Abstract Abstract
Female patients requiring treatment for pulmonary arterial hypertension (PAH) are advised to avoid pregnancy because of the high associated mortality rate. Oral contraception is one of the main methods of preventing pregnancy in this context, mandating pharmacokinetic and safety studies for new agents in this setting. Riociguat is a soluble guanylate cyclase stimulator approved for treatment of PAH and inoperable and persistent or recurrent chronic thromboembolic pulmonary hypertension. This single-center, randomized, nonblinded study involving healthy postmenopausal women investigated the effect of riociguat on plasma concentrations of levonorgestrel (0.15 mg) and ethinylestradiol (0.03 mg) in a combined oral contraceptive. Treatment A was a single oral tablet of levonorgestrel-ethinylestradiol. In treatment B, subjects received 2.5 mg riociguat 3 times daily for 12 days. On the eighth day, they also received a single oral tablet of levonorgestrel-ethinylestradiol. Subjects received both regimens in a crossover design. There was no change in area under the plasma concentration–time curves of levonorgestrel or ethinylestradiol or maximum concentration in plasma (Cmax) of levonorgestrel during combined administration versus levonorgestrel-ethinylestradiol alone. A 20% increase in the Cmax of ethinylestradiol was noted during coadministration; this is not anticipated to adversely impact the contraceptive efficacy or to require any dose adjustment for ethinylestradiol. Plasma concentrations and exposures of riociguat were within the expected range and were not influenced by coadministration with levonorgestrel-ethinylestradiol. Combined treatment was safe and well tolerated. In conclusion, riociguat did not change the exposure to levonorgestrel or ethinylestradiol relative to oral contraceptive administered alone.
Keywords: pulmonary hypertension, levonorgestrel-ethinylestradiol, drug-drug interaction, soluble guanylate cyclase stimulator, steady state, CYP3A4
Pulmonary hypertension (PH) is a progressive condition of the lung vasculature that is diagnosed by an elevated mean pulmonary artery pressure of ≥25 mmHg at rest and includes pulmonary arterial hypertension (PAH) and chronic thromboembolic PH (CTEPH), which, if left untreated, can be life threatening.1 Current treatment guidelines recommend that female patients with PAH avoid pregnancy because of the high mortality rate associated with pregnancy in this condition.1 Oral contraception is one of the main methods of preventing pregnancy in women with PAH. Therefore, drug-interaction evaluations with oral contraceptive agents are essential for agents likely to be used for the treatment of PAH in this context.
Riociguat is the first member of the soluble guanylate cyclase (sGC) stimulator class of therapeutics that has been approved for the treatment of PAH and inoperable and persistent or recurrent CTEPH,2,3 following positive results from the phase 3 studies PATENT and CHEST.4,5 Riociguat has a dual mode of action, sensitizing sGC to endogenous nitric oxide (NO) by stabilizing NO-sGC binding as well as directly stimulating sGC independent of NO. This restores the NO-sGC–cyclic guanosine monophosphate (cGMP) pathway, which is impaired in PH, leading to increased generation of cGMP and maintenance of pulmonary homeostatic pressure.6-8 Animal studies have shown reproductive toxicity and placental transfer of riociguat; therefore, riociguat is contraindicated during pregnancy, and women of reproductive potential must use an effective contraception during treatment with riociguat.2,3
In the United States, riociguat carries a black box warning for embryo-fetal toxicity, and a risk evaluation and mitigation strategy program is in place with the goal of reducing embryo-fetal toxicity in women of reproductive potential.2 Adequate contraception is a core component of any such risk mitigation strategy, and oral contraceptives (OCs) are one option for adequate contraception. Consequently, reassurance is required that riociguat does not reduce the efficacy of oral contraception.
Against this background, a pharmacokinetic (PK) drug interaction study was performed in a cohort of healthy postmenopausal women to investigate the effect of riociguat on the plasma concentrations of or exposure to levonorgestrel and ethinylestradiol in the combined OC levonorgestrel-ethinylestradiol (Microgynon; Bayer Pharma).
Methods
Study population
Healthy postmenopausal female subjects were eligible to enroll in the study if they were 54–65 years of age, had a body mass index ≥20 and ≤32 (calculated as weight in kilograms divided by height in meters squared), and were nonsmokers for at least 3 months before screening. Participants provided written informed consent to participate before any study procedure.
Subjects were excluded from participation if they had a resting heart rate of <50 or >95 bpm, systolic blood pressure of <110 or >145 mmHg, diastolic blood pressure of <60 or >95 mmHg, or medical conditions that could impair the subject’s ability to complete the study or if use of levonorgestrel or ethinylestradiol was contraindicated. Additional exclusion criteria comprised febrile illness in the week before the study and drug use in the preceding 2 weeks that could interfere with the study objectives.
Study design
This was a single-center, randomized, nonblinded, 2-fold crossover study without a placebo control. Subjects were randomized to receive either treatment A followed by treatment B or treatment B followed by treatment A. In both cases, treatment A and treatment B were separated by a 7-day washout period. Treatment A was a single oral tablet of levonorgestrel-ethinylestradiol (0.03 mg ethinylestradiol and 0.15 mg levonorgestrel) given in the fasting state. In treatment B, the subjects received oral tablets of 2.5 mg riociguat 3 times daily (tid) for a total of 12 days. On the eighth day, after 7 days of pretreatment with riociguat, subjects received an additional single oral tablet of levonorgestrel-ethinylestradiol (in the fasting state). Riociguat administration continued uninterrupted, with only a single dose on the morning of the final day. Pretreatment for 7 days with riociguat was selected to ensure that riociguat and M1 had reached steady state and to allow any potential induction of cytochrome P450 3A4 (CYP3A4) by riociguat to take place.
Drug plasma concentration analyses
In treatment A, blood samples for determination of ethinylestradiol and levonorgestrel were taken immediately before administration of levonorgestrel-ethinylestradiol and at the following times afterward: 15 and 30 minutes; 1, 1.5, 2, 3, 4, 7, and 12 hours; and once daily on the second to sixth days inclusive.
With treatment B, levonorgestrel-ethinylestradiol and the morning dose of riociguat were taken together on the eighth day. To permit evaluation of the effects of riociguat on PK of ethinylestradiol and levonorgestrel, samples for bioanalytical quantification were taken at the same times after dosing as for treatment A. For evaluation of riociguat and its metabolite M1, additional samples were taken. These were obtained once daily on the second, fourth, and sixth days; at short intervals on the eighth day (immediately before dosing, at 15 and 30 minutes, and at 1, 1.5, 2, 3, 4 and 7 hours after dose administration); and once daily up to and including the thirteenth day (the day after final administration of riociguat). These samples were collected immediately before riociguat intake and therefore gave trough values. Plasma concentrations of all analytes were measured using validated high-performance liquid chromatography–tandem mass spectroscopy methods.
PK analysis
Ethinylestradiol and levonorgestrel plasma concentration–time data were used to calculate PK parameters that included area under the plasma concentration–time curve (AUC), maximum concentration in plasma (Cmax), time to Cmax (tmax), and terminal elimination half-life (t1/2). These parameters were calculated using model-independent (compartment-free) methods with WinNonlin (ver. 5.3; Pharsight, Mountain View, CA) in conjunction with the Automation Extension (Bayer Pharma). Geometric mean values for plasma concentration–time courses were only calculated when at least two-thirds of the individual data were above the lower limit of quantification.
Safety and tolerability evaluation
Safety was assessed via electrocardiography, vital signs, clinical laboratory tests, gynecologic examination, and monitoring of treatment-emergent adverse events (TEAEs) at screening, with TEAEs monitored throughout the study and at a follow-up visit at the end of the study. TEAEs were assessed for intensity (mild, moderate, or severe) and relation to treatment and were summarized using Medical Dictionary for Regulatory Activities preferred terms.
Statistical analysis
Primary PK parameters AUC and Cmax of ethinylestradiol and levonorgestrel (assuming lognormally distributed data) were analyzed using analysis of variance (ANOVA), including sequence, subject (sequence) period, and treatment effects. On the basis of these analyses, point estimates (least squares means) and exploratory 90% confidence intervals (CIs) for the ratios “treatment B/treatment A” of ethinylestradiol and levonorgestrel were calculated by retransformation of the logarithmic data using the intraindividual standard deviation of the ANOVA.
Results
Study participants
Overall, 31 subjects were randomized. Before the start of the first treatment phase, two subjects were excluded because of an adverse event, and one subject withdrew from the study. Therefore, a total of 28 subjects received at least 1 dose of study drug and were included in the safety evaluation. An additional 4 subjects discontinued the study after randomization; 2 discontinued because of adverse events during the first treatment period, 1 withdrew consent, and 1 was withdrawn from the study because of noncompliance.
Twenty-four subjects completed both treatment periods and had valid PK profiles for ethinylestradiol and levonorgestrel and so were included in the PK evaluation. The demographic characteristics of the study population are shown in Table 1.
Table 1.
Demographic characteristics of the study population who were valid for the pharmacokinetic and safety analyses
| Characteristic | Pharmacokinetic analysis set (n = 24) |
Safety analysis set (n = 28) |
|---|---|---|
| Female sex | 24 (100) | 28 (100) |
| White | 24 (100) | 28 (100) |
| Age, years, mean (range) | 60.8 (54–65) | 60.1 (54–65) |
| Weight, kg, mean ± SD | 69.7 ± 9.2 | 70.3 ± 9.0 |
| Height, cm, mean ± SD | 161.9 ± 4.8 | 162.3 ± 4.7 |
| BMI, mean ± SD | 26.62 ± 3.41 | 26.71 ± 3.34 |
| Smoking history | ||
| Never smoker | 16 (66.7) | 19 (67.9) |
| Former smoker | 8 (33.3) | 9 (32.1) |
Data are no. (%) of patients, unless otherwise indicated. BMI: body mass index, (weight in kg)/(height in m)2; SD: standard deviation.
PK
The AUC of ethinylestradiol was comparable between administration of levonorgestrel-ethinylestradiol alone (treatment A) or with riociguat (treatment B). The estimated ratio for AUC between the 2 regimens was 101.83% (90% CI: 97.51%–106.34%; Fig. 1; Table 2). The Cmax of ethinylestradiol was increased by 20% with levonorgestrel-ethinylestradiol plus riociguat compared with levonorgestrel-ethinylestradiol alone (Fig. 1; Table 2), with an estimated ratio of 120.30% (90% CI: 111.35%–129.97%). AUC and Cmax of levonorgestrel were highly comparable between administration of levonorgestrel-ethinylestradiol alone or with riociguat (estimated ratio [90% CI]: 100.21% [96.75%–103.80%] and 104.53% [95.30%–114.64%] for AUC and Cmax, respectively; Fig. 1; Table 2). Plasma concentrations and exposures of riociguat and M1 were in the expected ranges and were not influenced by coadministration with levonorgestrel-ethinylestradiol.
Figure 1.
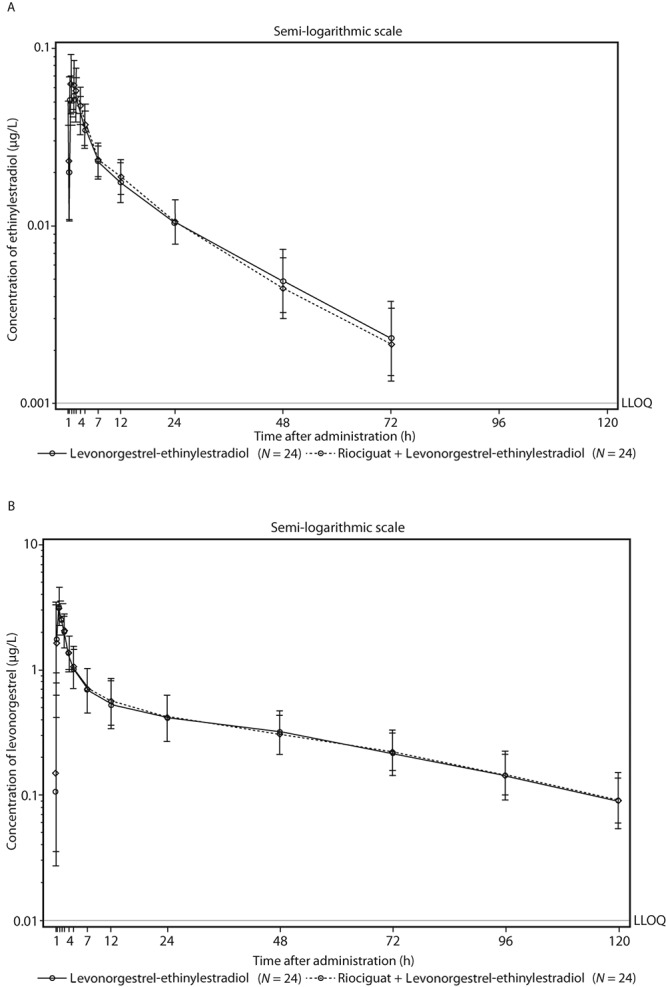
Plasma concentrations of ethinylestradiol (A) and levonorgestrel (B) after a single oral dose of levonorgestrel-ethinylestradiol (Microgynon; Bayer Pharma) with and without pretreatment and cotreatment with riociguat 2.5 mg 3 times daily (geometric means and geometric standard deviation, semilogarithmic scale; all subjects valid for pharmacokinetic analysis). LLOQ: lower limit of quantification.
Table 2.
Pharmacokinetic parameters of ethinylestradiol and levonorgestrel in plasma after single-dose administration of levonorgestrel-ethinylestradiol alone (treatment A) or with riociguat coadministration (treatment B) in the pharmacokinetic analysis set
| Ethinylestradiol | Levonorgestrel | |||
|---|---|---|---|---|
| Parameter | Levonorgestrel-ethinylestradiol alone | Levonorgestrel-ethinylestradiol plus riociguat | Levonorgestrel-ethinylestradiol alone | Levonorgestrel-ethinylestradiol plus riociguat |
| AUC, μg×h/L | ||||
| Geometric mean | 0.858 | 0.874 | 46.5 | 46.6 |
| %CV (range) | 30.1 (0.52–1.95) | 28.5 (0.57–1.63) | 40.1 (21.6–95.8) | 33.3 (26.6–106) |
| Cmax, μg/L | ||||
| Geometric mean | 0.0572 | 0.0688 | 3.26 | 3.41 |
| %CV (range) | 28.6 (0.04–0.102) | 30.6 (0.04–0.12) | 34.4 (1.8–5.3) | 29.7 (1.67–6.34) |
| tmax, hours, median (range) | 1.50 (1.00–3.00) | 1.00 (1.00–3.00) | 1.00 (1.00–1.50) | 1.00 (0.50–1.50) |
| t1/2, hours | ||||
| Geometric mean | 22.1 | 20.51 | 39.4 | 39.0 |
| %CV (range) | 22.1 (14.2–35.3) | 24.7 (12.2–34.2) | 23.5 (26.8–57.2) | 22.8 (26.5–63.3) |
AUC: area under plasma concentration–time curve; Cmax: maximum concentration; CV: coefficient of variation; tmax: time to Cmax; t1/2: terminal elimination half-life.
Safety and tolerability
A summary of TEAEs is presented in Table 3. TEAEs were of mild to moderate intensity, and none were rated severe. No serious adverse events or deaths occurred during the study.
Table 3.
Adverse events (AEs) in the safety analysis set
| AE | Levonorgestrel-ethinylestradiol alone (n = 27) |
Riociguat alone (n = 26) |
Levonorgestrel-ethinylestradiol plus riociguat (n = 25) |
|---|---|---|---|
| Any AE | 7 (25.9) | 22 (84.6) | 8 (32.0) |
| Any levonorgestrel-ethinylestradiol-related AE | 1 (3.7) | 0 | 2 (8.0) |
| Any riociguat-related AE | 1 (3.7)a | 21 (80.8) | 7 (28.0) |
| Most commonly reported levonorgestrel-ethinylestradiol-related AEs | |||
| Ocular discomfort | 1 (3.7) | 0 | 0 |
| Chills | 1 (3.7) | 0 | 0 |
| Headache | 1 (3.7) | 0 | 0 |
| Paresthesia | 1 (3.7) | 0 | 0 |
| Vaginal discharge | 0 | 0 | 1 (4.0) |
| Pruritus | 0 | 0 | 1 (4.0) |
| Most commonly reported riociguat-related AEs (reported by >1 subject) | |||
| Palpitations | 0 | 2 (7.7) | 0 |
| Abdominal distension | 0 | 4 (15.4) | 0 |
| Lower abdominal pain | 0 | 2 (7.7) | 0 |
| Dyspepsia | 1 (3.7)a | 14 (53.8) | 1 (4.0) |
| Nausea | 0 | 8 (30.8) | 1 (4.0) |
| Esophageal spasm | 0 | 2 (7.7) | 1 (4.0) |
| Vomiting | 0 | 6 (23.1) | 1 (4.0) |
| Pain in extremity | 0 | 3 (11.5) | 0 |
| Dizziness | 0 | 4 (15.4) | 0 |
| Headache | 0 | 14 (53.8) | 2 (8.0) |
| Restless leg syndrome | 0 | 3 (11.5) | 1 (4.0) |
| Somnolence | 0 | 4 (15.4) | 0 |
| Discontinuation of levonorgestrel-ethinylestradiol due to an AE | 1 (3.7)b | 1 (3.8)c | 0 |
| Discontinuation of riociguat due to an AE | 1 (3.7)b | 1 (3.8)c | 0 |
Data are no. (%) of patients.
Riociguat-related dyspepsia in treatment phase 2 (levonorgestrel-ethinylestradiol alone) was a reoccurrence of riociguat-related dyspepsia in treatment phase 1 (riociguat and levonorgestrel-ethinylestradiol).
One subject discontinued treatment with levonorgestrel-ethinylestradiol and riociguat due to AEs after a single dose of levonorgestrel-ethinylestradiol alone in treatment phase 1.
One subject discontinued treatment with levonorgestrel-ethinylestradiol and riociguat due to AEs after 2 doses of riociguat alone in treatment phase 1.
The tolerability of levonorgestrel-ethinylestradiol was not adversely affected when administered with riociguat; a similar frequency of levonorgestrel-ethinylestradiol-related TEAEs was observed in subjects receiving levonorgestrel-ethinylestradiol alone (26%) compared with levonorgestrel-ethinylestradiol in combination with ongoing treatment with riociguat 2.5 mg tid (32%). Similarly, the tolerability of riociguat was not affected by concomitant administration of levonorgestrel-ethinylestradiol. The most common riociguat-related TEAEs with levonorgestrel-ethinylestradiol plus riociguat treatment were headache, in 2 (8%) of the subjects, and dyspepsia, nausea, vomiting, restless leg syndrome, and esophageal spasm, each occurring in 1 (4%) of the subjects. The most common levonorgestrel-ethinylestradiol-related TEAEs in subjects receiving the combination were pruritus and vaginal discharge, each in 4% of subjects.
Two subjects discontinued treatment due to TEAEs. One subject withdrew after the first dose of levonorgestrel-ethinylestradiol was administered because of paresthesia of the chest and shivering, which were considered to be related to levonorgestrel-ethinylestradiol by the investigator. One subject withdrew after the second dose of riociguat was administered because of vomiting, which was not considered to be related to the study drug by the investigator.
Discussion
This study was undertaken to investigate the effect of riociguat 2.5 mg tid on the plasma concentration of and exposure to ethinylestradiol and levonorgestrel, as combined in the OC levornogestrel-ethinylestradiol, in healthy postmenopausal women. Our results have shown that riociguat 2.5 mg tid (the maximum recommended dose, administered so that steady state was reached) did not change the exposure to a combined OC containing levonorgestrel and ethinylestradiol (in terms of AUC) relative to OC administered alone.
When subjects received riociguat and then riociguat plus levonorgestrel-ethinylestradiol, there was no change in AUC of levonorgestrel and ethinylestradiol or Cmax of levonorgestrel compared with those seen with levonorgestrel-ethinylestradiol alone. A 20% increase in the Cmax of ethinylestradiol was noted when levonorgestrel-ethinylestradiol was coadministered with riociguat compared with levonorgestrel-ethinylestradiol alone. However, because this increase was smaller than the observed interindividual variability in the Cmax of ethinylestradiol—approximately 40%—the effect of riociguat is not anticipated to adversely impact contraceptive efficacy during coadministration.9 Plasma concentrations and exposures of riociguat were within the expected range and were not influenced by coadministration with levonorgestrel-ethinylestradiol.
The effects of other PAH-specific agents on OCs have been demonstrated in PK studies. Sildenafil increases the Cmax of ethinylestradiol, but no dose adjustment for coadministration with either levonorgestrel or ethinylestradiol is required.10 Tadalafil 40 mg at steady state increased ethinylestradiol exposure (AUC) by 26% and Cmax by 70% relative to OC administered with placebo but has no significant effect on levonorgestrel exposure.11 The prescribing information for tadalafil describes the consequences of the interaction with ethinylestradiol as uncertain and does not recommend dose adjustment. Bosentan is an inducer of CYP3A and CYP2C9; it reduces the levels of norethindrone and ethinylestradiol and has a black box warning for all hormonal contraceptives.12 An interaction study demonstrated that coadministration of bosentan and a combination oral hormonal contraceptive produced average decreases of norethindrone and ethinylestradiol levels of 14% and 31%, respectively. However, decreases in exposure were as much as 56% and 66%, respectively, in individual subjects.12 A recent study reported that macitentan has no significant PK interaction with a combined OC.13
The prescribing information for levonorgestrel-ethinylestradiol notes that interactions can occur with drugs that induce microsomal enzymes (especially CYP3A4), which can result in increased clearance of sex hormones.14 This may lead to breakthrough bleeding and/or contraceptive failure. The prescribing information also notes that strong and moderate CYP3A4 inhibitors can increase concentrations of the estrogen or progestin. Riociguat and M1 are neither inhibitors nor inducers of major CYP isoforms (including CYP3A4) in vitro at therapeutic plasma concentrations, as also shown in an earlier clinical interaction study with the sensitive CYP3A4 substrate midazolam, in which no change in exposure was observed.15 The lack of effect on CYP isoforms may explain the absence of a significant PK interaction in this study.
Riociguat exposure was not influenced by coadministration with levonorgestrel-ethinylestradiol. Combined treatment with riociguat as multiple doses of 2.5 mg tid over a 12-day period and single doses of levonorgestrel-ethinylestradiol (0.03 mg ethinylestradiol and 0.15 mg levonorgestrel) were safe and well tolerated. Concomitant administration of riociguat and levonorgestrel-ethinylestradiol did not adversely affect the safety and tolerability of any of the study drugs.
In conclusion, this study has shown that riociguat at steady state did not change the exposure to levonorgestrel or ethinylestradiol relative to OC administered alone. The combined regimen was safe and well tolerated.
Source of Support: This study was supported by Bayer Pharma. Editorial assistance was provided by Adelphi Communications, supported by Bayer Pharma.
Conflict of Interest: All authors are employees of Bayer Pharma.
References
- 1.Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, Simonneau G, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015;46(4):903–975. [DOI] [PubMed]
- 2.Bayer HealthCare Pharmaceuticals. Prescribing information [Adempas (riociguat) tablets]. Accessed June 27, 2014. http://labeling.bayerhealthcare.com/html/products/pi/Adempas_PI.pdf.
- 3.European Medicines Agency. Annex I: summary of product characteristics [Adempas (riociguat tablets)]. Published March 27, 2014. Accessed November 25, 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002737/WC500165034.pdf.
- 4.Ghofrani HA, Galiè N, Grimminger F, Grünig E, Humbert M, Jing ZC, Keogh AM, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013;369(4):330–340. [DOI] [PubMed]
- 5.Ghofrani HA, D’Armini AM, Grimminger F, Hoeper MM, Jansa P, Kim NH, Mayer E, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med 2013;369(4):319–329. [DOI] [PubMed]
- 6.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 2009;191:277–308. [DOI] [PubMed]
- 7.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123(20):2263–2273. [DOI] [PMC free article] [PubMed]
- 8.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol 2013;218:279–313. [DOI] [PubMed]
- 9.Bayer Pharma. Yasmin prescribing information. Revised May 2015. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021098s023lbl.pdf.
- 10.Pfizer. Revatio: US prescribing information. Revised March 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021845s011,022473s004,0203109s002lbl.pdf.
- 11.Eli Lilly. Adcirca prescribing information. Revised April 2015. http://www.drugs.com/pro/adcirca.html.
- 12.Actelion. Tracleer: highlights of prescribing information. Revised December 2015. https://www.tracleer.com/docs/Tracleer_Full_Prescribing_Information.pdf.
- 13.Hurst N, Pellek M, Dingemanse J, Sidharta PN. Lack of pharmacokinetic interactions between macitentan and a combined oral contraceptive in healthy female subjects. J Clin Pharmacol. doi:10.1002/jcph.639. [DOI] [PubMed]
- 14.Bayer PLC. Microgynon [summary of product characteristics]. Updated July 6, 2015. https://www.medicines.org.uk/emc/medicine/1827.
- 15.Becker C, Frey R, Unger S, Thomas D, Reber M, Wiemann G, Dietrich H, Arens ER, Mueck W. Pharmacokinetic interaction of ketoconazole, clarithromycin, and midazolam with riociguat. BMC Pharmacol Toxicol 2013;14(suppl. 1):P5. [DOI] [PMC free article] [PubMed]


