Abstract Abstract
Riociguat is a soluble guanylate cyclase stimulator for the treatment of pulmonary hypertension that is principally metabolized via the cytochrome P450 (CYP) pathway. Three studies in healthy males investigated potential pharmacokinetic interactions between riociguat and CYP inhibitors (ketoconazole, clarithromycin, and midazolam). In two studies, subjects were pretreated with either once-daily ketoconazole 400 mg or twice-daily clarithromycin 500 mg for 4 days before cotreatment with either riociguat 0.5 mg ± ketoconazole 400 mg or riociguat 1.0 mg ± clarithromycin 500 mg. In the third study, subjects received riociguat 2.5 mg 3 times daily (tid) for 3 days, followed by cotreatment with riociguat 2.5 mg tid ± midazolam 7.5 mg. Pharmacokinetic parameters, the effect of smoking on riociguat pharmacokinetics, safety, and tolerability were assessed. Pre- and cotreatment with ketoconazole and clarithromycin led to increased riociguat exposure. Pre- and cotreatment with riociguat had no significant effect on midazolam plasma concentrations. In all studies, the bioavailability of riociguat was reduced in smokers because its clearance to the metabolite M1 increased. Riociguat ± ketoconazole, clarithromycin, or midazolam was generally well tolerated. The most common treatment-emergent adverse events (TEAEs) across all studies were headache and dyspepsia. One serious TEAE was reported in the midazolam study. Owing to the potential for hypotension, concomitant use of riociguat with multipathway inhibitors, such as ketoconazole, should be approached with caution. Coadministration of riociguat with strong CYP3A4 inhibitors, for example, clarithromycin, does not require additional dose adjustment. No significant drug-drug interaction was revealed between riociguat and midazolam.
Keywords: pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, soluble guanylate cyclase, NO signaling, pharmacokinetics
Riociguat is the first member of a novel class of therapeutic agents, termed “soluble guanylate cyclase stimulators,”1,2 which has demonstrated robust efficacy in both pulmonary arterial hypertension (PAH) and inoperable chronic thromboembolic pulmonary hypertension (PH) or recurrent/persistent PH following surgical treatment,3,4 and it has recently been approved in the United States and Canada. Previous pharmacokinetic studies have shown riociguat to be rapidly absorbed, with high oral availability, dose-proportional pharmacokinetics, and a good safety profile.1,3-6
Cytochrome P450 (CYP)–mediated oxidative metabolism is a major riociguat clearance pathway, and in vitro data show that riociguat is a substrate of the transporter proteins P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP).7 Thus, riociguat may be subject to pharmacokinetic interactions when coadministered with strong inhibitors or inducers of CYP enzymes and/or transporter proteins.8,9 Such drugs include the fungicide ketoconazole, which is a multipathway CYP and P-gp/BRCP inhibitor, and the antibiotic clarithromycin, which is a potent inhibitor of CYP3A4, an important enzyme in phase 1 transformation in humans (and also of other CYP enzymes involved in riociguat metabolism, such as CYP1A1, CYP2J2, and CYP2C8). Given the possible concomitant administration of riociguat with drugs with similar inhibitory potential in patients with PH, the pharmacokinetic interactions between riociguat and the model CYP substrates ketoconazole and clarithromycin were investigated, in line with the recommendations of the US Food and Drug Administration (FDA) guidelines on drug-drug interaction studies.10 The effect of riociguat on the pharmacokinetics of midazolam, a sensitive CYP3A4 substrate, was also investigated; “sensitive CYP substrates” are defined in the FDA guidelines as drugs whose concentrations increase markedly in the presence of CYP inhibitors.10 The major biotransformation pathway of riociguat, leading to formation of the metabolite M1, is also catalyzed by CYP1A1. Extrahepatic CYP1A1 is highly inducible by tobacco smoke in pulmonary tissue; thus, smokers may have a considerably increased clearance of riociguat to M1. As part of the midazolam study, the influence of smoking on riociguat pharmacokinetics was assessed as a secondary objective.
Methods
Study population
Healthy white men aged 18–55 years (18–45 years in the midazolam study) were eligible for the studies if they had a body mass index of 18–30. Exclusion criteria included a resting heart rate of <45 or >90 bpm (>95 bpm in the midazolam study), systolic blood pressure (SBP) of <100 mmHg (<110 mmHg in the midazolam study) or >145 mmHg, diastolic blood pressure (DBP) of >95 mmHg, a positive drug screen, medical conditions that could impair the subject’s ability to participate in or complete the study, febrile illness in the week before the study, and pathological changes in electrocardiogram (ECG) findings, such as second- or third-degree atrioventricular block, prolongation of the QRS complex to >120 ms, or prolongation of the QT/QTc interval to >450 ms.
Subjects could not be enrolled in the study if they had participated in a clinical trial in the previous 3 months. Participants gave written informed consent to participate in the study.
Study design
Three separate single-center, open-label, randomized studies were conducted between July 2006 and July 2010. All studies were conducted in accordance with the Declaration of Helsinki and adhered to the International Conference on Harmonization guideline E6: Good Clinical Practice. Each study protocol was approved by the Ethics Committee of the North Rhine Medical Council, Düsseldorf, Germany. Randomization was carried out after completion of the prestudy examinations. Use of medicines other than the investigational product during the trial was not permitted in any study without consulting the investigator. Any concomitant medication was documented.
Ketoconazole
Subjects received two treatments in a sequential study carried out at ClinPharmCologne, MEDA Manufacturing, Cologne, Germany. Treatment A was a single dose of riociguat 0.5 mg. Treatment B was a single daily dose of ketoconazole 400 mg (Nizoral, Janssen-Cilag, 2 × 200-mg tablets) from day −4 to day −1, followed by a single dose of riociguat 0.5 mg plus ketoconazole 400 mg on day 0 (a profiling day). On the profiling day, samples were taken for pharmacokinetic analysis of riociguat up to 96 hours after dosing and of ketoconazole up to 48 hours after dosing. The washout phase between treatment periods was 3 days.
Clarithromycin
Subjects received two treatments in a crossover study carried out at Bayer Pharma, Wuppertal, Germany. Treatment A was a single dose of riociguat 1.0 mg. Treatment B was clarithromycin 500 mg (Klacid Forte, Abbott) twice daily from day −4 to day −1, followed by a single dose of riociguat 1.0 mg plus clarithromycin 500 mg on day 0 (a profiling day). On the profiling day, samples were taken for pharmacokinetic analysis of riociguat for up to 72 hours after dosing. The washout phase between treatment periods was 14 days.
Midazolam
Subjects received two treatments in a crossover study carried out at Bayer Pharma, Wuppertal, Germany. Treatment A was a single dose of midazolam 7.5 mg (Dormicum, Roche). In treatment B, subjects were pretreated with riociguat 2.5 mg 3 times daily (tid) from day −4 to day −1, followed by a single dose of midazolam 7.5 mg together with riociguat 2.5 mg tid on day 0 (a profiling day). On the profiling day, samples were taken for pharmacokinetic analysis of riociguat up to 8 hours after dosing and of midazolam up to 48 hours after dosing. The washout phase between treatment periods was at least 10 days.
Drug plasma concentration analyses
Ketoconazole
Riociguat and M1 plasma concentrations were analyzed by high-performance liquid chromatography coupled with mass spectrometry (HPLC/MS), using [2H3]riociguat as the internal standard. The calibration range was from the lower limit of quantification (LLOQ; 0.1 μg/L for parent drug and 0.2 μg/L for the metabolite) to 100.0 μg/L. Riociguat quality control (QC) samples were determined from 0.3 to 80.0 μg/L, with an accuracy of 97.8%–102.0% and a precision of 3.83%–7.83%. M1 QC samples were determined from 0.6 to 80.0 μg/L, with an accuracy of 90.7%–110.0% and a precision of 5.38%–11.1% (riociguat: n = 28; M1: n = 24–26). All samples were stored at ≤−15°C and analyzed within 7 weeks after sampling.
Quantitative analysis of urinary riociguat and M1 concentrations was performed in a similar manner. The calibration range was from 2 to 200 μg/L. QC samples were determined from 6 to 160 μg/L (for both analytes), with an accuracy of 94.6%–102.0% and 96.0%–98.4% and a precision of 4.29%–10.8% and 4.6%–10.9% for riociguat and M1, respectively (n = 18 for each analyte). All samples were stored at ≤−15°C and analyzed within 3.5 months after sampling.
Clarithromycin
Riociguat and M1 plasma concentrations were determined by HPLC/MS. The internal standards were [2H3]riociguat and [2H3]M1. The calibration range was from 0.5 μg/L (LLOQ) to 100.0 μg/L. Riociguat QC samples were determined from 1.5 to 80.0 μg/L, with an accuracy of 99.8%–102.0% and a precision of 6.62%–14.50% (n = 18 or 19 per concentration). M1 QC samples were determined from 1.50 to 80.0 μg/L, with an accuracy of 106%–108% and a precision of 6.08%–11.1% (n = 22 per concentration). All samples were stored at ≤−15°C and analyzed within 3 months after sampling.
Riociguat and M1 urinary concentrations were determined by HPLC/MS. The internal standards were [2H3]riociguat and [2H3]M1. The calibration range was from 10 μg/L (LLOQ) to 1000 μg/L. Riociguat QC samples from 30.0 to 800.0 μg/L were determined, with an accuracy of 99.4%–102.0% and a precision of 3.41%–6.75% (n = 12 per concentration). M1 QC samples from 30.0 to 800.0 μg/L were determined, with an accuracy of 96.0%–100.0% and a precision of 4.02%–6.66% (n = 12 per concentration). All samples were stored at ≤−15°C and analyzed within 4 months after sampling.
Midazolam
Plasma concentrations of midazolam and its metabolite 1-hydroxy-midazolam were determined with a fully validated HPLC/MS/MS assay. The internal standards were [13C6]midazolam and α-hydroxy-triazolam. The calibration range of the procedure was from 0.1 μg/L (LLOQ) to 200 μg/L. Midazolam QC samples were determined from 0.3 to 180 μg/L, with an accuracy of 92.2%–15.0% and a precision of 2.63%–10.40% (n = 4 per concentration). QC samples for 1-hydroxy-midazolam were determined from 0.3 to 180 μg/L, with an accuracy of 95.4%–110.0% and a precision of 5.79%–9.89% (n = 5 per concentration).
Riociguat and M1 plasma concentrations were determined by HPLC/MS. The internal standards were [methoxycarbonyl-2H3]riociguat and [2H3]M1. The calibration range was from 2 μg/L (LLOQ) to 500 μg/L. Riociguat QC samples were determined from 6 to 400 μg/L, with an accuracy of 94.4%–96.9% and a precision of 2.73%–3.75% (n = 4 per concentration). M1 QC samples were determined from 6 to 400 μg/L, with an accuracy of 97.1%–100.0% and a precision of 1.44%–3.10% (n = 4 per concentration). All samples were stored at ≤−15°C and analyzed within 5 months after sampling.
Pharmacokinetic analysis
In all studies, pharmacokinetic parameters were calculated with model-independent (compartment-free) methods in WinNonlin software (ver. 4.1.a, Pharsight, Mountain View, CA), in conjunction with Automation Extension (Bayer).
Ketoconazole and clarithromycin
Riociguat and M1 concentration-time data were used to calculate pharmacokinetic parameters, including area under the plasma concentration-time curve (AUC0-∞), maximum concentration in plasma (Cmax), proportion excreted via urine (Aeur), time to Cmax (tmax), terminal elimination half-life interval (t1/2), mean residence time (MRT), total body clearance of drug from plasma (CL/f), and apparent volume of distribution during terminal phase after oral administration (Vz/f).
Midazolam
Midazolam plasma concentration-time data were used to calculate the pharmacokinetic parameters AUC0-∞, Cmax, tmax, and t1/2 for midazolam. In addition, area under T1-T2 of the plasma concentration–time curve at steady state (AUC(T1-T2) ss), maximum plasma concentration at steady state (Cmax ss), and time to reach Cmax ss (tmax ss) were calculated for riociguat and M1.
Statistical analysis
Statistical analysis was performed in the SAS software package (SAS Institute, Cary, NC). The pharmacokinetic characteristics AUC0-∞ and Cmax were analyzed under the assumption of lognormally distributed data. The logarithms of these characteristics were analyzed with analysis of variance (ANOVA) including sequence, subject (sequence), period, and treatment effects. On the basis of these analyses, point estimates (least squares means) and exploratory 90% confidence intervals (CIs) for the ratios (ketoconazole + riociguat)∶riociguat, (ketoconazole + riociguat)∶ketoconazole, (clarithromycin + riociguat)∶riociguat, and (midazolam + riociguat)∶midazolam were calculated by retransformation of the logarithmic data using the intraindividual standard deviation of the ANOVA. Confirmatory statistical analyses were not performed in the ketoconazole and clarithromycin studies. In the midazolam study, a lack of pharmacokinetic interaction was confirmed if the 90% CI for the ratio was within the range 80%–125%.
Safety and tolerability
Safety and tolerability were evaluated with standard vital signs (blood pressure and heart rate, to estimate effects of riociguat on the cardiovascular system), ECGs, laboratory findings (hematology, clinical chemistry, and urinalysis), and physical examinations. Treatment-emergent adverse events (TEAEs) were assessed for severity (mild, moderate, or severe) and relation to treatment and summarized in Medical Dictionary for Regulatory Activities–preferred terms (ver. 9 in the ketoconazole study, ver. 12 in the clarithromycin study, and ver. 13.1 in the midazolam study).
Results
Demographics
The characteristics of the healthy subjects enrolled in each of the studies who were valid for pharmacokinetic analyses are shown in Table 1. All subjects were men.
Table 1.
Demographics of healthy subjects valid for pharmacokinetic analyses in the ketoconazole, clarithromycin, and midazolam studies
| Characteristic | Ketoconazole study (n = 16) |
Clarithromycin study (n = 15) |
Midazolam study (n = 22) |
|---|---|---|---|
| Male, no. (%) | 16 (100) | 15 (100) | 22 (100) |
| Age, mean (range), years | 42.3 (27.0–54.0) | 34.5 (26.0–45.0) | 36.5 (26.0–45.0) |
| Height, mean (SD), cm | 179.8 (7.9) | 181.3 (7.5) | 181.3 (7.1) |
| Weight, mean (SD), kg | 83.4 (10.8) | 80.6 (10.7) | 80.8 (9.6) |
| BMI, mean (SD) | 25.8 (2.5) | 24.5 (2.5) | 24.6 (2.4) |
BMI: body mass index; SD: standard deviation.
Ketoconazole
Sixteen subjects were enrolled, completed the study, and were valid for pharmacokinetic and safety analyses.
Clarithromycin
Fifteen subjects were enrolled, of whom 14 received the study drug and were valid for pharmacokinetic and safety analyses.
Midazolam
Twenty-four subjects were enrolled. One subject (a smoker) had a positive result for cannabinoids in the predose drug screening. He was withdrawn from the study and did not receive any study drug. Twenty-three subjects received the study drug and were valid for safety analysis; 22 subjects completed the study and were valid for pharmacokinetic analysis. One patient discontinued because of an adverse event (chest pain).
Pharmacokinetics
Ketoconazole
Pre- and cotreatment with ketoconazole led to an increase in riociguat bioavailability (Table 2; Fig. 1). Mean Cmax increased by approximately 46% and mean AUC0-∞ by 150%. Riociguat t1/2 increased from 7.4 to 9.2 hours, and CL/f decreased from 6.1 to 2.4 L/h when riociguat was given concomitantly with ketoconazole. Riociguat Aeur increased from 7.9% to 17.1%.
Table 2.
Pharmacokinetic parameters for riociguat following a single oral dose of riociguat 0.5 mg ± ketoconazole 400 mg
| Riociguat 0.5 mg (n = 16) | Riociguat 0.5 mg + ketoconazole 400 mg (n = 16) | (Riociguat + ketoconazole)∶riociguat | ||||
|---|---|---|---|---|---|---|
| Parameter | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Estimated ratio, %a | 90% CI |
| AUC0-∞, μg·h/L | 81.9 (19.6–218.7) | 78.6 | 204.9 (91.9–409.5) | 44.9 | 250.1 | 214.1–292.3 |
| Cmax, μg/L | 9.4 (5.2–14.0) | 29.9 | 13.7 (9.8–17.8) | 19.3 | 146.0 | 135.3–157.6 |
| tmax, hours | 2.0 (1.0–6.0) | … | 3.0 (1.0–4.0) | … | … | … |
| t1/2, hours | 7.4 (1.2–22.8) | 78.5 | 9.2 (3.6–22.6) | 57.1 | … | … |
| Aeur, % | 7.9 (1.6–13.8) | 4.0 | 17.1 (3.3–27.3) | 6.5 | … | … |
| MRT, hours | 10.2 (3.7–24.0) | 53.7 | 15.1 (7.3–29.5) | 39.4 | … | … |
| Vz/f, L | 64.7 (45.4–100.3) | 26.7 | 32.3 (19.0–50.8) | 29.7 | … | … |
| CL/f, L/h | 6.1 (2.3–25.6) | 78.6 | 2.4 (1.2–5.4) | 44.9 | … | … |
Data are means, except tmax, which is a median. Aeur: amount excreted via urine; ANOVA: analysis of variance; AUC0-∞: area under the plasma concentration–time curve; CI: confidence interval; CL/f: total riociguat clearance from plasma; Cmax: maximum riociguat plasma concentration; CV: coefficient of variation; MRT: mean residence time; tmax: time to reach Cmax; t1/2: elimination half-life; Vz/f: apparent volume of distribution during terminal phase after oral administration.
The logarithms of AUC0-∞/dose and Cmax/dose of riociguat were analyzed with an ANOVA including subject and treatment effects. On the basis of these analyses, point estimates (least square means) and exploratory 90% CIs for the (ketoconazole + riociguat)∶riociguat ratios of these kinetic parameters were calculated by retransformation of the logarithmic results (given by the ANOVA) using the intraindividual standard deviation.
Figure 1.
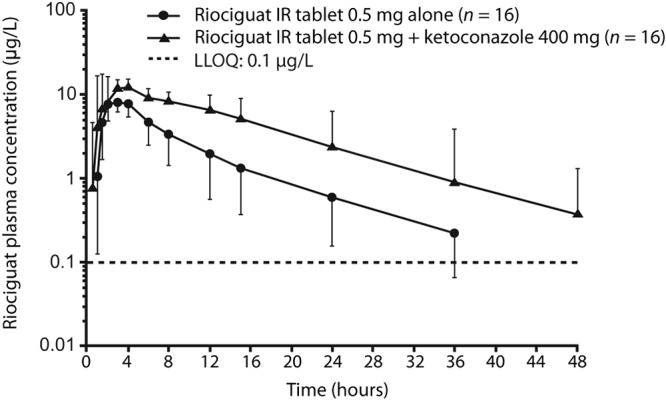
Plasma concentrations of riociguat after a single oral dose of riociguat 0.5 mg alone or in combination with ketoconazole 400 mg (geometric mean ± geometric standard deviation, semilogarithmic scale; all subjects valid for pharmacokinetic analysis; n = 16). IR: immediate release; LLOQ: lower limit of quantification.
Pre- and cotreatment with ketoconazole decreased M1 mean Cmax by approximately 49% and mean AUC0-∞ by 24%. M1 t1/2 increased from 16.2 to 18.3 hours when riociguat was given concomitantly with ketoconazole; M1 Aeur decreased from 15.0% to 10.7%. A single dose of riociguat did not affect the bioavailability of ketoconazole (data not shown).
Clarithromycin
Pre- and cotreatment with clarithromycin led to a marginal increase in riociguat bioavailability (Table 3; Fig. 2), with mean AUC0-∞ increased by 42% and no significant changes in mean Cmax values. Riociguat t1/2 increased from 6.4 to 7.9 hours, with a decrease in CL/f from 5.8 to 4.2 L/h and a decrease in tmax from 3.5 to 2.5 hours. Riociguat Aeur increased from 8.1% to 10.0%.
Table 3.
Clarithromycin study: pharmacokinetic parameters for riociguat following a single oral dose of riociguat 1 mg ± clarithromycin 500 mg
| Riociguat 1 mg (n = 14) | Riociguat 1 mg + clarithromycin 500 mg (n = 14) | (Riociguat + clarithromycin)∶riociguat | ||||
|---|---|---|---|---|---|---|
| Parameter | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Estimated ratio, %a | 90% CI |
| AUC0-∞, μg·h/L | 171.1 (38.5–535.5) | 97.0 | 240.0 (49.4–644.6) | 88.9 | 141.5 | 122.9–162.9 |
| Cmax, μg/L | 20.9 (10.1–32.5) | 37.7 | 21.6 (11.4–41.0) | 33.9 | 104.0 | 89.2–121.3 |
| tmax, hours | 3.5 (0.5–6.0) | … | 2.5 (0.5–4.0) | … | … | … |
| t1/2, hours | 6.4 (1.5–15.7) | 77.1 | 7.9 (2.5–15.1) | 54.6 | … | … |
| Aeur, % | 8.1 (1.8–20.7) | 6.1 | 10.0 (2.5–21.4) | 6.1 | … | … |
| MRT, hours | 9.5 (2.7–21.0) | 61.6 | 12.1 (4.9–25.2) | 53.3 | … | … |
| Vz/f, L | 54.3 (37.3–85.0) | 25.3 | 47.3 (29.9–102.3) | 37.6 | … | … |
| CL/f, L/h | 5.8 (1.9–26.0) | 97.0 | 4.2 (1.6–20.3) | 88.9 | … | … |
Data are means, except tmax, which is a median. Aeur: amount excreted via urine; ANOVA: analysis of variance; AUC0-∞: area under the plasma concentration–time curve; CI: confidence interval; CL/f: total riociguat clearance from plasma; Cmax: maximum riociguat plasma concentration; CV: coefficient of variation; MRT: mean residence time; tmax: time to reach Cmax; t1/2: elimination half-life; Vz/f: apparent volume of distribution during terminal phase after oral administration.
The logarithms of AUC0-∞/dose and Cmax/dose of riociguat were analyzed with an ANOVA including subject and treatment effects. On the basis of these analyses, point estimates (least square means) and exploratory 90% CIs for the (clarithromycin + riociguat)∶riociguat ratios of these kinetic parameters were calculated by retransformation of the logarithmic results (given by the ANOVA) using the intraindividual standard deviation.
Figure 2.

Plasma concentrations of riociguat after a single oral dose of riociguat 1 mg alone or in combination with clarithromycin 500 mg (geometric mean ± geometric standard deviation, semilogarithmic scale; all subjects valid for pharmacokinetic analysis; n = 14). IR: immediate release; LLOQ: lower limit of quantification.
Pre- and cotreatment with clarithromycin led to an increase in M1 bioavailability, with AUC0-∞ increased by 19% and t1/2 prolonged from 15.0 to 16.6 hours, although Cmax was unaffected. M1 Aeur was unchanged.
Midazolam
Pre- and cotreatment with riociguat had no significant effect on the pharmacokinetics of midazolam and its CYP3A4-mediated metabolite 1-hydroxy-midazolam compared with midazolam-only treatment (Table 4; Fig. 3). After oral administration of a single-tablet dose of 7.5 mg midazolam with or without concomitant administration of riociguat, midazolam was readily absorbed, with a median tmax of 1 hour. After reaching Cmax, plasma concentrations declined, with a t1/2 of 4.3 hours for midazolam only and 4.5 hours for midazolam plus riociguat (Table 4; Fig. 3).
Table 4.
Midazolam study: pharmacokinetic parameters of midazolam following oral riociguat 2.5 mg 3 times daily ± midazolam 7.5 mg
| Midazolam 7.5 mg (n = 22) | Midazolam 7.5 mg + riociguat 2.5 mg (n = 22) | (Midazolam + riociguat)∶midazolam | ||||
|---|---|---|---|---|---|---|
| Parameter | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Estimated ratio, %a | 90% CI |
| AUC, μg·h/L | 91.1 (48.8–169.0) | 34.3 | 98.2 (48.9–174.0) | 37 | 108.4 | 97.0–121.1 |
| Cmax, μg/L | 29.0 (17.2–70.6) | 45.1 | 29.5 (14.5–74.9) | 41.5 | 101.8 | 88.9–116.5 |
| tmax, hours | 1.0 (0.5–4.0) | … | 1.0 (0.5–4.0) | … | … | … |
| t1/2, hours | 4.5 (2.2–8.3) | 35.9 | 4.3 (2.6–9.4) | 34.9 | … | … |
Data are means, except tmax, which is a median. ANOVA: analysis of variance; AUC: area under the plasma concentration–time curve; CI: confidence interval; Cmax: maximum midazolam plasma concentration; CV: coefficient of variation; tmax: time to reach Cmax; t1/2: elimination half-life.
The logarithms of AUC0-∞/dose and Cmax/dose of riociguat were analyzed with an ANOVA including subject and treatment effects. On the basis of these analyses, point estimates (least square means) and exploratory 90% CIs for the (midazolam + riociguat)∶riociguat ratios of these kinetic parameters were calculated by retransformation of the logarithmic results (given by the ANOVA) using the intraindividual standard deviation.
Figure 3.
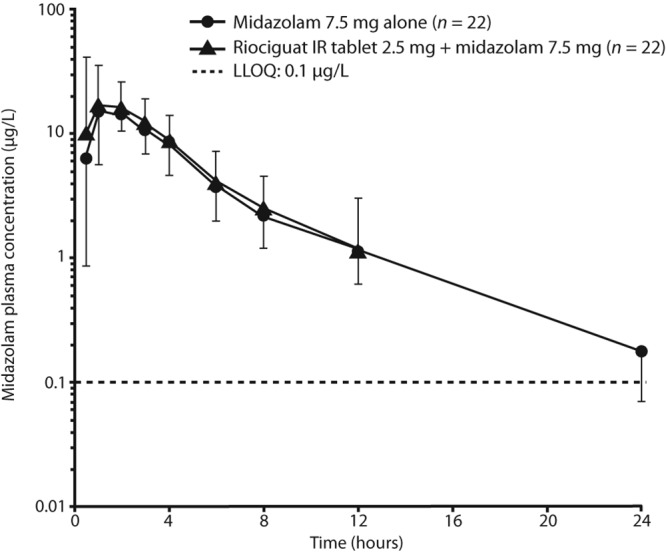
Plasma concentrations of midazolam after a single oral dose of midazolam 7.5 mg alone or in combination with riociguat 2.5 mg 3 times daily (geometric mean ± geometric standard deviation, semilogarithmic scale; all subjects valid for pharmacokinetic analysis; n = 22). IR: immediate release; LLOQ: lower limit of quantification.
After oral administration of a single tablet of 7.5 mg midazolam with or without concomitant administration of riociguat, 1-hydroxy-midazolam appeared in plasma with a median tmax of 1 hour. After reaching Cmax, plasma concentrations declined, with a mean t1/2 of 4.6 hours for midazolam only and 5.4 hours for midazolam plus riociguat (data not shown).
Effect of smoking on midazolam and riociguat pharmacokinetics
Smoking had little effect on midazolam plasma concentrations, with or without coadministration of riociguat (Table S1). However, smoking had a marked influence on the pharmacokinetics of riociguat and M1 (Table S2). In nonsmokers, riociguat mean AUC(T1-T2) ss was 692.8 μg·h/L versus 379.5 μg·h/L for M1, with a mean Cmax ss of 116.5 μg/L for riociguat versus 56.3 μg/L for M1. These patterns were reversed in smokers: riociguat mean AUC(T1-T2) ss was 215 μg·h/L versus 633.5 μg·h/L for M1, with a mean Cmax ss of 59.6 μg/L for riociguat versus 95.1 μg/L for M1 (Fig. S1). Riociguat mean t1/2 decreased from 3 hours in nonsmokers to 1 hour in smokers; M1 t1/2 remained the same in both groups (4 hours).
Safety and tolerability
Riociguat was well tolerated in all studies, and most TEAEs were of mild severity and as expected from the mechanism of action of riociguat. TEAEs for all studies are shown in Table 5.
Table 5.
Incidence of study drug-related TEAEs occurring in each arm of the study population in the three studies
| Ketoconazole study | Clarithromycin study | Midazolam study | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MedDRA preferred term | Riociguat 0.5 mg (n = 16) |
Ketoconazole 400 mg pretreatment (n = 16) |
Riociguat 0.5 mg + ketoconazole 400 mg (n = 16) |
Riociguat 1 mg (n = 14) |
Clarithromycin 500 mg pretreatment (n = 14) |
Riociguat 1 mg + clarithromycin 500 mg (n = 14) |
Midazolam 7.5 mg (n = 22) |
Riociguat 2.5 mg pretreatment (n = 23) |
Riociguat 2.5 mg + midazolam 7.5 mg (n = 23) |
| Any AE | 0 | 5 (31) | 3 (19) | 1 (7) | 0 | 9 (64) | 2 (9) | 20 (87) | 7 (30) |
| Abnormal sensation in eye | 0 | 0 | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 |
| Abdominal discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 (26) | 0 |
| Angina pectoris | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 |
| Chest discomfort | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 |
| Dizziness | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4) |
| Dyspepsia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 (43) | 1 (4) |
| Fatigue | 0 | 4 (25) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flatulence | 0 | 1 (6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flushing | 0 | 0 | 2 (13) | 1 (7) | 0 | 0 | 0 | 0 | 0 |
| Feeling hot | 0 | 0 | 0 | 1 (7) | 0 | 3 (21) | 0 | 1 (4) | 0 |
| Headache | 0 | 1 (6) | 3 (19) | 0 | 0 | 5 (36) | 2 (9) | 4 (17) | 4 (17) |
| Hyperhidrosis | 0 | 0 | 2 (13) | 0 | 0 | 0 | 0 | 0 | 0 |
| Micturition urgency | 0 | 0 | 0 | 0 | 0 | 1 (7) | 0 | 0 | 0 |
| Nasal congestion | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 (35) | 1 (4) |
| Nausea | 0 | 0 | 1 (6) | 0 | 0 | 1 (7) | 0 | 1 (4) | 0 |
| Palpitations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 |
| Spontaneous penile erection | 0 | 0 | 0 | 0 | 0 | 3 (21) | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4) | 0 |
Data are no. (%) of participants. AE: adverse event; MedDRA: Medical Dictionary for Regulatory Activities; TEAE: treatment-emergent adverse event.
Ketoconazole
TEAEs were reported in 4 subjects (25%) receiving riociguat only, in 6 (38%) receiving riociguat plus ketoconazole, and in 5 (31%) receiving ketoconazole only. No serious TEAEs occurred. All TEAEs were mild, apart from 1 case each of arthralgia, nasopharyngitis, and headache, which were reported as moderate. The most frequently reported TEAEs were fatigue (4 events in 4 subjects) and headache (4 events in 3 subjects), all of which were considered drug related. No clinically relevant laboratory abnormalities above the upper limit of normal or below the lower limit of normal were detected, apart from a constant creatine kinase increase in 1 subject, which was attributable to a coexisting tendonitis.
Mean heart rate increased slightly, with small, concomitant decreases in SBP and DBP, in line with the vasorelaxing properties of riociguat. Most subjects had normal ECGs or normal ECG variants. Some subjects had transient ECG abnormalities without clinical significance. All TEAEs had resolved at the end of the study, apart from 1 case of tendonitis, which was considered unrelated to the study drug and had insufficient follow-up.
Clarithromycin
TEAEs were reported in 4 subjects (29%) receiving riociguat only, in 9 (64%) receiving riociguat plus clarithromycin, and in 9 (64%) receiving clarithromycin only. No serious TEAEs occurred. All TEAEs were of mild intensity, apart from 2 events of moderate headache after clarithromycin plus riociguat and clarithromycin alone in 1 subject (1 drug related, 1 not drug related) and moderate back pain after riociguat alone in 1 subject, which was not drug related.
The most frequently reported TEAEs were headache (8 events in 7 subjects, 5 drug related), flatulence (5 events in 5 subjects, none drug related), and feeling hot (4 events in 4 subjects, all drug related). All TEAEs had resolved at the end of the study. No drug-induced laboratory parameter changes were observed. SBP, DBP, and heart rate increased after clarithromycin-plus-riociguat treatment, which was associated with a higher systemic exposure to riociguat and M1, in comparison with riociguat alone. No abnormal ECG findings, including QTc analysis, were reported.
Midazolam
TEAEs were reported in 20 subjects (87%) receiving riociguat only, in 11 (47.8%) receiving riociguat plus midazolam, and in 6 (27%) receiving midazolam only. The most frequently reported TEAEs were headache (11 events in 10 subjects) and dyspepsia (11 events in 10 subjects), 10 of which were drug related.
One serious TEAE occurred; 1 subject had increased blood creatine phosphokinase (CPK) concentrations (46 times the upper limit of normal) in the predose blood sample taken before administration of midazolam 7.5 mg. This was assessed as not related to study drug. No action was taken, and the subject completed the study according to the protocol. All TEAEs were mild, apart from 1 case of moderate chest pain associated with C-reactive protein (CRP) increase and not related to study drug. All TEAEs had resolved at the end of the study. One subject discontinued permanently because of moderate chest pain not related to study drug, and 1 subject was discontinued and restarted because of mild, drug-related vomiting. Beyond the 1 case of increased CRP and another of increased CPK, there were no clinically relevant changes in laboratory parameters during this study. Mean SBP and DBP decreased when riociguat was administered, as compared with midazolam alone, associated with higher mean pulse rates. No abnormal ECG findings, including QTc analysis, were reported.
Discussion
These clinical pharmacology studies in healthy men investigated the pharmacokinetic interactions between riociguat and ketoconazole, clarithromycin, and midazolam, all of which interact with CYP3A4, the principal enzyme of the riociguat clearance pathway. In patients with PH, especially those with left ventricular dysfunction, an evaluation of the potential influence of riociguat on CYP3A4 is important because almost all drugs used for the treatment of PAH are cleared via CYP3A metabolism. This patient population is often treated with concomitant drugs that have the potential for drug-drug interactions; therefore, these patients may be susceptible to uncontrolled changes in drug plasma concentrations. The studies also assessed safety and tolerability.
The pharmacokinetic results show that pre- and cotreatment with the model multipathway inhibitor ketoconazole led to an increase in riociguat bioavailability, with a mean AUC increase of 150% and a mean Cmax increase of approximately 46%, indicating a relevant pharmacokinetic interaction. Pre- and cotreatment with the model CYP3A inhibitor clarithromycin also resulted in increased riociguat bioavailability, with a mean AUC increase of 41%, although in this study riociguat Cmax was unchanged. These data indicate that ketoconazole and, to a lesser extent, clarithromycin inhibit CYP3A4-mediated metabolic clearance of riociguat. The potential inhibiting/inducing influence of riociguat on the activity of CYP3A4 was investigated by using midazolam as a probe. Concomitant administration of riociguat with midazolam did not reveal any significant change in midazolam pharmacokinetics, indicating that riociguat does not inhibit or induce CYP3A4 activity.
Smoking had a marked effect on the levels of riociguat and M1 in plasma, with an increased clearance of riociguat to M1 in smokers versus nonsmokers. In addition to CYP3A4, CYP1A1 also catalyzes the transformation of riociguat to M1. Consequently, titration to doses higher than 2.5 mg tid, if tolerated, may be necessary in patients who smoke; a dose decrease may be required in patients who stop smoking while receiving riociguat treatment.8,9,11 Treatment with ketoconazole had an enhanced effect on the pharmacokinetics of riociguat in smokers, with a 2.29-fold increase in riociguat AUC in nonsmokers, compared with a 3.65-fold increase in smokers (data not shown). The ketoconazole-mediated increase in riociguat AUC was also more pronounced in subjects exhibiting higher clearances of riociguat. This suggests that inhibition of CYP1A1 (normally induced by tobacco smoke, increasing the rate of metabolism of riociguat to M1), as well as CYP3A4, by ketoconazole resulted in increased riociguat exposure in smokers compared with nonsmokers.
These three studies found that riociguat had a favorable safety profile both with and without coadministration of ketoconazole, clarithromycin, or midazolam. Treatment with riociguat was generally well tolerated, although 3-day treatment with riociguat 2.5 mg tid was not well tolerated in the midazolam study; this was due to gastrointestinal disorders, which are known adverse events associated with riociguat.
As is standard for these types of studies, this pharmacokinetics study was conducted in one population, in this case healthy males. Previous assessment of the effect of age and gender on riociguat metabolism has revealed that there is no clinically relevant gender effect.11,12 Furthermore, the result of this age-and-gender study was confirmed in the pooled analyses of the phase 3 PATENT and CHEST studies.13
In conclusion, coadministration of riociguat did not alter the pharmacokinetics of midazolam, confirming that riociguat does not influence the metabolism of other drugs via CYP3A4. In contrast, concomitant use of riociguat with strong multipathway CYP inhibitors and P-gp/BCRP inhibitors, such as antimycotics (e.g., ketoconazole, itraconazole) or HIV protease inhibitors (e.g., ritonavir), increases riociguat exposure. Consequently, owing to the potential for hypotension, the European label states that concomitant use of such medications with riociguat is not recommended,8 while the US label suggests that a reduced starting dose of riociguat 0.5 mg tid should be considered for patients receiving strong CYP or P-gp/BCRP inhibitors and that these patients should be monitored for signs or symptoms of hypotension. However, general dose adaptation for patients with comedication inhibiting either the CYP3A4 pathway (e.g., clarithromycin) or the P-gp-/BCRP-mediated excretion of riociguat, beyond the individual dose-adjustment concept for riociguat,3,4 is not necessary.
Acknowledgements
Editorial assistance was provided by Adelphi Communications (Bollington, United Kingdom), sponsored by Bayer Pharma.
Appendix. Supplementary data
Figure S1.
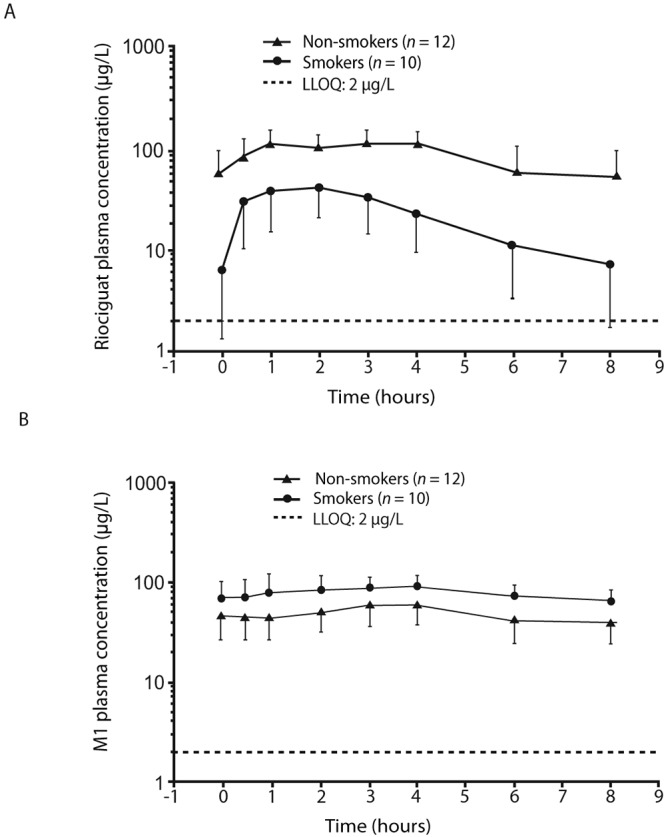
Midazolam study: plasma concentrations of riociguat (A) and M1 (B) in smokers (n = 10) and nonsmokers (n = 12; geometric mean concentrations ± geometric standard deviation, semilogarithmic scale; all subjects valid for pharmacokinetic analysis). LLOQ: lower limit of quantification.
Table S1.
Midazolam study: pharmacokinetic parameters of midazolam and midazolam + riociguat in smokers and nonsmokers
| Nonsmokers | Smokers | |||||||
|---|---|---|---|---|---|---|---|---|
| Midazolam 7.5 mg (n = 12) | Midazolam 7.5 mg + riociguat 2.5 mg (n = 12) | Midazolam 7.5 mg (n = 8) | Midazolam 7.5 mg + riociguat 2.5 mg (n = 10) | |||||
| Parameter | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % |
| AUC, μg·h/L | 83.5 (48.8–169.3) | 39.7 | 93.8 (48.9–174.0) | 43.7 | 103.8 (80.2–146.1) | 20.1 | 103.7 (67.5–147.1) | 28.9 |
| Cmax, μg/L | 24.5 (17.2–42.2) | 24.0 | 27.5 (14.5–48.1) | 36.4 | 35.4 (18.4–70.6) | 56.9 | 32.0 (18.5–74.9) | 47.6 |
| tmax, hours | 1.0 (0.5–4.0) | … | 1.0 (0.5–4.0) | … | 0.5 (0.5–2.0) | … | 1.0 (0.5–2.0) | … |
| t1/2, hours | 5.0 (3.3–7.9) | 29.1 | 4.1 (2.6–7.6) | 32.4 | 3.9 (2.2–8.3) | 41.7 | 4.6 (2.9–9.3) | 38.7 |
Data are means, except tmax, which is a median. AUC0-∞: area under the plasma concentration–time curve; Cmax: maximum plasma concentration; CV: coefficient of variation; tmax: time to reach Cmax; t1/2: elimination half-life.
Table S2.
Midazolam study: pharmacokinetic parameters of riociguat and M1 in smokers and nonsmokers
| Nonsmokers (n = 12) | Smokers (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Riociguat | M1 | Riociguat | M1 | |||||
| Parameter | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % | Geometric mean (range) |
CV, % |
| AUC(T1-T2) ss, μg·h/L | 692.8 (471.5–1,245.2) | 34.5 | 379.5 (132.0–747.5) | 45.0 | 215.0 (66.2–667.6) | 94.2 | 633.5 (445.6–1,015.2) | 29.3 |
| Cmax ss, μg/L | 116.5 (83.9–193.8) | 28.3 | 56.3 (20.4–104.4) | 43.7 | 59.6 (28.6–152.7) | 58.4 | 95.1 (67.2–160.4) | 28.5 |
| tmax ss, hours | 3.0 (1.0–4.0) | … | 4.0 (2.0–4.0) | … | 1.0 (0.5–4.0) | … | 4.0 (1.0–4.0) | … |
Data are means, except tmax ss which is a median. AUC(T1-T2) ss: area under T1-T2 of the plasma concentration–time curve at steady state; Cmax ss: maximum plasma concentration at steady state; CV: coefficient of variation; tmax ss: time to reach Cmax ss.
Source of Support: Bayer Pharma (Berlin, Germany).
Conflict of Interest: All authors are employees of Bayer Pharma.
Supplements
Appendix: Supplementary dataPulmCirc-006-S49.s001.pdf (490.9KB, pdf)
References
- 1.Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bölkow D, Weissmann N, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J 2009;33(4):785–792. [DOI] [PubMed]
- 2.Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, Kern A, et al. Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. ChemMedChem 2009;4(5):853–865. [DOI] [PubMed]
- 3.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369(4):319–329. [DOI] [PubMed]
- 4.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 5.Frey R, Mück W, Unger S, Artmeier-Brandt U, Weimann G, Wensing G. Single-dose pharmacokinetics, pharmacodynamics, tolerability, and safety of the soluble guanylate cyclase stimulator BAY 63-2521: an ascending-dose study in healthy male volunteers. J Clin Pharmacol 2008;48(8):926–934. [DOI] [PubMed]
- 6.Ghofrani HA, Hoeper MM, Halank M, Meyer FJ, Staehler G, Behr J, Ewert R, Weimann G, Neuser D, Grimminger F. Riociguat for chronic thromboembolic pulmonary hypertension and pulmonary arterial hypertension: long-term safety, tolerability, and efficacy. Am J Respir Crit Care Med 2012;185(MeetingAbstracts):A2370. doi:10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A2370.
- 7.Becker C, Frey R, Unger S, Thomas D, Reber M, Weimann G, Dietrich H, Arens ER, Mueck W. Pharmacokinetic interaction of ketoconazole, clarithromycin, and midazolam with riociguat. BMC Pharmacol Toxicol 2013;14(suppl. 1):P5. doi:10.1186/2050-6511-14-S1-P5. [DOI] [PMC free article] [PubMed]
- 8.European Medicines Agency. Annex I: summary of product characteristics [Adempas (riociguat tablets)]. Published March 27, 2014. Accessed November 25, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf.
- 9.US Food and Drug Administration. Highlights of prescribing information [Adempas (riociguat) tablets]. Published October 8, 2013. Accessed November 25, 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204819s000lbl.pdf.
- 10.US Food and Drug Administration. Guidance for industry: drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations [draft]. Published February 2012. Accessed October 17, 2014. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf.
- 11.Bayer HealthCare Pharmaceuticals. Briefing document for Cardiovascular and Renal Drugs Advisory Committee: riociguat (BAY 63-2521). Published August 6, 2013. Accessed November 10, 2014. http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/cardiovascularandrenaldrugsadvisorycommittee/ucm363543.pdf.
- 12.Frey R, Lettieri J, Nadel A, Becker C, Mueck W. Effects of age and gender on the pharmacokinetics of the soluble guanylate cyclase stimulator riociguat. BMC Pharmacol Toxicol 2013;14(suppl. 1):P23. doi:10.1186/2050-6511-14-S1-P23.
- 13.Frey R, Saleh S, Becker C, Mück W. Effects of age and sex on the pharmacokinetics of the soluble guanylate cyclase stimulator riociguat (BAY 63-2521). Pulm Circ 2016;6(S1):S58–S65. [DOI] [PMC free article] [PubMed]


