standards. The working ranges were 0.500–250 μg/L in plasma and 10.0–1,000 μg/L in urine. QC samples in the plasma concentration range 1.50–200 μg/L were determined with an accuracy of 98.3%–101% and a precision of 5.11%–6.97% for riociguat and with an accuracy of 95.4%–99.8% and a precision of 5.33%–8.15% for M1. QC samples in the urine concentration range 30.0–800 μg/L were determined with an accuracy of 95.8%–100% and a precision of 4.62%–5.47% for riociguat and with an accuracy of 99.0%–102% and a precision of 5.06%–9.06% for M1.
In both studies, determination of bound and unbound riociguat and M1 fractions was performed in vitro according to the method of Schuhmacher et al.,23 using [14C]-radiolabeled test substances. Noncompartmental methods, performed in WinNonlin, version 4.1a (Pharsight, Mountain View, CA), were used to calculate the following pharmacokinetic parameters for total (bound and unbound) riociguat and M1: area under the plasma concentration–time curve from time 0 to infinity (AUC), maximum concentration in plasma (Cmax), time to Cmax (tmax), terminal elimination half-life (t1/2), apparent oral clearance (CL/F), amount excreted into urine from time 0 to infinity, and renal clearance (CLR). AUC was obtained with the linear-logarithmic trapezoidal method, and t1/2 was calculated by linear least squares (LS) regression after logarithmic transformation of the (3 or more) terminal concentrations. Values normalized by dose of riociguat per kilogram body weight (AUCnorm and Cmax, norm) were also derived. Corresponding parameters for unbound riociguat and M1 (AUCu, AUCu, norm, Cmax, u, and Cmax, u, norm) were calculated with the fraction unbound (fu).
Statistical analysis
Statistical evaluation was performed with SAS software, releases 9.1 and 9.2 (SAS Institute, Cary, NC). An integrated analysis explored the pooled data from both studies to evaluate the effect of renal impairment on riociguat and M1 pharmacokinetics in a population considered to be representative of the target population (i.e., a population of smokers and nonsmokers, with an enrichment of nonsmokers). Individuals with severe renal impairment in study I received a 0.5-mg dose of riociguat rather than the 1.0-mg dose that was received by all other participants in both studies. Therefore, the pharmacokinetic concentrations recorded for these individuals were normalized to a 1.0-mg dose before commencement of the integrated analysis.
Descriptive statistics of pharmacokinetic results were calculated. The logarithms of parameters AUCnorm and Cmax, norm for riociguat and M1 were subjected to an analysis of variance, including a group effect to explore the pharmacokinetics between groups of differing renal function. Data were assumed to fit a lognormal distribution. On the basis of these results, point estimates (LS means) and exploratory 90% confidence intervals (CIs) were calculated for the pairwise comparisons of the mild-, moderate-, and severe-renal-impairment groups with the control group. Graphical descriptions of the relationships between renal function (CLCR) and pharmacokinetic parameters were carried out. Linear regression lines were fitted to describe the possible relationships between CLCR and each pharmacokinetic parameter.
Sample size was determined with nQuery Advisor 7.0 (Statistical Solutions, Cork, Ireland). Assuming that the standard deviation of CLCR is 39.4 mL/min24 and that the standard deviation of the residuals is 31.7 kg·h/L (study I data), a pooled sample size of 64 individuals would have 78% power to detect a difference between the null hypothesis (that the regression slope of CLCR and AUCnorm for riociguat is 0.00) and an alternative hypothesis (that the regression slope is −0.279 kg·h/L), using the 2-sided t test at α = 0.05.
Results
Study population
Baseline demographic characteristics of study participants are summarized in Table 2. A total of 72 individuals received riociguat in studies I (n = 32) and II (n = 40) and provided valid data for the safety evaluations. Sixty-three individuals (52 nonsmokers and 11 smokers; 40 men and 23 women; mean age: 61.3 years; range: 36–78 years) received riociguat and were eligible for inclusion in the per-protocol set and pharmacokinetic analyses. Eight individuals were excluded because of unstable impairment of renal function, indicated by an increase in CLCR exceeding 15 mL/min after riociguat administration compared with CLCR recorded at the prestudy (screening) visit. One individual was excluded from the pharmacokinetic analysis after administration of a prohibited concomitant medication (esomeprazole). The study population was generally representative of a nonobese population (mean BMI for total study population: 27.3).
Table 2.
Baseline demographic characteristics of healthy individuals (normal renal function) and patients with mild, moderate, or severe renal impairment in the mixed population of smokers and nonsmokers
| Demographic characteristic | Normal (CLCR > 80 mL/min) |
Mild (CLCR = 50–80 mL/min) |
Moderate (CLCR = 30–49 mL/min) |
Severe (CLCR < 30 mL/min) |
Total |
|---|---|---|---|---|---|
| Patients | 16 | 15 | 16 | 16 | 63 |
| Age, mean (range), years | 60.3 (44.0–69.0) | 63.9 (53.0–74.0) | 60.1 (36.0–78.0) | 61.2 (39.0–75.0) | 61.3 (36.0–78.0) |
| Female | 5 (31) | 8 (53) | 6 (38) | 4 (25) | 23 (37) |
| White | 16 (100) | 15 (100) | 16 (100) | 16 (100) | 63 (100) |
| BMI, mean (SD) | 27.5 (2.7) | 27.3 (4.1) | 28.3 (3.6) | 26.1 (3.4) | 27.3 (3.5) |
| Current smoker | 2 (13) | 3 (20) | 4 (25) | 2 (13) | 11 (17) |
Except where indicated, data are no. (%) of individuals. Means are arithmetic means. BMI: body mass index; CLCR, creatinine clearance.
Safety and tolerability
No serious AEs were reported during the studies, and there were no deaths. Treatment-emergent AEs were reported by 18 of 72 (25%) individuals, and the incidence was similar in individuals with renal impairment and healthy volunteers. All treatment-emergent AEs resolved by study completion. Twelve (17%) individuals experienced treatment-emergent, drug-related AEs; all were mild or moderate in severity. The most commonly reported drug-related AE was headache, reported in 2 (13%) healthy volunteers, 5 (25%) individuals with mild renal impairment, and 1 (5%) individual with moderate renal impairment. After riociguat administration, no clinically relevant changes in laboratory parameters were seen, and there were no consistent changes in vital signs that could be attributed to enhanced vasodilation. No clinically relevant changes in electrocardiogram parameters were observed.
Pharmacokinetics
Table 3 summarizes the pharmacokinetic parameters for riociguat and M1 in the study population valid for analysis. Riociguat was readily absorbed: the median tmax was 1.00 hour in each of the renal-function groups (Table 3; Fig. 1A). Riociguat plasma concentrations subsequently declined, with a mean t1/2 of 6.19 hours in healthy individuals. In comparison, mean t1/2 was prolonged in individuals with mild, moderate, or severe renal impairment, ranging from 9.52 to 11.4 hours (Table 3). In healthy individuals, the median tmax for total plasma M1 was 4.00 hours after riociguat administration, with a mean t1/2 of 13.9 hours (Table 3; Fig. 1B). Median tmax and mean t1/2 of total M1 were somewhat prolonged across the three groups of individuals with renal impairment (the extended t1/2 of M1 was consistent with the increased contribution of CLR to the overall excretion of M1 compared with riociguat; Table 3). Mean total riociguat and total M1 Cmax, norm values were similar in all four groups: the ratios (90% CIs) of LS means for Cmax, norm between renally impaired and healthy individuals ranged from 96.6 (81.7–114) to 115 (97.5–136) for riociguat and from 76.1 (55.2–105) to 86.4 (63.0–119) for M1 (Table 4).
Table 3.
Pharmacokinetic parameters for riociguat and metabolite M1 in healthy individuals and patients with mild, moderate, or severe renal impairment, following a single oral dose of riociguat 1.0 mg in the mixed population of smokers and nonsmokers
| Parameter | Normal (CLCR > 80 mL/min) |
Mild (CLCR = 50–80 mL/min) |
Moderate (CLCR = 30–49 mL/min) |
Severe (CLCR < 30 mL/min)a |
|---|---|---|---|---|
| Patients, no. | 16 | 15 | 16 | 16 |
| Riociguat | ||||
| AUC, μg·h/L | 246 (51) | 375 (111) | 499 (110) | 523 (70)b |
| AUCnorm, kg·h/L | 20.6 (56) | 29.4 (126) | 42.1 (109) | 29.7 (102) |
| Cmax, μg/L | 36.6 (17) | 44.2 (21) | 42.0 (32) | 40.6 (38)b |
| Cmax, norm, kg/L | 3.07 (17) | 3.48 (25) | 3.54 (30) | 2.97 (40) |
| tmax, hours | 1.00 (0.500–2.00) | 1.00 (0.500–3.00) | 1.00 (0.500–3.00) | 1.00 (0.500–4.00) |
| t1/2, hours | 6.19 (50) | 10.1 (116) | 11.4 (103) | 9.52 (75) |
| CL/F, L/h | 4.07 (51) | 2.67 (111) | 2.00 (110) | 2.61 (95) |
| fu, % | 3.40 (18) | 3.10 (21) | 3.62 (27) | 4.11 (26) |
| AUCu, norm, kg·h/L | 0.702 (53) | 0.912 (123) | 1.53 (129) | 1.22 (107) |
| Cmax, u, norm, kg/L | 0.104 (25) | 0.108 (34) | 0.128 (37) | 0.122 (37) |
| AE, ur, % | 8.73 ± 4.60 | 10.8 ± 7.17c | 6.21 ± 4.01 | 4.04 ± 2.93d |
| CLR, L/h | 0.306 (40) | 0.207 (35)c | 0.0995 (71) | 0.0564 (92)d |
| Metabolite M1 | ||||
| AUC, μg·h/L | 257 (22) | 278 (42) | 418 (44) | 547 (62)b |
| AUCnorm, kg·h/L | 22.3 (20) | 22.6 (40) | 36.5 (40) | 39.0 (50) |
| Cmax, μg/L | 9.85 (48) | 8.01 (94) | 7.94 (55) | 8.73 (52)b |
| Cmax, norm, kg/L | 0.855 (44) | 0.651 (84) | 0.694 (51) | 0.739 (51) |
| tmax, hours | 4.00 (4.00–24.0) | 10.0 (3.00–24.1) | 24.0 (4.00–48.0) | 20.0 (2.00–24.1) |
| t1/2, hours | 13.9 (27) | 22.6 (54) | 31.0 (54) | 30.3 (79) |
| CL/F, L/h | 3.76 (22) | 3.47 (42) | 2.32 (44) | 1.99 (55) |
| fu, % | 2.90 (14) | 2.69 (18) | 3.20 (23) | 3.86 (30) |
| AUCu, norm, kg·h/L | 0.646 (23) | 0.611 (41) | 1.17 (39) | 1.51 (53) |
| Cmax, u, norm, kg/L | 0.0248 (49) | 0.0175 (91) | 0.0222 (44) | 0.0286 (52) |
| AE, ur, % | 19.0 ± 6.85 | 11.5 ± 6.85 | 7.38 ± 3.60d | 4.00 ± 3.40e |
| CLR, L/h | 0.698 (53) | 0.363 (73) | 0.189 (74)d | 0.0645 (69)e |
Values are geometric means (percentage coefficient of variation), except for the number of patients; tmax, which is expressed as median (range); and AE, ur, which is expressed as arithmetic mean ± standard deviation. AE, ur: amount excreted into urine from time 0 to infinity; AUC: area under the plasma concentration–time curve from time 0 to infinity; AUCnorm: AUC divided by dose of riociguat per kilogram body weight for total riociguat/M1; AUCu, norm: AUC divided by dose of riociguat per kilogram body weight for unbound riociguat/M1; CLCR: creatinine clearance; CL/F: apparent oral clearance for total riociguat/M1; CLR: renal clearance of riociguat/M1; Cmax: maximum concentration in plasma; Cmax, norm, Cmax divided by dose of riociguat per kilogram body weight for total riociguat/M1; Cmax, u, norm, Cmax divided by dose of riociguat per kilogram body weight for unbound riociguat/M1; fu, fraction unbound; tmax, time to Cmax of total riociguat/M1; t1/2, terminal elimination half-life for total riociguat/M1.
In study I, individuals with severe renal impairment received riociguat 0.5 mg, and therefore pharmacokinetic concentrations recorded for these individuals were normalized to a 1.0-mg dose before analysis; all other participants in both studies received riociguat 1.0 mg.
AUC and Cmax values shown for individuals with severe renal impairment are taken from study II (n = 8), in which individuals with severe renal impairment received riociguat 1.0 mg.
Data available from 14 patients.
Data available from 11 patients.
Data available from 8 patients.
Figure 1.
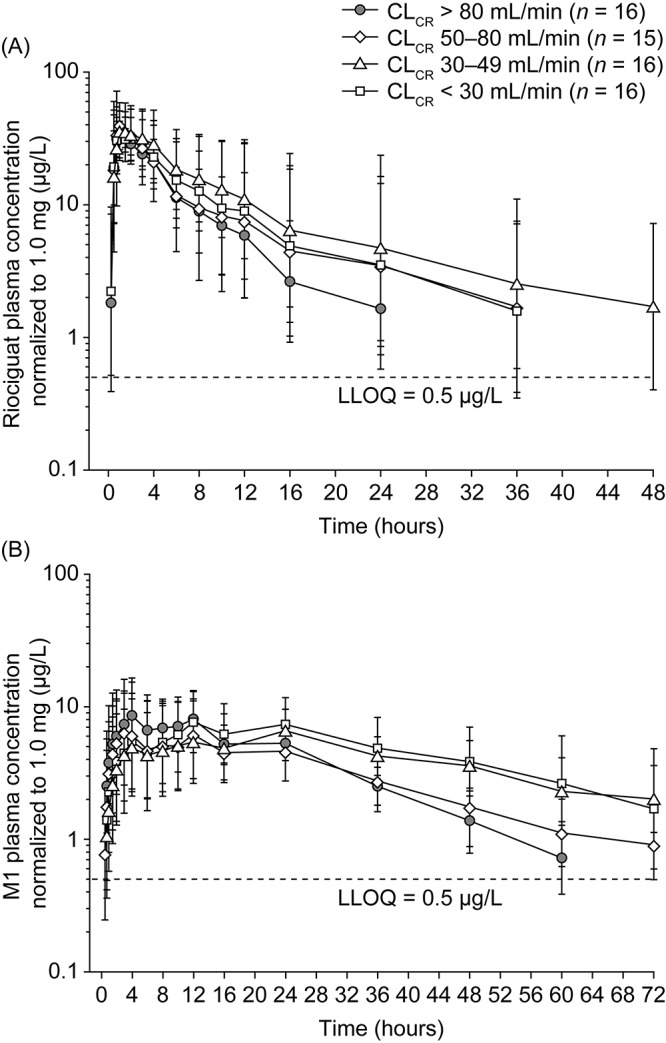
Geometric mean plasma concentration–time courses of total riociguat (A) and metabolite M1 (B) after a single oral dose of riociguat 1.0 mg in the mixed population (smokers and nonsmokers). In study I, individuals with severe renal impairment received riociguat 0.5 mg; plasma concentrations in these subjects were normalized to a 1.0-mg riociguat dose. Bars denote the geometric standard deviation; semilogarithmic scale, N = 63 (all individuals valid for pharmacokinetic evaluation). Individual data values below the LLOQ ceased to be measured. Mean values at any one time were calculated only if at least two-thirds of individual data were above the LLOQ. Values below the LLOQ were substituted with half of this limit for calculation of mean values. CLCR: creatinine clearance; LLOQ: lower limit of quantification.
Table 4.
Point estimates (least squares means) and 2-sided 90% CIs of selected pharmacokinetic parameters for total and unbound riociguat and metabolite M1 in plasma from patients with mild, moderate, or severe renal impairment versus those in plasma from individuals with normal renal function, following a single oral dose of riociguat 1.0 mg in the mixed population of smokers and nonsmokers
| Comparison, parameter | n | CV, % | Estimated ratio, % | 90% CI |
|---|---|---|---|---|
| Riociguat (total) | ||||
| Mild vs. normal | ||||
| AUCnorm | 31 | 98 | 143 | 87.1–234 |
| Cmax, norm | 31 | 29 | 113 | 95.4–134 |
| Moderate vs. normal | ||||
| AUCnorm | 32 | 98 | 204 | 126–332 |
| Cmax, norm | 32 | 29 | 115 | 97.5–136 |
| Severea vs. normal | ||||
| AUCnorm | 32 | 98 | 144 | 88.6–234 |
| Cmax, norm | 32 | 29 | 96.6 | 81.7–114 |
| Metabolite M1 (total) | ||||
| Mild vs. normal | ||||
| AUCnorm | 31 | 39 | 101 | 81.0–127 |
| Cmax, norm | 31 | 58 | 76.1 | 55.2–105 |
| Moderate vs. normal | ||||
| AUCnorm | 32 | 39 | 163 | 131–204 |
| Cmax, norm | 32 | 58 | 81.1 | 59.1–111 |
| Severea vs. normal | ||||
| AUCnorm | 32 | 39 | 175 | 140–218 |
| Cmax, norm | 32 | 58 | 86.4 | 63.0–119 |
| Riociguat (unbound) | ||||
| Mild vs. normal | ||||
| AUCu, norm | 31 | 103 | 130 | 78.0–217 |
| Cmax, u, norm | 31 | 34 | 103 | 84.8–126 |
| Moderate vs. normal | ||||
| AUCu, norm | 32 | 103 | 218 | 132–360 |
| Cmax, u, norm | 32 | 34 | 123 | 101–149 |
| Severea vs. normal | ||||
| AUCu, norm | 32 | 103 | 174 | 105–288 |
| Cmax, u, norm | 32 | 34 | 117 | 96.2–142 |
| Metabolite M1 (unbound) | ||||
| Mild vs. normal | ||||
| AUCu, norm | 31 | 40 | 94.6 | 75.1–119 |
| Cmax, u, norm | 31 | 59 | 70.7 | 50.9–98.2 |
| Moderate vs. normal | ||||
| AUCu, norm | 32 | 40 | 181 | 144–227 |
| Cmax, u, norm | 32 | 59 | 89.7 | 64.9–124 |
| Severea vs. normal | ||||
| AUCu, norm | 32 | 40 | 233 | 186–293 |
| Cmax, u, norm | 32 | 59 | 115 | 83.4–159 |
AUCnorm: area under the plasma concentration–time curve from time 0 to infinity divided by dose of riociguat per kilogram body weight for total riociguat/M1; AUCu, norm: area under the plasma concentration–time curve from time 0 to infinity divided by dose of riociguat per kilogram body weight for unbound riociguat/M1; Cmax, norm: maximum concentration in plasma divided by dose of riociguat per kilogram body weight for total riociguat/M1; Cmax, u, norm: maximum concentration in plasma divided by dose of riociguat per kilogram body weight for unbound riociguat/M1; CI: confidence interval; CV: coefficient of variation.
In study I, individuals with severe renal impairment received riociguat 0.5 mg, and therefore pharmacokinetic concentrations recorded for these individuals were normalized to a 1.0-mg dose before analysis; all other participants in both studies received riociguat 1.0 mg.
Mean CL/F of riociguat was higher in healthy individuals (4.07 L/h) than in each of the three groups of patients with renal impairment but did not decrease strictly according to progression of renal impairment (mild renal impairment: 2.67 L/h; moderate renal impairment: 2.00 L/h; severe renal impairment: 2.61 L/h; Table 3). However, mean CLR of riociguat decreased in line with decreasing renal function: compared with healthy volunteers, riociguat CLR was lower by 32%, 67%, and 82% in individuals with mild, moderate, and severe renal impairment, respectively. Mean M1 CLR followed a trend similar to yet more pronounced than that observed for riociguat (Table 3). Total CL/F of M1 was lower in individuals with renal insufficiency than in healthy controls and decreased in line with renal function (Table 3). The CLR values of riociguat increased alongside those observed for CLCR on the day of study drug administration, displaying a monotonically increasing relationship (R2 [coefficient of determination] = 0.62 for riociguat; Fig. 2). A similar trend was observed for CLR of M1 (R2 = 0.76).
Figure 2.
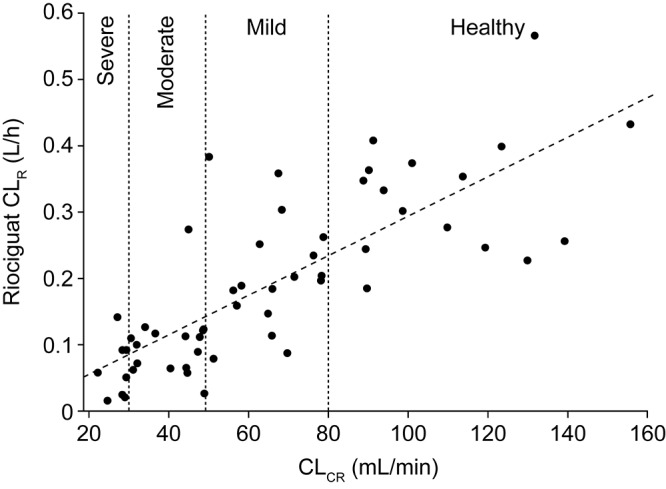
Individual values of CLR of riociguat versus individual CLCR levels (indicative of renal-function group, as shown) on the day of study drug administration in the mixed population (smokers and nonsmokers), with the corresponding fitted regression line (R2 = 0.62; N = 63). CLR: renal clearance; CLCR: creatinine clearance.
Exposure to riociguat and M1 (AUCnorm) was greater in individuals with renal impairment than in matched healthy controls (Table 3; Fig. 3). Compared with that in individuals with normal renal function, mean AUCnorm of total riociguat was 43% higher in individuals with mild renal impairment, 104% higher in individuals with moderate renal impairment, and 44% higher in individuals with severe renal impairment. Thus, riociguat exposure did not increase strictly in proportion with renal insufficiency and decreasing CLR. Furthermore, observed riociguat exposure in individuals with renal impairment was highly variable and overlapped values observed for healthy individuals (Fig. 3A). M1 exposure (AUCnorm) was similar in individuals with normal renal function and those with mild renal impairment (Table 3; Fig. 3B). In individuals with moderate or severe renal impairment, mean exposure to M1 (AUCnorm) was increased by approximately 63% and 75%, respectively, compared with that in individuals with normal or mildly impaired renal function. However, substantial interindividual variability in M1 exposure was observed, irrespective of renal-function status. Values for M1 exposure in individuals with mild, moderate, or severe renal impairment overlapped those in healthy controls (Fig. 3B).
Figure 3.
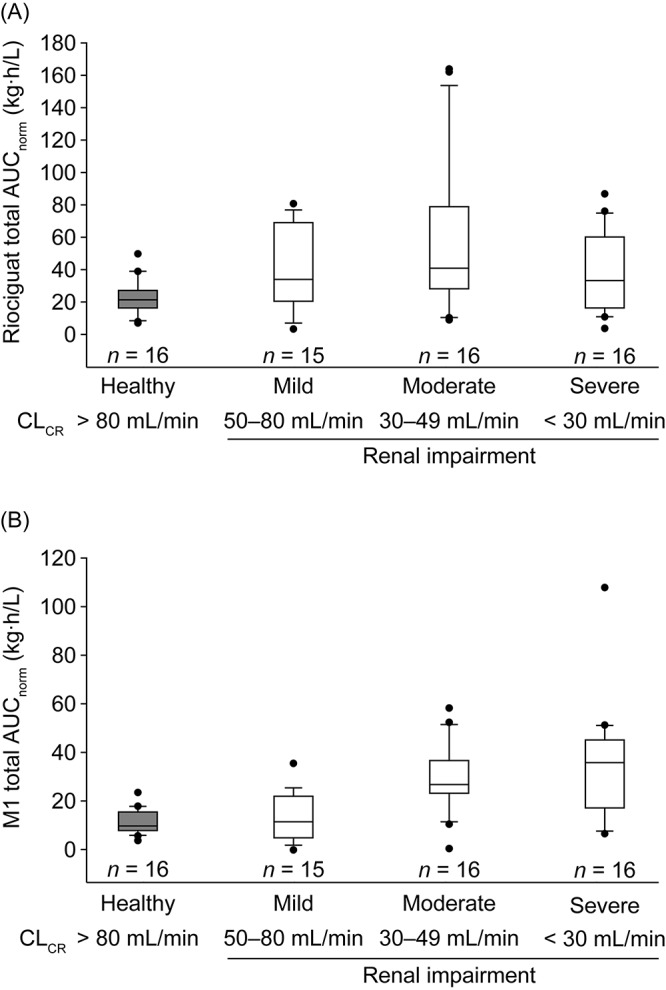
Box-and-whisker plots of exposure to total riociguat (A) and metabolite M1 (B), normalized for riociguat dose and body weight, following a single oral dose of riociguat in a mixed population of smokers and nonsmokers (N = 63). In study I, individuals with severe renal impairment received riociguat 0.5 mg; all other participants in both studies received riociguat 1.0 mg. Boxes signify the twenty-fifth to seventy-fifth percentiles; vertical lines show the tenth to ninetieth percentiles; the horizontal bisecting line represents the median. More extreme values are plotted as points. AUCnorm: area under the plasma concentration–time curve from time 0 to infinity divided by dose of riociguat per kilogram of body weight for total riociguat; CLCR: creatinine clearance.
The fu of riociguat was similar in all four renal-function groups, accounting for 3.10%–4.11% of total riociguat (Table 3). Results for unbound riociguat and unbound M1 were similar to those for total riociguat and total M1, and the ratios of LS means of AUCu, norm and Cmax, u, norm followed a trend similar to those for total riociguat and total M1 (Table 4).
To investigate the pharmacokinetics of riociguat and M1 while excluding the influence of smoking as a confounding variable, a separate pooled analysis restricted to a population of nonsmoking individuals (n = 52) was conducted (Table S1). Trends in exposure (total AUCnorm) for riociguat and M1 in individuals with varying degrees of renal impairment were similar in the population of nonsmokers to those in the total population of nonsmokers and smokers (Figs. 3, 4). As expected, nonsmokers had a lower rate of metabolism of riociguat to M1 than the total population of smokers and nonsmokers, as demonstrated by lower mean Cmax, norm values of total M1 in nonsmokers (Tables 3, S1). Consequently, median exposure to total riociguat appeared to be greater in the nonsmoking population than in the total population of patients with renal impairment (median AUCnorm of nonsmokers vs. that of whole population: mild renal impairment: 40.4 vs. 33.9 kg·h/L; moderate renal impairment: 65.2 vs. 40.9 kg·h/L; severe renal impairment: 40.4 vs. 33.6 kg·h/L; Figs. 3A, 4A). This effect was observed irrespective of renal status. Thus, in nonsmoking individuals, impaired renal function resulted in a mean exposure to riociguat higher than that in the respective groups of individuals with mild, moderate, or severe renal impairment in the total population.
Figure 4.
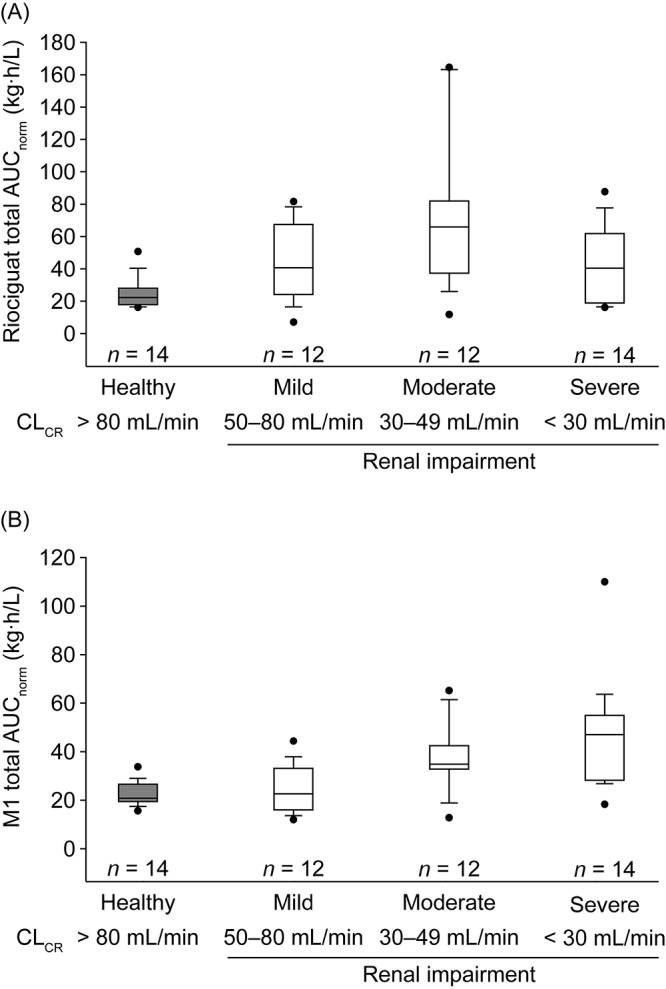
Box-and-whisker plots of exposure to total riociguat (A) and metabolite M1 (B), normalized for riociguat dose and body weight, following a single oral dose of riociguat in a population of nonsmokers only (n = 52). In study I, individuals with severe renal impairment received riociguat 0.5 mg; all other participants in both studies received riociguat 1.0 mg. Boxes signify the twenty-fifth to seventy-fifth percentiles; vertical lines show the tenth to ninetieth percentiles; the horizontal bisecting line represents the median. More extreme values are plotted as points. AUCnorm: area under the plasma concentration–time curve from time 0 to infinity divided by dose of riociguat per kilogram of body weight for total riociguat; CLCR: creatinine clearance.
Discussion
This joint evaluation of two studies provides pharmacokinetic and safety data for riociguat in individuals with mild, moderate, or severe renal impairment compared with healthy individuals. A single oral dose of riociguat 0.5 or 1.0 mg was generally well tolerated in all study participants, and the safety profile of riociguat was similar in healthy volunteers and individuals with renal impairment. The rate of reported treatment-emergent AEs was similar across all groups, despite increased exposure to riociguat in individuals with renal impairment.
After study I, the importance of extrahepatic metabolism of riociguat was recognized, specifically, the role of CYP1A1 in the metabolism of riociguat to the primary metabolite M1. In nonsmoking adults, CYP1A1 is expressed at constitutively low levels25 in the liver26,27 and in extrahepatic tissues, notably the lungs.28 However, CYP1A1 transcription is highly inducible by polycyclic aromatic hydrocarbons found in tobacco smoke.29 Active smokers display increased CYP1A1 activity and therefore have an elevated rate of N-demethylation of riociguat to M1 compared with nonsmoking individuals. In addition, a time-dependent decrease in CYP1A1 gene expression is seen in the pulmonary tissue of former smokers after cessation of smoking.20
This study presents primarily the integrated analysis of pharmacokinetic data from two analyses conducted overall in a mixed population of nonsmokers and smokers, with an enrichment of nonsmokers. The main findings therefore reflect the effect of renal impairment on the pharmacokinetics of riociguat and M1, with limited influence of smoking as a confounder.
Riociguat was readily absorbed, and overall the pharmacokinetic characteristics in healthy individuals were consistent with those previously reported.12 The influence of body weight on riociguat was minimal.30,31 As would be expected for a drug that is eliminated partially by renal excretion, there was a consistent and substantial effect of renal impairment on CLR of riociguat, which decreased in line with progressive renal impairment, indicated by decreasing CLCR. There was evidence for a monotonically increasing relationship between CLCR observed on the day of riociguat administration and CLR of both riociguat and M1.
The increase in mean exposure to riociguat with renal impairment that was seen in this study was greater than anticipated, after a previous mass-balance study in which approximately 4%–19% of the administered dose was found to be eliminated as unchanged riociguat (excluding M1) via renal excretion, primarily by glomerular filtration.19 Given that there is substantial elimination of unchanged riociguat and metabolites via the biliary/fecal route in healthy volunteers (48%–59%), the elevation of riociguat exposure in patients with disrupted renal function may be explained, at least in part, by the interaction of renal impairment with hepatic/biliary processes.19 Loss of renal function is known to influence considerably the nonrenal clearance and systemic bioavailability of drugs that are predominantly metabolized by the liver.32,33 Furthermore, the activities of several drug-metabolizing enzymes and drug transmembrane transporters in the liver and intestine are known to be perturbed in chronic renal failure.34,35 Reduced CLR can lead to inhibition of CYP-mediated drug metabolism, including that of CYP3A4-related isozymes, because of high levels of circulating uremic factors.19,32,36-39 The expression and activity of the hepatic and intestinal transporters are also modulated by the accumulation of uremic factors, which could in turn contribute to the increase in exposure of various drugs in patients with renal impairment.32,40 Although the exact underlying mechanisms for the unexpectedly large effect of loss of renal function on riociguat exposure remain to be elucidated, a need for careful dose titration of riociguat in individuals with renal impairment is apparent.
The requirement for individualized dosing of riociguat was identified in phase 1 studies in healthy volunteers and patients with unimpaired renal function12,13 and confirmed in a phase 2 study.14 The first phase 3 clinical studies used this dose-titration scheme.16-18 Long-term extensions to these trials (PATENT-2 and CHEST-2) demonstrated a sustained clinical effect of riociguat over a treatment period of 2 years, in addition to a favorable long-term safety profile in patients with PAH and CTEPH.41,42 Knowledge of riociguat dosing in patients with poor renal function is extremely important, given the close association of PH and consequent right heart failure with increased central venous pressure, which has in turn been associated with the development of renal impairment.43 Renal dysfunction is indeed common in patients with PH: the US Pulmonary Hypertension Connection registry found the prevalence of chronic kidney disease among patients with PAH to be 12%.44
In the total population of smokers and nonsmokers in this study, riociguat exposure was 43%–104% higher with renal impairment than with normal renal function. An inverse relationship between the degree of renal failure and CLR of riociguat was observed, but this was not strictly proportional with respect to either total clearance or plasma exposure of riociguat. Patients with mild or moderate renal impairment had higher average riociguat exposure than patients with severe renal impairment in this single-dose study. This effect may be explained by differences in riociguat excretion mechanisms, with patients who have severe renal impairment perhaps having an additional clearance pathway. Indeed, the breast cancer resistance protein, an adenosine triphosphate–binding cassette protein found in the intestine and known to include riociguat as one of its substrates, could account for some of the variation that was observed in riociguat exposure.45 In patients with renal impairment, exposure was highly variable, and the ranges of exposure in individuals with mild, moderate, and severe renal impairment overlapped. For the treatment of patients with renal impairment, country-specific guidance on riociguat labels should be followed. The US label recommends that riociguat not be used in patients with CLCR < 15 mL/min or in patients receiving dialysis, because safety and efficacy have not been demonstrated in these populations (riociguat is not expected to be dialyzable because of high plasma protein binding).31 Particular care should be exercised during individual dose titration in patients with renal impairment and especially in patients with severe renal dysfunction. This individual dose titration is supported by the availability of a broad range of riociguat tablet strengths, from 0.5 mg to 2.5 mg.
In the homogenous pooled nonsmoking population in this study, riociguat exposure in patients with renal impairment was increased by 53%–139% compared to that in healthy individuals.30 Because of the higher risks associated with elevated riociguat exposure in nonsmoking individuals than in smoking individuals, it was decided to use the exposure data from nonsmokers for inclusion in the European Summary of Product Characteristics and the US product label for riociguat.30,31 Here, we present data on exposure in both the mixed population of smokers and nonsmokers and the homogenous nonsmoking population to help inform physicians performing individual dose titrations with riociguat.
In conclusion, individuals with renal impairment had reduced riociguat clearance compared with controls; however, riociguat exposure was highly variable, and ranges in the renal-impairment groups overlapped those in healthy controls. Irrespective of renal function, riociguat exposure was greater in nonsmokers than in the combined population of smokers and nonsmokers. Successful population evaluations of the efficacy and safety of riociguat in patients with PH have been performed in phase 3 studies and their long-term extensions, which included patients with renal impairment. It is recommended with riociguat that dose titration be performed to avoid hypotension while maximizing effectiveness; particular care should be exercised during individual dose titration in patients with renal impairment, and dose adjustment may be necessary in patients who start or stop smoking during treatment with riociguat.30,31
Acknowledgments
The studies were performed by Dr. Atef Halabi, Medical Director of CRS Clinical Research Services Kiel, Lornsenstrasse 7, 24105 Kiel, Germany. Selected data included in this manuscript have previously been presented at the 6th International Conference on cGMP, Erfurt, Germany, June 28–30, 2013,46 and the European Respiratory Society Annual Congress 2013, Barcelona, Spain, September 7–11, 2013.
Appendix. Supplementary table
Table S1.
Pharmacokinetic parameters for riociguat and metabolite M1 in nonsmoking healthy individuals and patients with mild, moderate, or severe renal impairment, following a single oral dose of riociguat 1.0 mg
| Parameter | Normal (CLCR > 80 mL/min) |
Mild (CLCR = 50–80 mL/min) |
Moderate (CLCR = 30–49 mL/min) |
Severe (CLCR < 30 mL/min)a |
|---|---|---|---|---|
| Patients, no. | 14 | 12 | 12 | 14 |
| Riociguat | ||||
| AUC, μg·h/L | 283 (32.3) | 466 (73.2) | 691 (80.7) | 523 (70)b |
| AUCnorm, kg·h/L | 24.0 (34.9) | 36.7 (79.8) | 57.4 (83.9) | 37.0 (66.6) |
| Cmax, μg/L | 37.7 (16.3) | 46.0 (21.1) | 42.9 (37.0) | 40.6 (38)b |
| Cmax, norm, kg/L | 3.20 (13.5) | 3.62 (24.4) | 3.57 (34.3) | 3.13 (29.2) |
| tmax, hours | 1.00 (0.500–2.00) | 1.00 (0.750–3.00) | 1.25 (0.750–3.00) | 1.00 (0.500–4.00) |
| t1/2, hours | 7.00 (36.9) | 12.2 (72.6) | 15.8 (71.8) | 11.1 (60.5) |
| CL/F, L/h | 3.54 (32.3) | 2.15 (73.2) | 1.45 (80.7) | 2.13 (65.0) |
| fu, % | 3.39 (19.4) | 3.14 (22.5) | 4.00 (22.8) | 4.21 (27.0) |
| AUCu, norm, kg·h/L | 0.813 (32.6) | 1.15 (80.6) | 2.30 (80.4) | 1.56 (63.7) |
| Cmax, u, norm, kg/L | 0.108 (24.5) | 0.114 (36.4) | 0.143 (35.5) | 0.132 (22.6) |
| AE, ur, % | 9.63 ± 4.19 | 11.4 ± 7.28 | 7.65 ± 3.55 | 4.04 ± 2.93c |
| CLR, L/h | 0.312 (42.6) | 0.208 (37.8) | 0.105 (55.2) | 0.0564 (92.1)c |
| Metabolite M1 | ||||
| AUC, μg·h/L | 256 (23.7) | 285 (43.8) | 407 (50.2) | 547 (62)b |
| AUCnorm, kg·h/L | 22.5 (21.5) | 23.2 (44.0) | 35.0 (44.8) | 40.9 (48.9) |
| Cmax, μg/L | 8.92 (40.8) | 7.09 (74.4) | 6.85 (55.0) | 8.73 (52)b |
| Cmax, norm, kg/L | 0.783 (37.8) | 0.577 (69.7) | 0.589 (46.0) | 0.679 (45.5) |
| tmax, hours | 8.00 (4.00–24.0) | 11.0 (3.00–24.1) | 24.0 (4.00–48.0) | 24.0 (2.00–24.1) |
| t1/2, hours | 14.7 (24.3) | 25.04 (44.3) | 31.4 (60.0) | 34.0 (74.7) |
| CL/F, L/h | 3.77 (23.7) | 3.39 (43.8) | 2.38 (50.2) | 1.93 (55.7) |
| fu, % | 2.86 (15.1) | 2.65 (18.9) | 3.47 (19.9) | 3.96 (30.6) |
| AUCu, norm, kg·h/L | 0.643 (24.4) | 0.619 (43.5) | 1.21 (44.8) | 1.62 (50.1) |
| Cmax, u, norm, kg/L | 0.0224 (40.9) | 0.0153 (70.5) | 0.0204 (47.4) | 0.0269 (51.5) |
| AE, ur, % | 18.7 ± 7.29 | 11.0 ± 6.24 | 8.87 ± 2.98d | 4.16 ± 3.64e |
| CLR, L/h | 0.688 (56.9) | 0.367 (67.3) | 0.256 (39.9)d | 0.0670 (75.0)e |
Values are geometric means (percentage coefficient of variation), except for the number of patients; tmax, which is expressed as median (range); and AE, ur, which is expressed as arithmetic mean ± standard deviation. AE, ur: amount excreted into urine from time 0 to infinity; AUC: area under the plasma concentration–time curve from time 0 to infinity; AUCnorm: AUC divided by dose of riociguat per kilogram body weight for total riociguat/M1; AUCu, norm: AUC divided by dose of riociguat per kilogram body weight for unbound riociguat/M1; CLCR: creatinine clearance; CL/F: apparent oral clearance for total riociguat/M1; CLR: renal clearance of riociguat/M1; Cmax: maximum concentration in plasma; Cmax, norm: Cmax divided by dose of riociguat per kilogram body weight for total riociguat/M1; Cmax, u, norm: Cmax divided by dose of riociguat per kilogram body weight for unbound riociguat/M1; fu: fraction unbound; tmax: time to Cmax of total riociguat/M1; t1/2: terminal elimination half-life for total riociguat/M1.
In study I, individuals with severe renal impairment received riociguat 0.5 mg, and therefore pharmacokinetic concentrations recorded for these individuals were normalized to a 1.0-mg dose before analysis; all other participants in both studies received riociguat 1.0 mg.
AUC and Cmax values shown for individuals with severe renal impairment are taken from study II (n = 8), in which individuals with severe renal impairment received riociguat 1.0 mg.
Data available from 11 patients.
Data available from 8 patients.
Data available from 7 patients.
Source of Support: The study was funded by a research grant from Bayer Pharma. Medical writing support and editorial assistance were provided by Dr. Bernd Sierakowski of Sierakowski Medical Writing and by Oxford PharmaGenesis, Oxford, United Kingdom, and were funded by Bayer Pharma.
Conflict of Interest: All authors are employees of Bayer Pharma. In addition, RF, WM, AS, SU, and GW own stock in Bayer but are not paid in stock or stock options.
Supplements
Appendix: Supplemental tablePulmCirc-006-S15.s001.pdf (428.2KB, pdf)
References
- 1.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006;5(9):755–768. [DOI] [PMC free article] [PubMed]
- 2.McLaughlin V, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53(17):1573–1619. [DOI] [PubMed]
- 3.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 4.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, et al. ASPIRE Registry: assessing the spectrum of pulmonary hypertension identified at a referral centre. Eur Respir J 2012;39(4):945–955. [DOI] [PubMed]
- 5.Tonelli A, Haserodt S, Aytekin M, Dweik R. Nitric oxide deficiency in pulmonary hypertension: pathobiology and implications for therapy. Pulm Circ 2013;3(1):20–30. [DOI] [PMC free article] [PubMed]
- 6.Dasgupta A, Bowman L, D’Arsigny C, Archer S. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther 2015;97(1):88–102. [DOI] [PMC free article] [PubMed]
- 7.Kaneko FT, Arroliga A, Dweik R, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 1998;158(3):917–923. [DOI] [PubMed]
- 8.Dweik RA. The lung in the balance: arginine, methylated arginines, and nitric oxide. Am J Physiol Lung Cell Mol Physiol 2007;292(1):L15–L17. [DOI] [PubMed]
- 9.Schermuly RT, Stasch JP, Pullamsetti SS, Middendorff R, Müller D, Schlüter KD, Dingendorf A, et al. Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension. Eur Respir J 2008;32(4):881–891. [DOI] [PubMed]
- 10.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123(20):2263–2273. [DOI] [PMC free article] [PubMed]
- 11.Ghofrani HA, Grimminger F. Soluble guanylate cyclase stimulation: an emerging option in pulmonary hypertension therapy. Eur Respir Rev 2009;18(111):35–41. [DOI] [PubMed]
- 12.Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521; an ascending-dose study in healthy male volunteers. J Clin Pharmacol 2008;48(8):926–934. [DOI] [PubMed]
- 13.Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bölkow D, Weissmann N, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009;33(4):785–792. [DOI] [PubMed]
- 14.Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, Ewert R, Weimann G, Grimminger F. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 2010;36(4):792–799. [DOI] [PubMed]
- 15.Hoeper MM, Halank M, Wilkens H, Günther A, Weimann G, Gebert I, Leuchte HH, Behr J. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J 2013;41(4):853–860. [DOI] [PubMed]
- 16.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 17.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369(4):319–329. [DOI] [PubMed]
- 18.Kim NH, D’Armini AM, Grünig E, Hoeper MM, Jansa P, Mayer E, Simonneau G, et al. Hemodynamic assessment of patients with inoperable chronic thromboembolic pulmonary hypertension (CTEPH) in the phase III CHEST-1 study. Am J Respir Crit Care Med 2013;187(MeetingAbstracts):A3529.
- 19.Bayer HealthCare Pharmaceuticals. Briefing document for Cardiovascular and Renal Drugs Advisory Committee: riociguat (BAY 63-2521). Published August 6, 2013. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm363543.pdf.
- 20.McLemore TL, Adelberg S, Liu MC, McMahon NA, Yu SJ, Hubbard WC, Czerwinski M, et al. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst 1990;82(16):1333–1339. [DOI] [PubMed]
- 21.US Food and Drug Administration. Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. Published May 1998. Accessed February 21, 2013. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072127.pdf.
- 22.European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Note for guidance on the evaluation of the pharmacokinetics of medicinal products in patients with impaired renal function. Published June 23, 2004. Accessed February 21, 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003123.pdf.
- 23.Schuhmacher J, Bühner K, Witt-Laido A. Determination of the free fraction and relative free fraction of drugs strongly bound to plasma proteins. J Pharm Sci 2000;89(8):1008–1021. [DOI] [PubMed]
- 24.Kubitza D, Becka M, Mueck W, Halabi A, Maatouk H, Klause N, Lufft V, Wand DD, Philipp T, Bruck H. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol 2010;70(5):703–712. [DOI] [PMC free article] [PubMed]
- 25.Whitlock JP Jr. Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol 1999;39:103–125. [DOI] [PubMed]
- 26.Drahushuk AT, McGarrigle BP, Larsen KE, Stegeman JJ, Olson JR. Detection of CYP1A1 protein in human liver and induction by TCDD in precision-cut liver slices incubated in dynamic organ culture. Carcinogenesis 1998;19(8):1361–1368. [DOI] [PubMed]
- 27.Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi 2003;123(5):369–375. [DOI] [PubMed]
- 28.Wei C, Caccavale RJ, Kehoe JJ, Thomas PE, Iba MM. CYP1A2 is expressed along with CYP1A1 in the human lung. Cancer Lett 2001;171(1):113–120. [DOI] [PubMed]
- 29.Anttila S, Tuominen P, Hirvonen A, Nurminen M, Karjalainen A, Hankinson O, Elovaara E. CYP1A1 levels in lung tissue of tobacco smokers and polymorphisms of CYP1A1 and aromatic hydrocarbon receptor. Pharmacogenetics 2001;11(6):501–509. [DOI] [PubMed]
- 30.European Medicines Agency. Annex I: summary of product characteristics [Adempas (riociguat tablets)]. Published March 27, 2014. Accessed November 5, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf.
- 31.US Food and Drug Administration. Prescribing information [Adempas (riociguat) tablets]. Revised May 2014. Accessed February 25, 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204819s002lbl.pdf.
- 32.Dreisbach AW. The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther 2009;86(5):553–556. [DOI] [PubMed]
- 33.Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther 2006;109(1–2):1–11. [DOI] [PubMed]
- 34.Verbeeck R, Musuamba F. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol 2009;65(8):757–773. [DOI] [PubMed]
- 35.Pichette V, Leblond FA. Drug metabolism in chronic renal failure. Curr Drug Metab 2003;4(2):91–103. [DOI] [PubMed]
- 36.Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol 2008;4(8):1065–1074. [DOI] [PMC free article] [PubMed]
- 37.Guévin C, Michaud J, Naud J, Leblond FA, Pichette V. Down-regulation of hepatic cytochrome P450 in chronic renal failure: role of uremic mediators. Br J Pharmacol 2002;137(7):1039–1046. [DOI] [PMC free article] [PubMed]
- 38.Leblond F, Guévin C, Demers C, Pellerin I, Gascon-Barré M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol 2001;12(2):326–332. [DOI] [PubMed]
- 39.Leblond FA, Petrucci M, Dubé P, Bernier G, Bonnardeaux A, Pichette V. Downregulation of intestinal cytochrome P450 in chronic renal failure. J Am Soc Nephrol 2002;13(6):1579–1585. [DOI] [PubMed]
- 40.Naud J, Michaud J, Leblond FA, Lefrancois S, Bonnardeaux A, Pichette V. Effects of chronic renal failure on liver drug transporters. Drug Metab Dispos 2008;36(1):124–128. [DOI] [PubMed]
- 41.Simonneau S, D’Armini A-M, Ghofrani H-A, Grimminger F, Hoeper MM, Jansa P, Kim NH, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension (CTEPH): 2-year results from the CHEST-2 long-term extension. Eur Respir J 2014;44(suppl. 58):1802. [DOI] [PubMed]
- 42.Rubin L, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh A, et al. Riociguat for the treatment of pulmonary arterial hypertension (PAH): 2-year results from the PATENT-2 long-term extension. Eur Respir J 2014;44(suppl. 58):1803. [DOI] [PubMed]
- 43.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009;53(7):582–588. [DOI] [PubMed]
- 44.Shah SJ, Thenappan T, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Association of serum creatinine with abnormal hemodynamics and mortality in pulmonary arterial hypertension. Circulation 2008;117(19):2475–2483. [DOI] [PubMed]
- 45.Rickert V, Haefeli WE, Weiss J. Pharmacokinetic interaction profile of riociguat, a new soluble guanylate cyclase stimulator, in vitro. Pulm Pharmacol Ther 2014;28(2):130–137. [DOI] [PubMed]
- 46.Frey R, Becker C, Unger S, Schmidt A, Wensing G, Mueck W. Pharmacokinetics of the soluble guanylate cyclase stimulator riociguat in individuals with renal impairment. BMC Pharmacol Toxicol 2013;14(suppl. 1):P22. doi:10.1186/2050-6511-14-S1-P22.


