Abstract Abstract
Pulmonary arterial hypertension (PAH) is a known complication of rheumatologic diseases, but it is only rarely associated with adult-onset Still’s disease (AOSD). We describe the case of a 30-year-old woman who presented in a pulmonary hypertension crisis and was found to have underlying AOSD with PAH and nonspecific interstitial pneumonia (NSIP) with a course complicated by macrophage activation syndrome (MAS). She dramatically improved with steroids, cyclosporine A, and anakinra, with total resolution of the MAS and significant improvement of her pulmonary arterial pressures. While there are only select case reports of AOSD associated with PAH, this is the first reported case of (1) AOSD complicated by both PAH and MAS and (2) AOSD complicated by biopsy-proven NSIP. Clinically, this case highlights the efficacy of immunosuppressive agents in the treatment of PAH and MAS from underlying AOSD and supports their use in this setting.
Keywords: anakinra, pulmonary arterial hypertension, secondary hemophagocytic lymphohistiocytosis
Adult-onset Still’s disease (AOSD) is a rare multisystemic autoinflammatory disorder that affects young adults. It is characterized by major criteria of quotidian temperatures >39°C, arthralgias/arthritis, evanescent salmon-pink rash, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Laboratory features can include leukocytosis, abnormal liver function tests (LFTs), and negative antinuclear antibody and rheumatoid factor tests. Diagnosis requires the exclusion of other possible etiologies, including infection, malignancy, or other rheumatic diseases. Pulmonary and cardiac manifestations of AOSD most commonly include pleuritis, pericarditis with effusion, or, in some cases, transient pulmonary infiltrates. Case reports have reported acute respiratory distress syndrome, restrictive lung disease, and pulmonary arterial hypertension (PAH).1-7
PAH (World Health Organization [WHO] group 1) has been associated with several connective tissue diseases (CTDs), such as systemic sclerosis, mixed CTD, and systemic lupus erythematosus. CTD-associated PAH (CTD-PAH) may be biologically distinct from other forms of WHO group 1 PAH with respect to pathogenesis, prognosis, and response to therapy.8 WHO group 1 PAH is caused by an endothelial injury in a genetically predisposed individual, which leads to intimal proliferation and medial hypertrophy, causing luminal narrowing of the pulmonary vessels. Up-regulation of endothelin 1 and thromboxane A2 and down-regulation of prostacyclin and nitric oxide leads to pulmonary vessel vasoconstriction, while chronic hypoxia leads to further vasoconstriction and vascular remodeling.8 The role played by inflammation in the pathophysiology of PAH is suggested by the presence of perivascular inflammatory cells in remodeled vessels of human and animal models, including macrophages, dendritic cells, T and B lymphocytes, and mast cells. Further evidence includes associations between increased cytokine levels and severity of disease and improvements in CTD-PAH with immunosuppressive treatment.9 CTD-PAH has a mortality rate that is up to four times higher than that of idiopathic PAH (IPAH), with poorer responses to traditional therapies of prostanoids, endothelin receptor antagonists, and phosphodiesterase 5 (PDE-5) inhibitors.8 While outcomes of CTD-PAH vary on the basis of the specific CTD, patients with systemic sclerosis–associated PAH have demonstrated worse outcomes, including short-term survival rates.10 Although data on the treatment of CTD-PAH are limited, several studies have shown promise in the use of these traditional IPAH therapies to improve hemodynamics and exercise capacities in patients with systemic sclerosis–associated PAH.8,11-13 However, due to the worse prognosis of CTD-PAH, there may be a role for immunomodulatory agents in its treatment. Currently, the data for the use of immunosuppressive therapies for treatment of CTD-PAH are controversial. Small cohorts of patients with systemic lupus erythematosus and mixed CTD with PAH have shown improved outcomes with immunosuppressive therapy, although there is no evidence to support its use in patients with systemic sclerosis.14,15
Macrophage activation syndrome (MAS) is another serious complication of rheumatological disorders. It is considered a subtype of secondary hemophagocytic lymphohistiocytosis (HLH), a cluster of conditions characterized by an excessive and unregulated immune response with expansion of CD8+ T lymphocytes and hyperactive macrophages. MAS is a known but infrequent complication of several autoimmune diseases, most commonly associated with systemic juvenile idiopathic arthritis (sJIA) and AOSD, with an incidence of overt MAS in ∼10%16,17 of patients and of subclinical MAS in another 30%–40% of patients. Mortality rates ranging from 20% to 30% have been reported.17 The diagnosis is challenging but involves early recognition of suspicious signs and symptoms, including recurrent fever, progressive cytopenias, hyperferritinemia, hypofibrinogenemia, and elevated liver enzymes. While there is no established, validated treatment protocol, the typical regimen involves steroids with the addition of other agents in steroid-refractory disease.
We describe here a case of a 30-year-old woman with no known previous diagnosis who presented in a pulmonary hypertension (PH) crisis and was found to have AOSD with PAH and nonspecific interstitial pneumonia (NSIP), with a course complicated by MAS.
Case description
A 30-year-old woman of Pakistani descent was admitted to the cardiac intensive care unit as a transfer from an outside hospital for features of acute decompensated heart failure. The patient initially presented to an outside hospital with 3 weeks of progressive dyspnea. She reported an unusual medical history. She was well until 2009, when she was found to have a pericardial effusion and diffuse lymphadenopathy, with biopsy showing nonspecific follicular hyperplasia. Over the next few months, she began developing intermittent severe urticarial reactions to multiple foods and medications, and she was treated with steroids and hydroxyzine. She also began experiencing periods of days to weeks of quotidian fevers with associated rash, weakness, alopecia, and bilateral joint pain localized to her knees, wrists, and proximal interphalangeal joints. A complete rheumatologic workup was negative at that time. She was seen by an allergist and was treated with intravenous (IV) immunoglobulin.
A few months prior to presentation during an outpatient visit, she was found to be tachycardic with a heart rate of ∼150 bpm. An echocardiogram at this time was reportedly normal. She reported elevated transaminases, with aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels of ∼200 U/L. In the months prior to presentation, she saw an herbal specialist who recommended an unknown herbal cocktail that she took for 1 week, with no improvement in her symptoms.
She presented to the outside hospital with persistent dyspnea for several weeks. An echocardiogram showed right ventricular (RV) and right atrial dilatation, a mildly hypokinetic right side, severe tricuspid regurgitation, and a moderate pericardial effusion. Initial laboratory findings were notable for elevated D-dimer, lactic acidosis, elevated transaminases, elevated international normalized ratio (INR), and leukocytosis. Electrocardiogram showed sinus tachycardia, right axis deviation, and incomplete right bundle branch block. A computed tomography (CT) study of the chest showed a pericardial effusion, a small right pleural effusion, a small right lower lobe patchy infiltrate, and bilateral axillary, paratracheal, supraclavicular, and hilar lymphadenopathy, enlarged from a prior study. She was started on aztreonam and IV methylprednisolone. Her venous lactate continued to rise, peaking at 16.1 mmol/L. Her transaminases peaked at AST of 4,207 U/L and ALT of 11,836 U/L, and the leukocytosis peaked at 32.3 × 109/L. The patient was started on a milrinone drip and transferred to our cardiac care unit for further management.
On presentation to our medical center, she complained of continued dyspnea and lower extremity edema. On initial exam, she was afebrile, was mildly tachycardic (111 bpm), had blood pressure of 96/65 mmHg, and was tachypneic (25 breaths/min), with oxygen saturation of 97% on supplemental oxygen by nasal cannula. Exam was notable for jugular venous distension to the mandible, a III/VI systolic murmur heard loudest at the left lower sternal border, a loud pulmonary component of the second heart sound, and an S3 gallop. Pulmonary exam was significant for decreased breath sounds bilaterally at the bases and inspiratory crackles. Abdominal exam showed hepatomegaly. Her lower extremities had trace pitting edema. She had a palpable nontender, mobile left axillary lymph node. Her laboratory findings on admission were notable for a leukocytosis and elevated transaminases, creatinine, ferritin, and INR. D-dimer was >20 μg/mL (normal range: <0.5 μg/mL). A thrombophilia workup, including factor V, factor VII, factor VIII, and anti-cardiolipin antibodies, was unremarkable. CT angiogram and ventilation-perfusion scan were negative for thromboembolism. CT of the chest showed mild pulmonary edema with septal thickening, enlarged pulmonary artery, bilateral pleural effusions, scattered ground-glass opacities, and mediastinal and axillary lymphadenopathy.
Transthoracic echocardiogram showed severely increased right atrial and RV size, flattening of the interventricular septum throughout the cardiac cycle, and severely reduced RV function. Right heart catheterization (RHC) showed right atrial pressure of 28/33 (25) mmHg, pulmonary arterial pressure (PAP) of 59/31 (42) mmHg, pulmonary capillary wedge pressure (PCWP) of 2 mmHg, pulmonary arterial saturation of 27%, and pulmonary vascular resistance (PVR) of 1,142 dyn-s/cm5. Fick cardiac output was calculated to be 2.80 L/min (cardiac index: 1.6 L/min/m), and transpulmonary gradient was calculated to be 40 mmHg.
The working diagnosis on presentation was IPAH with severe RV failure leading to cardiogenic shock. The patient was started on vasopressin (0.04 U/L) for blood pressure support, milrinone and digoxin (0.125 mg) for RV support, and epoprostenol (2 ng/kg/min) and inhaled nitric oxide (5 ppm) for confirmed PH.
On nitric oxide, PVR decreased to 941 dyn-s/cm5, and mean PAP decreased to 35 mmHg. Transaminitis and creatinine levels improved significantly. Following this initial improvement, she showed a variable response to epoprostenol titration. Repeat RHC 1 week after hospitalization showed PAPs of 81/38 (52) mmHg and PCWP of 9 mmHg. She also developed persistent tachycardia and fevers, along with persistent pericardial effusion with respirophasic variation on echocardiogram.
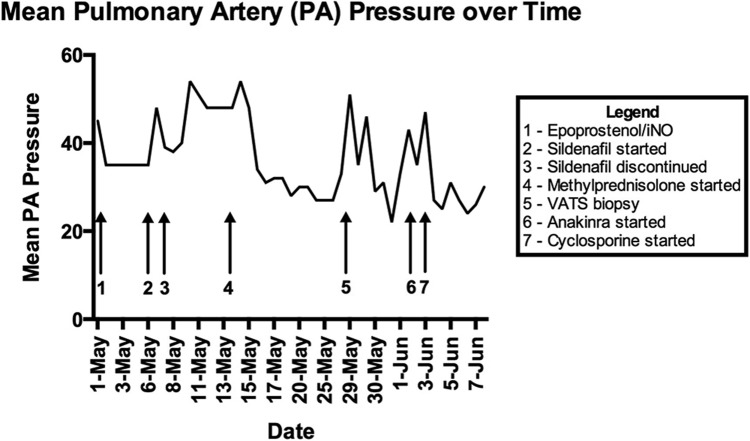
Given concern for an underlying unidentified rheumatologic condition or malignancy, she was started on methylprednisolone (60 mg IV daily) on hospital day 14. Bone marrow biopsy was normocellular, without evidence of malignancy or hemophagocytosis. A lymph node biopsy was performed that showed nonspecific, reactive inflammatory changes, with no evidence of a malignant process. After 2 days of methylprednisolone therapy, her PAPs (measured via pulmonary artery catheter) stabilized in the low 30s for 12 days (Fig. 1). At this point, there was significant concern for pulmonary venoocclusive disease, given CT findings and poor response to pulmonary vasodilators. To confirm this and make management decisions regarding pulmonary vasodilator therapy and the possibility of lung transplant, she underwent video-assisted thorascopic surgery (VATS) lung biopsy. She simultaneously underwent a diagnostic and therapeutic pericardial window, given reaccumulation of significant pericardial effusion and concern for tamponade.
Figure 1.
Timeline of mean pulmonary arterial pressures. Shown is a graphical representation of the patient’s mean pulmonary arterial pressures (mmHg) over time, measured by pulmonary artery catheter. Arrows indicate major events and changes in treatment.
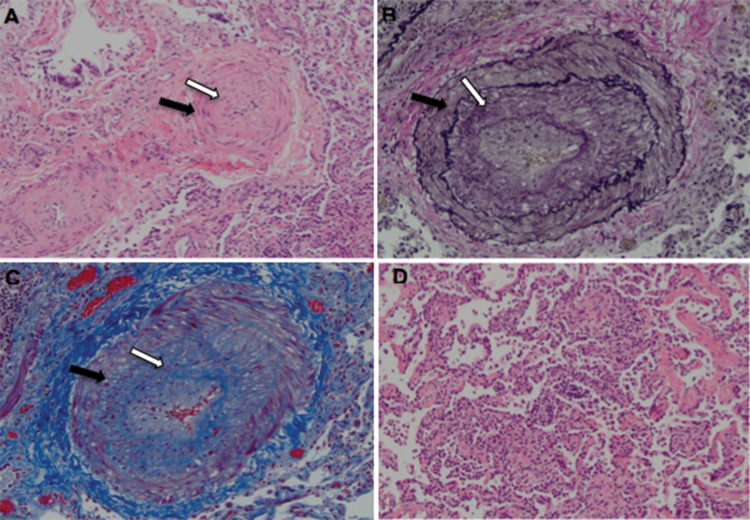
The lung biopsy findings were consistent with severe PAH with prominent medial smooth muscle hypertrophy and intimal fibroplasia of the small pulmonary artery branches and arterioles (Fig. 2). Surrounding lung parenchyma showed patchy but scarce NSIP, with moderate T cell inflammatory infiltrates. No interstitial fibrosis was seen. Venous thrombi were absent. Pericardial biopsy showed inflammatory infiltrates, and pericardial fluid showed no malignant cells.
Figure 2.
Lung biopsy demonstrating pulmonary arterial hypertension and patchy nonspecific interstitial pneumonia. A, Hematoxylin-eosin stain (×200) showing a nearly occluded pulmonary artery branch with smooth muscle hypertrophy (black arrow) and intimal proliferation (white arrow). B, Elastin stain (×200) showing widening of the space between the internal and external elastica by smooth muscle hypertrophy (black arrow) as well as intimal proliferation (white arrow). C, Trichrome stain (×200) showing red smooth muscle hypertrophy (black arrow) and blue fibrous tissue in the intima (white arrow). D, Hematoxylin-eosin stain (×200) showing a segment of interstitial inflammation. No interstitial fibrosis was seen, and no venous thrombi or plexiform lesions were seen.
The VATS procedure was complicated by a PH crisis, with elevated PAPs, RV failure, shock, and temperature to 40.6°C. She required significant inotropic support as well as inhaled nitric oxide, epoprostenol, and an elevated dose of methylprednisolone (500 mg IV daily), which stabilized her PAPs. In the days immediately following the procedure, she developed quotidian fevers with associated bilateral, symmetric joint pain and minimal swelling in her knees, wrists, and proximal interphalangeal joints as well as sore throat and malaise. After an initial postbiopsy leukocytosis (18.7 × 109/L), she developed transaminitis and pancytopenia, with a concurrent drop in fibrinogen and rise in ferritin (Table 1). Given the constellation of findings concerning for MAS, an interleukin 2 (IL-2) receptor level was ordered and later found to be markedly elevated at 27,600 IU/mL.
Table 1.
Pertinent laboratory values before and after video-assisted thorascopic surgery
| Before biopsy | After biopsy | |
|---|---|---|
| AST, U/L | 34 | 938 |
| ALT, U/L | 35 | 895 |
| WBC count, ×109/L | 6.9 | 0.5 |
| Hemoglobin, g/dL | 9.6 | 6.9 |
| Platelets, ×109/L | 188 | 28 |
| Fibrinogen, mg/dL | 492 | 138 |
| Ferritin, ng/L | 212 | 16,829 |
| CRP, mg/L | 65 | 106 |
| ESR, mm/hr | 17 | 10 |
| IL-2a | … | 27,600 |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; WBC: white blood cell.
Interleukin 2 (IL-2) receptor level.
Given the high concern for MAS, the patient was started on the IL-1 receptor antagonist anakinra (100-mg subcutaneous injection twice per day) and continuous cyclosporine infusion; methylprednisolone dosage was adjusted to 80 mg IV daily. Over the following days, her laboratory values began to normalize. Her platelets, LFTs, ferritin, fibrinogen, and C-reactive protein (CRP) normalized. Her hemoglobin stabilized after transfusion. Her white blood cell count stabilized, but she remained leukopenic (ranging from 0.3 to 0.9 × 109/L), and she responded appropriately to three doses of filgastrim.
The patient was started on iloprost (5 μg of nebulized solution every 3 hours) and ambrisentan (5 mg daily) to wean off nitric oxide and epoprostenol. During this time, she was maintained on cyclosporine, anakinra, and methylprednisolone for systemic inflammation and MAS. She was successfully weaned off both the inhaled epoprostenol and the nitric oxide as well as the high-flow nasal cannula. Repeat echocardiograms showed RV systolic pressures stable at 45 mmHg with systolic function mild to moderately reduced, which was improved from prior. She was transitioned from inhaled iloprost to inhaled treprostinil and discharged on a regimen that included treprostinil, ambrisentan, cyclosporine, anakinra, and prednisone. On follow-up visit 1 month after discharge, her PAPs remained stable. She no longer required the use of oxygen, with saturation levels of 98% on room air.
Discussion
We have described a patient with AOSD who developed severe PH and NSIP years after the initial onset of undiagnosed AOSD, with a course complicated by MAS. Other etiologies of PH, including pulmonary venoocclusive disease, were considered but were ruled out with biopsy and imaging, including echocardiogram, ventilation-perfusion scan, and CT angiogram. The elevated D-dimer in the absence of pulmonary embolism was attributed to systemic inflammation from flare of AOSD.
Suspicion for AOSD was first raised during this hospitalization on the basis of her medical history of recurrent episodes of daily fevers with associated joint pain and rash over days to weeks. Her underlying illness eventually became apparent after her VATS biopsy when she became most symptomatic. While multiple different sets of diagnostic criteria have been proposed for AOSD, the most validated and most commonly used set is the Yamaguchi criteria18 (Table 2), found to have a sensitivity of 96.2% and a specificity of 92.1%.18 In a comparison among six different classification criteria, the Yamaguchi criteria were found to be the most sensitive for AOSD.19 The Yamaguchi criteria require a patient to meet five criteria, with at least two major criteria. This patient met three major criteria (fever, arthralgia, and leukocytosis) and four minor criteria (sore throat, lymphadenopathy, LFT abnormality, and negative antinuclear antibody test), leading to a likely diagnosis of AOSD. Furthermore, the presence of MAS is strongly suggestive of this diagnosis, given that it is most strongly associated with sJIA and AOSD among rheumatologic disorders.17
Table 2.
Yamaguchi criteria for diagnosis of adult-onset Still’s disease16
| Major criteria | Minor criteria |
|---|---|
| Temperature ≥39°C, lasting ≥1 week | Sore throat |
| Arthralgias, lasting ≥2 weeks | Lymphadenopathy |
| Typical rash | Hepato- or splenomegaly |
| Leukocytosis (≥10,000/μL), ≥80% granulocytes | Abnormal LFTs (AST/ALT/LDH) |
| Negative ANA and RF tests |
ALT: alanine aminotransferase; ANA: antinuclear antibody; AST: aspartate aminotransferase; LDH: lactate dehydrogenase; LFT: liver function test; RF: rheumatoid factor.
To our knowledge, there are only five reported cases of AOSD associated with PH. These cases were all classified as WHO group 1 PAH and occurred after a known diagnosis of AOSD. The first case was reported in 1990, describing a woman in Japan who, 2 years after the onset of AOSD, developed PH without any associated restrictive or obstructive lung disease.7 In another case, a 29-year-old woman with a 9-year history of AOSD developed severe PH with a good response to vasodilator infusion but died 2 months later of RV failure. A lung biopsy did not show any parenchymal involvement.5 A case report in India described an 18-year-old Indian woman who presented with severe PH 2 weeks after diagnosis of AOSD with no lung biopsy performed.6 Another case reported was that of a 27-year-old Middle Eastern woman who developed PAH after 7 years of AOSD refractory to glucocorticoids. Her PAH initially improved with amlodipine and completely resolved with the use of the IL-1 antagonist anakinra.3 The most recent case report is of a 38-year-old African American woman with diagnosed AOSD with PAH that responded rapidly to an IL-6 antagonist, tocilizumab.4 We are not aware of any reported cases of AOSD complicated by both biopsy-confirmed NSIP and WHO group 1 PAH.
This patient’s lung biopsy demonstrated grade 2 PAH as well as patchy NSIP (Fig. 2). Other causes of interstitial lung disease (ILD)—specifically NSIP—were ruled out, including recurrent aspiration, HIV infection, drug toxicity, and heart failure. Histopathological findings for AOSD are not well documented, given its low prevalence and generally benign prognosis. However, a review of reports of the pathological changes in cases of AOSD showed an association with interstitial inflammation and fibrosis of visceral organs, including the lungs.20 AOSD has not yet been reported to be associated with NSIP specifically, although NSIP is known to be the most common pathological pattern of ILD in CTD other than rheumatoid arthritis.21
When a patient with CTD presents with both ILD and PH, it may be challenging to determine the appropriate classification of WHO group 1 or 3 PH with significant biologic overlap between inflammatory lung and vascular pathologies. In some patients, CTD can cause ILD, which can then lead to hypoxic pulmonary vasoconstriction and subsequent PH. However, patients may alternatively have separate, concurrent processes of both PAH and ILD. Many would consider such patients as WHO group 1/3 overlap or define the disease simply as precapillary PH with active inflammatory disease and marked responsiveness to O2. Given the biopsy findings of scarce, patchy interstitial inflammation with no interstitial fibrosis, we are confident that the degree of PAH is disproportionate to the severity of the ILD and thus is not adequately explained by it. Using the current nomenclature, our patient is best described as having WHO group 1 PAH associated with AOSD.
Most impressive in this case was the dramatic response to immune-modulating agents, further suggesting WHO group 1 CTD-PAH. While the PH showed a variable response to standard treatments of PH (prostacyclin analogs, endothelin-receptor antagonists, and PDE-5 inhibitors), she demonstrated a marked improvement in her PAPs with the addition of steroids, cyclosporine, and anakinra. She remained stable with the addition of these immune-modulating agents, suggesting effective treatment of the inflammatory component of her PH. The patient was effectively weaned off nitric oxide and IV epoprostenol with successful treatment of the underlying autoimmune-mediated systemic inflammation as well as the addition of an endothelin receptor antagonist (ambristentan) and inhaled prostanoid (iloprost).
This patient’s course was further complicated by MAS. The pathogenesis of MAS is not fully understood but is thought to involve a prolonged and hyperactive immune response with an unregulated expansion of CD8+ T cells and macrophages and a subsequent cytokine storm.22 While the syndrome is often triggered by a viral infection, MAS is also known to occur with episodes of particularly active disease with changes in therapy or as an adverse effect of medications.16,17,22 We believe that following the VATS biopsy, this patient developed a systemic inflammatory response and a subsequent episode of high disease activity of the underlying AOSD, which then likely triggered the MAS and a PH crisis.
Given its high mortality, MAS requires prompt recognition and treatment. Patients typically present with fevers, progressive cytopenias, hyperferritinemia, transaminitis, neurological symptoms, elevated CRP, and a normal erythrocyte sedimentation rate. It is well established that hemophagocytosis on bone marrow biopsy is neither sensitive nor specific and thus is not diagnostic.23 The diagnosis of MAS can be challenging, as the signs and symptoms of MAS can be very similar to that of systemic inflammation, sepsis, or the underlying autoimmune disease process itself. Because of this difficulty, efforts have been made to establish criteria specifically for MAS (Table 3). The Ravelli criteria24 use clinical and laboratory features for diagnosis and were found to be more capable of identifying MAS than the previously used HLH-2004 criteria.25 An alternative diagnostic criteria was recently proposed for detection of early MAS using only laboratory values, given that the presence of clinical symptoms is often delayed.26 Additionally, a multinational effort by the MAS Study Group is in progress to establish new diagnostic criteria.16,27
Table 3.
Proposed criteria for diagnosing macrophage activation syndrome
| HLH-2004 criteria32 | Ravelli et al.24 | Kostik et al.26 |
|---|---|---|
| Either criterion 1 or 2 fulfilled | ≥2 laboratory criteria or ≥2 clinical and/or laboratory criteria | ≥3 laboratory criteria |
| 1. Molecular diagnosis consistent with primary HLH | Laboratory criteria: | • Low platelet count (≤211 × 109/L) |
| 2. At least 5 of 8 diagnostic criteria fulfilled | • Low platelet count (<262 × 109/L) | • Low WBC count (≤9.9 × 109/L) |
| • Persistent fever (>38.5°C) for at least 7 days | • Elevated AST (>59 U/L) | • Elevated AST (>59.7 U/L) |
| • Splenomegaly | • Low WBC count (≤4.0 × 109/L) | • Elevated LDH (>882 U/L) |
| • Cytopenias of ≥2 of 3 cell lines (hemoglobin <9.0 g/dL, platelets <100,000/μL, neutrophils <1,000/μL) | • Low fibrinogen (≤2.5 g/L) | • Low albumin (≤2.9 g/dL) |
| • Hypertrigyceridemia (fasting triglycerides ≥160mg/dL) or low fibrinogen (≤150 mg/dL) | Clinical criteria: | • Elevated ferritin (>400 μg/L) |
| • Serum ferritin ≥500 μg/L | • Central nervous system dysfunction (i.e., headache, seizures, coma, irritability, lethargy) | • Low fibrinogen (≤1.8 g/L) |
| • Low or absent NK cell activity | • Hemorrhages (mucosal bleeding, bruising, purpura) | • Proteinuria |
| • Elevated serum sIL-2R >2,400 IU/mL | • Hepatomegaly (≥3 cm below costal margin) | |
| • Hemophagocytosis in bone marrow, spleen, or lymph nodes |
AST: aspartate aminotransferase; HLH: hemophagocytic lymphohistiocytosis; LDH: lactate dehydrogenase; NK: natural killer; sIL-2R: soluble interleukin 2 receptor; WBC: white blood cell.
This patient’s presentation fulfilled all the proposed criteria for diagnosing MAS. Given the high clinical suspicion and the rapidly evolving course while already on methylprednisolone, cyclosporine A and anakinra were added prior to obtaining the soluble IL-2 receptor value. While there is no established protocol for MAS, treatment typically involves pulse IV methylprednisolone (30 mg/kg for 3 days) followed by 2–3 mg/kg/day in two to four divided doses.17 Other therapies can be added if steroids are inadequate. Cyclosporine A (2–7 mg/kg/day) is the typical second-line agent used in conjunction with methylprednisolone, as it is known to rapidly control MAS and avoid the excessive use of steroids.17 If this combination is insufficient, less well-established agents can be added. There is growing evidence that anakinra, an IL-1 receptor antagonist, can be used to control MAS after an inadequate response to steroids and cyclosporine, with several case studies documenting response.28-31 This patient drastically improved with the addition of anakinra to steroids and cyclosporine A, with liver enzymes, cell counts, ferritin, and inflammatory markers returning to normal levels. This case emphasizes the importance of maintaining a high clinical suspicion for MAS and initiating treatment early, even before all diagnostic values have been obtained. Furthermore, this case demonstrates the efficacy of the combination of methylprednisolone, cyclosporine A, and anakinra in controlling MAS and PAH in a patient with underlying AOSD.
Conclusion
In conclusion, we report the first case of AOSD complicated by both PAH and MAS and the second reported case of anakinra-responsive PAH-AOSD. Furthermore, this is the first reported case of AOSD complicated by biopsy-proven NSIP. We believe that the etiology of this patient’s acute decompensation with PH crisis and MAS was likely an exaggerated inflammatory response from the VATS biopsy, given the strong temporal relationship and negative extensive infectious workup. We would like to highlight that, in this case, both the PAH and the MAS resolved with a regimen of methylprednisolone, cyclosporine A, and anakinra, in combination with pulmonary vasodilators. We believe that these immune-modulating agents dampened a hyperactive generalized immune response, which manifested as both a PH crisis and MAS. We agree with earlier literature that PAH should be considered a rare but increasingly recognized pulmonary manifestation of AOSD and that clinicians should consider screening for this complication as appropriate. We also suggest that immunosuppressive agents, such as cyclosporine A and anakinra in addition to steroids, may play a role in the treatment of PAH-AOSD as well as MAS.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Corbett AJ, Zizic TM, Stevens MB. Adult-onset Still’s disease with an associated severe restrictive pulmonary defect: a case report. Ann Rheum Dis 1983;42:452–454. [DOI] [PMC free article] [PubMed]
- 2.Hirohata S, Kamoshita H, Taketani T, Maeda S. Adult Still’s disease complicated with adult respiratory distress. Arch Intern Med 1986;146:2409–2410. [PubMed]
- 3.Campos M, Schiopu E. Pulmonary arterial hypertension in adult-onset Still’s disease: rapid response to anakinra. Case Rep Rheumatol 2012;2012:537613. [DOI] [PMC free article] [PubMed]
- 4.Kadavath S, Zapantis E, Zolty R, Efthimiou P. A novel therapeutic approach in pulmonary arterial hypertension as a complication of adult-onset Still’s disease: targeting IL-6. Int J Rheum Dis 2014;17:336–340. [DOI] [PubMed]
- 5.Mubashir E, Ahmed MM, Hayat S, Heldmann M, Berney SM. Pulmonary hypertension in a patient with adult-onset Stills disease. Clin Rheumatol 2007;26:1359–1361. [DOI] [PubMed]
- 6.Thakare M, Habibi S, Agrawal S, Narsimulu G. Pulmonary arterial hypertension complicating adult-onset Still’s disease. Clin Rheumatol 2013;32(suppl. 1):S1–S2. [DOI] [PubMed]
- 7.Zen A, Yamashita N, Ueda M, et al. A case of adult Still’s disease with pulmonary hypertension [in Japanese]. Ryumachi 1990;30:45–52. [PubMed]
- 8.Shahane A. Pulmonary hypertension in rheumatic diseases: epidemiology and pathogenesis. Rheumatol Int 2013;33:1655–1667. [DOI] [PubMed]
- 9.Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest 2012;141:210–221. [DOI] [PubMed]
- 10.Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease–associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest 2010;138:1383–1394. [DOI] [PMC free article] [PubMed]
- 11.Fagan KA, Badesch DB. Pulmonary hypertension associated with connective tissue disease. Prog Cardiovasc Dis 2002;45:225–234. [DOI] [PubMed]
- 12.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903. [DOI] [PubMed]
- 13.Mittoo S, Jacob T, Craig A, Bshouty Z. Treatment of pulmonary hypertension in patients with connective tissue disease and interstitial lung disease. Can Respir J 2010;17:282–286. [DOI] [PMC free article] [PubMed]
- 14.Sanchez O, Sitbon O, Jaïs X, Simonneau G, Humbert M. Immunosuppressive therapy in connective tissue diseases–associated pulmonary arterial hypertension. Chest 2006;130:182–189. [DOI] [PubMed]
- 15.Tanaka E, Harigai M, Tanaka M, Kawaguchi Y, Hara M, Kamatani N. Pulmonary hypertension in systemic lupus erythematosus: evaluation of clinical characteristics and response to immunosuppressive treatment. J Rheumatol 2002;29:282–287. [PubMed]
- 16.Minoia F, Davi S, Horne A, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol 2014;66:3160–3169. [DOI] [PubMed]
- 17.Schulert GS, Grom AA. Macrophage activation syndrome and cytokine-directed therapies. Best Pract Res Clin Rheumatol 2014;28:277–292. [DOI] [PMC free article] [PubMed]
- 18.Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol 1992;19:424–430. [PubMed]
- 19.Masson C, Le Loet X, Liote F, et al. Comparative study of 6 types of criteria in adult Still’s disease. J Rheumatol 1996;23:495–497. [PubMed]
- 20.Zhao DB, Dai SM, Liu XP, Xu H. Interstitial inflammation in visceral organs is a pathologic feature of adult-onset Still’s disease. Rheumatol Int 2011;31:923–927. [DOI] [PubMed]
- 21.Gutsche M, Rosen GD, Swigris JJ. Connective tissue disease–associated interstitial lung disease: a review. Curr Respir Care Rep 2012;1:224–232. [DOI] [PMC free article] [PubMed]
- 22.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med 2015;66:145–159. [DOI] [PMC free article] [PubMed]
- 23.Gupta A, Weitzman S, Abdelhaleem M. The role of hemophagocytosis in bone marrow aspirates in the diagnosis of hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2008;50:192–194. [DOI] [PubMed]
- 24.Ravelli A, Magni-Manzoni S, Pistorio A, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr 2005;146:598–604. [DOI] [PubMed]
- 25.Davi S, Minoia F, Pistorio A, et al. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol 2014;66:2871–2880. [DOI] [PubMed]
- 26.Kostik MM, Dubko MF, Masalova VV, et al. Identification of the best cutoff points and clinical signs specific for early recognition of macrophage activation syndrome in active systemic juvenile idiopathic arthritis. Semin Arthritis Rheum 2015;44:417–422. [DOI] [PubMed]
- 27.Davi S, Consolaro A, Guseinova D, et al. An international consensus survey of diagnostic criteria for macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol 2011;38:764–768. [DOI] [PubMed]
- 28.Bruck N, Suttorp M, Kabus M, Heubner G, Gahr M, Pessler F. Rapid and sustained remission of systemic juvenile idiopathic arthritis–associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J Clin Rheumatol 2011;17:23–27. [DOI] [PubMed]
- 29.Durand M, Troyanov Y, Laflamme P, Gregoire G. Macrophage activation syndrome treated with anakinra. J Rheumatol 2010;37:879–880. [DOI] [PubMed]
- 30.Kelly A, Ramanan AV. A case of macrophage activation syndrome successfully treated with anakinra. Nat Clin Pract Rheumatol 2008;4:615–620. [DOI] [PubMed]
- 31.Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease–associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford) 2011;50:417–419. [DOI] [PubMed]
- 32.Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–131. [DOI] [PubMed]