Abstract Abstract
In preclinical studies, drugs that increase cyclic guanosine monophosphate levels have been shown to influence platelet function/aggregation; however, the effect of riociguat on human platelets is unclear. Aspirin, a platelet inhibitor, is likely to be given concomitantly in patients receiving riociguat. It is therefore important to establish clinically whether (1) riociguat affects platelet function and (2) aspirin and riociguat interact. This randomized, open-label, crossover study investigated potential pharmacodynamic and pharmacokinetic interactions between these drugs in healthy male volunteers (N = 18). There were 3 treatment regimens: a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, and both treatments together, with riociguat given on the second morning. Fifteen participants were available for pharmacodynamic/pharmacokinetic analysis. There was no effect of riociguat alone on bleeding time, platelet aggregation, and serum thromboxane B2 levels. The effects of aspirin on these parameters were not influenced by concomitant administration of riociguat. The pharmacokinetic profile of riociguat showed interindividual variability, which was independent of aspirin coadministration. Six of 17 participants available for safety evaluation reported at least 1 treatment-emergent adverse event. All adverse events were of mild severity, apart from 1 report of moderate headache. No serious adverse events occurred. In conclusion, riociguat demonstrated no clinically relevant pharmacodynamic or pharmacokinetic interactions with aspirin at the doses used in this study in healthy men; coadministration of riociguat and aspirin should therefore not require any dose adjustment for either drug.
Keywords: soluble guanylate cyclase, salicylic acid, platelet aggregation, safety, bleeding time
Riociguat (BAY 63-2521), a drug used in the treatment of pulmonary hypertension (PH), targets the nitric oxide (NO)–soluble guanylate cyclase (sGC)–cyclic guanosine monophosphate (cGMP) pathway.1 It increases cGMP production through a novel dual mode of action: acting as a direct NO-independent stimulator of sGC and also increasing the sensitivity of sGC to low levels of NO.2 On the basis of phase 3 studies,3,4 riociguat titrated to a maximum dose of 2.5 mg 3 times daily has been approved by regulatory authorities (including the US Food and Drug Administration5 and the European Medicines Agency6) for the treatment of chronic thromboembolic PH (CTEPH) and pulmonary arterial hypertension (PAH).
Patients with PAH may receive a variety of supportive treatments to reduce the severity of common signs and symptoms of the condition. Data from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) indicate that about 16% of patients with PAH are taking aspirin.7 In such patients, aspirin may commonly be taken at high doses (≥500 mg) to provide pain relief as well as at low doses for anticoagulant activity. Low-dose aspirin is known to reduce the synthesis of the vasoconstrictive agent thromboxane A2 and platelet aggregation in vivo.8 Platelet aggregation is also inhibited by increased cGMP levels.9 The phosphodiesterase-5 inhibitor sildenafil potentiates platelet inhibition in human platelets and inhibits collagen-induced platelet aggregation ex vivo.9,10 Predecessors of riociguat showed antiplatelet activity in preclinical studies.2,11 YC-1 inhibited platelet aggregation in vitro,12 and BAY 41-2272 inhibited platelet aggregation in washed rat platelets in the presence of reduced sGC and NO.13 BAY 41-8543 inhibited collagen-mediated platelet aggregation in plasma and washed human platelets through activation of the vasodilator-stimulated phosphoprotein pathway14 and also prolonged rat tail bleeding time.14 However, the effect of riociguat on human platelet aggregation is not well established in the scientific literature. It is therefore important to evaluate both the safety risk of riociguat in terms of its in vivo effect on platelet aggregation in humans and whether clinically relevant interactions between riociguat and aspirin might occur in individuals who take aspirin while receiving riociguat. This clinical pharmacology study is the first to investigate both the potential effects of riociguat on platelet function and any pharmacodynamic interactions between riociguat and aspirin in healthy male volunteers.
Methods
Study design and treatments
This randomized, open-label, 3-arm crossover, nonblinded, single-center study was conducted from March to June 2009 at an in-house clinical research unit of Bayer Pharma (Clinical Pharmacology, Pharma Research Center, Wuppertal, Germany). The study consisted of 3 treatment regimens: a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, and aspirin 500 mg on 2 consecutive mornings with a single concomitant dose of riociguat 2.5 mg on the second day. These doses were selected because they are at the high end of the spectrum of commonly used doses (aspirin 500 mg for pain relief; riociguat 2.5 mg 3 times daily for PAH) and are considered to have the greatest potential for pharmacodynamic interaction. Participants fasted on the profile day of treatment (for 2-day regimens, this was the second day) until 4 hours after each regimen was completed. A washout period of at least 14 days separated crossover between treatment regimens. Before study drug administration, participants underwent medical examination, were admitted to the ward at least 12 hours before dosing, and remained at the study center for 5 days. Participants underwent a final examination approximately 1 week after the last administration of the study drug.
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guidelines, and the German drug law (Arzneimittelgesetz). The protocol was approved by the Ethics Committee of the North-Rhine Medical Council (Düsseldorf). Randomization was carried out after the prestudy examination, and treatment sequence was determined by random-number generation. All individuals gave written informed consent to participate in the study.
Study population
Healthy white men aged 18–45 years were eligible to participate if they had a body mass index of 18–30, a resting heart rate of 45–90 beats/min, a systolic blood pressure of 100–145 mmHg, a diastolic blood pressure no higher than 95 mmHg, and a negative illicit drug (cannabinoids, benzodiazepines, opiates, barbiturates, amphetamines, or cocaine) screen. Exclusion criteria included a history of medical disorders or conditions that could impair a volunteer’s ability to participate in or complete the study, febrile illness in the week before the study, and pathological changes in the electrocardiogram, such as a second- or third-degree atrioventricular block, prolongation of the QRS complex to more than 120 ms, or prolongation of the QT/corrected QT (QTc) interval to more than 450 ms. Volunteers could not be enrolled if they had participated in a clinical study in the previous 3 months, donated more than 100 mL of blood in the preceding 4 weeks or 500 mL in the preceding 3 months, or had a regular daily consumption of more than 40 g (5 units) of alcohol, 1 L of xanthine-containing beverages, or 25 cigarettes. Use of medicinal products other than the study drugs was not permitted during the study without consulting the investigator. Any concomitant medication was documented.
Safety and tolerability
Safety and tolerability were evaluated by monitoring standard vital signs and hematology, urinalysis, and biochemical tests. Adverse events were identified by questioning of participants and through spontaneous self-reporting.
Pharmacodynamic evaluation
Bleeding time was assessed and blood samples for platelet aggregation tests and serum thromboxane B2 (TxB2) levels were taken before the initial aspirin dose or at the equivalent time if receiving only riociguat (t = −24 hours), before administration of the second aspirin dose and/or riociguat (t = 0), and at 1, 2, 4, and 24 hours after the last dose of each study drug treatment. Bleeding time was also assessed at 72 hours, and additional blood samples for measurement of serum TxB2 levels were taken at 48 and 72 hours. Bleeding time was assessed using the Surgicutt Adult I device (ITC Nexus, Edison, NJ).17-19 Platelet aggregation was quantified in platelet-rich plasma by turbidimetry with a wavelength of 650 nm, using the Born method.20 Maximum aggregation, which is proportional to maximum light transmittance, was evaluated over 5 minutes following induction of platelet-rich plasma samples with collagen (100 μg/mL) or arachidonic acid (303 μg/mL). Platelet-rich plasma was obtained as the supernatant after a citrated blood sample was centrifuged for 10 minutes at 100 g. Platelet-poor plasma was obtained as the supernatant after the remaining citrated blood was centrifuged for 10 minutes at 2,000 g. To correct for intersample differences in light transmission, 100% aggregation was defined for each sample as corresponding to the light transmission through the platelet-poor plasma obtained from that sample. TxB2 was measured in serum by radioimmunoassay following in vitro conversion of metabolic precursors to TxB2 in whole blood.
Pharmacokinetic evaluations
For the analysis of riociguat and metabolite M1 (BAY 60-4552) pharmacokinetic parameters, plasma samples were taken before and up to 72 hours after riociguat administration. Samples were stored below −15°C.
Plasma riociguat and M1 concentrations were determined by high-performance liquid chromatography coupled with mass spectrometry, using [2H3]riociguat and [2H3]M1 as the internal standards. The working range was 2–500 μg/L. Quality control samples in the concentration range of 6–400 μg/L were determined with an accuracy of 99.5%–105% and a precision of 3.52%–9.81% for riociguat and with an accuracy of 98.4%–106% and a precision of 2.85%–6.10% for M1.
Area under the plasma concentration–time curve from time 0 to infinity (AUC), maximum concentration in plasma (Cmax), time to Cmax (tmax), and terminal elimination half-life (t1/2) for riociguat and M1 were calculated using noncompartmental methods in WinNonlin (ver. 4.1a; Pharsight, Mountain View, CA). Plasma samples were taken at 1 and 8 hours after riociguat administration, with and without aspirin coadministration, for determination of the unbound riociguat and M1 fractions. Determination of protein binding was performed ex vivo by spiking erythrocyte/plasma suspensions with about 100 μg/L of radiolabeled [14C]riociguat or [14C]M1.21
Statistics
Statistical evaluation was performed using SAS software (SAS Institute, Cary, NC). The primary statistical analysis was of bleeding time measured 4 hours after administration of each treatment regimen. Owing to the results of a previous study,22 normally distributed observations were assumed. An analysis of covariance (ANCOVA) was performed on bleeding time with fixed treatment, sequence, and period effects and random participant (sequence) effects, as well as with the baseline value taken immediately before administration in each period as a covariate. Treatment effect was tested at the 0.05 level of significance. On the basis of these ANCOVAs, point estimates (least squares means) and 2-sided 95% confidence intervals (CIs) for (riociguat plus aspirin treatment) minus (aspirin treatment) and (riociguat plus aspirin treatment) minus (riociguat treatment) bleeding times were calculated.
The pharmacokinetic characteristics AUC, Cmax, and fraction unbound for riociguat and M1 were analyzed in an exploratory manner assuming lognormal distribution of data. The logarithms of these characteristics were evaluated using an analysis of variance (ANOVA) with treatment, period, sequence, and participation (sequence) as factors. On the basis of these analyses, point estimates (least squares means) and exploratory 90% CIs for the ratio (riociguat plus aspirin treatment)/(riociguat treatment) were calculated by retransformation of the logarithmic data using the intraindividual standard deviation (SD) of the ANOVA.
A sample size of 12 was deemed adequate on the basis of normally distributed bleeding time. The value for the intraindividual SD observed in a previous study was 1.6 minutes.22 Assuming this variability, a statistical difference in bleeding time could be detected with a power of 83% if the true difference was 2.1 minutes on the basis of 12 participants with paired observations (SAS Proc Power, 2-sided paired t test of mean difference with normal data, α = 0.05).
Results
Study population
Eighteen healthy white men (mean age: 35.2 years; range: 23.0–45.0 years) were enrolled into the study. Fifteen participants received the study drug as scheduled and completed all 3 study periods without major deviation from the study protocol. One participant was withdrawn before the first administration of the study drug because of a protocol violation (platelet aggregation could not be measured). Another participant received riociguat plus aspirin in the first study period but terminated the study prematurely after administration of 1 dose of aspirin in the second study period as a result of an adverse event (increase in blood creatine phosphokinase detected in a blood sample taken at the start of the second study period before the first aspirin dose), considered by the investigator to be related to fitness training/bodybuilding. An additional participant was withdrawn from the study because of noncompliance after administration of aspirin in the first study period. Thus, in total, data from 15 participants were valid for pharmacodynamic and pharmacokinetic analyses. The mean age of these 15 participants was 34.3 years (range: 23.0–45.0 years), mean weight (±SD) was 85.5 ± 10.4 kg, mean height was 182.1 ± 6.30 cm, and mean body mass index was 25.7 ± 2.40. Three (20%) individuals were smokers at the time of the study, but an effect on exposure to riociguat was demonstrated in only 1 of these individuals, and this was not considered to be a clinically relevant influence on the results.
Safety and tolerability
Six (35%) of the 17 participants with valid data for the safety evaluation reported at least 1 treatment-emergent adverse event. No serious adverse events occurred. All treatment-emergent adverse events were of mild severity, apart from moderate headache after riociguat treatment in 1 participant (Table 1). Three participants experienced at least 1 adverse event considered to be related to riociguat and/or aspirin, as follows: headache (n = 2, after riociguat alone), nasal congestion (n = 2, after riociguat alone), and dyspepsia (n = 1, after riociguat plus aspirin; Table 1). Bleeding was not reported as a treatment-emergent adverse event.
Table 1.
Incidence of treatment-emergent adverse events in participants receiving a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, or both treatments concomitantly
| No. (%) of participants | ||||
|---|---|---|---|---|
| Category | Description | Riociguat (n = 15) | Aspirin (n = 17) | Riociguat plus aspirin (n = 16) |
| Cardiac disorders | Ventricular extrasystoles | 1 (6) | ||
| Eye disorders | Conjunctivitis | 1 (6) | ||
| Gastrointestinal disorders | Dyspepsia | 1 (6)a | ||
| General disorders | Injection site swelling | 1 (7) | ||
| General disorders | Vessel puncture site reaction | 1 (7) | ||
| Infections | Rhinitis | 1 (6) | ||
| Investigations | Blood creatine phosphokinase increased | 1 (6) | ||
| Nervous system disorders | Headache | 2 (13)b | ||
| Respiratory disorders | Nasal congestion | 2 (13)a | ||
| Skin disorders | Scar | 1 (6) | ||
Considered to be drug related.
Moderate in 1 of the 2 incidents, the other was mild; both cases considered to be drug related.
Apart from 1 participant in whom creatine phosphokinase became elevated at the end of the washout phase as a result of physical exercise (considered to be unrelated to the study drugs), all treatment-emergent adverse events had resolved spontaneously by the end of the study. All other laboratory values above the upper limit of normal were minor, transient, and without clinical relevance. No drug-induced changes in laboratory parameters were observed.
Because of the vasorelaxing properties of riociguat, mean heart rate increased from 59.9 beats/min before riociguat administration to a maximum of 66.9 beats/min 1 hour after administration and from 57.8 beats/min before riociguat plus aspirin administration to a maximum of 69.8 beats/min 1 hour after administration. Concomitantly, mean diastolic blood pressure decreased from 65.5 to 59.2 mmHg with riociguat and from 64.6 to 62.1 mmHg with riociguat plus aspirin. No abnormal electrocardiogram findings, including QTc assessments, were reported.
Pharmacodynamics
No changes in mean bleeding time were observed after a single oral dose of riociguat. Bleeding time after aspirin treatment alone increased from 5.58 minutes before the first dose to 7.63 minutes immediately before the second dose; there was no further prolongation of mean bleeding time after the second dose of aspirin. A similar bleeding time profile was reported following combined treatment with riociguat and aspirin. Adding riociguat to aspirin did not result in any significant prolongation of bleeding time compared with bleeding time after aspirin treatment alone (Tables 2, S1). In contrast, bleeding time after combined treatment with riociguat and aspirin was prolonged significantly in comparison with bleeding time after riociguat alone (Tables 2, S1).
Table 2.
Differences in baseline-adjusted point estimates (least squares means) and associated 2-sided 95% confidence intervals (CIs) of bleeding time (minutes) measured 4 hours after administration of a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, or both treatments concomitantly (n = 15)
| Difference | Point estimate | 95% CI | P |
|---|---|---|---|
| (Riociguat plus aspirin treatment) minus (aspirin treatment) | 1.30 | −0.229 to 2.82 | 0.0922 |
| (Riociguat plus aspirin treatment) minus (riociguat treatment) | 2.86 | 1.28 to 4.44 | 0.0010 |
Following administration of riociguat alone, the maximal platelet aggregation remained virtually unchanged after stimulation with collagen or arachidonic acid (Fig. 1). However, following administration of either aspirin alone or aspirin in combination with riociguat, mean maximal platelet aggregation observed after arachidonic acid stimulation decreased to less than 1% (Fig. 1B), whereas mean maximal platelet aggregation after collagen stimulation decreased by only approximately 10% compared with pretreatment values (Fig. 1A). Adding riociguat to aspirin treatment did not result in any significant change in platelet aggregation compared with treatment with aspirin alone (−4.48% [95% CI: −10.1% to 1.12%]; P = 0.1118). By contrast, platelet aggregation after combined treatment with riociguat and aspirin was significantly decreased when compared with treatment with riociguat alone (−18.2% [95% CI: −25.3% to −11.2%]; P < 0.0001).
Figure 1.
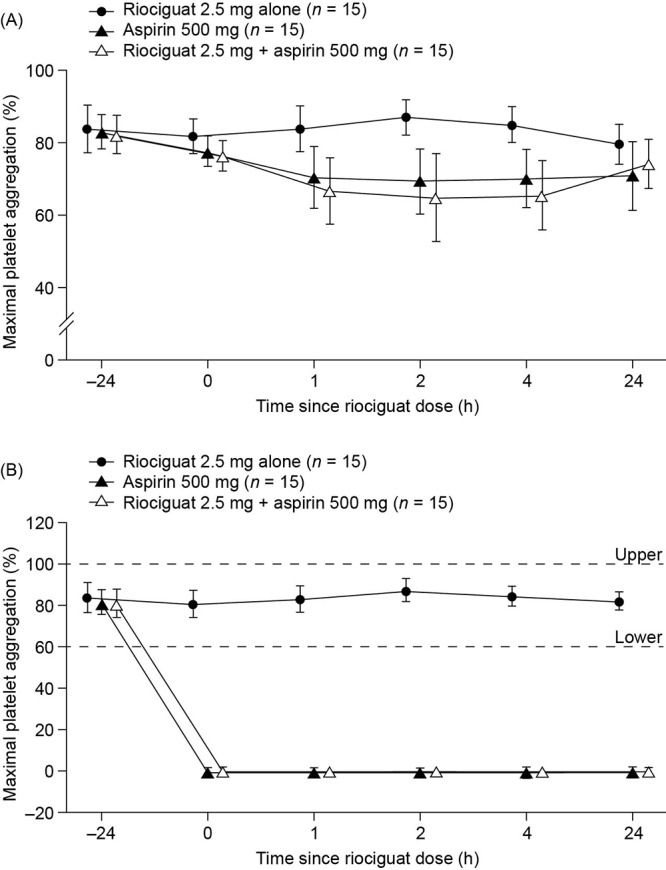
Percentage of maximal platelet aggregation following stimulation with collagen (A) and arachidonic acid (B) to assess the effects of administration of a single oral dose of riociguat 2.5 mg, 2 daily doses of aspirin 500 mg, or 2 daily doses of aspirin with coadministration of riociguat and the second daily dose of aspirin. Measurements were taken before the initial aspirin dose (t = −24 hours); before administration of riociguat and/or a second aspirin dose (t = 0; baseline); and 1, 2, 4, and 24 hours after completion of each treatment regimen. Data are shown as mean ± standard deviation. Data points have been staggered to avoid overlap of error bars. In B, dashed lines indicate upper and lower limits of normal for maximal platelet aggregation.
A pronounced decrease of TxB2 in serum was observed in the 24 hours after the first dose of aspirin in both the aspirin and aspirin plus riociguat regimens (Fig. 2). However, adding riociguat to aspirin did not result in any significant change in serum TxB2 levels compared with adding aspirin alone (−0.0857 ng/mL [95% CI: −28.7 to 28.5 ng/mL]; P = 0.9951). No change in serum TxB2 levels was observed in individuals treated with riociguat alone; however, TxB2 levels after combined treatment with riociguat and aspirin were significantly decreased compared with riociguat alone (−108 ng/mL [95% CI: −181 to −34.3 ng/mL]; P = 0.0057).
Figure 2.
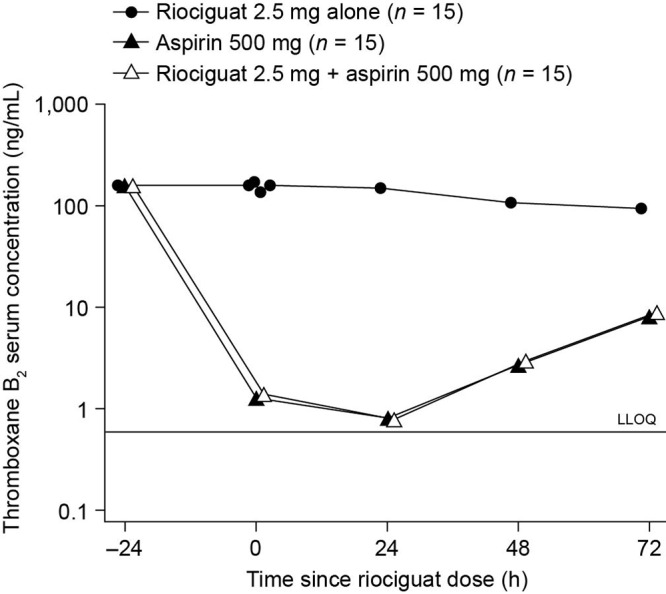
Serum concentration of thromboxane B2 after a single oral dose of riociguat 2.5 mg, 2 daily doses of aspirin 500 mg, or 2 daily doses of aspirin with coadministration of riociguat and the second daily dose of aspirin. Measurements were taken before the initial aspirin dose (t = −24 hours); before administration of riociguat and/or a second aspirin dose (t = 0; baseline); and 24, 48, and 72 hours after completion of each treatment regimen. Data are shown as geometric means and plotted on a semilogarithmic scale. LLOQ: lower limit of quantification.
Pharmacokinetics
The pharmacokinetic parameters of riociguat and its metabolite M1 were very similar after administration of riociguat with and without coadministration of aspirin (Fig. 3; Table 3). For the ratio (riociguat plus aspirin treatment)/(riociguat treatment), the 90% CIs for AUC and Cmax for riociguat and M1 were fully contained in the bioequivalence range (0.80–1.25), apart from the lower boundary of the 90% CI for Cmax for riociguat (0.780; Table 4).
Figure 3.
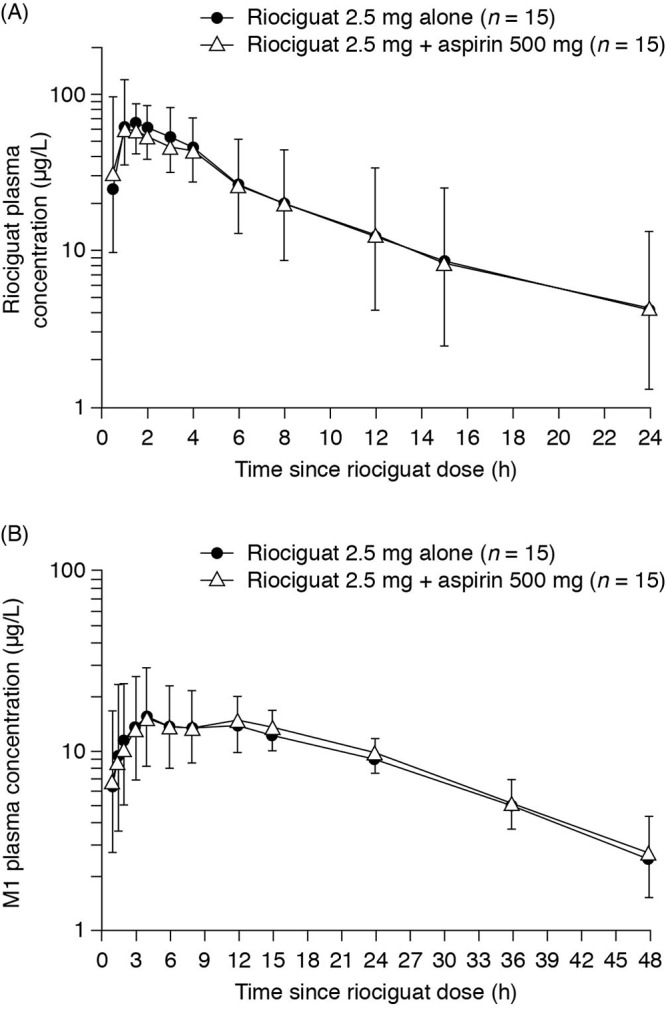
Plasma concentration–time course of riociguat (A) and metabolite M1 (B) following a single oral dose of riociguat 2.5 mg or coadministration of riociguat 2.5 mg with the second daily dose of aspirin 500 mg. Data are shown as geometric means; upward bars denote geometric mean × geometric standard deviation, and downward bars denote geometric mean/geometric standard deviation.
Table 3.
Pharmacokinetic parameters of riociguat and metabolite M1 after a single oral dose of riociguat 2.5 mg with and without coadministration of aspirin 500 mg (n = 15)
| Parameter | Riociguat | Riociguat plus aspirin |
|---|---|---|
| Riociguat | ||
| AUC, μg h/L | 566 (70.8) | 545 (80.1) |
| Cmax, μg/L | 82.6 (21.5) | 70.4 (31.9) |
| tmax, hours | 1.00 (0.500–3.00) | 1.00 (0.500–3.00) |
| t1/2, hours | 6.80 (59.8) | 6.39 (73.2) |
| Metabolite M1 | ||
| AUC, μg h/L | 504 (22.6) | 526 (28.9) |
| Cmax, μg/L | 17.3 (53.9) | 17.9 (53.2) |
| tmax, hours | 4.00 (3.00–24.0) | 6.00 (3.00–24.0) |
| t1/2, hours | 14.7 (30.2) | 14.7 (34.1) |
Values are geometric means (percent coefficient of variation), except for tmax, which is given as median (range). AUC: area under the plasma concentration–time curve from time 0 to infinity; Cmax: maximum concentration in plasma; tmax: time to Cmax; t1/2: terminal elimination half-life.
Table 4.
Point estimates (least squares means) and 2-sided 90% confidence intervals (CIs) for the ratio (riociguat plus aspirin treatment)/(riociguat treatment) calculated for selected pharmacokinetic parameters in participants receiving a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, or both treatments concomitantly (n = 15)
| Analyte and parameter | Point estimate | 90% CI | CV, % |
|---|---|---|---|
| Riociguat | |||
| AUC | 0.964 | 0.868–1.07 | 16 |
| Cmax | 0.853 | 0.780–0.933 | 14 |
| Metabolite M1 | |||
| AUC | 1.04 | 0.960–1.14 | 13 |
| Cmax | 1.04 | 0.928–1.16 | 17 |
AUC: area under the plasma concentration–time curve from time 0 to infinity; Cmax: maximum concentration in plasma; CV: coefficient of variation (intraindividual).
At 1 hour after coadministration of riociguat and aspirin, the mean increase in fraction unbound was 19% for riociguat and 24% for metabolite M1 when compared with riociguat administered alone (Table 5). This corresponds to the well-known tmax of salicylic acid, the main metabolite of aspirin (1–2 hours). At 1 hour, the 90% CIs for both riociguat and M1 exceeded the upper boundary of the bioequivalence range (0.80–1.25; Table 5). In contrast, 8 hours after coadministration, the 90% CIs were fully contained in the bioequivalence range (Table 5); at that time after dosing, salicylic acid plasma concentrations would be expected to decrease substantially.
Table 5.
Point estimates (least squares means) and 2-sided 90% confidence intervals (CIs) of fraction unbound for the ratio (riociguat plus aspirin treatment)/(riociguat treatment) in participants receiving a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, or both treatments concomitantly (n = 15)
| Analyte and time point | Point estimate | 90% CI |
|---|---|---|
| Riociguat | ||
| 1 | 1.19 | 1.07–1.33 |
| 8 | 1.06 | 0.966–1.17 |
| Metabolite M1 | ||
| 1 | 1.24 | 1.12–1.37 |
| 8 | 1.05 | 0.978–1.13 |
Time points are hours after riociguat administration.
Discussion
This clinical pharmacological study in healthy young men investigated the safety and potential pharmacodynamic and pharmacokinetic interactions of riociguat and aspirin because these 2 drugs are likely to be coadministered in the treatment of patients with PH. Riociguat at a single oral dose of 2.5 mg was well tolerated with and without concomitant administration of aspirin. Pharmacodynamic effects of aspirin were not affected by concomitant administration of riociguat.
Before this study, the effect of riociguat on platelet aggregation and bleeding time had not been fully established. A precursor to riociguat, BAY 41-8543, showed antiplatelet activity in plasma and washed human platelets in a preclinical in vitro study but only when high concentrations—equivalent to supratherapeutic riociguat doses—were used.14 In vivo, BAY 41-8543 is known to prolong rat tail bleeding time; however, it remains unclear whether this is the result of increased vasodilatation or impaired hemostasis.14
In this clinical study, riociguat clearly did not affect bleeding time or platelet aggregation. Consistent with this, in a dose-escalation study in healthy young men, which used the same collagen-induced platelet aggregation assay in platelet-rich plasma that was employed in this study and the same turbidometric method that was used in the preclinical study, no effect on platelet aggregation was demonstrated, even at the highest dose of riociguat 5 mg.15 Furthermore, safety data from 2 other clinical studies suggest that riociguat does not increase bleeding risk in patients with PAH or CTEPH.23,24 We conclude, therefore, that at therapeutic doses riociguat does not influence platelet aggregation or bleeding time in vivo in humans.
It is of interest that the phosphodiesterase type-5 inhibitor sildenafil has been found to inhibit collagen-induced platelet aggregation and adenosine diphosphate–mediated platelet activation in healthy volunteers.10,25 A dose of 100 mg was associated with a significantly prolonged bleeding time (+72%) 1 hour after sildenafil administration compared with baseline, with recovery after 4 hours.10 Other treatments for PAH—including the prostacyclin analogs epoprostenol and iloprost—have also been shown to have antiplatelet activity in vitro and in humans.19,26-28 At the time of writing, there is little or no information on the potential for interaction between aspirin and other treatments for PAH; however, the possibility that some of these drugs inhibit platelet aggregation and thus prolong bleeding means that pharmacodynamic and pharmacokinetic interaction studies for these agents and aspirin would be timely and of interest.
The NO-sGC-cGMP pathway regulates platelet aggregation as well as vascular tone. Thus, it is of interest that in this study, interindividual variability in bleeding time after aspirin administration was high, regardless of riociguat coadministration. By contrast, interindividual variability in platelet aggregation and serum TxB2 after aspirin administration was low, regardless of riociguat coadministration. Therefore, concomitant administration of aspirin and riociguat is unlikely to require any dose adjustment for either drug.
Intraindividual pharmacokinetic variability in healthy young men receiving a single dose of riociguat 0.5–2.5 mg has previously been reported as low (generalized coefficient of variation: Cmax: <20%; AUC: <20%), and interindividual variability is known to be moderate to high (generalized coefficient of variation: Cmax: ∼65%; AUC: ∼100%).29 In this 3-way crossover study, interindividual variability of riociguat pharmacokinetics was low (Cmax: ∼20%–30%) to moderate (AUC: ∼70%–80%), and this variation was mainly independent of aspirin coadministration. Intraindividual variability was minimal (generalized coefficient of variation: AUC: 16%; Cmax: 14%), consistent with existing data.29
At 1 hour after coadministration of riociguat and aspirin, the mean increase in fraction unbound compared with that for administration of riociguat alone was 19% for riociguat and 24% for M1, corresponding to a mild displacement by salicylic acid. These results are in line with in vitro experiments (C. Kohlsdorfer, unpublished data [Bayer Pharma, Wuppertal, Germany]). Displacement studies at the protein-binding site revealed a mild displacement of riociguat by salicylic acid at supratherapeutic concentrations (200 and 1,000 mg/L). Thus, 2 daily doses of aspirin 500 mg did not influence riociguat and M1 pharmacokinetics to a clinically relevant extent.
A potential limitation of this study is the use of a single dose of riociguat (2.5 mg) and 2 daily doses of aspirin (500 mg), even though patients with PH are intended to receive long-term treatment with riociguat. As described in a previous single-dose, pharmacodynamic study in healthy young men, a single riociguat dose of 2.5 mg is pharmacologically effective,15 which makes extrapolation possible. Moreover, in the current study, pharmacodynamic effects of aspirin were not increased by the second aspirin dose. Thus, the exposure to aspirin was deemed appropriate. A possible limitation is that the study was performed in healthy volunteers, who have a lower exposure to riociguat than patients with PH. However, the pooled safety analyses of the phase 3 PATENT and CHEST studies revealed no increased safety risk in patients taking aspirin.3,4
In conclusion, a single dose of riociguat (2.5 mg) demonstrated no pharmacodynamic or pharmacokinetic interaction with aspirin (500 mg on 2 consecutive mornings). The results of this study therefore suggest that coadministration of riociguat and aspirin does not require dose adjustment of either drug.
Acknowledgments
We thank the whole study team for their dedicated support.
Selected data included in this manuscript have previously been presented at the Fifth International Conference on cGMP: Generators, Effectors, and Therapeutic Implications (Halle, Germany, June 24–26, 2011).
Appendix. Supplementary table
Table S1.
Time course of bleeding time (minutes) in participants receiving a single morning dose of riociguat 2.5 mg, aspirin 500 mg on 2 consecutive mornings, or both treatments concomitantly (n = 15)
| Treatment and time point, hours | Mean | SD |
|---|---|---|
| Riociguat | ||
| −24 | 6.17 | 2.81 |
| 0 | 5.73 | 1.85 |
| 4 | 5.39 | 1.55 |
| 24 | 6.11 | 2.22 |
| 72 | 5.92 | 2.04 |
| Aspirin | ||
| −24 | 5.58 | 1.18 |
| 0 | 7.63 | 2.44 |
| 4 | 7.28 | 1.83 |
| 24 | 7.39 | 2.27 |
| 72 | 6.62 | 1.27 |
| Riociguat plus aspirin | ||
| −24 | 6.10 | 1.01 |
| 0 | 6.93 | 1.91 |
| 4 | 8.45 | 2.76 |
| 24 | 7.96 | 2.87 |
| 72 | 7.08 | 2.66 |
Time points are as follows: −24 hours: before the initial aspirin dose/at the equivalent time if receiving only riociguat; 0 hours: before administration of the second aspirin dose and/or riociguat; 4, 24, and 72 hours: after the last dose of each study drug treatment. SD: standard deviation.
Source of Support: The study was funded by a research grant from Bayer Pharma. Dr. Bernd Sierakowski of Sierakowski Medical Writing and Oxford PharmaGenesis provided medical writing support funded by Bayer Pharma.
Conflict of Interest: All authors are or have been employees of Bayer. In addition, RF, JK, WM, SU, and GW have stock in Bayer but are not paid in stock or stock options.
Supplements
Appendix: Supplemental tablePulmCirc-006-S35.s001.pdf (419.3KB, pdf)
References
- 1.Dasgupta A, Bowman L, D’Arsigny C, Archer S. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther 2015;97:88–102. [DOI] [PMC free article] [PubMed]
- 2.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123:2263–2273. [DOI] [PMC free article] [PubMed]
- 3.Ghofrani H-A, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369:319–329. [DOI] [PubMed]
- 4.Ghofrani H-A, Galiè N, Grimminger F, Grünig E, Humbert M, Jing Z-C, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369:330–340. [DOI] [PubMed]
- 5.US Food and Drug Administration. Prescribing information [Adempas (riociguat) tablets]. Revised May 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204819s002lbl.pdf.
- 6.European Medicines Agency. Annex I: summary of product characteristics [Adempas (riociguat tablets)]. Accessed November 5, 2014. http://ec.europa.eu/health/documents/community-register/2014/20140327128191/anx_128191_en.pdf.
- 7.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387. [DOI] [PubMed]
- 8.Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, Loscalzo J, Kimmel SE, Christman BW, Barst RJ. A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J 2006;27:578–584. [DOI] [PubMed]
- 9.Gresele P, Momi S, Falcinelli E. Anti-platelet therapy: phosphodiesterase inhibitors. Br J Clin Pharmacol 2011;72:634–646. [DOI] [PMC free article] [PubMed]
- 10.Berkels R, Klotz T, Sticht G, Englemann U, Klaus W. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol 2001;37:413–421. [DOI] [PubMed]
- 11.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 2006;5:755–768. [DOI] [PMC free article] [PubMed]
- 12.Tulis DA, Bohl Masters KS, Lipke EA, Schiesser RL, Evans AJ, Peyton KJ, Durante W, West JL, Schafer AI. YC-1-mediated vascular protection through inhibition of smooth muscle cell proliferation and platelet function. Biochem Biophys Res Commun 2002;291:1014–1021. [DOI] [PubMed]
- 13.Roger S, Badier-Commander C, Paysant J, Cordi A, Verbeuren TJ, Feletou M. The anti-aggregating effect of BAY 41-2272, a stimulator of soluble guanylyl cyclase, requires the presence of nitric oxide. Br J Pharmacol 2010;161:1044–1058. [DOI] [PMC free article] [PubMed]
- 14.Stasch JP, Dembowsky K, Perzborn E, Stahl E, Schramm M. Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vivo studies. Br J Pharmacol 2002;135:344–355. [DOI] [PMC free article] [PubMed]
- 15.Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Single-dose pharmacokinetics, pharmacodynamics, tolerability and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol 2008;48:926–934. [DOI] [PubMed]
- 16.Frey R, Mück W, Kirschbaum N, Krätzschmar J, Weimann G, Wensing G. Riociguat (BAY 63-2521) and warfarin: a pharmacodynamic and pharmacokinetic interaction study. J Clin Pharmacol 2011;51:1051–1060. [DOI] [PubMed]
- 17.Mielke CH Jr., Ramos JC, Britten AF. Aspirin as an antiplatelet agent: template bleeding time as a monitor of therapy. Am J Clin Pathol 1973;59:236–242. [DOI] [PubMed]
- 18.Mielke CH Jr. Aspirin prolongation of the template bleeding time: influence of venostasis and direction of incision. Blood 1982;60:1139–1142. [PubMed]
- 19.Adaikan PG, Karim SM, Lau LC, Tai MY, Kottegoda SR. Inhibition of platelet aggregation and antagonism of vasopressin-induced ECG changes in primates by a carboprostacyclin analogue, ZK 36374. Thromb Res 1984;33:333–340. [DOI] [PubMed]
- 20.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962;194:927–929. [DOI] [PubMed]
- 21.Schuhmacher J, Buhner K, Witt-Laido A. Determination of the free fraction and relative free fraction of drugs strongly bound to plasma proteins. J Pharm Sci 2000;89:1008–1021. [DOI] [PubMed]
- 22.Kubitza D, Becka M, Mück W, Zuehlsdorf M. Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban—an oral, direct factor Xa inhibitor—are not affected by aspirin. J Clin Pharmacol 2006;46:981–990. [DOI] [PubMed]
- 23.Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bölkow D, Weissmann N, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009;33:785–792. [DOI] [PubMed]
- 24.Ghofrani H-A, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, Ewert R, Weimann G, Grimminger F. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: a phase II study. Eur Respir J 2010;36:792–799. [DOI] [PubMed]
- 25.Halcox JP, Nour KR, Zalos G, Mincemoyer RA, Waclawiw M, Rivera CE, Willie G, Ellahham S, Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 2002;40:1232–1240. [DOI] [PubMed]
- 26.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996;334:296–302. [DOI] [PubMed]
- 27.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med 1998;338:273–277. [DOI] [PubMed]
- 28.Belch JJ, Greer I, McLaren M, Saniabadi AR, Miller S, Sturrock RD, Forbes CD. The effects of intravenous ZK36-374, a stable prostacyclin analogue, on normal volunteers. Prostaglandins 1984;28:67–77. [DOI] [PubMed]
- 29.Becker C, Frey R, Hesse C, Unger S, Reber M, Mueck W. Absorption behavior of riociguat (BAY 63-2521): bioavailability, food effects, and dose-proportionality. Eur Respir J 2012;40(suppl. 56):167s. [DOI] [PMC free article] [PubMed]


